Abstract
Objective
To describe caregiver burden among those whose children survive in-hospital cardiac arrest and have high risk of neurologic disability, and explore factors associated with burden during the first year post-arrest.
Methods
The study is a secondary analysis of the Therapeutic Hypothermia after Paediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial. 329 children who had an in-hospital cardiac arrest, chest compressions for >2 minutes, and mechanical ventilation after return of circulation were recruited to THAPCA-IH. Of these, 155 survived to one year, and caregivers of 138 were assessed for burden. Caregiver burden was assessed at baseline, and 3 and 12 months post-arrest using the Infant Toddler Quality of Life Questionnaire for children <5 years old and the Child Health Questionnaire for children >5 years. Child functioning was assessed using the Vineland Adaptive Behaviour Scales Second Edition (VABS-II), the Paediatric Overall Performance Category (POPC) and Paediatric Cerebral Performance Category (PCPC) scales, and caregiver perception of global functioning.
Results
Of 138 children, 77 (55.8%) were male, 77 (55.8%) were white, and 109 (79.0%) were <5 years old at the time of arrest. Caregiver burden was greater than reference norms at all time points. Worse POPC, PCPC and VABS-II scores at 3 months post-arrest were associated with greater caregiver burden at 12 months. Worse global functioning at 3 months was associated with greater burden at 12 months for children <5 years.
Conclusions
Caregiver burden is substantial during the first year after paediatric in-hospital cardiac arrest, and associated with the extent of the child’s neurobehavioural dysfunction.
Keywords: In-hospital cardiac arrest, Paediatric, Caregiving, Neurobehavioural outcome
INTRODUCTION
Caregiver burden is a multidimensional concept that reflects the stress and time demands of providing care for another person [1–3]. Parents providing care for children with disabilities often experience caregiver burden, which can lead to impaired parental health and decreased ability to care for the child. Children who survive a cardiac arrest may have disabilities due to the underlying condition that led to the arrest as well as neurologic injury incurring during the arrest [4–7]. The extent of a child’s disability has been associated with caregiver burden after out-of-hospital cardiac arrest and other chronic complex childhood conditions [8–14]. Out-of-hospital and inhospital arrest in children have different aetiologies, treatments and outcomes, and the associated degree and determinants of caregiver burden may be different for these two conditions [5–7]. For example, most children with out-of-hospital cardiac arrest are healthy before the arrest whereas most children with in-hospital cardiac arrest have pre-existing illness that led to the hospitalisation in which the arrest occurred. Greater knowledge about caregiver burden after a child’s in-hospital cardiac arrest would guide development of supportive interventions tailored to meet families’ needs.
The Therapeutic Hypothermia after Paediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial was a randomised trial comparing the efficacy of two targeted temperature management interventions on survival with good neurobehavioural function in children one year after in-hospital cardiac arrest [15]. All children recruited to the THAPCA-IH trial were comatose, required mechanical ventilation after return of circulation, and were at high risk for neurologic injury. Results of the trial showed that neither treatment arm conferred a significant benefit on survival with good neurobehavioural function. As part of the THAPCA-IH trial, we evaluated caregiver burden at baseline (reflecting pre-arrest burden), and 3 and 12 months post-arrest. We hypothesised that caregiver burden is substantial during the first year after a child’s in-hospital cardiac arrest and that the extent of the child’s disability is associated with caregiver burden. Our objective was to describe caregiver burden among those whose children survived in-hospital cardiac arrest and were at high risk of neurologic injury, and explore factors associated with burden during the first year post-arrest.
METHODS
Study Design
This study is a secondary analysis of the THAPCA-IH trial. Thirty-seven children’s hospitals in the United States, Canada, and the United Kingdom participated between September 1, 2009 and February 27, 2015. Details of the trial were previously published [15–17]. The study was approved by the institutional review boards at all sites and the Data Coordinating Centre at the University of Utah. Parent/guardian permission was obtained for all participants.
Study Population
The THAPCA-IH trial recruited 329 children [15]. Inclusion criteria were age 48 hours to 18 years, occurrence of an in-hospital cardiac arrest with compressions for >2 minutes, and dependence on mechanical ventilation after return of circulation. Major exclusion criteria were a Glasgow Coma Scale motor subscale score of 5 or 6 [18], inability to be randomised within 6 hours of return of circulation, pre-existing terminal illness with life expectancy <12 months, and a decision by the clinical team to withhold aggressive treatment. Of those recruited, 155 survived to one year. Caregivers (i.e., parents and/or guardians) of 138 were assessed for burden.
Outcomes
The primary outcome was caregiver burden 12 months after in-hospital cardiac arrest. Caregiver burden was assessed using two scales from the Infant and Toddler Quality of Life Questionnaire (ITQOL) [19, 20] for children <5 years old, and three scales from the Child Health Questionnaire (CHQ) [21] for children >5 years. The two scales of the ITQOL used to assess caregiver burden were (1) parent impact-emotion, and (2) parent impact-time [19]. The parent impact-emotion scale assesses how much parental anxiety or worry are caused by the child’s physical and psychological problems. The parent impact-time scale assesses how much the parent’s personal time is limited by the child’s problems. The mean of the responses for each ITQOL scale is transformed to 0–100 with higher scores indicating lesser burden. Normative reference data from a U.S. population are not available; however, Dutch reference data exist [20].
