Abstract
Objective
To evaluate hospital-level variability in resource utilization and mortality in children with new leukemia who require intensive care unit (ICU) support, and identify factors associated with variation.
Design
Retrospective cohort study.
Setting
Children’s hospitals contributing to the Pediatric Health Information Systems administrative database from 1999–2011.
Patients
Inpatients <25 years old with newly diagnosed acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML) requiring ICU support (n=1,754).
Interventions, Measurements, and Main Results
Evaluated exposures included leukemia type, year of diagnosis, and hospital-wide proportion of patients with public insurance. The main outcome was hospital mortality. Wide variability existed in the ICU resources used across hospitals. Combined ALL and AML mortality varied by hospital from 0% (95% CI 0–14.8%) to 42.9% (95% CI 17.7–71.1%). A mixed effects model with a hospital-level random effect suggests significant variation across hospitals in mortality (P=0.007). When including patient and hospital factors as fixed effects into the model, younger age, AML vs. ALL diagnosis, leukemia diagnosis prior to 2005, hospital-wide proportion of public insurance patients, and hospital-level proportion of leukemia patients receiving ICU care are significantly associated with mortality. The variation across hospitals remains significant with all patient factors included (P=0.021), but is no longer significant after adjusting for the hospital-level factors proportion of public insurance and proportion receiving ICU care (P=0.48).
Conclusions
Wide hospital-level variability in ICU resource utilization and mortality exists in the care of children with leukemia requiring ICU support. Hospital payer mix is associated with some mortality variability. Additional study into how ICU support could be standardized through clinical practice guidelines, impact of payer mix on hospital resources allocation to the ICU, and subsequent impact on patient outcomes is warranted.
Keywords: Intensive care units, hospital mortality, lymphoid leukemia, myeloid leukemia, child, resource allocation
INTRODUCTION
Leukemia accounts for one third of childhood cancers, with an incidence of 4.4 per 100,000 children age 0–19.(1) Outcomes for children with leukemia continue to improve,(2, 3) in part due to standardized cancer treatment protocols.(4) Despite improvements in outcomes, due to the high incidence, childhood leukemia remains a leading causing of death from childhood cancer.(1)
Outcomes for critically ill children with leukemia requiring intensive care unit (ICU) support have also improved, with recently reported ICU mortality rates of 25–30% for children with leukemia or other cancers and respiratory failure.(5–7) However, single center studies, limited by variability in study design, inclusion criteria, and insufficient sample sizes, have reported a wide range of mortality rates for children with leukemia who require ICU support.(8–11) It is not known if this outcome variability across centers is borne out when examining a large national dataset, and whether factors such as differences in resource utilization, patient factors, or hospital factors contribute to such variability.
Wide variability in resource utilization and outcomes is a persistent theme across childhood diseases.(12–17) Several studies have used large pediatric hospital databases to study variation in outcome across hospitals for specific diseases and general pediatric populations, standardizing to patient mix and hospital-level variables. Identified hospital and therapeutic factors contributing to variability in outcomes in these populations include Medicaid insurance status, nursing staffing ratios,(18) use of clinical practice guidelines,(15, 19) and therapeutic and resource utilization variability.(17, 20) Use of clinical practice guidelines can decrease organ dysfunction and length of stay in pediatric septic shock,(21–23) demonstrating the potential benefit of standardizing hospital care for specific conditions. Children with leukemia represent a high-risk population with frequent need for ICU care, and variability in ICU resource use in this population across centers is not well described. Limited data exist on how much mortality in critically ill children with leukemia truly varies across hospitals, whether ICU resource utilization varies with mortality rates across hospitals, and factors contributing to variability in mortality. Understanding this variability and factors contributing to it could provide a step toward development of clinical practice guidelines to help standardize care, potentially resulting in further incremental improvement in outcomes for children with leukemia. We evaluated the ICU resource utilization and hospital mortality of children with newly diagnosed acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML) who required ICU support in a large nationally representative sample of tertiary children’s hospitals in the United States. We hypothesized that there would be large variability in the ICU resource utilization and mortality by center among children with leukemia receiving ICU care, and that patient-level and hospital-level factors would contribute to this variability.
MATERIALS AND METHODS
Study Design and Setting
A retrospective cohort study was used with data from the Pediatric Health Information System (PHIS) database, an administrative dataset containing data for over 18 million inpatient encounters from 45 tertiary children’s hospitals affiliated with the Children’s Hospital Association (CHA, Overland Park, Kansas). The PHIS database contains patient demographic information, admission and discharge dates, International Classification of Diseases-9-Clinical Modification (ICD-9-CM) diagnosis and procedure codes, financial and resource utilization data (pharmacy, imaging, and clinical services), and discharge disposition. Data quality oversight of the database has been described. (24) Forty-two centers had usable data on patients that contributed to the study cohort. The Institutional Review Board at The Children’s Hospital of Philadelphia approved the proposed research and waived informed consent.
