Abstract
Hepatitis B virus (HBV) infection is a worldwide health problem because of its potential to cause liver cirrhosis and hepatocellular carcinoma. Silibinin is a constituent of an extract of milk thistle, which is empirically used as a herbal medicine for the protection of liver, but its detailed effects on HBV are unknown. Because a previous study reported that silibinin hinders clathlin-mediated endocytosis (CME), we aimed to test whether silibinin inhibits the entry of HBV into hepatocytes. Using HepG2-NTCP-C4 cells, which overexpress sodium taurocholate cotransporting polypeptide (NTCP), it was shown that silibinin inhibited HBV infection dose-dependently. Similar effects were observed using human primary hepatocytes (PXB-cells). Additionally, a combination of silibinin and entecavir reduced HBV DNA in the culture supernatant more than either mono-treatment alone in HepG2-NTCP-C4 cells that had already been infected with HBV. Silibinin decreased transferrin uptake but did not affect the interaction between the HBV envelope and NTCP, suggesting that silibinin might inhibit HBV infection by hindering CME. In conclusion, this study showed that silibinin inhibits HBV entry in vitro.
Keywords: HBV, Clathrin-mediated endocytosis, Herbal medicine
Highlights
-
•
This study shows that silibinin inhibits HBV infection in vitro.
-
•
Silibinin hinders clathrin-mediated endocytosis to inhibit HBV entry.
-
•
Continuous addition of silibinin or combination with entecavir is effective.
1. Introduction
Hepatitis B virus (HBV) infects humans acutely or chronically and is associated with severe diseases including liver cirrhosis and hepatocellular carcinoma (HCC). It is estimated that 240 million people are chronically infected with HBV worldwide and that the chronic infection rate is almost 1% in Japan [1]. HBV infection is one of major health problems in the world because almost one million patients die due to HBV-associated HCC or liver cirrhosis every year [2], [3]. HBV-infected patients are treated with interferon (IFN) or nucleotide analogues (NAs) that can suppress HBV replication and inflammation in the liver, but it is difficult to clear covalently closed circular DNA (cccDNA), which is a replication template of HBV, in the nucleus of hepatocytes [4], [5], [6]. So far, no drug that eradicates HBV completely is available and the development of anti-HBV drugs with novel mechanisms is required for the termination of HBV infection.
It is considered that endocytosis is essential for the uptake of HBV in hepatocytes. A previous report showed that the endocytosis of HBV might be caveolin-dependent [7], but another showed that it might be clathrin-dependent [8]. After the identification of sodium taurocholate cotransporting polypeptide (NTCP) as an HBV receptor [9], it has been considered that clathrin-mediated endocytosis (CME) is required because HepG2 cells, which lack caveolin-1 expression, are susceptible to HBV after the expression of NTCP [10]. There are few reports of HBV entry inhibitors that work via CME inhibition.
A recent report showed that silibinin, which had mainly been used for chronic liver diseases as a herbal medicine in Europe and China, inhibits the entry of hepatitis C virus (HCV) into hepatocytes via the inhibition of CME [11]. However, there is no report of this drug on the anti-HBV effects in vitro. In this study, we aimed to evaluate the inhibitory effects of silibinin on HBV entry using cell culture systems.
2. Materials and methods
2.1. Reagents
Silibinin was purchased from Sigma-Aldrich (St. Louis, MO), and was dissolved in dimethyl sulfoxide (DMSO) (Wako, Osaka, Japan). Anti-HBs antibody S14 was purchased from Abcam (Cambridge, England). Anti-NTCP antibody and anti-FLAG antibody was purchased from Sigma-Aldrich. Entecavir (ETV) was obtained from Bristol-Myers Squibb (New York City, NY).
2.2. Cell culture
HepG2-NTCP-C4 cells were kindly provided by Dr. Koichi Watashi (National Institute of Infectious Diseases, Tokyo, Japan). The cells were cultured with DMEM/F-12,GlutaMAX (Thermo Fisher Scientific, Waltham, MA), 10% fetal bovine serum (Thermo Fisher Scientific), 100 μg/ml penicillin/streptomycin (Sigma-Aldrch), 5.0 μg/ml insulin (Sigma-Aldrich), 10 mM HEPES (Sigma-Aldrich), and 400 μg/ml G418 (Sigma-Aldrich). HepG2.2.15 cells, which stably express HBV of genotype D, were cultured with the same medium. PXB-cells, which were isolated from chimeric mice with humanized livers, were purchased from PhoenixBio (Higashihiroshima, Japan) and were cultured with dHCGM medium (PhoenixBio).