The three scales of the CHQ used to assess caregiver burden are (1) parent impact-emotion, (2) parent impact-time, and (3) family activities [21]. The parent impact-emotion and parent impact-time scales are similar to the corresponding scales of the ITQOL. The family activities scale assesses how often the child’s health or behaviour interferes with family activities. The mean of the responses for each CHQ scale is transformed to 0–100 with higher scores indicating lesser burden. Normative reference data from a U.S. population exist [21]. Details of the ITQOL and CHQ can be found at https://www.healthactchq.com/surveys.php
Independent variables
Independent variables included child and caregiver sociodemographics, child clinical characteristics, family functioning and child functioning. Child clinical characteristics included pre-existing conditions, presence of congenital heart disease, occurrence of arrest post-cardiac surgery, primary aetiology of arrest, and use of extracorporeal membrane oxygenation at THAPCA-IH treatment initiation. Family functioning was assessed using the General Functioning Scale of the Family Assessment Device (FAD-GF) [22]. Child functioning was assessed using the Vineland Adaptive Behaviour Scales Second Edition (VABS-II) [23], the Paediatric Overall Performance Category (POPC) and Paediatric Cerebral Performance Category (PCPC) scales [24], and caregiver’s perception of global functioning.
The FAD-GF is a 12-item measure used to distinguish healthy and unhealthy family functioning [22]. Each item is rated using a 4-point scale. Total scores are the mean of the item responses. A score >2 indicates unhealthy functioning.
The VABS-II is a measure of adaptive behaviour from birth through adulthood [23]. Adaptive behaviour refers to a person’s performance on daily life activities necessary for personal and social independence. VABS-II domains include communication, daily living, socialisation, and motor skills. The number of items that can be performed in each domain is standardised for the child’s age. In a normative U.S. population, the mean VABS-II score is 100 and standard deviation is 15. Higher scores indicate better functioning.
POPC and PCPC scales are used to assess overall health and neurological functioning, respectively [24]. Both are 6-point scales of increasing disability. Scores are 1 for good/normal, 2 for mild disability, 3 for moderate disability, 4 for severe disability, 5 for coma or vegetative state, and 6 for death.
Caregiver perception of global child functioning was assessed using items developed by the investigators. At baseline, caregivers were asked, “Compared with children of the same age, were your child’s home, school or social activities limited before his/her cardiac arrest? Response choices were, “not limited, limited a little, or limited a lot.” At 3 and 12 months, caregivers were asked (1) “Compared with children of the same age, are your child’s home, school or social activities limited now?” Response choices were, “not limited, limited a little, or limited a lot.” (2) “Thinking about your child since his/her cardiac arrest, has he/she gained a lot of new skills, gained a few new skills, stayed the same, lost a few skills, or lost a lot of skills.
Procedures
Trained research coordinators at the local sites assisted caregivers with completing baseline measures (ITQOL, CHQ, FAD-GF, VABS-II, and global functioning) within 24 hours of recruitment. Baseline measures were intended to reflect pre-arrest status. Research coordinators rated POPC and PCPC scores at baseline and hospital discharge. At 3 and 12 months post-arrest, caregivers completed measures (ITQOL, CHQ, VABS-II, POPC, PCPC, and global functioning) via telephone with an interviewer from the Kennedy Krieger Institute.
Statistical Analyses
Baseline characteristics were summarised using frequencies and percentages for categorical variables and medians and quartiles for continuous variables. The caregiver burden scales and VABS-II were summarised at baseline, 3 months, and 12 months using the mean and standard deviation. The caregiver burden scales at each time point were compared to reference values using the t-test, and the difference from baseline to 3 and 12 months as well as the difference from 3 to 12 months were examined using the paired t-test. Spearman correlations were used to assess associations between the caregiver burden scales and the independent variables. The reference values for each caregiver burden scale were used to calculate z-scores at month 12. These z-scores were used to categorise caregiver burden as mildly elevated/normal (z-score −1.5 to 1.5), moderately elevated (z-score −3 to −1.5) or highly elevated (z-score < -3). All analyses were completed using SAS software v9.4 (Cary, NC).
RESULTS
Of 138 children, 77 (55.8%) were male, 77 (55.8%) were white, and 109 (79.0%) were <5 years old at the time of arrest (Table 1). One hundred and twenty-five (90.6%) had a pre-existing condition. Eighty-two (59.4%) had congenital heart disease and 51 (37.0%) had cardiac surgery. Aetiology of arrest was cardiac for 85 (61.6%), respiratory for 46 (33.3%) and other/unknown for 7 (5.1%). Pre-arrest adaptive behaviour was similar to reference norms for both age groups. Pre-arrest POPC and PCPC were in the good/normal to mild disability range for 107 (77.5%) and 116 (84.1%), respectively. Children’s global functioning was assessed as not limited or limited a little for 106 (76.8%). Among caregivers, 114 (82.6%) had at least a high school diploma. Family functioning was assessed as healthy for 119 (86.2%).
Table 1.
