Abstract
We examined mechanisms underlying infants’ ability to categorize human biological motion stimuli from sex-typed walk motions, focusing on how visual attention to dynamic information in point-light displays (PLDs) contributes to infants' social category formation. We tested for categorization of PLDs produced by women and men by habituating infants to a series of female or male walk motions and then recording posthabituation preferences for new PLDs from the familiar or novel category (Experiment 1). We also tested for intrinsic preferences for female or male walk motions (Experiment 2). We found that infant boys were better able to categorize PLDs than were girls, and that male PLDs were preferred overall. Neither of these effects were found to change with development across the observed age range (about 4 to 18 months). We conclude that infants’ categorization of walk motions in PLDs is constrained by intrinsic preferences for higher motion speeds and higher spans of motion and, relatedly, by differences in walk motions produced by men and women.
Keywords: biological motion perception, visual social attention, sex differences
One important attentional mechanism available to infants is the tendency to orient toward motion patterns that are specific to animate entities (Frankenhuis, Barrett, & Johnson, 2013; Frankenhuis, House, Barrett, & Johnson, 2013). Perception of biological motion was initially demonstrated in classic experiments by Johansson (1973, 1976), who showed that adults quickly and spontaneously recognize human figures in point-light displays (PLDs) consisting of a small number of illuminated moving dots that were affixed to the joints of a human actor. Adult observers readily recognize actions (Dittrich, 1993; Norman, Payton, Long, & Hawkes, 2004), emotions (Johnson, McKay, & Pollick, 2011), intentions (Blakemore & Decety, 2001), gender (Kozlowski & Cutting, 1977; Mather & Murdoch, 1994; Troje, 2002), and identity (Cutting & Kozlowski, 1977; Fani, Prasad, Harber, & Shiffrar, 2005; Troje, Westhoff, & Lavrov, 2005) solely from PLDs. Perception of biological motion offers vital insights into the nature of the social brain (Adolphs, 1999; Anderson et al., 2013; Lieberman, 2013), social behavior (Grossman, Blake, & Kim, 2004; Shi, Weng, He, & Jiang, 2010), and social cognition (Blake & Shiffrar, 2007; Klin et al., 2009). As such, insights into mechanisms of attention for PLDs and their development in infancy might aid in understanding social development more broadly, perhaps including the perceptual origins of social categorization, the question we address in the present paper.
Visual mechanisms that support the perception of biological motion seem to be in place early in development (Bertenthal, 1993). For example, newborns look longer at upright human PLDs relative to foil stimuli consisting of the same number of dots moving randomly (Bidet-Ildei, Kitromilides, Orliaguet, Pavlova, & Gentaz, 2014). By 3 months, infants differentiate walking from running motions in PLDs (Booth, Pinto, & Bertenthal, 2002), and by 5–6 months, infants distinguish a PL hand from a foil stimulus (Fox & McDaniel, 1982), recognize walk direction in sagittal PLDs (Kuhlmeier, Troje, & Lee, 2010), and discriminate canonical PLDs from those in which rigidity of the limbs (Bertenthal, Proffitt, & Kramer, 1987a)) or bilateral symmetry of gait (Booth et al., 2002) is disrupted. By 7–9 months, even more complex human actions may be perceived veridically, including PL versions of infants’ own leg motions (Schmuckler & Fairhall, 2001), emotional expression in PL faces (Soken & Pick, 1992), and timing of self-occlusion of limbs in PL walkers (Bertenthal, Proffitt, Spetner, & Thomas, 1985). It is unknown, however, whether infants perceive PLDs as providing information relevant to social categories such as sex.
In the current paper we report two experiments that examine infants’ categorization and discrimination of PLDs produced by men and women. Categorization is critical for the systematization and stability of cognition, serving to organize low-level structure and prepare cognitive resources for identification of more abstract relations (Bruner, 1957). Social categorization, in particular, is regarded as an obligatory aspect of social life (Allport, 1954). Informed by visible cues in the face and body of others, social categorization allows observers to readily and rapidly parse the social world in terms of sex, race, age, and even sexual orientation. Once categorized, social categorizations and the percepts that inform them elicit knowledge structures (i.e., stereotypes) that impinge on adults’ judgments of fairness, discrimination, and distribution of reward (Billig & Tajfel, 1973; Freeman & Johnson, 2016).
It is not known whether infants can detect sex differences in PLDs, but adults use information in biological motion to discriminate between and categorize men’s and women’s walk motions (Lick, Johnson, & Gill, 2013; Pollick, Kay, Heim, & Stringer, 2005; Troje, 2002), and infants use information in bodies and faces to distinguish social categories. For example, there is an advantage in processing characteristics of female faces (vs. male faces) by 3- 4 months (Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002; Ramsey, Langlois, & Marti, 2005), a preference for own-race faces by 3 months (Kelly et al., 2005), a decline in the ability to discriminate faces from other races by 9 months (Kelly et al., 2007), a progressive narrowing of intersensory matching of face and voice by 10 months (Lewkowicz & Ghazanfar, 2006), and a preference for minority-race faces by 11 months (Liu et al., 2015; Singarajah et al., 2017). Moreover, infants prefer sex-congruent (face and body of the same sex) versus sex-incongruent images by 5 months, and thus discriminate and match sex differences in bodies and faces (Hock, Kangas, Zieber, & Bhatt, 2015); by 6.5 months, infants discriminate and match emotions in body movements and voices (Zieber, Kangas, Hock, & Bhatt, 2014a, 2014b). Such effects are presumed to stem in part from infants’ typical day-to-day exposure to bodies and faces (Bhatt, Hock, White, Jubran, & Galati, 2016; Scott, Pascalis, & Nelson, 2007).
If similar developmental processes were operational for perception of biological motion, they would require a growing sensitivity to structural information in PLDs, because a substantial proportion of information for individuals’ identities is found in second-order relations among visual features (i.e., global structure; Troje et al., 2005). Some sensitivity to global structure is presumably in place at birth, contributing to discrimination of upright PLDs and random or scrambled dot motion (Bidet-Ildei et al., 2014; Simion, Regolin, & Bulf, 2008), but not until 5 months did infants respond to disruption of dots’ spatial organization and temporal phase in human PL walkers (Bertenthal et al., 1987a), and not until 9 months did infants appear to recognize disruptions of canonical points of dot occlusion during cyclical walk motion (Bertenthal et al., 1985). Taken together, these findings imply an emerging sensitivity to structure in human PLDs across the first year after birth. The means by which this sensitivity translates into higher order social cognitive processing such as infants’ processing of social categories (e.g., sex) in PLDs remain to be investigated.