2.3. Cell viability assay
HepG2-NTCP-C4 cells were treated with 0–200 μM silibinin for 2 h and cell viability was determined with MTS assay using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI).
2.4. HBV inoculum for the infection experiments
The culture medium from HepG2.2.15 cells was collected and was concentrated using Amicon Ultra-15 (Merck Millipore, Billerica, MA). The final concentration of HBV DNA was adjusted to 6.0 × 109 copies/ml for the infection experiments with HepG2-NTCP-C4 cells. For the infection experiments with PXB-cells, HBV derived from human hepatocyte chimeric mouse serum (Phoenix Bio) was used at a concentration of 2.0 × 106 copies/well (24-well plate).
2.5. HBV infection
HepG2-NTCP-C4 cells were cultured in 6-well plates with 0–200 μM silibinin for 2 h, and were inoculated with HBV in the presence of 4% PEG8000 at 37 °C for 16 h. The cells were washed three times with fresh medium every 3 days, and the culture supernatant was collected until 13 days after the infection. PXB-cells, which were purchased in a 24-well plate, were cultured with 0–100 μM silibinin for 2 h. Then the cells were inoculated with HBV in the presence of 4% PEG8000 at 37 °C for 16 h. Every 5 days, the cells were washed three times with phosphate buffered saline (PBS, Thermo Fisher Scientific) and fresh medium was added. The culture supernatant was collected until 17 days after the infection.
2.6. Assay of HBV DNA and HBsAg
Total DNA, which was extracted from the 1000 μl culture supernatant using QIAamp UltraSense Virus Kit (QIAGEN, Hilden, Germany) or from the 200 μl culture supernatant using QIAamp Blood Mini Kit (QIAGEN), was used as a template for real-time PCR analysis using StepOnePlus (Thermo Fisher Scientific) with TaqMan Universal PCR Master Mix (Thermo Fisher Scientific). The primers HBV-S-Forward (5′-ACT CAC CAA CCT CTT GTC CT-3′) and HBV-S-Reverse (5′-GAC AAA CGG GCA ACA TAC CT-3′), and TaqMan probe (5′-FAM-TAT CGC TGG ATG TGT CTG CGG CGT-TAMRA-3′) were used. After the incubation at 50 °C for 2 min and 95 °C for 10 min, PCR was carried out for 40 cycles (95 °C for 15 s, 60 °C for 60 s). A standard curve was made with a plasmid containing the full HBV genome of 104 to 107 copies. HBsAg in the culture supernatant was quantified using the chemiluminescent enzyme immunoassay measurement reagent Lumipulse HBsAg-HQ (Fujirebio Inc, Shinjuku, Japan).
2.7. Immunofluorescence microscopy
HepG2-NTCP-C4 cells were cultured on sterilized cover glass with 100 μM silibinin for 2 h. The cells were inoculated with HBV in the presence of 4% PEG8000 at 37 °C for 16 h. Thereafter, the cells were washed three times with fresh medium every 3 days until 13 days after the infection. After fixation with fixative (0.1 M PIPES, 1 mM EGTA, 3 mM MgSO4, 2.5% paraformaidehyde), the cells were stained with anti-HBs antibody. The cells were mounted with ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific) and were observed by confocal microscopy LSM780 (Zeiss, Oberkochen, Germany). Cells were counted at randomly selected fields with or without silibinin, and the percentages of HBs-positive cells in more than 150 cells were evaluated.
2.8. Observation of transferrin uptake
HepG2-NTCP-C4 cells were cultured with 100 μM silibinin for 2 h, and cultured with 5 μg/ml Alexa Fluor 488 transferrin (Thermo Fisher Scientific) with 2% albumin for 15 min. They were fixed and observed as described above. Similarly, after adding transferrin, the cells were collected after treatment with 0.25% trypsin (Thermo Fisher Scientific), and quantification of fluorescence-labeled transferrin in the cells was performed by flow cytometry using FACS Canto II (BD Biosciences, Franklin Lakes, NJ).
2.9. Immunoprecipitation
HepG2 cells were transfected with FLAG-LHBs plasmid that expressed FLAG-tagged large hepatitis B s protein (LHBs) [12] using Transit-LT1 (Mirus Bio, Madison, WI). Three days after transfection, the total protein was collected with NP-40 buffer (20 mM Tris-HCl, 137 mM NaCl, 10% Glycerol, 1% NP-40, 2 mM EDTA). The protein was combined with that collected from HepG2-NTCP-C4 cells and was rocked with anti-NTCP antibody at 4 °C for 24 h, with or without 600 μM silibinin. Protein A (Sigma-Aldrich) was added and the mixture was rocked at 4 °C for 2 h. The pellet after 4000 rpm centrifugation for 1 min was washed, and was subjected to western blotting analysis using anti-FLAG antibody.