Descriptive Characteristics
| Pre-arrest Caregiver Burden Measure | ||
|---|---|---|
| ITQOL (N = 109) | CHQ (N = 29) | |
| Age at cardiac arrest (years), median [IQR] | 0.4 [0.1–1.3] | 11.5 [9.0–14.3] |
| Male, No. (%) | 61 (56.0) | 16 (55.2) |
| Race, No. (%) | ||
| Black or African American | 30 (27.5) | 9 (31.0) |
| White | 59 (54.1) | 18 (62.1) |
| Other/Unknown | 20 (18.3) | 2 (6.9) |
| Ethnicity, No. (%) | ||
| Hispanic or Latino | 24 (22.0) | 5 (17.2) |
| Not Hispanic or Latino | 79 (72.5) | 24 (82.8) |
| Unknown | 6 (5.5) | 0 (0.0) |
| Caregiver’s highest education received, No. (%) | ||
| Some high school or less | 15 (13.8) | 8 (27.6) |
| High school graduate or General Equivalency Diploma | 32 (29.4) | 3 (10.3) |
| Vocational school or some college | 31 (28.4) | 7 (24.1) |
| College degree | 17 (15.6) | 10 (34.5) |
| Graduate or doctoral degree | 13 (11.9) | 1 (3.4) |
| Question not answered | 1 (0.9) | 0 (0.0) |
| Pre-existing conditions, No. (%) | ||
| Any pre-existing condition | 100 (91.7) | 25 (86.2) |
| Cardiac condition | 82 (75.2) | 13 (44.8) |
| Respiratory condition | 33 (30.3) | 9 (31.0) |
| Neurologic condition | 26 (23.9) | 14 (48.3) |
| Gastrointestinal condition | 32 (29.4) | 3 (10.3) |
| Prenatal condition | 32 (29.4) | 4 (13.8) |
| Other pre-existing condition | 43 (39.4) | 11 (37.9) |
| Congenital heart disease, No. (%) | 75 (68.8) | 7 (24.1) |
| Arrest post-cardiac surgery, No. (%) | 47 (43.1) | 4 (13.8) |
| Primary aetiology of arrest, No. (%) | ||
| Cardiac | 69 (63.3) | 16 (55.2) |
| Respiratory | 37 (33.9) | 9 (31.0) |
| Other/Unknown | 3 (2.8) | 4 (13.8) |
| ECMO at treatment initiation, No. (%) | 53 (48.6) | 15 (51.7) |
| Pre-cardiac arrest VABS-II Composite Score, median [IQR] | 92.0 [78.0–101.0] | 98.0 [82.0–119.0] |
| Pre-cardiac arrest POPC, No. (%) | ||
| Good = 1 | 37 (33.9) | 16 (55.2) |
| Mild disability = 2 | 48 (44.0) | 6 (20.7) |
| Moderate disability = 3 | 19 (17.4) | 5 (17.2) |
| Severe disability = 4 | 5 (4.6) | 2 (6.9) |
| Pre-cardiac arrest PCPC, No. (%) | ||
| Normal = 1 | 61 (56.0) | 21 (72.4) |
| Mild disability = 2 | 32 (29.4) | 2 (6.9) |
| Moderate disability = 3 | 13 (11.9) | 4 (13.8) |
| Severe disability = 4 | 3 (2.8) | 2 (6.9) |
| Pre-cardiac arrest global child functioning: Parent perception of limitations, No. (%) | ||
| Not limited | 57 (52.2) | 20 (69.0) |
| Limited a little | 23 (21.1) | 6 (20.7) |
| Limited a lot | 16 (14.6) | 3 (10.3) |
| Missing | 13 (11.9) | 0 (0.0) |
| Pre-cardiac arrest FAD, No. (%) | ||
| Healthy family functioning | 92 (84.4) | 27 (93.1) |
| Unhealthy family functioning | 15 (13.7) | 2 (6.9) |
| Missing | 2 (1.8) | 0 (0.0) |
Abbreviations: ITQOL, Infant Toddler Quality of Life; CHQ, Child Health Questionnaire; IQR, interquartile range; ECMO, extracorporeal membrane oxygenation; VABS-II, Vineland Adaptive Behaviour Scale, Second Edition; POPC, Paediatric Overall Performance Category; PCPC, Paediatric Cerebral Performance Category; FAD, Family Assessment Device.
For children <5 years old, pre-arrest caregiver burden was greater (i.e., lower ITQOL scores) than reference values (Table 2). Caregiver burden at 3 months post-arrest was similar to pre-arrest; caregiver burden at 12 months was improved compared to pre-arrest and 3-month values. For children >5 years, pre-arrest caregiver burden was greater (i.e., lower CHQ parent impact-emotion and family activity scores) than reference values (Table 3). Caregiver burden at 3 months post-arrest was greater (i.e., lower parent impact-time and family activity scores) than pre-arrest values. Caregiver burden at 12 months post-arrest was improved compared to 3-months and was similar to pre-arrest. In both age groups, mean caregiver burden was greater at all times compared to reference values.
Table 2.
Infant Toddler Quality of Life Caregiver Burden Measures over Time
| Time point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Reference | Pre-cardiac arrest | P-valuea | Month 3 | P-valuea | P-valueb | Month 12 | P-valuea | P-valueb | P-valuec | |
|
| ||||||||||
| ITQOL Parent Impact - Emotional | ||||||||||
| N | 106 | 109 | 107d | |||||||
| Standardized score, mean (SD) | 92.1 (10.84) | 73.7 (25.56) | <.001 | 76.3 (23.65) | <.001 | 0.431 | 85.5 (16.67) | <.001 | <.001 | <.001 |
|
| ||||||||||
| ITQOL Parent Impact - Time | ||||||||||
| N | 107 | 109 | 107d | |||||||
| Standardized score, mean (SD) | 93 (10.92) | 81.5 (22.71) | <.001 | 80.4 (22.45) | <.001 | 0.590 | 87.4 (18.76) | 0.003 | 0.025 | 0.003 |
|
| ||||||||||
| VABS-II Composite Score | ||||||||||
| N | 109 | 109 | 107 | |||||||
| Standardized score, mean (SD) | 100 (15) | 89.8 (16.44) | 80.0 (18.63) | 80.3 (17.17) | ||||||
Abbreviations: ITQOL, Infant Toddler Quality of Life, VABS-II, Vineland Adaptive Behaviour Scale, Second Edition.
P-value from t-test comparing caregiver burden measure to reference.
P-value from paired t-test comparing caregiver burden measure to pre-arrest.
P-value from paired t-test comparing the change in caregiver burden measure from month 3 to month 12.
Two children whose caregivers completed the ITQOL at 3 months were administered the CHQ at 12 months because the children turned 5 years between the 3- and 12-month assessments.