Can infants, like adults, categorize and discriminate between men’s and women’s walk motions in PLDs? We see several possibilities. First, sensitivity to social category information in PLDs may become shaped by experience observing walk motions. Infants begin postnatal life with an apparent preference for biological motion (Bardi, Regolin, & Simion, 2011; Bidet-Ildei et al., 2014; Simion et al., 2008), as well as face-like stimuli (Morton & Johnson, 1991; Reid et al., 2017). Infants receive substantial exposure to faces early in postnatal life (Fausey, Jayaraman, & Smith, 2016), apparently sufficient to induce preferences for faces from familiar social categories by 3–4 months (Kelly et al., 2005; Quinn et al., 2002). It is unknown, however, if young infants have similar kinds of exposure to human walk motions as they do to human faces, nor is it clear whether infants are sensitive to the dynamic characteristics that differentiate female and male walk motions, in which case positive evidence for biological motion categorization might emerge later in development.
Second, there may be asymmetries in discriminability of PLD targets. Female PLDs may be easier to detect than male PLDs, for example, if individual females’ walk motions are more distinct or familiar (as may be true of female faces; Ramsey et al., 2005), thus generating a female preference. Alternatively, asymmetries in adults’ sex categorization from body shape favoring males—specifically, a bias to perceive ambiguous targets as male (Johnson, Iida, & Tassinary, 2012)—implies that there may be an advantage for discrimination of, and preference for, male PLDs. In addition, differences in variability in PLDs may yield asymmetric category learning. If items in a category are too distinct (e.g., if males’ walk motions are more variable; Johnson et al., 2012), they may be difficult to organize into a common group (Quinn, Eimas, & Rosenkrantz, 1993),
Finally, there may be differences in infant girls’ vs. boys’ responses to PLDs. For example, girls might form social categories from PLDs earlier than boys, given that (a) parents may socialize girls and boys differently, even in infancy (Constantinescu, Moore, Johnson, & Hines, in press; Clearfield & Nelson, 2006; Sung, Fausto-Sterling, García Coll, & Seifer, 2013), which may promote attention to social information from walk motions, (b) girls, relative to boys, may show an increased interest in social stimuli from an early age (Alexander & Wilcox, 2012; Proverbio, 2016; cf. Simpson et al., 2016), and (c) PLD stimuli activate cortical regions involved in social perception (amygdala, medial temporal gyrus, and the temporal pole) more strongly in females at least from childhood (Anderson et al., 2014). On the other hand, there might be a male advantage for processing motion information in PLDs, given that (a) boys often prefer toys that afford physical activity (such as vehicles), relative to toys that foster social play, more than do girls (Berenbaum & Hines, 1992; Hines, 2010; cf. Alexander & Hines, 2002), and (b) males attend more to global vs. local stimulus properties across a range of visual attributes at least from childhood (Kramer, Ellenberg, Leonard, & Share, 1996; Roalf, Lowery, & Turetsky, 2006; cf. Pomerantz, 1983); this may stem from the facilitating effects of androgens on development of the magnocellcular visual pathway, also implicated in motion processing (Vanston & Strother, 2017; cf. Salyer, Lund, Fleming, Lephart, & Horvath, 2001).
Design and Measures
Our questions require designs that can establish categorization and discrimination of sex differences in human biological motion. We chose a simple design for Experiment 1 that meets the first requirement for testing category formation by habituating infants with a series of individual PLDs depicting men’s or women’s walk motions, followed by a test phase in which infants saw a new item from the habituated, familiar category (i.e., a new man or a new woman), alternating with a new item from the novel category. Looking times were recorded by a trained observer. Longer looking at one of the test stimuli is taken as evidence that infants recognize the correspondence between PLDs in the familiar category and the new test item from the same category, and identify the new PLD as distinct. The dependent variable is posthabituation looking times, operationalized as total looking to the familiar vs. novel test items. A posthabituation preference score was also computed, operationalized as total looking at the novel test PLD divided by total looking at novel + familiar displays (novelty preference > .50; familiarity preference < .50). Because posthabituation looking patterns can be influenced by factors such as habituation time, infant age, and stimulus complexity (Hunter & Ames, 1988; Hunter, Ames, & Koopman, 1983; Roder, Bushnell, & Sasseville, 2000), it was not possible to predict a priori which direction infants’ preferences might take.
In Experiment 2, we then tested for discrimination of male and female walk motions by presenting infants with side-by-side pairs of PLDs as we recorded infants’ eye movements with an eye tracker. Greater attention to one PLD sex is taken as evidence for discrimination (i.e., a bias or preference). Visual attention was operationalized as dwell times, or accumulated visual fixations. In addition, we recorded infant attention to specific regions, or areas of interest (AOIs) of each PLD—top (head and shoulders), middle (waist and arms), and bottom (legs and feet)— and we quantified the speed and extent of motion of dots in these AOIs, to better understand how attention may have been guided by particular stimulus features. Sensitivity to horizontal translation, for example, has been suggested as a possible basis for the early bias toward animate motion (Bidet-Ildei et al., 2014), and there are sex differences in human gait yielding distinct motion patterns in shoulder and waist regions (Johnson & Tassinary, 2005). The dependent variable is dwell times to the three AOIs composing each PLD.
In both experiments we observed infants in three age groups, approximately 6, 10, and 15 months. Bidet-Ildei et al. (2014) found that newborns look longer at translating groups of dots, whether they be biological motion or random; by 3 months infants appeared to discriminate biological motion from foil stimuli based on differences in coherence of dot groupings, but not differences in translation (Bertenthal, Proffitt, Kramer, & Spetner, 1987b). This indicates that development of infants’ perception of biological motion in some ways is nonlinear, and so we analyzed for age differences in categorization and discrimination to capture developmental changes in PLD perception. Finally, in both experiments we analyzed for differences in performance between female and male infants.
Experiment 1
We habituated infants to a series of PLDs representing walk motions of either women or men, and then tested for differences in looking times toward a new PLD from the familiar category (i.e., women or men) vs. a new PLD from the novel category. Evidence for categorization would take the form of posthabituation looking time differences to the novel and the familiar PLDs.