Study Population
The study cohort included patients < 25 years old (at the time of index admission) with newly diagnosed ALL or AML defined by ICD-9-CM codes and chemotherapy review (5, 25) hospitalized in 42 PHIS-contributing hospitals between January 1, 1999 and September 30, 2011 for ALL patients or December 31, 2011 for AML patients. Patients were excluded if hospital data were deemed invalid or incomplete by PHIS administrative standards. All included patients required ICU support defined by ICU resources used during the follow-up period (Supplemental Digital Content Table) in order to exclude any in-hospital, non-ICU deaths from the analysis, as these patients likely represent a very different population of patients with refractory leukemia receiving palliative care. To limit the cohort to newly diagnosed leukemia, and thus a cohort with similar baseline risk profile, patients who received hematopoietic cell transplant (HCT) within 60 days of the index admission date were excluded, as this typically only occurs in the secondary leukemia or relapsed setting.(25, 26) Patients entered the cohort on the first day of the index admission and were followed through all admissions that started within 9 months of the index admission. As standard upfront AML therapy typically lasts 6–9 months, a 9-month study period was chosen to capture all courses of AML therapy. ALL therapy lasts up to 3.5 years, with the most intensive treatment cycles typically occurring in the first 9 months after diagnosis. For patients receiving HCT > 90 days from the index admission, data were censored at the time of the HCT admission.(5)
Patient Variables
Demographic data including age, gender, race, and insurance status were ascertained for each patient at the first day of the index admission. Age on admission (in years) was analyzed both as a continuous and a categorical variable (<1 year, 1–5 years, 5–10 years, 10–15 years, and >15 years). The number of ICU days for each patient was defined as any day during the study period when there was a code for use of an ICU resource. A patient-level variable to indicate severity of illness at leukemia presentation was defined as receipt of ICU care in the first three days of index admission.(27)
Hospital-level Variables
An association between hospital-wide proportion of patients with public insurance and ALL induction mortality has previously been reported as a hospital factor contributing to center variability in mortality.(28) Thus, the proportion of all patients with public insurance cared for at each hospital was calculated and considered as a hospital-level variable. Hospital-level ICU volume and experience with critically ill leukemia patients was evaluated with the hospital-level variable proportion of leukemia patients receiving ICU care, defined as the proportion of newly diagnosed ALL or AML patients receiving ICU care in the follow-up period at each hospital.
Outcome Measures
ICU resources evaluated included vasoactive infusions (composite variable of days of dopamine, intravenous epinephrine, norepinephrine, dobutamine, and milrinone), invasive mechanical ventilation (composite variable of invasive mechanical ventilation and high frequency ventilation), non-invasive mechanical ventilation, inhaled nitric oxide, hemodialysis, extracorporeal membrane oxygenation, cardiopulmonary resuscitation, and chest radiography. Inpatient mortality during the study follow-up period was determined.
Statistical Analysis
Patient demographics were summarized by descriptive statistics. The number of ICU days per patient was summarized by median and interquartile range (IQR). Rates of ICU days were calculated in two ways: number of ICU days per 100 days spent hospitalized as an inpatient during the study follow-up period (hospital days), and number of ICU days per 100 total days in the study follow-up period (study days). Rates of different ICU resources utilized were calculated as number of resource days per 100 ICU days. Rates were compared between AML and ALL using Poisson regressions with Pearson scale to adjust for potential over-dispersion. Rates of various ICU resources were summarized for each hospital.
Inpatient mortality was estimated with 95% exact confidence intervals (CIs) and compared between AML and ALL using Fisher’s exact test. To evaluate whether there was significant variation in inpatient mortality across hospitals and explore the factors that are associated with mortality, we utilized mixed-effect (ME) logistic regression models. The base ME model included no fixed effects and only a hospital level random effect. The estimated variance of the random effect reflected the magnitude of the mortality variation across hospitals and a significant test of variance greater than zero suggested the between-hospital variation is statistically significant. We then added patient and hospital factors to the base model as fixed effects to explore if the variation remains significant after adjusting for these factors. Patient factors (age, leukemia diagnosis before 2005) that have previously been associated with mortality in ALL and/or AML(5, 28, 29) were added as fixed effects into the ME model. Diagnosis of ALL vs. AML was included as a patient factor because AML has been reported to be an independent risk factor for ICU mortality in children with hematologic malignancies(7) and because of the large mortality difference in ALL and AML patients in this cohort. ICU care at leukemia diagnosis was also added as a patient factor to account for initial patient severity of illness contributing to mortality risk. We evaluated two hospital factors, the proportion of total patients cared for at that center with public insurance status, since hospital-wide proportion of patients with public insurance has been reported as a hospital-level factor contributing to variability in ALL induction mortality,(28) and the proportion of leukemia patients at each center receiving ICU care.
RESULTS
During the study period, 1,339 patients with newly diagnosed ALL and 415 patients with AML required ICU support at 42 hospitals. Five percent (93/1,754) required ICU care within the first three days of diagnosis. Demographic characteristics of the patients are summarized in Table 1. The overall cohort mortality was 15% (259/1,754): 170/1,339 (13%) of ALL patients died and 89/415 (21%) of AML patients died (P<0.001). Of the 259 patients who died, 185 (71%) received ICU resources in the 2 days prior to death.