2.10. Statistical analysis
For statistical analysis, JMP software (SAS Institute Inc., Cary, NC) was used. The data presented in this study are expressed as mean ± standard error (SE) and statistical significance was determined by the Student's t-test. P-values were considered statistically significant when less than 0.05.
3. Results
3.1. HBV infection of HepG2-NTCP-C4 cells
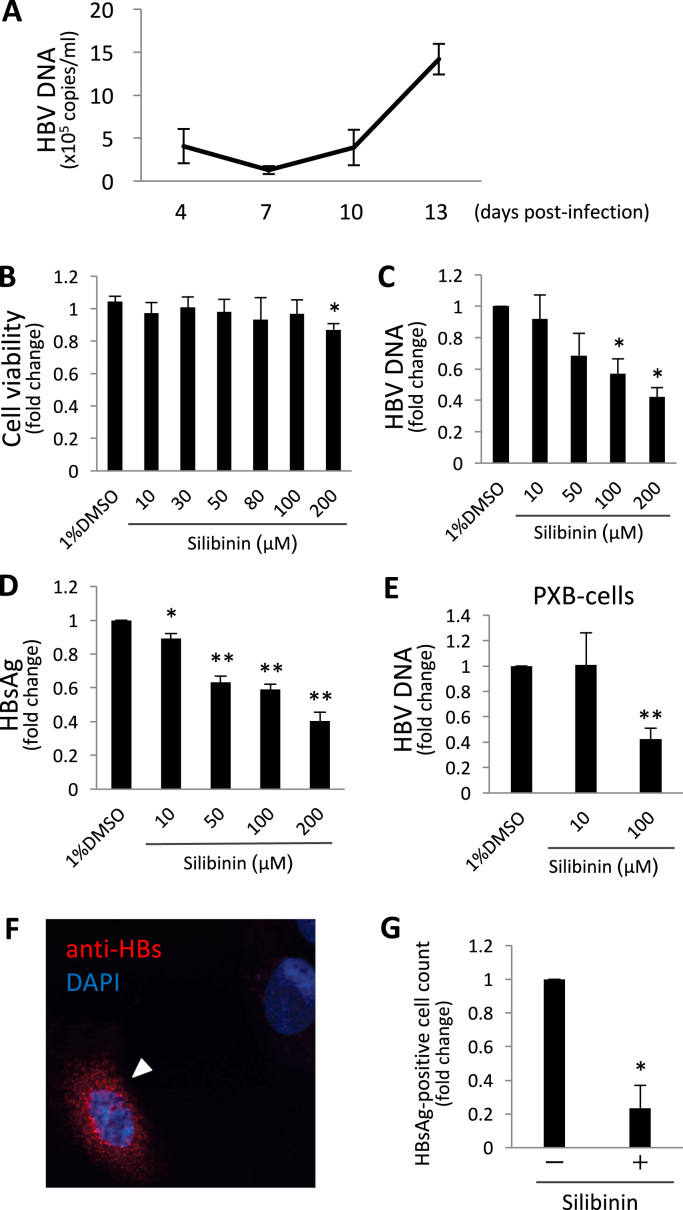
At first, we confirmed the infectivity of HBV from HepG2.2.15 cells to HepG2-NTCP-C4 cells. When the HBV DNA level in the culture supernatant was evaluated 4, 7, 10, and 13 days after the infection, it decreased slightly from 4.1 × 105 copies/ml at day 4 to 1.3 × 105 copies/ml at day 7, and then increased to 1.4 × 106 copies/ml at day 13 (Fig. 1A). Therefore, we considered that HBV infected successfully to HepG2-NTCP-C4 cells.
Fig. 1.
Effect of silibinin on the inhibition of HBV infection in vitro. (A) HepG2-NTCP-C4 cells were exposed to HBV and washed, and HBV DNA levels in the culture supernatant 4, 7, 10, and 13 days after infection were evaluated. (B) Quantification of cell viability after the addition of silibinin. HepG2-NTCP-C4 cells were incubated with 0–200 μM silibinin for 2 h and cell viability was measured with MTS assay in 3 independent experiments. (C, D) After the incubation of HepG2-NTCP-C4 cells with 0–200 μM silibinin for 2 h, the cells were exposed to HBV and washed. Thirteen days after infection, the culture supernatant was harvested and HBV DNA (C) and HBsAg (D) were quantified in 5 independent experiments. (E) The levels of HBV DNA in the supernatant from PXB-cells that were exposed to HBV with 0–100 μM silibinin and cultured for 17 days. (F) HepG2-NTCP-C4 cells that were exposed to HBV with or without silibinin were stained with anti-HBs antibody and observed by immunofluorescence microscopy. (G) HBs-positive cells were quantified and positive rates are shown in 3 independent experiments. Error bars represent SE. Statistical significance was determined using Student's t-test (*p < 0.05, **p < 0.01).