Method
Participants
A sample of 66 healthy full-term infants completed the habituation paradigm (7 were parent-reported as Asian/Pacific Islander, 26 Caucasian, 6 Hispanic/Latino, 26 Multiracial, and 1 unreported). We tested three age groups (M age = 6.5 months, SD = 1.1, range = 3.5 – 8.3 months, N = 22, 10 females; M age = 10.0 months, SD = .7, range 8.8 – 11.5 months, N = 22, 13 females; M age = 14.5 months, SD = 1.2, range = 12.0 – 16.6 months, N = 22, 13 females). Two additional infants were observed but excluded from the final dataset due to excessive fussiness. Parents and caregivers were contacted by telephone from birth records and infants were given a small gift (a toy or a t-shirt) for their participation. Participants in both experiments were treated in accordance with University IRB #10-000619, “Brain Mechanisms of Visual Development.”
Stimuli
Stimuli consisted of 20 PLDs depicting the walk motions of 10 men and 10 women, who were undergraduates recruited for purposes of recording walk motions. We used a Vicon MX 3D motion-capture system to record each walker’s body motion from reflective markers affixed to the head, shoulders, elbows, wrists, hips, knees, and ankles as he or she walked on a treadmill at a self-selected comfortable speed. The first 10 s of the spatial coordinates for each marker were transformed into PLDs using custom software. The midpoint of each PLD's hips was aligned with the display’s midpoint. Each PLD was sized 12.9 cm (12.2° visual angle at the infant’s 60 cm viewing distance) by 7.1 cm (6.8°) and edited to seamlessly loop through walk cycles. PLDs were presented in the frontal plane so as to maximize availability of information that can be used to discriminate women from men, namely the structure and dynamics of shoulder and hip regions (Johnson et al., 2012).
The 20 PLD stimuli were shown to 15 undergraduates naïve to the purposes of the study. Participants were asked to indicate whether each PLD was female or male via button press. Choice and latency to respond were recorded and no feedback was provided. Participants chose the correct sex on M = 7.93 out of 10 trials (SD = 2.34) with female PLDs and M = 8.07 out of 10 trials (SD = 1.16) with male PLDs. We used signal detection to analyze these data, arbitrarily coding the correct categorization of female PLDs as “hits” and incorrect categorization of male PLDs as “false alarms.” Not surprisingly, participants’ accuracy corresponded to a strong and significant d’ = 1.29 (SD = .73), p < .0001. Participants exhibited no significant response bias, c’ = -0.027 (SD = 0.218), ns. Participants answered after M = 3.05 s (SD = 1.85) for female PLDs and after M = 3.43 s (SD = 1.76) for males, not significantly different, t(14) = -.78, ns. In general, therefore, the sex of our PLD stimuli was readily apparent to adult observers.
Apparatus and Procedure
PLDs were presented on a 61.5 cm monitor using an Apple G5 computer. The experimental protocol was scripted using Experiment Builder (SR Research, Ltd). Each infant sat on a caregiver’s lap approximately 60 cm from the display. An observer in the next room recorded looking times by watching the infant on a monitor and pressing a key on the computer when the infant looked at the screen, and letting go when the infant looked away. A trial resumed if the infant returned attention to the screen within 2 s; otherwise the trial was terminated and an attention-getter (a small animation) reoriented the infant’s gaze to the screen before the next trial.
Infants were randomly assigned to view either female or male PLDs during habituation. Infants viewed multiple PLD exemplars until habituation of looking occurred or 12 trials elapsed. A different PLD was shown during each trial, and the walk motion looped until the trial terminated. Each habituation and test trial continued until the infant looked away from the monitor for more than 2 s or until 60 s had elapsed. Nine PLDs from each sex were randomly selected for presentation during habituation, and the other for test as the familiar stimulus. The novel test stimulus was randomly selected from the set of 10 of the other sex. If an infant took longer than nine trials to habituate, previously-seen PLDs were presented until habitation occurred or 12 trials elapsed. The habituation criterion was defined as a decline in looking times across a block of four trials that summed to less than 50% of looking times during the first four trials. Test trials began immediately following habituation. The six test trials consisted of pseudo-randomized presentation of familiar and novel PLDs (i.e., three female and three male, with each sex presented no more than twice consecutively). Of the infants who successfully completed experimental procedures, 31 infants were habituated to female PLDs (14 boys, 17 girls) and 35 were habituated to male PLDs (16 boys, 19 girls).
Results and Discussion
Preliminary analyses indicated that there were no effects involving test display order (familiar vs. novel first after habituation), and so subsequent analyses collapsed over this variable. A 2 (infant gender)×3 (age group)×2 (habituation stimulus: male PLD or female PLD)×2 (test display: familiar vs. novel)×3 (trial: first, second, or third) mixed ANOVA with posthabituation looking time as the dependent variable revealed interactions between infant gender and test display, F(1, 54) = 6.24, p = .016, ηp 2 = 0.10 (Figure 1a), and between PLD sex and test display, F(1, 61) = 6.16, p = .016, ηp 2 = 0.10 (Figure 2a). There were no other significant effects. We computed a post hoc power analysis to examine the age effect using G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007). With effect size f = .39, alpha = .05, and sample size = 66, achieved power = .80. Therefore we can be confident that the lack of an age effect was not due to low power.
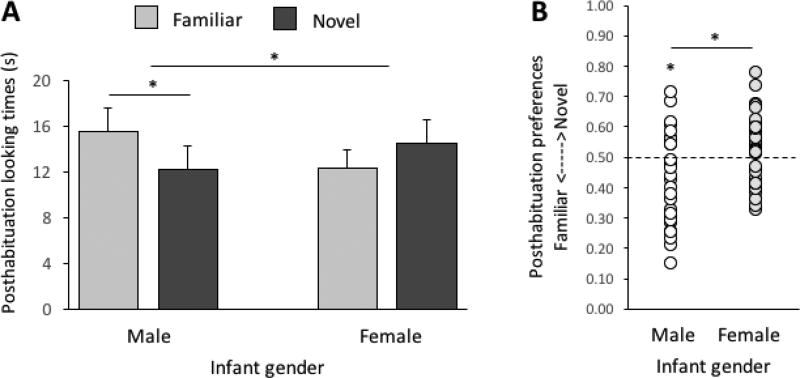
Figure 1.