Table 1.
Characteristics of Leukemia Patients Receiving ICUa Support
| Patient Characteristics | ALLb (n=1,339) | AMLc (n=415) |
|---|---|---|
| Male gender, No. (%) | 767 (57) | 205 (49) |
| Age, median (IQR) | 7.9 (3.3–13.5) | 11.0 (3.0–15.0) |
| Race, No. (%) | ||
| White | 1000 (75) | 280 (68) |
| Black | 109 (8) | 54 (13) |
| Asian/Pacific Islander | 38 (3) | 11 (3) |
| American Indian | 16 (1) | 5 (1) |
| Other | 137 (10) | 54 (13) |
| Unknown | 39 (3) | 11 (3) |
| Insurance, No. (%) | ||
| Private | 494 (37) | 154 (37) |
| Government | 543 (41) | 167 (40) |
| Self-pay | 35 (3) | 8 (2) |
| Other | 257 (19) | 78 (19) |
| Unknown | 10 (1) | 8 (2) |
| Mean No. ICUa days per patient (range) | 6.1 (1–115) | 9.5 (1–193) |
| No. ICU days per 100 hospital days (95% CI) | 10.4 (10.1–10.6) | 7.8 (7.6–8.1) |
| No. ICU days per 100 study days (95% CI) | 3.0 (2.9–3.0) | 5.2 (5.0–5.4) |
| No. Deaths (%) | 170 (13) | 89 (21) |
ICU: Intensive care unit,
ALL: Acute lymphocytic leukemia,
AML: Acute myeloid leukemia
In patients receiving ICU care, ALL patients had an overall mean of 6.1 days of ICU resource utilization per patient (range 1–115 days) and AML patients had a mean of 9.5 days of ICU resource utilization per patient (range 1–193 days). ALL patients had a mean of 3.0 (95% CI 2.9–3.0) ICU days per 100 study days and AML patients had a mean of 5.2 (95% CI 5.0–5.4) ICU days per 100 study days (P<0.001). The number of ICU days per 100 hospital days was higher in ALL patients, likely due to fewer total hospital days in the study period related to differences in intensity of chemotherapy regimens in ALL vs. AML patients (Table 1).
The most common ICU resources utilized were vasoactive infusions followed by invasive mechanical ventilation and hemodialysis (Table 2). The rate of use of hemodialysis and inhaled nitric oxide was lower in AML compared to ALL patients, while the rate of non-invasive mechanical ventilation was higher in AML patients (Table 2).
Table 2.
Rate of ICUa Resource Utilization (in days of resource use per 100 ICU days)
| ICUa Resource | ALLb (n=1,339) |
AMLc (n=415) |
P value for comparing rates of resource |
||
|---|---|---|---|---|---|
|
| |||||
| No. Patients (%) |
Days of resource used per 100 ICUa days |
No. Patients (%) |
Days of resource used per 100 ICUa days |
||
| Vasoactive Infusion | 813 (61) | 40.1 | 285 (69) | 42.8 | 0.1559 |
| Invasive Mechanical Ventilation | 290 (22) | 35.3 | 138 (33) | 32.1 | 0.1296 |
| Hemodialysis | 227 (17) | 13.9 | 48 (12) | 10.9 | 0.0365 |
| Non-invasive Mechanical Ventilation | 151 (11) | 9.6 | 91 (22) | 15.3 | <0.0001 |
| Nitric Oxide | 64 (5) | 6.2 | 31 (8) | 4.3 | 0.0253 |
| CPRd | 42 (3) | 0.6 | 20 (5) | 0.7 | 0.8029 |
| ECMOe | 14 (1) | 1.6 | 7 (2) | 2.0 | 0.5360 |
ICU: Intensive care unit,
ALL: Acute lymphocytic leukemia,
AML: Acute myeloid leukemia,
CPR: Cardiopulmonary resuscitation,
ECMO: Extracorporeal membrane oxygenation
Hospital Variation in ICU Resources