3.2. Inhibition of HBV infection by silibinin
At first, we evaluated the cytotoxicity of silibinin in HepG2-NTCP-C4 cells with the MTS assay. When compared with the drug-free control, 100 μM of silibinin caused no significant change in cell viability. Because 200 μM showed a small (0.87-fold) but statistically significant effect (p = 0.0038) on the viability (Fig. 1B), we used silibinin at concentrations of 100 μM or lower in the later experiments.
Next, the inhibition of HBV infection with silibinin was evaluated with the same cells. Silibinin was added to the culture medium at the time of infection but it was not added after the wash with HBV inoculum. When the HBV DNA levels in the culture supernatants 13 days after the infection were assayed, the levels were decreased dose-dependently by silibinin, with 100 μM of silibinin showing a significant decrease (0.57-fold, p = 0.0101, Fig. 1C). The levels of HBsAg were also decreased similarly and 100 μM of silibinin showed a significant decrease (0.59-fold, p = 0.0002, Fig. 1D). These results suggest that silibinin may inhibit HBV infection at concentrations lacking cytotoxicity.
The inhibitory effect by silibinin was also confirmed using PXB-cells, primary human hepatocytes. Then, it was shown that the HBV DNA levels in the culture supernatant 17 days after infection were significantly decreased at 100 μM of silibinin in comparison with the control (0.43-fold, p < 0.0001, Fig. 1E).
HepG2-NTCP-C4 cells infected with HBV were observed by immunofluorescent microscopy. When HBsAg-positive cells, which are considered to be HBV-infected cells, were counted, 2.0 ± 0.7% cells were positive in 3 independent experiments. It was shown that 100 μM of silibinin decreased HBsAg-positive cells in comparison with the drug-free control (0.23-fold, p = 0.026, Fig. 1G,H).
3.3. Evaluation of effects on the HBV of silibinin release from hepatocytes
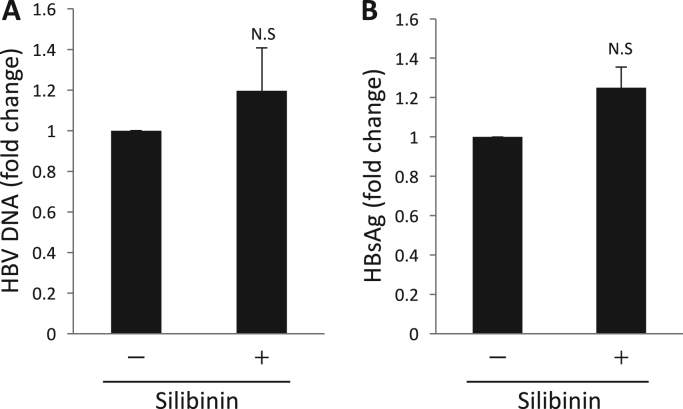
To determine whether silibinin has effects on HBV replication or release, HepG2.2.15 cells, which express HBV stably, were used. Silibinin was added to the culture medium of HepG2.2.15 cells and the culture supernatants were collected at 2 days after the addition of silibinin. There were no significant changes in the levels of HBV DNA and HBsAg (Fig. 2A,B), and silibinin appeared to have no effects on HBV replication or release.
Fig. 2.
Silibinin had no effect on HBV release. (A, B) HepG2.2.15 cells were incubated with 100 μM silibinin for 2 days and HBV DNA (A) and HBsAg (B) in the culture supernatant were quantified in 6 independent experiments. Error bars represent SE. Statistical significance was determined using Student's t-test.