Table 3.
Child Health Questionnaire Caregiver Burden Measures over Time
| Time point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Reference | Pre-cardiac arrest | P-valuea | Month 3 | P-valuea | P-valueb | Month 12 | P-valuea | P-valueb | P-valuec | |
|
| ||||||||||
| CHQ Parent Impact - Emotional | ||||||||||
| N | 29 | 29 | 31d | |||||||
| Standardized score, mean (SD) | 80.3 (19.1) | 54.3 (28.92) | <.001 | 44.3 (34.08) | <.001 | 0.230 | 61.6 (28.52) | <.001 | <.336 | <.008 |
|
| ||||||||||
| CHQ Parent Impact - Time | ||||||||||
| N | 29 | 29 | 31d | |||||||
| Standardized score, mean (SD) | 87.8 (19.9) | 77.0 (29.09) | <.056 | 50.6 (35.58) | <.001 | <.001 | 68.5 (33.59) | 0.003 | 0.140 | 0.033 |
|
| ||||||||||
| CHQ Family Activities | ||||||||||
| N | 29 | 29 | 31d | |||||||
| Standardized score, mean (SD) | 89.7 (18.6) | 70.3 (27.59) | <.001 | 54.5 (26.49) | <.001 | 0.031 | 65.9 (24.28) | <.001 | 0.380 | 0.035 |
|
| ||||||||||
| VABS-II Composite Score | ||||||||||
| N | 29 | 29 | 31d | |||||||
| Standardized score, mean (SD) | 100 (15) | 96.3 (28.17) | 75.4 (22.76) | 82.3 (25.68) | ||||||
Abbreviations: CHQ, Child Health Questionnaire, VABS-II, Vineland Adaptive Behaviour Scale, Second Edition.
P-value from t-test comparing caregiver burden measure to reference.
P-value from paired t-test comparing caregiver burden measure to pre-arrest.
P-value from paired t-test comparing the change in caregiver burden measure from month 3 to month 12.
Two children whose caregivers completed the ITQOL at 3 months were administered the CHQ at 12 months because the children turned 5 years between the 3- and 12-month assessments.
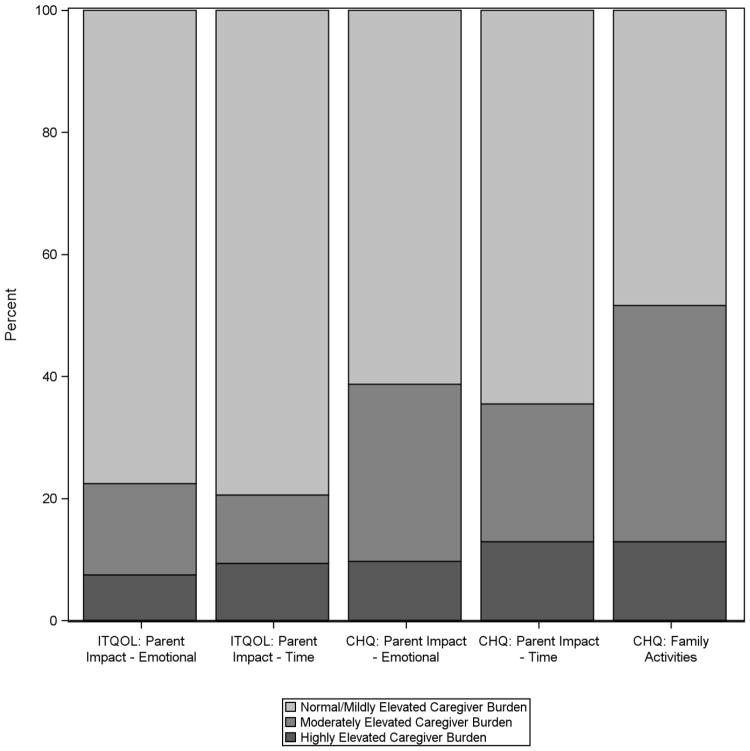
For caregivers of children <5 years old, 24 (22.4%) reported moderate to highly elevated burden for the parent impact-emotion domain and 22 (20.6%) the parent impact-time domain at 12 months post-arrest (Figure 1). For caregivers of children >5 years old, 12 (38.7%) reported moderate to highly elevated burden for the parent impact-emotion domain, 11 (35.4%) for the parent impact-time domain, and 16 (51.6%) for the family activities domain.
Figure 1. Caregiver Burden 12 Months after a Child’s In-Hospital Cardiac Arrest.
Percent of caregivers reporting normal/mild, moderate, and high levels of burden for each assessed caregiver burden domain12 months after their child’s cardiac arrest. Abbreviations: ITQOL, Infant Toddler Quality of Life; CHQ, Child Health Questionnaire.
Several measures of child function prior to 3 months post-arrest correlated moderately with caregiver burden at 3 months (Table 4). Worse pre-arrest POPC and PCPC correlated with greater caregiver worry (i.e., parent impact-emotion) for children <5 years old. Worse POPC and/or PCPC at hospital discharge correlated with greater caregiver worry and limitation in personal time (i.e., parent-impact-time) for children in both age groups. Worse POPC, PCPC, VABS-II and global functioning at 3 months post-arrest correlated with greater caregiver worry and limitation in personal time for children in both age groups. Higher caregiver education correlated with greater caregiver worry for children <5 years old and greater caregiver burden in all domains for children >5 years old. Healthy family functioning correlated with greater caregiver worry and limitation in personal time for children <5 years old. Race, ethnicity, and the presence of pre-existing conditions did not correlate with caregiver burden (Data not shown).
Table 4.