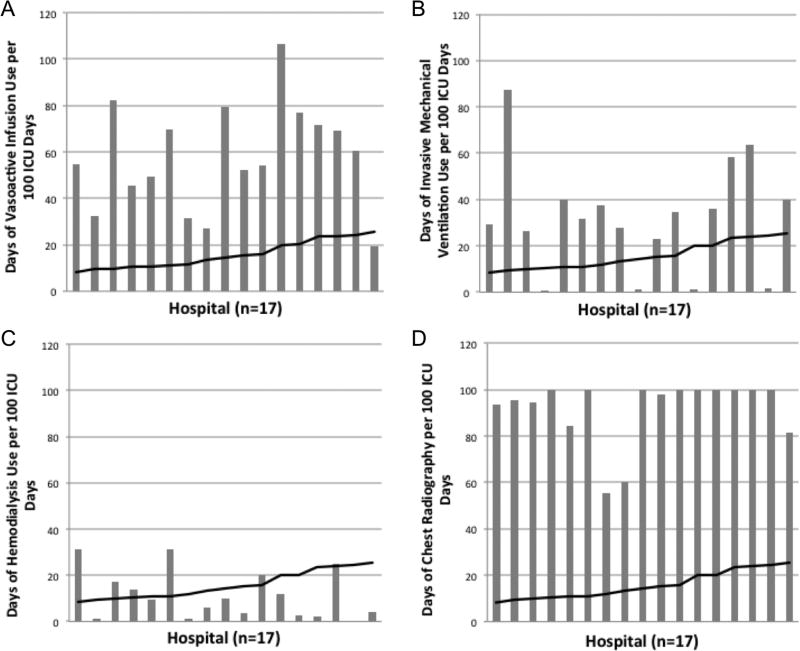
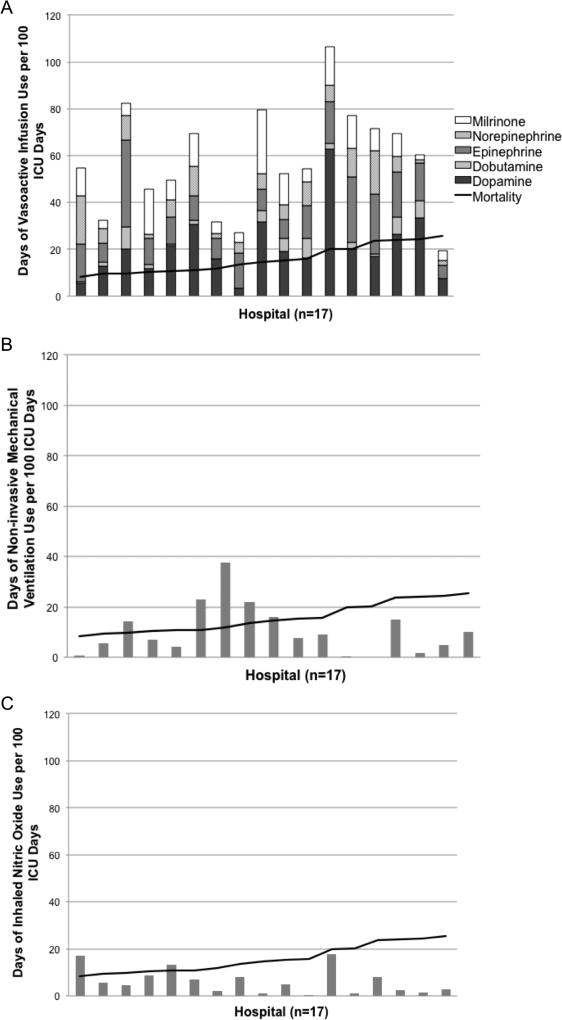
The number of days of different ICU resource utilization per 100 ICU days for the patients in the cohort at each hospital was plotted against hospital mortality for the patients in the cohort (Fig. 1). Data are shown for higher volume hospitals (the 17 hospitals caring for at least 40 leukemia patients requiring ICU support), but similar results are seen when including all hospitals (data not shown). The number of days of vasoactive infusion use (Fig. 1a), invasive mechanical ventilation use (Fig. 1b), and hemodialysis (Fig. 1c) varied widely by center and had no clear relationship with mortality. Similar wide variation without clear relationship to mortality across hospitals was seen in the types of vasoactive infusions used and in the use of non-invasive ventilation and inhaled nitric oxide (Fig. 2). The number of chest radiographs used per 100 ICU days at each hospital (Fig. 1d) was also evaluated as a baseline resource utilization and had much lower variability than the other ICU resources evaluated.
Figure 1.
Days of ICU resources used per 100 ICU days by hospital. Panel A: days of vasoactive infusion support. Panel B: days of invasive mechanical ventilation. Panel C: days of hemodialysis. Panel D: days of chest radiography. The black line represents the mortality at each hospital.
Figure 2.
Days of ICU resources used per 100 ICU days by hospital. Panel A: days of vasoactive infusion support broken down by name of infusion. Panel B: days of non-invasive mechanical ventilation. Panel C: days of inhaled nitric oxide support. The black line represents mortality at each hospital.
Hospital Variation in Mortality
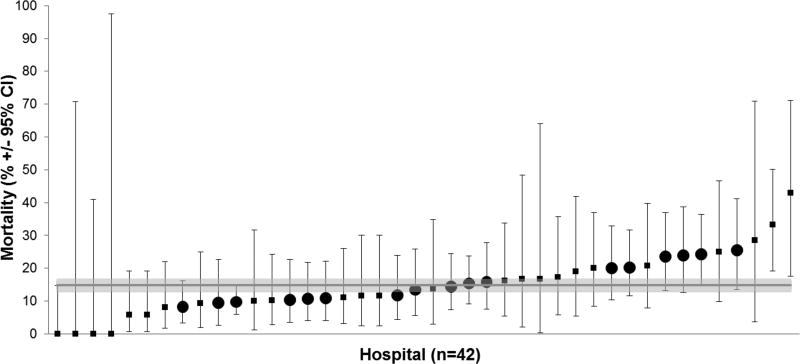
Mortality in the cohort varied by hospital (Fig. 3) from 0% (95% CI 0–14.8%) to 42.9% (95% CI 17.7–71.1%). This variation was also evaluated using mixed-effect (ME) logistic regression models. As ICU resource utilization had no clear association with mortality, this was not included as a variable in the final models. In the base ME model, the estimated variance of the random effect is significantly different from zero suggesting significant variation in mortality across hospitals (P=0.007, Table 3). When adding patient factors (age, type of leukemia, year of diagnosis, receipt of ICU care in the first three days of index admission) as fixed effects into the ME model, the variation in mortality across hospitals remained significant (P=0.021). Hospitals had a median proportion of patients with public insurance of 41.1% (IQR 32.7%, 52.6%) and a median of 13.6% (IQR 11.6%, 16.1%) of leukemia patients required ICU care at each hospital. When the hospital variables proportion of total patients with public insurance status and proportion of leukemia patients receiving ICU care were added as a fixed effects to the ME model, the mortality variation across hospitals was no longer significant (P =0.48).