3.4. Anti-HBV effect with continuous addition of silibinin
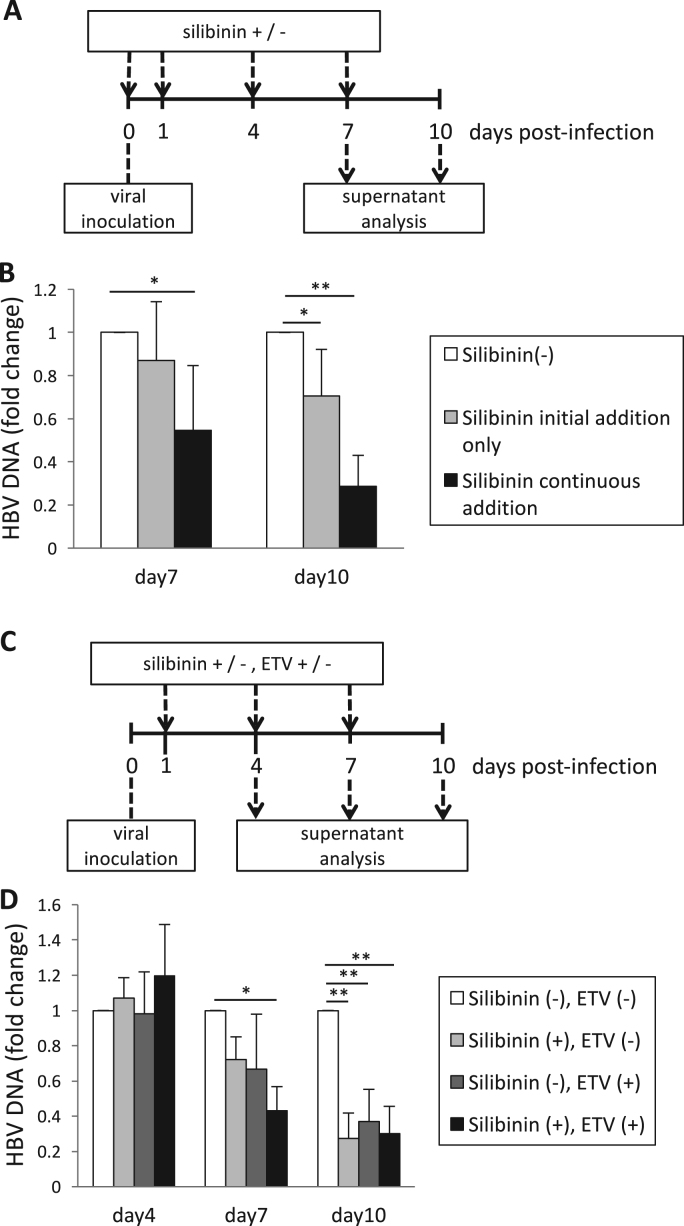
We also evaluated the anti-viral effects of silibinin, not only when the drug was added at the time of HBV infection, but also when it was added continuously. Ten days after the infection, the HBV DNA levels were decreased more in the continuously treated cells than in the temporarily treated cells (0.29-fold vs. 0.71-fold, Fig. 3B). Supplementary Fig. 1 A shows the analysis of HBV DNA change compared with the drug-free control on day 7. Therefore, it was considered that continuous treatment of silibinin might suppress the spread of HBV infection from the infected cells to other uninfected cells.
Fig. 3.
Inhibition of HBV infection with continuous addition of silibinin and that with the combination of silibinin and entecavir (ETV). (A) Time course of cell culture with continuous addition of silibinin. HepG2-NTCP-C4 cells were exposed to HBV for 16 h and washed, and the culture supernatant was harvested 7 and 10 days after the infection. (B) Levels of HBV DNA in the culture supernatant obtained in 6 independent experiments of (A). The white bars indicate the results of no addition of silibinin, the gray bars indicate the results of addition of silibinin only before and during infection, and the black bars indicate the results of the addition of silibinin before and after infection. (C) Time course of cell culture with silibinin and/or ETV. These were added continuously after infection, and the culture supernatant was collected 4, 7, and 10 days after the infection. (D) Levels of HBV DNA in the culture supernatant obtained in 4 independent experiments of (C). The white bars indicate the results of no addition of silibinin and ETV, the light gray bars indicate the results of addition of silibinin alone, the dark gray bars indicate the results of addition of ETV alone, and the black bars indicate the results of the addition of both silibinin and ETV. Error bars represent SE. Statistical significance was determined using Student's t-test (*p < 0.05, **p < 0.01).
3.5. Combination treatment with silibinin and nucleoside analogue
As a model of HBV chronic infection, silibinin was added in combination with ETV, a nucleoside analogue that is widely used for chronic infection of HBV. ETV was used at a concentration of EC50, 3.75 nM. The changes in the HBV DNA levels in the culture supernatants were compared among the cells with silibinin treatment on and after the next day of infection, those with ETV treatment, and those with silibinin and ETV in combination. When the HBV DNA levels in the culture supernatant were compared 7 days after infection, they were decreased more by the combination treatment than by ETV treatment or silibinin treatment alone (0.30-fold vs. 0.67-fold vs. 0.72-fold, Fig., 3D). This result suggested that the combination therapy might have stronger effects than monotherapies. Supplementary Fig. 1B shows the analysis of HBV DNA change compared with the drug-free control on day 4.