Month 3 Caregiver Burden Measure Correlations
| Covariate | ITQOL Parent Impact - Emotion | ITQOL Parent Impact - Time | CHQ Parent Impact - Emotion | CHQ Parent Impact - Time | CHQ Family Activities |
|---|---|---|---|---|---|
| Pre-cardiac arrest | |||||
| Age at randomization (years) | 0.006 | −0.030 | −0.154 | −0.039 | −0.142 |
| Caregiver's highest education received | −0.248* | −0.125 | −0.431* | −0.496* | −0.568* |
| Global child functioning: Parent perception of limitations | −0.090 | −0.139 | −0.146 | −0.114 | −0.144 |
| Average FAD score | 0.197* | 0.194* | −0.346 | −0.306 | −0.180 |
| VABS-II Composite Score | 0.162 | −0.034 | 0.088 | −0.009 | −0.171 |
| POPC | −0.198* | −0.114 | −0.016 | 0.060 | 0.140 |
| PCPC | −0.192* | −0.091 | −0.114 | 0.050 | 0.081 |
| Hospital discharge | |||||
| POPC | −0.403* | −0.204* | −0.330 | −0.214 | −0.060 |
| PCPC | −0.346* | −0.142 | −0.484* | −0.369* | −0.163 |
| Month 3 | |||||
| VABS-II Composite Score | 0.444* | 0.363* | 0.640* | 0.591* | 0.409* |
| POPC | −0.465* | −0.469* | −0.706* | −0.574* | −0.327 |
| PCPC | −0.431* | −0.341* | −0.644* | −0.590* | −0.424* |
| Global child functioning: Parent perception of limitations | −0.492* | −0.383* | −0.603* | −0.415* | −0.265 |
| Global child functioning: Parent perception of skills | −0.235* | −0.339* | −0.610* | −0.420* | −0.342 |
Abbreviations: ITQOL, Infant Toddler Quality of Life; CHQ, Child Health Questionnaire; FAD, Family Assessment Device; VABS-II, Vineland Adaptive Behaviour Scale, Second Edition; POPC, Paediatric Overall Performance Category; PCPC, Paediatric Cerebral Performance Category.
Indicates p-value < 0.05
Several measures of child function prior to 12 months post-arrest correlated moderately with caregiver burden at 12 months (Table 5). Worse pre-arrest POPC and PCPC correlated with greater limitation in caregiver personal time for children >5 years old. Worse POPC and/or PCPC at hospital discharge correlated with greater caregiver worry and limitation in personal time for children in both age groups. Worse POPC, PCPC, VABS-II and global functioning at 3 months and 12 months post-arrest correlated with greater caregiver burden in all domains for children <5 years old, and with greater burden in most domains for children >5 years old. Higher caregiver education correlated with greater limitation in caregiver personal time for children <5 years old and greater caregiver worry and interference with family activities for children >5 years old. Family functioning did not correlate with caregiver burden at 12 months. Race, ethnicity and the presence of pre-existing conditions also did not correlate with burden (Data not shown).
Table 5.
Month 12 Caregiver Burden Measure Correlations
| Covariate | ITQOL Parent Impact - Emotion | ITQOL Parent Impact - Time | CHQ Parent Impact - Emotion | CHQ Parent Impact - Time | CHQ Family Activities |
|---|---|---|---|---|---|
| Pre-cardiac arrest | |||||
| Age at randomization (years) | −0.045 | −0.055 | −0.322 | −0.186 | −0.253 |
| Caregiver's highest education received | −0.173 | −0.218* | −0.390* | −0.148 | −0.362* |
| Global child functioning: Parent perception of limitations | −0.104 | −0.086 | −0.176 | −0.080 | −0.106 |
| Average FAD score | −0.054 | 0.004 | −0.237 | −0.208 | −0.241 |
| VABS-II Composite Score | 0.144 | 0.085 | 0.157 | 0.314 | 0.196 |
| POPC | −0.126 | −0.076 | −0.139 | −0.423* | −0.232 |
| PCPC | −0.151 | −0.137 | −0.246 | −0.456* | −0.344 |
| Hospital discharge | |||||
| POPC | −0.311* | −0.316* | −0.250 | −0.303 | −0.191 |
| PCPC | −0.254* | −0.215* | −0.364* | −0.427* | −0.291 |
| Month 3 | |||||
| VABS-II Composite Score | 0.431* | 0.300* | 0.548* | 0.504* | 0.429* |
| POPC | −0.456* | −0.402* | −0.318 | −0.532* | −0.377* |
| PCPC | −0.382* | −0.311* | −0.456* | −0.568* | −0.346 |
| Global child functioning: Parent perception of limitations | −0.305* | −0.243* | −0.115 | −0.150 | −0.092 |
| Global child functioning: Parent perception of skills | −0.203* | −0.207* | −0.220 | −0.165 | −0.174 |
| Corresponding caregiver burden measure | 0.571* | 0.356* | 0.555* | 0.361 | 0.460* |
| Month 12 | |||||
| VABS-II Composite Score | 0.466* | 0.265* | 0.432* | 0.587* | 0.466* |
| POPC | −0.482* | −0.420* | −0.310 | −0.556* | −0.365* |
| PCPC | −0.397* | −0.287* | −0.439* | −0.611* | −0.431* |
| Global child functioning: Parent perception of limitations | −0.367* | −0.311* | −0.417* | −0.548* | −0.453* |
| Global child functioning: Parent perception of skills | −0.233* | −0.211* | −0.502* | −0.419* | −0.470* |
Abbreviations: ITQOL, Infant Toddler Quality of Life; CHQ, Child Health Questionnaire; FAD, Family Assessment Device; VABS-II, Vineland Adaptive Behaviour Scale, Second Edition; POPC, Paediatric Overall Performance Category; PCPC, Paediatric Cerebral Performance Category.