Figure 3.
Mortality in leukemia patients requiring ICU support by hospital. Large circles denote hospitals caring for ≥ 40 patients requiring ICU support.
Table 3.
Mixed-Effect Models for Hospital Mortality
| Mixed-Effect Models Used to Evaluate Mortality of ALLa and AMLb Patients Across Hospitals | |||
|---|---|---|---|
| Fixed Effects Included in Model | Variance of Hospital Level Random Effect |
95% CI of the Variance |
P value for testing if variance > 0 |
| Hospital alone | 0.109 | 0.032–0.374 | 0.007 |
| Hospital plus patient factorsc | 0.096 | 0.024–0.388 | 0.021 |
| Hospital plus patient and hospital-level factorsd | 0.003 | <0.001–3.6x1014 | 0.48 |
| Details of Covariate Association with Mortality in Full Mixed-Effect Model | |||
| Covariate | Odds Ratio for Mortality | 95% CI | P value |
| Age <1 year (reference) | |||
| Age 1–5 years | 0.38 | 0.23–0.62 | <0.001 |
| Age 5–10 years | 0.54 | 0.33–0.89 | 0.02 |
| Age 10–15 years | 0.55 | 0.35–0.87 | 0.01 |
| Age >15 years | 0.92 | 0.58–1.46 | 0.72 |
| Diagnosis during or after 2005 | 0.65 | 0.48–0.87 | 0.005 |
| AMLb diagnosis | 1.87 | 1.37–2.55 | <0.001 |
| ICUe care at diagnosis | 1.24 | 0.70–2.21 | 0.45 |
| Hospital proportion of patients with public insurance status (10% increasef) | 1.12 | 1.03–1.22 | 0.01 |
| Hospital proportion of leukemia patients requiring ICU care (10% increase) | 0.74 | 0.55–0.99 | 0.05 |
ALL: Acute lymphocytic leukemia.
AML: Acute myeloid leukemia.
Patient factors included age category, disease category (acute lymphoblastic leukemia or acute myeloid leukemia), and diagnosis after 2005.
Hospital-level factor was a 10% increase in the proportion of patients with public insurance cared for at the hospital.
ICU: Intensive care unit.
A 10% increase represents approximately the difference between quartiles in median proportion of patients with public insurance.
In the final ME model, patient age, AML diagnosis, leukemia diagnosis prior to 2005, and increase in hospital proportion of public insurance patients are all associated with mortality (Table 3). A 10% increase in the hospital proportion of patients with public insurance is associated with 12% higher odds of inpatient mortality for this leukemia cohort (OR 1.12, 95% CI 1.03–1.22, Table 4); a 20% increase in the hospital proportion of patients with public insurance is associated with 26% higher odds of inpatient mortality (OR 1.26, 95% CI 1.06–1.49, Table 4). The proportion of leukemia patients receiving ICU care at a hospital was inversely associated with mortality, with a 5% increase in proportion of leukemia patients receiving ICU care associated with a 14% lower odds of inpatient mortality (OR 0.86, 95% CI 0.74–0.99), and a 10% increase in proportion of leukemia patients receiving ICU care associated with a 26% lower odds of inpatient mortality (OR 0.74, 95% CI 0.55–0.99).
Table 4.
Impact of Increase in Hospital Proportion of Patients with Public Insurance on Mortality of ALLa and AMLb Patients Requiring ICUc care from Full Mixed Effects Model
| Hospital Proportion of Patients with Public Insurance |
Odds Ratio for Mortality |
95% CId |
|---|---|---|
| 10% Increase | 1.12 | 1.03–1.22 |
| 20% Increase | 1.26 | 1.06–1.49 |
| 30% Increase | 1.41 | 1.10–1.82 |
| 40% Increase | 1.58 | 1.13–2.22 |
| 50% Increase | 1.77 | 1.16–2.70 |
ALL: Acute lymphocytic leukemia,
AML: Acute myeloid leukemia,
ICU: Intensive care unit,
CI: Confidence interval
DISCUSSION
Despite continued improvement in outcomes for children with leukemia, leukemia remains a leading cause of death from childhood cancer.(1) Our results show wide variation in the types of ICU resources used and the hospital-specific mortality rates for critically ill children with newly diagnosed leukemia. While no appreciable pattern emerged between ICU resources utilized and center-level mortality, patient factors such as age, type of leukemia, and year of diagnosis contribute to the observed center-level variability in mortality. However, patient factors do not account for all of the observed variability in mortality across hospitals. We found that a higher overall proportion of patients hospital-wide with public insurance and fewer leukemia patients requiring ICU care at a hospital are also associated with higher center-level mortality for children with ALL or AML treated in the ICU.