3.6. Evaluation of the inhibitory effect of silibinin on the interaction between HBV receptor and envelope
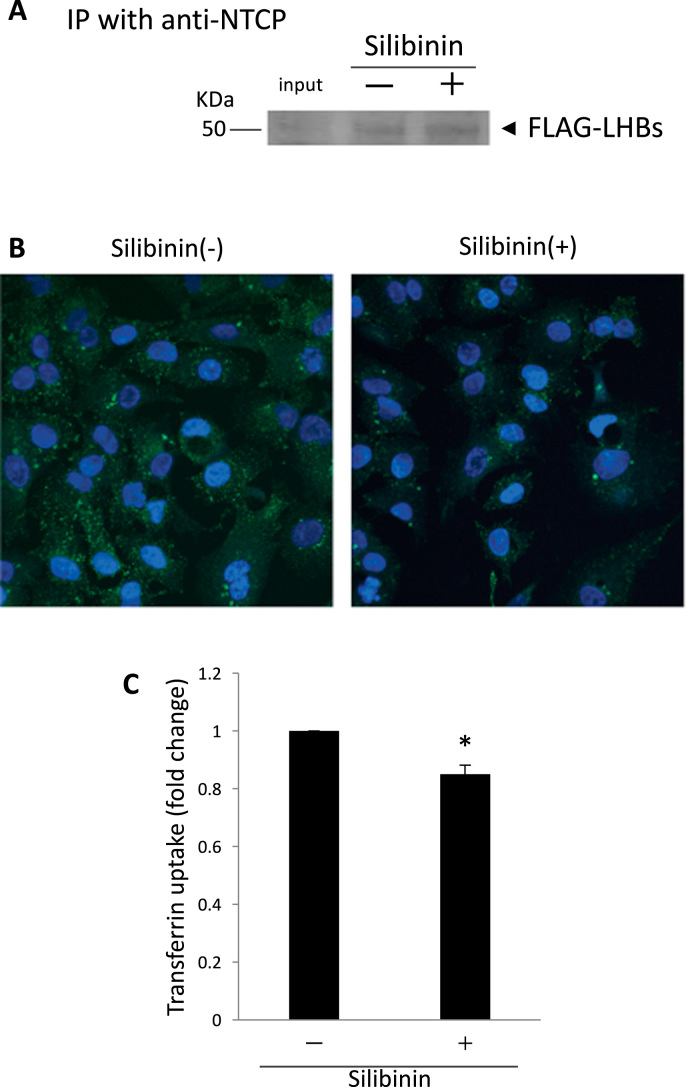
It is considered that HBV binds to NTCP and then enters cells via CME [13] to infect hepatocytes. Then, we tested whether silibinin inhibits the interaction between NTCP and the envelope protein LHBs, which is required for binding with the receptor [14]. Although the interaction between FLAG-tagged LHBs and NTCP was confirmed by immunoprecipitation, the addition of silibinin did not alter the interaction (Fig. 4A).
Fig. 4.
Investigation of the mechanisms of how silibinin inhibits HBV infection. (A) A representative result of immunoprecipitation to determine whether silibinin inhibits the binding of HBV envelope protein and the receptor, NTCP. Total protein from HepG2 cells that were transfected with FLAG-LHBs and that from HepG2-NTCP-C4 cells were combined with or without 600 μM silibinin. Immunoprecipitation with anti-NTCP antibody and western blot with anti-FLAG antibody were performed. (B) Analysis of the effect of silibinin on clathrin-mediated endocytosis (CME) using transferrin. HepG2-NTCP-C4 cells were cultured with or without 100 μM silibinin for 2 h, and 5 μg/ml Alexa Fluor 488 transferrin were added. Fifteen min later, the cells were fixed and observed by fluorescence microscopy. (C) Quantification of transferrin uptake by flow cytometry. Fifteen min after the addition of Alexa Fluor 488 transferrin, HepG2-NTCP-C4 cells were collected using 0.25% trypsin and the uptake level of transferrin was analyzed with FACS Canto II in 3 independent experiments. Error bars represent SE. Statistical significance was determined using Student's t-test (*p < 0.05).
3.7. Effect of silibinin on CME
We evaluated the effect of silibinin on the inhibition of CME using transferrin. When HepG2-NTCP-C4 cells were observed by immunofluorescent microscopy, silibinin reduced the uptake of fluorescence-labeled transferrin (Fig. 4B). Flow cytometric analysis showed that silibinin reduced the transferrin uptake significantly in comparison with the control (0.85-fold, p = 0.0084). Therefore, it was suggested that silibinin might not affect the binding of the HBV envelope and receptor, but might act on the next step, CME, and inhibit the entry of HBV into hepatocytes.