(A) Posthabituation looking times and (B) preferences as a function of infant gender differences in Experiment 1. Error bars = SEM.
Figure 2.
(A) Posthabituation looking times and (B) preferences as a function of habituation stimulus (male vs. female) in Experiment 1. Error bars = SEM.
We computed tests for simple effects to interpret the infant gender×test display interaction (Figure 1a). These analyses revealed longer looking overall at the familiar test stimulus by male infants, F(1, 29) = 7.73, p = .009, ηp 2 = 0.21; in contrast, female infants looked about equally at the two test displays, F(1, 35) = 1.38, ns. We also computed tests for simple effects to examine the PLD sex×test display interaction (Figure 2a). These analyses revealed longer looking overall at the familiar test stimulus after habituation to male PLDs, F(1, 34) = 7.85, p = .008, ηp 2 = 0.19; in contrast, infants looked about equally at the two kinds of test display after habituation to female PLDs, F(1, 35) = 1.38, ns.
Analyses of posthabituation preferences corroborated these findings. Male infants looked more overall at the familiar test display (M preference = .42, SD = .15) vs. chance, t(29) = -2.74, p = .010, but preferences for females (M = .52, SD = .11) were not different than chance, t(35) = 1.15, ns (Figure 1b). Posthabituation preferences for male and female infants were significantly different, t(64) = 4.96, p = .030. Finally, infants overall exhibited a familiarity preference after habituation to male PLDs (M novelty preference = .43, SD = .14) vs. chance, t(34) = -2.76, p = .009, but preferences following habituation to female PLDs (M = .53, SD = .12) were not different than chance, t(30) = 1.21, ns (Figure 2b). Posthabituation preferences for male and female infants were significantly different, t(64) = 2.83, p = .006.
Taken together, these results support three conclusions. First, infant males showed an overall familiarity preference, implying that infant boys are better able than girls to categorize PLDs from motion-based information, perhaps because boys are particularly attuned to motion parameters of PLD stimuli. Second, infants overall provided evidence for categorizing male, but not female, PLDs. If infants are biased toward male PLDs, however, such a bias would interact with posthabituation preferences such that a male bias and a posthabituation familiarity preference would work at odds and thus yield a null result for infants habituated to female PLDs. (We address the possibility of a male bias in Experiment 2.) Third, we observed no reliable effects of age, implying that at least some of the processes that support infant learning of social categories from human walk motions are in place by 6.5 months (cf. Ramsey et al., 2005).
An interesting question concerns the outcomes of the two habituation stimulus conditions plotted in Figure 2. Infants in both conditions (habituated to male vs. female PLDs) subsequently looked longer at male PLDs, a significant familiarity preference for infants habituated to male PLDs, and a nonsignificant novelty preference for infants habituated to female PLDs. On the whole, therefore, infants looked more overall at test at male PLDs, a conclusion confirmed by analysis (male M = 14.85 s, SD = 11.13; female M = 12.35 s, SD = 10.86; t(65) = 2.57, p = .013). This finding implies a bias or preference for male vs. female walk motions.
Experiment 2
In Experiment 2 we used a side-by-side preferential viewing paradigm to (a) more firmly establish infants’ perceptual discrimination of sex differences in PLDs, (b) provide a second test of a possible bias for male PLDs, and (c) further examine gender differences in infants’ processing information in PLDs. We recorded infants’ eye movements for evidence that preferences can be interpreted in light of detailed patterns of visual attention.
Method
Participants
A sample of 42 healthy full-term infants participated (5 were parent-reported as Asian/Pacific Islander, 18 Caucasian, 4 Hispanic/Latino, and 15 Multiracial). As in Experiment 1, we tested three age groups (M age = 5.5 months, SD = 1.0, range = 4.2 – 7.1 months, N = 15, 9 females; M age = 10.1 months, SD = 1.8, range = 7.5 – 12.6 months, N = 13, 7 females; M age = 14.7 months, SD = 1.5, range = 12.9 – 17.9 months, N = 14, 6 females). An additional 8 infants were observed but excluded from the final dataset due to side bias (i.e. looking at one side for > 90% of the time, n = 3), a medical condition that compromised vision (i.e., cataracts; n = 1), or failure to meet calibration criteria (n = 4). None of the infants recruited for Experiment 2 participated in Experiment 1. Parents and caregivers were contacted in the same fashion as described previously, and also given a small gift for their participation.
Stimuli
The same PLD stimuli as described previously were used in Experiment 2.
Stimulus motion parameters
We quantified motion parameters within three “areas of interest,” or AOIs (described in detail in the “Apparatus and Procedure” section; see Figure 3), of each PLD using custom Matlab scripts. Speed was operationalized as the M Euclidean distance in pixels traversed by the set of reflective markers in each AOI across consecutive frames (pixels per frame, px/fr). Motion span was operationalized as the M Euclidean distance in pixels traversed by the markers in each AOI during a complete walking cycle (i.e., two steps).
Figure 3.
Still image of side-by-side PLDs (female at left, male at right) with superimposed AOIs for coding eye movements. AOIs were not visible during the experiment.
Stimulus motion parameters
We analyzed the motion parameters of the stimuli to provide a foundation for understanding infants’ allocation of visual attention. A 3 (AOI: top, middle, or bottom)×2 (PLD sex) mixed-measures ANOVA with Speed as the dependent measure yielded a significant main effect of AOI, F(2, 18) = 274.44, p < .001, ηp 2 = 0.97, due to differences in marker speeds within AOIs (top M = .93 px/fr, SD = .21; middle M = 1.17 px/fr, SD = .24; bottom M = 2.01 px/fr, SD = .31). There was also a significant interaction between PLD sex and AOI, F(2, 18) = 8.25, p = .003, ηp 2 = 0.48. Tests for simple effects revealed greater speed in the male top AOI (M = 1.11 px/fr, SD = .31) relative to the female top AOI (M = .76 px/fr, SD = .28), F(1, 9) = 7.24, p = .025, ηp 2 = 0.45, but no PLD sex differences in middle and bottom regions, ps > .55. A similar ANOVA with Motion span as the dependent measure yielded significant main effects of PLD sex, F(1, 18) = 10.31, p = .005, ηp 2 = 0.36, and AOI, F(2, 18) = 192.94, p < .001, ηp 2 = 0.92, and a reliable PLD sex by AOI interaction, F(2, 18) = 3.53, p = .040, ηp 2 = 0.16. Tests for simple effects revealed greater motion span in the male top AOI (M = 56.69 px, SD = 10.35) relative to the female top AOI (M = 32.50 px, SD = 8.63), F(1, 18) = 32.16, p < .001, ηp 2 = 0.64. Motion span measures were also higher in the male vs. female middle (male M = 61.20 px, SD = 15.61; female M = 52.67 px, SD = 13.67) and bottom (male M = 111.99 px, SD = 17.66; female M = 103.48 px, SD = 9.40) AOIs, but the differences were not statistically significant, ps > .20.