We have previously reported an association between hospital payer mix and ALL induction mortality,(28) and adverse events in pediatric hospitalizations have been associated with hospital Medicaid reimbursement reliance.(30) Our data corroborate this link between hospital payer mix and outcome in this specific patient population. While the reason underlying this association is unclear, hospital financial stress, staffing ratios, overall hospital patient population acuity, and availability of hospital resources may all contribute. Specific hospital-level factors such as nursing staffing ratios, nursing education level, and open versus closed ICUs and intensivist staffing have been implicated in patient outcomes in the ICU and hospital-wide.(18, 31–33) These care models are influenced by hospital resources and culture, and are frequently targeted for quality improvement efforts to align resources to provide better patient care. Failure to rescue in adult surgical patients in high Medicaid burden hospitals has been associated with lower nurse:patient ratios, lower proportion of nurses with Registered Nursing degrees, and lack of intensivists providing care.(34) Data from the Hospital Cost Report Information System indicates that the percentage of critical care medicine days used by Medicaid is increasing, along with annual rises in critical care medicine costs, while the proportional allocation of hospital costs and national health expenditures for critical care is decreasing.(35) This has important health policy implications, as Medicaid cuts and reimbursement denials for adverse events could potentially augment the association of hospital payer mix and mortality in patients requiring ICU support. Further research regarding the impact of hospital payer mix and hospital resources on the outcomes of high risk pediatric admissions is warranted to identify strategies to optimize hospital resource allocation, such as improved nurse staffing and availability of intensivists. Alternatively, cultural differences regarding end of life decision making, Do Not Resuscitate orders, withdrawal of life sustaining therapies, and associated ICU resources may exist and may contribute to mortality differences across institutions with different proportions of patients with public insurance.
We also found an inverse relationship between proportion of leukemia patients receiving ICU care at a center and hospital-level mortality among those receiving ICU care, with the odds of mortality decreasing as the proportion of leukemia patients receiving ICU care at a center increased. This may reflect differences in thresholds for ICU admission, with some centers admitting lower acuity patients to the ICU, however, our assessment of ICU care was not merely admission to the ICU but rather receipt of ICU resources so the impact of this should have been minimized. Furthermore, the lack of association of ICU resource utilization with center mortality does not support a conclusion that hospitals with higher ICU volume were treating more lower acuity patients. It is plausible that a higher volume of critically ill leukemia patients may result in improved familiarity with this population and increased collaboration between the critical care and oncology care teams, resulting in improved ICU care and subsequent outcomes for these patients.
While we found that patient demographic factors and hospital factors contributed to hospital-level variation in mortality, we observed wide variation in ICU resource utilization for these patients without a clear association with mortality. Over 70% of children who died with leukemia were treated with ICU resources close to the time of death, suggesting that most children who died in this cohort received intensive care and not palliative care. This presents an important opportunity for further outcome improvement in this inpatient population by studying and standardizing the types of ICU care provided. A survey of pediatric oncologists and pediatric intensivists described large variability in medical decision-making and potential resource utilization for hypothetical patients in this population, and called for increased study of the actual variability in patient care and clearer recommendations to decrease this variability.(36) Our results confirm this postulated variability in resource utilization, and we showed large variability in patient outcome, revealing a prospect for improvement in care. Standardized hospital treatment guidelines have led to outcome improvements in other pediatric diseases such as sepsis and urinary tract infections with decreased length of stay(19, 21, 22, 37) and possible survival benefits.(38) We found wide variability in this cohort across hospitals in the rates of ICU resource utilization for supportive therapies such as vasoactive infusion and ventilation, and further variation in the specific types of resources used within these broader categories (e.g. type of vasoactive infusion, mode of ventilation). As standardizing practice guidelines has demonstrated outcome improvement in other pediatric diseases and for pediatric cancer treatment, studying methods to standardize supportive ICU care for children with leukemia may lead to additional improvements in outcome. The large variability in supportive care seen here establishes an excellent foundation for studying the impact of treatment guidelines. Future directions should include evaluation of factors contributing to differences in ICU resource utilization in this population which may inform development of practice guidelines.
These data support the findings of single center studies that report wide hospital-level variability in mortality for children with leukemia who require ICU support, yet the types of support provided do not clearly account for the observed variability in mortality. Use of the PHIS database to study this question has several advantages. PHIS provides data on children cared for at a large number of sizeable freestanding pediatric hospitals throughout the United States, allowing comparisons across multiple centers within a single dataset without the problem of confounding by study design when comparing single center studies. The patients in PHIS represent a large proportion of children treated for leukemia in the United States.(28) Furthermore, the cohort of children with leukemia was rigorously defined, both through manual chemotherapy review and through the use of ICU resources, rather than physical location in the ICU, limiting the impact of variability in ICU admission criteria across hospitals. Finally, the granularity of the data allow for detailed calculations of daily rates of ICU resource utilization at the patient and hospital level.