4. Discussion
Silibinin is a major constituent of silymarin, an extract from the seeds of the milk thistle plant Silybum marianum, which have been long thought to have a liver-protective effect. Silibinin is used as a Chinese herbal medicine and is approved as a medicine for alcoholic liver cirrhosis in Germany. It was reported that silibinin shows anti-oxidative and anti-inflammatory effects on various tissues and modulatory effects on apoptosis [15], [16]. Therefore, in addition to the entry inhibition of HBV shown in this study, silibinin may have potential effects in attenuating hepatitis due to HBV infection. Because the molecular structure of silibinin has been determined, it might be possible to construct derivatives of silibinin that have stronger effects on HBV with anti-inflammatory effects.
CME plays important roles in hepatocytes, which uptake various materials physiologically [17], and it is thought that hepatitis viruses utilize this system. A previous report showed that silibinin attenuated HCV infection via the inhibition of CME, and this study showed it also inhibits HBV infection. Because the virological characteristics of HBV and HCV are quite different but both have hepatotropism, there must be common mechanisms in their viral life cycles. The results of this study suggest that the common mechanisms could be targets for anti-viral treatment for different viruses. It is known that, in cases infection with both HBV and HCV, suppression of one virus leads to an increase of another virus [18], [19], but a drug that suppresses both viruses could overcome this phenomenon.
At first, we confirmed the infection of HBV to HepG2-NTCP-C4 cells (Fig. 1A). HBV DNA in the culture supernatant decreased 7 days after the infection once, and then it increased. It was considered that the transient decrease was due to the lower replication of HBV in the cells with active proliferation [20].
In this study, the rate of HBV-infected cells was decreased after the addition of silibinin to 23% of control, but the decrease of transferrin uptake was 85% of control (Fig. 1, Fig. 4). As a possible reason for this, transferrin uptake might proceed during the incubation with trypsin treatment without silibinin before the flow cytometry. Attention should be paid to the evaluation of transferrin uptake in a similar method.
In the experiments using HepG2.2.15 cells, it was shown that silibinin had no effect on HBV replication and release. Therefore, combination therapy with nucleos(t)ide analogues, which inhibit reverse transcription, could be a reasonable option. The result of this study showed that the combination of silibinin and ETV reduced the HBV DNA levels in HepG2-NTCP-C4 cells that had already been infected with HBV, suggesting the possible effects of combination therapy for patients with chronic HBV infection. Although this could not be evaluated in this study, the combination of silibinin with IFN, which modulates immune responses, could be another option. With the combination of these available drugs, the achievement of clinical goals such as hepatitis B e antigen (HBeAg) seroconversion or HBsAg clearance could be improved.
So far, HBV genotypes are classified into ten genotypes (A-J) [21], and the infection of genotype A HBV was reported to have increased recently in acute hepatitis patients in Japan, where genotype B and C had previously prevailed. A higher chronicity rate of genotype A HBV than genotype B and C was reported [22], but silibinin administration in the early phase of infection could reduce the chronicity rate due to the inhibition of HBV spreading to uninfected hepatocytes. Also, silibinin could help in preventing cases of mother-to-infant infections, which cannot be prevented completely by vaccines and hepatitis B immunoglobulin (HBIG).
In conclusion, this in vitro study showed that silibinin inhibits HBV entry into hepatocytes via the inhibition of CME. Further investigation in future studies is required for the clinical application, including the construction of silibinin derivatives.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K08981).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.03.003.