Apparatus and Procedure
Apparatus used to present stimuli were the same as described previously. We used an EyeLink 1000 eye tracker (SR Research, Ltd.) to record infants’ eye movements. Each infant sat on a caregiver’s lap approximately 60 cm from the display while viewing 24 trials of paired PLD stimuli. Each 10 s trial consisted of two PLDs— one female, the other male—side-by-side on the screen (Figure 3). PLDs were the same size as in Experiment 1, and their centers were separated by 22.20 cm (21.24°). The left/right placement of male and female PLDs was randomized with the constraint that one sex could not be presented on the same side for more than three trials in a row. An attention-getter reoriented the infant’s gaze to the center of the computer screen for each trial. Prior to testing, each infant’s point of gaze was calibrated to coordinates on the screen with a standard 5-point calibration routine.
To code eye movements, we created three AOIs for each PLD surrounding the head and shoulders (three markers), torso and arms (six markers), and legs (four markers; see Figure 3). The accumulated visual fixations within these three AOIs are reported subsequently as dwell times. Total height of the three AOIs was 13.5 cm (12.7°) and the width was 7.4 cm (7.1°). Horizontal boundaries of the AOIs were necessarily placed differently for each PLD to accommodate differences in marker position intrinsic to individual walkers.
Results and Discussion
We analyzed for gender and age differences in performance, a bias for male PLDs, and patterns of attention across AOIs with a 2 (infant gender)×3 (age group)×2 (PLD sex: female vs. male)×3 (AOI: top, middle, or bottom) mixed ANOVA with M dwell time as the dependent measure. This analysis yielded a significant main effect of infant gender, F(1, 36) = 4.25, p = .046, ηp 2 = 0.11, due to greater dwell times overall by infant males (see Figure 4a), a significant main effect of PLD sex, F(1, 36) = 7.92, p = .008, ηp 2 = 0.18, confirming an overall preference for male PLDs (male PLD M = 67.38 s, SD = 24.99; female PLD M = 60.34 s, SD = 20.30), and a significant main effect of AOI, F(1, 36) = 7.87, p = .008, ηp 2 = 0.18, stemming from differences in dwell times within AOIs (top M = 8.30 s, SD = 9.01; middle M = 40.40 s, SD = 17.28; bottom M = 15.43 s, SD = 9.98). These effects were modulated by a significant PLD sex×AOI interaction, F(2, 36) = 8.78, p = .005, ηp 2 = 0.20, which is plotted in Figure 4b. There were no additional significant effects. As in Experiment 1, we computed a post hoc power analysis to examine the age effect using G*Power 3.1 (Faul et al., 2007). With effect size f = .03, alpha = .05, and sample size = 42, achieved power = .05. Unlike Experiment 1, therefore, Experiment 2 may have been underpowered to reveal possible effects of age on PLD preferences.
Figure 4.
(A) Differences in dwell times as a function of infant gender and (B) AOIs in male and female PLDs in Experiment 2. Error bars = SEM.
Tests for simple effects revealed reliably greater dwell times in male top and middle regions, but somewhat lower dwell times in the bottom region, Fs(1, 41) = 7.72, p = .008, ηp 2 = 0.16; 11.35, p = .002, ηp 2 = 0.22; and 3.37, p = .074, ηp 2 = 0.08, respectively. Patterns of visual attention to AOIs within each PLD, therefore, largely followed stimulus motion parameters described previously: Attention overall was greatest to male PLDs, in particular top and middle regions, mostly likely due to greater speeds and spans of dot motion.
Taken together, the results of Experiment 2 corroborate three key findings from Experiment 1: First, infant boys exhibit greater visual attention to PLDs than do infant girls; second, infants exhibit a bias toward male vs. female gait patterns when viewing PLDs; and third, neither of these effects appears to undergo significant developmental change between 6 and 15 months under tested circumstances (although the study may have been insufficiently powered to detect an age effect).
General Discussion
We report two experiments investigating infants' categorization of human biological motion displays from differences in walk motions produced by men and women, and infants' intrinsic preferences for such motions when point-light displays are placed side-by-side. We found positive evidence for categorization in infant boys, but not girls (Experiment 1); in addition, male PLDs were preferred to female PLDs (Experiments 1 & 2). These effects appear to stem from differences in the walk motions themselves—dot speed and motion span were higher in male vs. female PLDs, particularly in the top region (the head and shoulders)—and from the propensity for infant boys to be especially attuned to such motion. We observed infants from about 6 to 15 months in both studies, and found no age-related differences in performance in any of our measures, though the possibility remains that our study of PLD preferences was underpowered to detect age differences.
These data provide little evidence that development of biological motion perception in infancy has strict parallels with infant face perception, in that infants become increasingly sensitive to features of social stimuli that mark social categories, such as emotions, race, and sex, over the first year after birth (Hoehl, 2014; Lee, Quinn, & Pascalis, 2017; Ramsay et al., 2005). We found no evidence for improvements in categorization performance with age or for developments in motion processing that might facilitate categorization. As such, our findings imply that for infants' formation of sex categories from biological motion, there does not seem to be a process of perceptual tuning akin to those invoked to account for emergence of "expertise" at processing information in faces (Scott et al., 2007) or bodies (Bhatt et al., 2016). However, such a possibility remains for other kinds of social category that might be induced by PLDs, a question for future investigations.
We reasoned that infant attention to distinct regions in the stimuli, perhaps due to differences in marker speeds or extent of motion, might shed light on infant preferences and categorization (cf. Deng & Sloutsky, 2015; Quinn, Doran, Reiss, & Hoffman, 2009; see also Johnson & Tassinary, 2005; Johnson, Lurye, & Tassinary, 2010 for studies of sex discrimination in PLDs by adults and children). Male and female walk motions were likely made perceptually distinct by differences in low-level stimulus features, and it is possible that the “right” variability in dot motions and spans supported infant boys’ recognition as individuals, yet with shared features.