Indicates p-value < 0.05
DISCUSSION
Our findings demonstrate that caregiver burden is substantial during the first year after paediatric in-hospital cardiac arrest. The extent of the child’s disability 3 months post-arrest was associated with caregiver burden at 12 months. Higher caregiver education was associated with greater burden 3 and 12 months post-arrest. Healthy family functioning was associated with greater burden 3 months post-arrest for caregivers of children <5 years old, but not associated with burden by 12 months. Race, ethnicity and the presence of pre-existing conditions were not associated with caregiver burden.
Although caregiver burden persists throughout the first year after paediatric in-hospital cardiac arrest, the temporal pattern of burden was different for caregivers of children <5 years and >5 years old. For caregivers of children <5 years old, burden at 3 months post-arrest was similar to pre-arrest baseline, and declined to less than baseline by 12-months. Many of these younger children had congenital heart disease and were post-operative (43.1%) at the time of arrest. At baseline, the high level of burden may reflect caring for a child with an unrepaired heart defect. By 12-months, these children would have recovered from their surgery and despite the cardiac arrest during the hospitalisation, their care at 12 months resulted in less burden than prior to their cardiac repair. Prior research has shown that young children with congenital heart disease have functional disabilities and that their parents have increased illness-related stress, although the time course of parental stress, coping and adaptation have not been well characterised [25, 26].
For caregivers of children >5 years old, burden was greater at 3 months post-arrest than pre-arrest baseline, and declined to baseline by 12 months. The decline in burden at 12 months post-arrest to baseline suggests that for many caregivers the child’s arrest did not contribute to burden in the long-term beyond that already present due to the illness that led to the arrest. It is also possible that the small number of children in the >5 year age group (n=29) made differences in burden difficult to demonstrate. Importantly, caregiver burden was significantly more than reference norms at all time points in both age groups.
Consistent with results exploring caregiver burden in the Therapeutic Hypothermia after Paediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial [8], caregiver-reported disability (VABS-II) and global functioning were moderately associated with caregiver burden. In contrast to the THAPCA-IH trial, most children in THAPCA-OH were healthy pre-arrest [27] and many survivors had severe disability post-arrest [28, 29]; caregiver burden at pre-arrest baseline was similar to reference norms and persistently elevated above baseline at 3 and 12 months post-arrest. The greater burden perceived by caregivers after a child’s out-of-hospital arrest may be related to a greater degree of post-arrest disability and the experience of caring for a disabled child when the child was previously healthy.
A higher level of caregiver education was associated with greater caregiver burden after paediatric in-hospital cardiac arrest. Highly educated caregivers may have greater recognition of their child’s cognitive disabilities; and hence, greater anxiety, worry, and time committed to their child’s care. Among parents of children with cancer, both higher and lower levels of education have been associated with increased parental distress and caregiving demands [30–33]. These findings suggest that general education may not protect against caregiver burden and that supportive interventions may be needed regardless of education level.
Healthy family functioning pre-arrest was associated with greater caregiver burden at 3 months post-arrest for children <5 years old. The relationship between family functioning and caregiver burden is complex [2]. Greater burden can lead to unhealthy family functioning and vice versa. On the other hand, the challenges of managing a chronic condition can improve aspects of family functioning such as problem solving [2, 34]. Thus, caregiver burden may be associated with families learning to function together better. Neither caregiver education nor family functioning were associated with burden after paediatric out-of-hospital arrest [8]. Children with out-of-hospital arrest have a greater degree of disability post-arrest which may be a more important determinant of burden, potentially masking other factors.
Limitations
Limitations of this study include using different caregiver burden measures for younger and older children. Separating the primary outcome by age group could have reduced the statistical power to detect some meaningful associations. Normative reference data from a U.S. population are not available for the ITQOL. Other limitations include the select population of children included in THAPCA-IH. However, because these children were at high risk for neurologic injury, knowledge about burden for this population will be most useful to caregivers and clinicians. Other potential risk factors for caregiver burden exist that were not evaluated. Caregiving can have a positive impact on caregivers [35] which was also not evaluated.
CONCLUSIONS
Caregiver burden is substantial during the first year after paediatric in-hospital cardiac arrest. Standardised measures of the child’s neurobehavioural function as well as caregivers’ subjective perceptions of their child’s global function were associated with caregiver burden.