Appendix A. Transparency document
Supplementary material
References
- 1.Hyun S., Lee S., Ventura W.R., McMenamin J. Knowledge, awareness, and prevention of hepatitis B virus infection among Korean American Parents. J. Immigr. Minor. Health. 2017 doi: 10.1007/s10903-017-0609-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganem D., Prince A.M. Hepatitis B virus infection--natural history and clinical consequences. N. Engl. J. Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Ott J.J., Stevens G.A., Groeger J., Wiersma S.T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 4.Kayaaslan B., Akinci E., Ari A., Tufan Z.K., Alpat S.N., Gunal O., Tosun S., Guner R., Tabak F. A long-term multicenter study: entecavir versus Tenofovir in treatment of nucleos(t)ide analogue-naive chronic hepatitis B patients. Clin. Res. Hepatol. Gastroenterol. 2018;42:40–47. doi: 10.1016/j.clinre.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Li G., Zhu Y., Shao D., Chang H., Zhang X., Zhou D., Gao Y., Lan K., Deng Q. Recombinant covalently closed circular DNA of hepatitis B virus induces long-term viral persistence with chronic hepatitis in a mouse model. Hepatology. 2018;67:56–70. doi: 10.1002/hep.29406. [DOI] [PubMed] [Google Scholar]
- 6.Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 7.Macovei A., Radulescu C., Lazar C., Petrescu S., Durantel D., Dwek R.A., Zitzmann N., Nichita N.B. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J. Virol. 2010;84:243–253. doi: 10.1128/JVI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H.C., Chen C.C., Chang W.C., Tao M.H., Huang C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J. Virol. 2012;86:9443–9453. doi: 10.1128/JVI.00873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., Huang Y., Qi Y., Peng B., Wang H., Fu L., Song M., Chen P., Gao W., Ren B., Sun Y., Cai T., Feng X., Sui J., Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamoto M., Watashi K., Tsukuda S., Aly H.H., Fukasawa M., Fujimoto A., Suzuki R., Aizaki H., Ito T., Koiwai O., Kusuhara H., Wakita T. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem. Biophys. Res. Commun. 2014;443:808–813. doi: 10.1016/j.bbrc.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Blaising J., Levy P.L., Gondeau C., Phelip C., Varbanov M., Teissier E., Ruggiero F., Polyak S.J., Oberlies N.H., Ivanovic T., Boulant S., Pécheur E.I. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell. Microbiol. 2013;15:1866–1882. doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
- 12.Inoue J., Krueger E.W., Chen J., Cao H., Ninomiya M., McNiven M.A. HBV secretion is regulated through the activation of endocytic and autophagic compartments mediated by Rab7 stimulation. J. Cell Sci. 2015;128:1696–1706. doi: 10.1242/jcs.158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H.C., Tao M.H., Hung T.M., Chen J.C., Lin Z.J., Huang C. (-)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir. Res. 2014;111:100–111. doi: 10.1016/j.antiviral.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Tsukuda S., Watashi K., Hojima T., Isogawa M., Iwamoto M., Omagari K., Suzuki R., Aizaki H., Kojima S., Sugiyama M., Saito A., Tanaka Y., Mizokami M., Sureau C., Wakita T. A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology. 2017;65:1104–1116. doi: 10.1002/hep.28952. [DOI] [PubMed] [Google Scholar]
- 15.Federico A., Dallio M., Loguercio C. Silymarin/Silybin and chronic liver disease: a marriage of many years. Molecules. 2017;22 doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyak S.J., Morishima C., Lohmann V., Pal S., Lee D.Y., Liu Y., Graf T.N., Oberlies N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA. 2010;107:5995–5999. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder B., McNiven M.A. Importance of endocytic pathways in liver function and disease. Compr. Physiol. 2014;4:1403–1417. doi: 10.1002/cphy.c140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bersoff-Matcha S.J., Cao K., Jason M., Ajao A., Jones S.C., Meyer T., Brinker A. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. food and drug administration adverse event reporting system. Ann. Intern. Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand S.B., Jaroszewicz J., Potthoff A., Honer Zu Siederdissen C., Maasoumy B., Deterding K., Manns M.P., Wedemeyer H., Cornberg M. Dominance of hepatitis C virus (HCV) is associated with lower quantitative hepatitis B surface antigen and higher serum interferon-gamma-induced protein 10 levels in HBV/HCV-coinfected patients. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015;21(710):e711–e719. doi: 10.1016/j.cmi.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Allweiss L., Volz T., Giersch K., Kah J., Raffa G., Petersen J., Lohse A.W., Beninati C., Pollicino T., Urban S., Lutgehetmann M., Dandri M. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. 2018;67:542–552. doi: 10.1136/gutjnl-2016-312162. [DOI] [PubMed] [Google Scholar]
- 21.Lin C.L., Kao J.H. Hepatitis B virus genotypes and variants. Cold Spring Harbor Perspect. Med. 2015;5:a021436. doi: 10.1101/cshperspect.a021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugauchi F., Orito E., Ohno T., Tanaka Y., Ozasa A., Kang J.H., Toyoda J., Kuramitsu T., Suzuki K., Tanaka E., Akahane Y., Ichida T., Izumi N., Inoue K., Hoshino H., Iino S., Yotsuyanagi H., Kakumu S., Tomita E., Okanoue T., Nishiguchi S., Murawaki Y., Hino K., Onji M., Yatsuhashi H., Sata M., Miyakawa Y., Ueda R., Mizokami M. Spatial and chronological differences in hepatitis B virus genotypes from patients with acute hepatitis B in Japan. Hepatol. Res.: Off. J. Jpn. Soc. Hepatol. 2006;36:107–114. doi: 10.1016/j.hepres.2006.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material