Despite these strengths, the analyses presented here have several limitations. PHIS data is subject to numerous quality control measures and has been shown to capture chemotherapy exposures accurately.(39) However, as with all administrative/billing data, there may be inaccuracies in billing data, and billed resources may not have been administered to the patient. Some variability in ICU resource utilization may be attributable to different indications for ICU admission, which is unavailable in PHIS, thus adjustment for ICU admitting diagnosis is not possible. Hospital-level variables describing staffing ratios are unavailable in PHIS, and severity of illness scores at ICU admission are not available, limiting the ability to adjust mortality across centers for illness severity. The mortality analysis only includes inpatient deaths, as PHIS only provides information on the inpatient admissions, so children who died outside of the hospital are considered survivors in this analysis. Furthermore, children who died of leukemia prior to receiving any chemotherapy cannot be included in this analysis as the eligibility criteria included induction chemotherapy treatment consistent with a diagnosis of ALL or AML. These patients represent a very small proportion of children dying from leukemia,(40) nonetheless, these patients likely received ICU support prior to death.
CONCLUSIONS
Wide variability in ICU resource utilization and mortality exists in the care of children with ALL and AML who require ICU support across U.S. children’s hospitals. Both patient mix and overall hospital payer mix may explain some of the observed variability in mortality, highlighting the importance of health insurance policy decisions for vulnerable pediatric patient populations. Clinical practice guidelines for ICU therapies and strategies to improve hospital resources dedicated to the ICU should be studied as ways to further improve survival in this patient population. Additionally, qualitative center-level differences in the approach to end of life decision making in this patient population, particularly in the context of improvements in population outcomes over time, may warrant further investigation.
Supplementary Material
Acknowledgments
None
Funding Source: Supported by the National Institutes of Health (1R01CA165277 [to RA]) and The Alex’s Lemonade Stand Foundation. The study sponsors did not have a role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript.
Drs. Fitzgerald and Aplenc received support for article research from the National Institutes of Health (NIH). Dr. Fitzgerald’s institution received funding from the NIH and the Alex's Lemonade Stand Foundation. Dr. Fisher’s institution received funding from Pfizer, Merck, and Ansun Biopharma. Dr. Seif’s institution received research grant funding from the American Cancer Society, the Hyundai Hope on Wheels Foundation, and the Alex's Lemonade Stand Foundation (all unrelated to this project). Dr. Thomas’s institution received funding from GeneFluidics, and he received funding from Therabron and CareFusion.
Footnotes
Copyright form disclosure: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: Apr, 2014. based on November 2013 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 4.Unguru Y. The successful integration of research and care: how pediatric oncology became the subspecialty in which research defines the standard of care. Pediatr Blood Cancer. 2011;56(7):1019–1025. doi: 10.1002/pbc.22976. [DOI] [PubMed] [Google Scholar]
- 5.Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr Crit Care Med. 2014;15(2):112–120. doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9(3):270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 7.Zinter MS, DuBois SG, Spicer A, et al. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive care medicine. 2014;40(10):1536–1544. doi: 10.1007/s00134-014-3389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Abraham R, Toren A, Ono N, et al. Predictors of outcome in the pediatric intensive care units of children with malignancies. J Pediatr Hematol Oncol. 2002;24(1):23–26. doi: 10.1097/00043426-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ha EJ, Kim S, Jin HS, et al. Early changes in SOFA score as a prognostic factor in pediatric oncology patients requiring mechanical ventilatory support. J Pediatr Hematol Oncol. 2010;32(8):e308–313. doi: 10.1097/MPH.0b013e3181e51338. [DOI] [PubMed] [Google Scholar]
- 10.Heying R, Schneider DT, Korholz D, et al. Efficacy and outcome of intensive care in pediatric oncologic patients. Crit Care Med. 2001;29(12):2276–2280. doi: 10.1097/00003246-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Keengwe IN, Stansfield F, Eden OB, et al. Paediatric oncology and intensive care treatments: changing trends. Arch Dis Child. 1999;80(6):553–555. doi: 10.1136/adc.80.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690. doi: 10.1001/jama.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta P, Tang X, Gall CM, et al. Epidemiology and outcomes of in-hospital cardiac arrest in critically ill children across hospitals of varied center volume: a multi-center analysis. Resuscitation. 2014;85(11):1473–1479. doi: 10.1016/j.resuscitation.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Tang X, Gossett JM, et al. Variation of ventilation practices with center volume after pediatric heart surgery. Clin Cardiol. 2015;38(3):178–184. doi: 10.1002/clc.22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneier AJ, Shields BJ, Hostetler SG, et al. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118(2):483–492. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- 16.Tieder JS, McLeod L, Keren R, et al. Variation in resource use and readmission for diabetic ketoacidosis in children's hospitals. Pediatrics. 2013;132(2):229–236. doi: 10.1542/peds.2013-0359. [DOI] [PubMed] [Google Scholar]