Evidence for categorization of PLDs by sex was observed only in infant boys—contrary to our expectation that girls might exhibit stronger social categorization skills—and infant boys showed heightened attention overall to PLDs relative to girls. These findings imply that the process of PLD category formation we observed stemmed largely from differences in motion patterns between men and women, and differences in attention to motion between infant boys and girls, rather than from recognition of PLD stimuli as representations of women and men (i.e., as distinct social categories). Although it is unknown whether infant boys' motion discrimination matures earlier in infancy, some visual functions such as binocularity (e.g., vergence eye movements, stereopsis; Held, Thorn, Gwiazda, & Bauer, 1996) and acuity (Makrides, Neumann, & Gibson, 2001) may develop earlier in girls, whereas other functions such as accommodation (Horwood & Ridell, 2008) and contrast sensitivity (Dobkins, Bosworth, & McCleery (2009) may develop earlier in boys. Some of these effects have been suggested to arise from differential rates of maturation of subcortical pathways responsible for coding low-level visual attributes (Dobkins et al., 2009; Vanston & Strother, 2017). Similar effects may be operational in infants' PLD learning and discrimination, to the extent that attention is directed to differences in motion cues that characterized sex-typed walk motions; if this is the case, it might explain the differences in performance between infant girls and boys, although ties with underlying maturational processes remain speculative.
Finally, we consider implications of our findings for biases in sex categorization reported in adults: When observers viewed computer-generated human figures with no cues to sexual dimorphism except waist-to-hip ratio (WHR) and were asked to judge whether each was a woman or a man, judgments tended to be biased toward males (Johnson et al., 2012). That is, even for some WHRs that characterize the female form in real life, observers judged them as male. (Moreover, some extreme WHRs that do not exist in real life were judged as acceptable for females.) The male bias was reduced when participants first viewed a film clip intended to induce a positive emotion (happiness) and exaggerated after viewing a clip to induce fear. Together these effects were suggested to arise from differences in physical formidability between men and women coupled with the costs associated with unwanted contacts with potentially more threatening individuals, implying a functional basis for the bias. Yet the origins of a male bias in adults' sex categorization remain unknown. Our findings are consistent with the possibility that adults' cognitive bias to perceive walk motion as male has its developmental origins in infants' perceptual bias to prefer walk motions with more motion and a greater span of motion across head, shoulders, and limbs—that is, male walk motions. As noted previously, the perceptual bias for male PLDs we have documented in infancy (Experiments 1 and 2) may provide an explanation for why infants in Experiment 1 habituated to males exhibited a strong familiarity preference, whereas infants habituated to females exhibited no consistent preference; a similar effect typifies differences in response latency for male and female categorizations among adults (Johnson et al., 2012). As infants accrue more experience viewing such motions (rooted in intrinsic preferences), this may lead to the establishment of the structural and dynamic signatures of male motion as the default for humans (a developmental process that might extend beyond infancy; cf. Johnson et al., 2010). It is not clear, however, how such an experience-dependent mechanism is constrained by differences in exposure to distinct individuals or groups.
Highlights.
We investigated infants' categorization of human biological motion from differences in men’s and women’s walk motions, and intrinsic preferences for such motions when point-light displays (PLDs) are placed side-by-side.
We found positive evidence for categorization in infant boys, but not girls, perhaps due to gender differences in infants’ motion processing.
Male PLDs were preferred to female PLDs.
We observed infants from about 4 to 18 months and found no age-related differences in performance in any of our measures.
Acknowledgments
This work was supported by a National Defense Science and Engineering Graduate Fellowship to T. Tsang and by NIH grant R01 HD-082844 to K. L. Johnson and S. P. Johnson. The authors would like to thank the UCLA Baby Lab crew for assistance with recruiting and testing, and especially the infants and parents who participated in our study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–479. doi: 10.1016/S1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Hines M. Sex differences in response to children’s toys in nonhuman primates (Cercopithecus aethiops sabaeus) Evolution and Human Behavior. 2002;23:467–479. doi: 10.1016/S1090-5138(02)00107-1. [DOI] [Google Scholar]
- Alexander GM, Wilcox T. Sex differences in early infancy. Child Development Perspectives. 2012;6:400–406. doi: 10.1111/j.1750-8606.2012.00247.x. [DOI] [Google Scholar]
- Allport GW. The nature of prejudice. Reading, MA: Addison Wesley; 1954. [Google Scholar]
- Anderson LC, Bolling DZ, Schelinski S, Coffman MC, Pelphrey KA, Kaiser MD. Sex differences in the development of brain mechanisms for processing biological motion. NeuroImage. 2013;83:751–760. doi: 10.1016/j.neuroimage.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi L, Regolin L, Simion F. Biological motion preference in humans at birth: Role of dynamic and configural properties. Developmental Science. 2011;14:353–359. doi: 10.1111/j.1467-7687.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3:203–206. doi: 10.1111/j.1467-9280.1992.tb00028.x. [DOI] [Google Scholar]
- Bertenthal BI. Perception of biomechanical motions by infants: Intrinsic image and knowledge-based constraints. In: Granrud C, editor. Carnegie symposium on cognition: Visual perception and cognition in infancy. Hillsdale, NJ: Erlbaum; 1993. pp. 175–214. [Google Scholar]
- Bertenthal BI, Proffitt DR, Kramer SJ. Perception of biomechanical motions by infants: Implementation of various processing constraints. Journal of Experimental Child Psychology: Human Perception and Performance. 1987a;13:577–585. doi: 10.1037/0096-1523.13.4.577. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Proffitt DR, Kramer SJ, Spetner NB. Infants’ encoding of kinetic displays varying in relative coherence. Developmental Psychology. 1987b;23:171–178. doi: 10.1037/0012-1649.23.2.171. [DOI] [Google Scholar]
- Bertenthal BI, Proffitt DR, Spetner NB, Thomas MA. The development of sensitivity to biomechanical motions. Child Development. 1985;56:531–543. www.jstor.org/stable/1129742. [PubMed] [Google Scholar]