Acknowledgments
ROLE OF FUNDING SOURCE
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Hypothermia after Paediatric Cardiac Arrest (THAPCA) trial investigators participated in this study: Frank W. Moler, MD, C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor; Kathleen L. Meert, MD, Children’s Hospital of Michigan, Detroit; Jamie S. Hutchinson, MD, Hospital for Sick Children, Toronto, Ontario, Canada; Christopher J. L. Newth, MD, Children’s Hospital Los Angeles, Los Angeles, California; Kimberly S. Bennett, MD, MPH, Primary Children’s Hospital, Salt Lake City, Utah; John T. Berger, MD, Children’s National Medical Centre, Washington, DC; Alexis A. Topjian, MD, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Jose A. Pineda, MD, Washington University, St Louis, Missouri; Joshua D. Koch, MD, Children’s Medical Centre Dallas, University of Texas Southwestern Medical School; Charles L. Schleien, MD, MBA, Morgan Stanley Children’s Hospital–Columbia University Medical Centre, New York, New York; Heidi J. Dalton, MD, Phoenix Children’s Hospital, Phoenix, Arizona; George Ofori-Amanfo, MB, ChB, Duke Children’s Hospital, Durham, North Carolina; Denise M. Goodman, MD, Anne and Robert Lurie Children’s Hospital of Chicago, Chicago, Illinois; Ericka L. Fink, MD, University of Pittsburgh Medical Centre, Pittsburgh, Pennsylvania; Patrick McQuillen, MD, University of California, San Francisco Benioff Children’s Hospital; Jerry J. Zimmerman, MD, PhD, Seattle Children’s Hospital, Seattle, Washington; Neal J. Thomas, MD, Penn State Children’s Hospital, Hershey, Pennsylvania; Elise W. van der Jagt, MD, MPH, University of Rochester Medical Centre/Golisano Children’s Hospital, Rochester, New York; Melissa B. Porter, MD, Kosair Charities Paediatric Clinical Research Unit, Department of Paediatrics, University of Louisville and the Kosair Children’s Hospital, Louisville, Kentucky; Michael T. Meyer, MD, Medical College of Wisconsin, Milwaukee; Rick Harrison, MD, Mattel Children’s Hospital UCLA (University of California, Los Angeles); Nga Pham, MD, Children’s Healthcare of Atlanta, Atlanta, Georgia; Adam J. Schwarz, MD, Children’s Hospital of Orange County, Orange, California; Jeffrey E. Nowak, MD, Children’s Hospitals and Clinics of Minnesota, Minneapolis; Jeffrey Alten, MD, The Children’s Hospital of Alabama, Birmingham; Derek S. Wheeler, MD, Cincinnati Children’s Hospital, Cincinnati, Ohio; Utpal S. Bhalala, MD, Johns Hopkins Children’s Centre, Baltimore, Maryland; Karen Lidsky, MD, Rainbow Babies and Children’s Hospital, Cleveland, Ohio; Eric Lloyd, MD, Nationwide Children’s Hospital, Columbus, Ohio; Mudit Mathur, MD, Loma Linda University Children’s Hospital, Loma Linda, California; Samir Shah, MD, University of Tennessee Health Science Centre, Memphis; Theodore Wu, MD, University of Texas Health Sciences Centre at San Antonio; Andreas A. Theodorou, MD, Diamond Children’s Medical Centre, Tucson, Arizona; Ronald C. Sanders Jr, MD, Arkansas Children’s Hospital, Little Rock; Faye S. Silverstein, MD, C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor; James R. Christensen, MD, and Beth S. Slomine, PhD, Outcome Centre, Kennedy Krieger Institute, and Johns Hopkins University, School of Medicine, Baltimore, Maryland; Victoria L. Pemberton, RNC, MS, National Heart, Lung, and Blood Institute, Bethesda, Maryland; and Brittan Browning, MS, RD, CCRC, Richard Holubkov, PhD, and J. Michael Dean, MD, MBA, Data Coordinating Centre, University of Utah, Salt Lake City.
Footnotes
CONFLICT OF INTEREST STATEMENT
Primary support for the conduct of the THAPCA-IH Trial was funding from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute, Bethesda, MD. HL094345 (FWM) and HL094339 (JMD). Additional support from the following federal planning grants contributed to the planning of the THAPCA Trials: NIH, Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), Bethesda, MD. HD044955 (FWM) and HD050531 (FWM). In part support was obtained from the participation of the following research networks: Paediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Paediatric Critical Care Research Network (CPCCRN) from cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934. At several centres (as indicated below), clinical research support was supplemented by the following grants or Cooperative Agreements: UL1 RR 024986, UL1 TR 000433, U54 HD087011, UL1TR000003, and P30HD040677. The National Emergency Medical Services for Children (EMSC) Data Analysis Resource Centre Demonstration grant U07MC09174 provided for educational study materials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen Meert, Children’s Hospital of Michigan, Wayne State University, 3901 Beaubien Boulevard, Detroit, MI 48201, USA.
Beth S. Slomine, Kennedy Krieger Institute, Johns Hopkins University, 707 North Broadway, Baltimore, MD 21205, USA.
James R. Christensen, Kennedy Krieger Institute, Johns Hopkins University, 707 North Broadway, Baltimore, MD 21205, USA.
Russell Telford, University of Utah, 295 Chipeta Way, P. O. Box 581289, Salt Lake City, UT 84158, USA.
Richard Holubkov, University of Utah, 295 Chipeta Way, P. O. Box 581289, Salt Lake City, UT 84158, USA.
J. Michael Dean, University of Utah, 295 Chipeta Way, P. O. Box 581289, Salt Lake City, UT 84158, USA.
Frank W. Moler, University of Michigan, CS Mott Children’s Hospital, 1540 East Hospital Drive, Ann Arbor, MI 48109, USA.