- 17.Weiss PF, Klink AJ, Hexem K, et al. Variation in inpatient therapy and diagnostic evaluation of children with Henoch Schonlein purpura. J Pediatr. 2009;155(6):812–818. e811. doi: 10.1016/j.jpeds.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubbs-Cooley HL, Cimiotti JP, Silber JH, et al. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735–742. doi: 10.1136/bmjqs-2012-001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789–796. doi: 10.1016/j.jpeds.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Goldin AB, Sawin RS, Garrison MM, et al. Aminoglycoside-based triple-antibiotic therapy versus monotherapy for children with ruptured appendicitis. Pediatrics. 2007;119(5):905–911. doi: 10.1542/peds.2006-2040. [DOI] [PubMed] [Google Scholar]
- 21.Akcan Arikan A, Williams EA, Graf JM, et al. Resuscitation Bundle in Pediatric Shock Decreases Acute Kidney Injury and Improves Outcomes. J Pediatr. 2015;167(6):1301–1305. e1301. doi: 10.1016/j.jpeds.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 22.Paul R, Neuman MI, Monuteaux MC, et al. Adherence to PALS Sepsis Guidelines and Hospital Length of Stay. Pediatrics. 2012;130(2):e273–280. doi: 10.1542/peds.2012-0094. [DOI] [PubMed] [Google Scholar]
- 23.Balamuth F, Weiss SL, Fitzgerald JC, et al. Protocolized Treatment Is Associated With Decreased Organ Dysfunction in Pediatric Severe Sepsis. Pediatr Crit Care Med. 2016;17(9):817–822. doi: 10.1097/PCC.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narus SP, Srivastava R, Gouripeddi R, et al. Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. AMIA Annu Symp Proc. 2011;2011:994–1003. [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher BT, Harris T, Torp K, et al. Establishment of an 11-year cohort of 8733 pediatric patients hospitalized at United States free-standing children's hospitals with de novo acute lymphoblastic leukemia from health care administrative data. Med Care. 2012 doi: 10.1097/MLR.0b013e31824deff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavcic M, Fisher BT, Torp K, et al. Assembly of a cohort of children treated for acute myeloid leukemia at free-standing children's hospitals in the United States using an administrative database. Pediatr Blood Cancer. 2013;60(3):508–511. doi: 10.1002/pbc.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winestone LE, Getz KD, Miller TP, et al. The role of acuity of illness at presentation in early mortality in black children with acute myeloid leukemia. Am J Hematol. 2017;92(2):141–148. doi: 10.1002/ajh.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seif AE, Fisher BT, Li Y, et al. Patient and hospital factors associated with induction mortality in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(5):846–852. doi: 10.1002/pbc.24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavcic M, Fisher BT, Li Y, et al. Induction mortality and resource utilization in children treated for acute myeloid leukemia at free-standing pediatric hospitals in the United States. Cancer. 2013;119(10):1916–1923. doi: 10.1002/cncr.27957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RB, Dynan L, Fairbrother G, et al. Medicaid, hospital financial stress, and the incidence of adverse medical events for children. Health Services Research. 2012;47(4):1621–1641. doi: 10.1111/j.1475-6773.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiken LH, Sloane DM, Bruyneel L, et al. Nurse staffing and education and hospital mortality in nine European countries: a retrospective observational study. Lancet. 2014;383(9931):1824–1830. doi: 10.1016/S0140-6736(13)62631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh MD, Rochman MF, Sloane DM, et al. Better nurse staffing and nurse work environments associated with increased survival of in-hospital cardiac arrest patients. Med Care. 2016;54(1):74–80. doi: 10.1097/MLR.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pronovost PJ, Angus DC, Dorman T, et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288(17):2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 34.Wakeam E, Hevelone ND, Maine R, et al. Failure to rescue in safety-net hospitals: availability of hospital resources and differences in performance. JAMA Surg. 2014;149(3):229–235. doi: 10.1001/jamasurg.2013.3566. [DOI] [PubMed] [Google Scholar]
- 35.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 36.Randolph AG, Zollo MB, Egger MJ, et al. Variability in physician opinion on limiting pediatric life support. Pediatrics. 1999;103(4):e46. doi: 10.1542/peds.103.4.e46. [DOI] [PubMed] [Google Scholar]
- 37.Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6):e1585–1592. doi: 10.1542/peds.2010-3513. [DOI] [PubMed] [Google Scholar]
- 38.Paul R, Melendez E, Stack A, et al. Improving adherence to PALS septic shock guidelines. Pediatrics. 2014;133(5):e1358–1366. doi: 10.1542/peds.2013-3871. [DOI] [PubMed] [Google Scholar]
- 39.Miller TP, Troxel AB, Li Y, et al. Comparison of administrative/billing data to expected protocol-mandated chemotherapy exposure in children with acute myeloid leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62(7):1184–1189. doi: 10.1002/pbc.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slats AM, Egeler RM, van der Does-van den Berg A, et al. Causes of death--other than progressive leukemia--in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia. 2005;19(4):537–544. doi: 10.1038/sj.leu.2403665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.