- Bhatt RS, Hock A, White H, Jubran R, Galati A. The development of body structure knowledge in infancy. Child Development Perspectives. 2016;10:45–52. doi: 10.1111/cdep.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Ildei C, Kitromilides E, Orliaguet J, Pavlova M, Gentaz E. Preference for point-light human biological motion in newborns: Contribution of translational displacement. Developmental Psychology. 2014;50:113–120. doi: 10.1037/a0032956. [DOI] [PubMed] [Google Scholar]
- Billig M, Tajfel H. Social categorization and similarity in intergroup behavior. European Journal of Social Psychology. 1973;3:27–52. doi: 10.1002/ejsp.2420030103. [DOI] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2:561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Booth AE, Pinto J, Bertenthal BI. Perception of the symmetrical patterning of human gait by infants. Developmental Psychology. 2002;38:554–563. doi: 10.1037/0012-1649.38.4.554. [DOI] [PubMed] [Google Scholar]
- Bruner JS. On perceptual readiness. Psychological Review. 1957;64:123–152. doi: 10.1037/h0043805. [DOI] [PubMed] [Google Scholar]
- Constantinescu M, Moore DS, Johnson SP, Hines M. Early contributions to infants’ mental rotation abilities. Developmental Science. doi: 10.1111/desc.12613. (in press) [DOI] [PubMed] [Google Scholar]
- Clearfield MW, Nelson NM. Sex differences in mothers’ speech and play behavior with 6-, 9-, and 14-month-old infants. Sex Roles. 2006;54:127–137. doi: 10.1007/s11199-005-8874-1. [DOI] [Google Scholar]
- Cutting JE, Kozlowski LT. Recognizing friends by their walk: Gait perception without familiarity cues. Bulletin of the Psychonomic Society. 1977;9:353–356. [Google Scholar]
- Dittrich WH. Action categories and the perception of biological motion. Perception. 1993;22:15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Deng W, Sloutsky VM. The development of categorization: Effects of classification and inference training on category representation. Developmental Psychology. 2015;51:392–405. doi: 10.1037/a0038749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkins KR, Bosworth RG, McCleery JP. Effects of gestational length, gender, postnatal age, birth order on visual contrast sensitivity in infants. Journal of Vision. 2009;19:1–21. doi: 10.1167/9.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani L, Prasad S, Harber K, Shiffrar M. Recognizing people from their movements. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:210–220. doi: 10.1037/0096-1523.31.1.210. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Fausey CM, Jayaraman S, Smith LB. From faces to hands: Changing visual input in the first two years. Cognition. 2016;152:101–107. doi: 10.1016/j.cognition.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Frankenhuis WE, Barrett HC, Johnson SP. Developmental origins of biological motion perception. In: Johnson KL, Shiffrar M, editors. Visual perception of the human body in motion. New York: Oxford University Press; 2013. pp. 121–138. [Google Scholar]
- Frankenhuis WE, House B, Barrett HC, Johnson SP. Infants’ perception of chasing. Cognition. 2013;126:224–233. doi: 10.1016/j.cognition.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Johnson KL. More than meets the eye: Split second social perception. Trends in Cognitive Sciences. 2016;20:362–374. doi: 10.1016/j.tics.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held R, Thorn F, Gwiazda J, Bauer J. Development of binocularity and its sexual differentiation. In: Vital-Durand F, Atkinson J, Braddick O, editors. Infant Vision. Oxford: Oxford Scientific Press; 1996. pp. 265–274. [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends in Cognitive Sciences. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock A, Kangas A, Zieber N, Bhatt RS. The development of sex category representation in infancy: Matching of faces and bodies. Developmental Psychology. 2015;51:346–352. doi: 10.1037/a0038743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S. Emotion processing in infancy. In: Lagattuta KH, editor. Children and emotion: New insights into developmental affective sciences. Basel: Karger Press; 2014. pp. 1–12. [Google Scholar]
- Horwood AM, Riddell PM. Gender differences in early accommodation and vergence development. Ophthalmic and Physiological Optics. 2008;28:115–126. doi: 10.1111/j.1475-1313.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Hunter M, Ames E. A multifactor model of infant preferences for novel and familiar stimuli. Advances in Infancy Research. 1988;5:69–95. [Google Scholar]
- Hunter MA, Ames EW, Koopman R. Effects of stimulus complexity and familiarization time on infant preferences for novel and familiar stimuli. Developmental Psychology. 1983;19:338–352. doi: 10.1037/0012-1649.19.3.338. [DOI] [Google Scholar]
- Grossman ED, Blake R, Kim C. Learning to see biological motion: Brain activity parallels behavior. Journal of Cognitive Neuroscience. 2004;16:1669–1679. doi: 10.1162/0898929042568569. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–211. [Google Scholar]
- Johansson G. Spatio-temporal differentiation and integration in visual motion perception: An experimental and theoretical analysis of calculus-like functions in visual data processing. Psychological Research. 1976;38:379–393. doi: 10.1007/BF00309043. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Iida M, Tassinary LG. Person (mis)perception: functionally biased sex categorization of bodies. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4982–4989. doi: 10.1098/rspb.2012.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Lurye LE, Tassinary LG. Sex categorization among preschool children: Increasing utilization of sexually dimorphic cues. Child Development. 2010;81:1346–1355. doi: 10.1111/j.1467-8624.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- Johnson KL, McKay LS, Pollick FE. He throws like a girl (but only when he’s sad): Emotion affects sex-decoding of biological motion displays. Cognition. 2011;119:265–280. doi: 10.1016/j.cognition.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Tassinary LG. Perceiving sex directly and indirectly: Meaning in motion and morphology. Psychological Science. 2005;16:890–897. doi: 10.1111/j.1467-9280.2005.01633.x. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Senju A, Tomalski P. The two-process theory of face processing: Modifications based on two decades of data from infants and adults. Neuroscience and Biobehavioral Reviews. 2015;50:169–179. doi: 10.1016/j.neubiorev.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, Ge L, Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Ellenberg L, Leonard J, Share LJ. Developmental sex differences in global-local perceptual bias. Neuropsychology. 1996;10:402–407. doi: 10.1037/0894-4105.10.3.402. [DOI] [Google Scholar]