References
- 1.Raina P, O’Donnell M, Schwellnus H, Rosenbaum P, King G, Brehaut J, et al. Caregiving process and caregiver burden: Conceptual models to guide research and practice. BMC Pediatr. 2004;4:1. doi: 10.1186/1471-2431-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilapil M, Coletti DJ, Rabey C, DeLaet D. Caring for the caregiver: Supporting families of youth with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:190–9. doi: 10.1016/j.cppeds.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HG, Chambers JA. Effects of caregiving on the families of children and adults with disabilities. Phys Med Rehabil Clin N Am. 2015;26:1–19. doi: 10.1016/j.pmr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Phillips RS, Scott B, Carter SJ, Taylor M, Peirce E, Davies P, et al. Systematic review and meta-analysis of outcomes after cardiopulmonary arrest in childhood. PloS One. 2015;10:e0130327. doi: 10.1371/journal.pone.0130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moler FW, Meert K, Donaldson AE, Nadkarni V, Brilli RJ, Dalton HJ, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study. Crit Care Med. 2009;37:2259–67. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meert KL, Donaldson A, Nadkarni V, Tieves KS, Schleien CL, Brilli RJ, et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544–53. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moler FW, Donaldson AE, Meert K, Brilli RJ, Nadkarni V, Shaffner DH, et al. Multicenter cohort study of pediatric out-of-hospital cardiac arrest. Crit Care Med. 2011;39:141–9. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meert KL, Slomine BS, Christensen JR, Telford R, Holubkov R, Dean JM, et al. Family burden after out-of-hospital cardiac arrest in children. Pediatr Crit Care Med. 2016;17:498–507. doi: 10.1097/PCC.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashid M, Goez HR, Mabood N, Damanhoury S, Yager JY, Joyce AS, et al. The impact of pediatric traumatic brain injury (TBI) on family functioning: A systematic review. J Pediatr Rehabil Med. 2014;7:241–54. doi: 10.3233/PRM-140293. [DOI] [PubMed] [Google Scholar]
- 10.Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020–6. doi: 10.1001/archpediatrics.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stacin T, Wade SL, Walz NC, Yeates KO, Taylor HG. Family adaptation 18 months after traumatic brain injury in early childhood. J Dev Behav Pediatr. 2010;31:317–25. doi: 10.1097/DBP.0b013e3181dbaf32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aitken ME, McCarthy ML, Slomine BS, Ding R, Durbin DR, Jaffe KM, et al. Family burden after traumatic brain injury in children. Pediatrics. 2009;123:199–206. doi: 10.1542/peds.2008-0607. [DOI] [PubMed] [Google Scholar]
- 13.Wade SL, Gerry Taylor H, Yeates KO, Drotar D, Stancin T, Minich NM, et al. Long-term parental and family adaptation following pediatric brain injury. J Pediatr Psychol. 2006;31:1072–83. doi: 10.1093/jpepsy/jsj077. [DOI] [PubMed] [Google Scholar]
- 14.Guyard A, Fauconnier J, Mermet MA, Cans C. Impact on parents of cerebral palsy in children: a literature review. Arch Pediatr. 2011;18:204–14. doi: 10.1016/j.arcped.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376:318–29. doi: 10.1056/NEJMoa1610493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moler FW, Silverstein FS, Meert KL, Clark AE, Holubkov R, Browning B, et al. Rationale, timeline study design, and protocol overview of the Therapeutic Hypothermia after Paediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2013;14:e304–15. doi: 10.1097/PCC.0b013e31828a863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holubkov R, Clark AE, Moler FW, Slomine BS, Christensen JR, Silverstein FS, et al. Efficacy outcome selection in the Therapeutic Hypothermia after Paediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2015;16:1–10. doi: 10.1097/PCC.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 19.HealthActCHQ. Infant and Toddler Quality of Life Questionnaire-97 (ITQOL-97) Boston, MA: HealthActCHQ; 2008. Confidential Scoring Rules. [Google Scholar]
- 20.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–60. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HealthActCHQ. The CHQ Scoring and Interpretation Manual. Boston, MA: HealthActCHQ; 2008. [Google Scholar]
- 22.Epstein N, Baldwin L, Bishop D. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9:171–80. [Google Scholar]
- 23.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales. 2. Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- 24.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 25.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C, et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. 2001;108:1325–31. doi: 10.1542/peds.108.6.1325. [DOI] [PubMed] [Google Scholar]
- 26.Caris EC, Dempster N, Wernovsky G, Butz C, Neely T, Allen R, et al. Anxiety scores in caregivers of children with hypoplastic left heart syndrome. Congenit Heart Dis. 2016;11:727–32. doi: 10.1111/chd.12387. [DOI] [PubMed] [Google Scholar]
- 27.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slomine BS, Silverstein FS, Christensen JR, Holubkov R, Page K, Dean JM, et al. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3412. pii:e20153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverstein FS, Slomine BS, Christensen J, Holubkov R, Page K, Dean JM, et al. Functional outcomes trajectories after out-of-hospital pediatric cardiac arrest. Crit Care Med. 2016;44:e1165–74. doi: 10.1097/CCM.0000000000002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klassen A, Raina P, Reineking S, Dix D, Pritchard S, O’Donnell M. Developing a literature base to understand the caregiving experience of parents of children with cancer: a systematic review of factors related to parental health and well-being. Support Care Cancer. 2007;15:807–18. doi: 10.1007/s00520-007-0243-x. [DOI] [PubMed] [Google Scholar]
- 31.Choi EK, Yoon SJ, Kim JH, Park HJ, Kim JY, Yu ES. Depression and distress in caregivers of children with brain tumors undergoing treatment: psychosocial factors as moderators. Psychooncology. 2016;25:544–50. doi: 10.1002/pon.3962. [DOI] [PubMed] [Google Scholar]
- 32.Mu PF, Ma FC, Hwang B, Chao YM. Families of children with cancer: the impact of anxiety experienced by fathers. Cancer Nurs. 2002;25:66–73. doi: 10.1097/00002820-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Svavarsdottir EK. Surviving childhood cancer: parents’ perceptions of their child’s health. J Pediatr Oncol Nurs. 2005;22:80–8. doi: 10.1177/1043454204273812. [DOI] [PubMed] [Google Scholar]
- 34.Blair C, Freeman C, Cull A. The families of anorexia nervosa and cystic fibrosis patients. Psychol Med. 1995;25:985–93. doi: 10.1017/s0033291700037478. [DOI] [PubMed] [Google Scholar]
- 35.Murphy NA, Christian B, Caplin DA, Young PC. The health of caregivers for children with disabilities: caregiver perspectives. Child Care Health Dev. 2007;33:180–7. doi: 10.1111/j.1365-2214.2006.00644.x. [DOI] [PubMed] [Google Scholar]