- Kuhlmeier VA, Troje NF, Lee V. Young infants detect the direction of biological motion in point-light displays. Infancy. 2010;15:83–93. doi: 10.1111/j.1532-7078.2009.00003.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Quinn PC, Olivier P. Other-race effect and racial bias: A Perceptual-Social Linkage hypothesis. Current Directions in Psychological Science. 2017;26:256–262. doi: 10.1177/0963721417690276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social: Why our brains are wired to connect. New York: Crown Publishers; 2013. [Google Scholar]
- Liu S, Xiao WS, Xiao NG, Quinn PC, Zhang Y, Chen H, Ge L, Pascalis O, Lee K. Development of visual preference for own- vs. other-race faces in infancy. Developmental Psychology. 2015;51:500–511. doi: 10.1037/a0038835. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. The decline of cross-species intersensory perception in human infants. Proceedings of the National Academy of Sciences (USA) 2006;103:6771–6774. doi: 10.1073/pnas.0602027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lick DJ, Johnson KL, Gill SV. Deliberate changes to gendered body motion influence basic social perceptions. Social Cognition. 2013;31:656–671. doi: 10.1521/soco.2013.31.6.656. [DOI] [Google Scholar]
- Makrides M, Neumann MA, Gibson RA. Perinatal characteristics may influence the outcome of visual acuity. Lipids. 2001;36:897–900. doi: 10.1007/s11745-001-0799-0. [DOI] [PubMed] [Google Scholar]
- Mather G, Murdoch L. Gender discrimination in biological motion displays based on dynamic cues. Proceedings of the Royal Society of London B: Biological Sciences. 1994;258:273–279. doi: 10.1098/rspb.1994.0173. [DOI] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295X.98.2.164. [DOI] [PubMed] [Google Scholar]
- Norman JF, Payton SM, Long JR, Hawkes LM. Aging and the perception of biological motion. Psychology and Aging. 2004;19:219–225. doi: 10.1037/0882-7974.19.1.219. [DOI] [PubMed] [Google Scholar]
- Pollick FE, Kay J, Heim K, Stringer R. Gender recognition from point-light walkers. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1247–1265. doi: 10.1037/0096-1523.31.6.1247. [DOI] [PubMed] [Google Scholar]
- Pomerantz JR. Global and local precedence: Selective attention in form and motion perception. Journal of Experimental Psychology: General. 1983;112:516–540. doi: 10.1037/0096-3445.112.4.516. [DOI] [PubMed] [Google Scholar]
- Proverbio AM. Sex differences in social cognition: The case of face processing. Journal of Neuroscience Research. 2016;95:222–234. doi: 10.1002/jnr.23817. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Doran MM, Reiss JE, Hoffman JE. Time course of visual attention in infant categorization of cats versus dogs: Evidence for a head bias as revealed through eye tracking. Child Development. 2009;80:151–161. doi: 10.1111/j.1467-8624.2008.01251.x. http://www.jstor.org/stable/29738603. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Eimas PD, Rosenkrantz SL. Evidence for representations of perceptually similar natural categories by 3-month-old and 4-month-old infants. Perception. 1993;22:463–475. doi: 10.1068/p220463. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Ramsey JL, Langlois JH, Marti NC. Infant categorization of faces: Ladies first. Developmental Review. 2005;25:212–246. doi: 10.1016/j.dr.2005.01.001. [DOI] [Google Scholar]
- Reid VM, Dunn KJ, Young RJ, Amu J, Donovan T, Reissland N. The human fetus preferentially engages with face-like visual stimuli. Current Biology. 2017;27:1825–1828. doi: 10.1016/j.cub.2017.05.044. [DOI] [PubMed] [Google Scholar]
- Roalf D, Lowery N, Turetsky BI. Behavioral and physiological findings of gender differences in global-local visual processing. Brain and Cognition. 2006;60:32–42. doi: 10.1016/j.bandc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Roder BJ, Bushnell EW, Sasseville AM. Infant’s preferences for familiarity and novelty during the course of visual processing. Infancy. 2000;1:491–507. doi: 10.1207/S15327078IN0104_9. [DOI] [PubMed] [Google Scholar]
- Salyer DL, Lund TD, Fleming DE, Lephart ED, Horvath TL. Sexual dimorphism and aromatase in the rat retina. Developmental Brain Research. 2001;126:131–136. doi: 10.1016/S0165-3806(00)00147-4. [DOI] [PubMed] [Google Scholar]
- Schmuckler MA, Fairhall JL. Visual-proprioceptive intermodal perception using point light displays. Child Development. 2001;72:949–962. doi: 10.1111/1467-8624.00327. [DOI] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16:197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Weng X, He S, Jiang Y. Biological motion cues trigger reflexive attentional orienting. Cognition. 2010;117:348–354. doi: 10.1016/j.cognition.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences (USA) 2008;105:809–813. doi: 10.1073/pnas.0707021105. 10.1073 pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Nicolini Y, Shetler M, Soumi SJ, Ferrari PF, Paukner A. Experience-independent sex differences in newborn macaques: Females are more social than males. Scientific Reports. 2016;6:19669. doi: 10.1038/srep19669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singarajah A, Chanley J, Gutierrez Y, Cordon Y, Nguyen B, Burakowski L, Johnson SP. Infant attention to same- and other-race faces. Cognition. 2017;159:76–84. doi: 10.1016/j.cognition.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soken NH, Pick AD. Intermodal perception of happy and angry expressive behaviors by seven- month-old infants. Child Development. 1992;63:787–795. doi: 10.1111/j.1467-8624.1992.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Sung J, Fausto-Sterling A, García Coll C, Seifer R. The dynamics of age and sex in the development of mother-infant vocal communication between 3 and 11 months. Infancy. 2013;18:1135–1158. 10:1111/infa.12019. [Google Scholar]
- Troje NF. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. Journal of Vision. 2002;2:371–387. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Troje NF, Westhoff C, Lavrov M. Person identification from biological motion: Effects of structural and kinematic cues. Perception & Psychophysics. 2005;67:667–675. doi: 10.3758/bf03193523. [DOI] [PubMed] [Google Scholar]
- Vanston JE, Strother L. Sex differences in the human visual system. Journal of Neuroscience Research. 2017;95:617–625. doi: 10.1002/jnr.23895. [DOI] [PubMed] [Google Scholar]
- Zieber N, Kangas A, Hock A, Bhatt RS. The development of intermodal emotion perception from bodies and voices. Journal of Experimental Child Psychology. 2014;126:68–79. doi: 10.1016/j.jecp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Zieber N, Kangas A, Hock A, Bhatt RS. Infants’ perception of emotion from body movements. Child Development. 2014;85:675–684. doi: 10.1111/cdev.12134. [DOI] [PubMed] [Google Scholar]