Abstract
αB-Crystallin, ubiquitously expressed in many tissues including the ocular lens, is a small heat shock protein that can prevent protein aggregation. A number of post-translation modifications are reported to modify αB-crystallin function. Recent studies have identified αB-crystallin lysine residues are modified by acetylation and ubiquitination. Therefore, we sought to determine the effects of lysine to alanine substitution on αB-crystallin functions including chaperone activity and modulation of actin polymerization. Analysis of the ten substitution mutants as recombinant proteins indicated all the proteins were soluble and formed oligomeric complexes similar to wildtype protein. Lysozyme aggregation induced by chemical treatment indicated that K82, K90, K121, K166 and K174/K175 were required for efficient chaperone activity. Thermal induction of γ-crystallin aggregation could be prevented by all αB-crystallin substitution mutants. These αB-crystallin mutants also were able to mediate wildtype levels of actin polymerization. Further analysis of two clones with either enhanced or reduced chaperone activity on individual client substrates or actin polymerization indicated both retained broad chaperone activity and anti-apoptotic activity. Collectively, these studies show the requirements for lysine residues in αB-crystallin function.
Keywords: Small heat shock proteins, αB-crystallin, Ocular lens, sHSP
Highlights
-
•
αB-crystallin Lysine-to-alanine mutation yields soluble recombinant protein.
-
•
αB-crystallin mutants form oligomeric complexes similar to wildtype.
-
•
αB-crystallin mutants prevent thermal aggregation of γ-crystallin.
-
•
αB-crystallin mutants have disperse activity in chemical aggregation assays.
-
•
αB-crystallin mutants retain ability to modulate actin polymerization.
1. Introduction
αB-Crystallin, along with αA-crystallin, make up about ~ 40% of the proteins found in the ocular lens [1]. These proteins are involved in the refractive properties of the lens [1]. Additionally, α-crystallins have chaperone activity to prevent protein aggregation which allows for maintaining the lens transparency [2]. These proteins also function in protein kinase signaling cascades [3], [4]. It is also well documented that α-crystallin proteins undergo post-translationally modifications (PTM), some of which are reported to affect function [5], [6], [7], [8]. Moreover, mutations to α-crystallins often result in cataract indicating changes to function have detrimental effect on the ocular lens [9], [10].
The requirements for many of the PTM that occur on αB-crystallin are unknown. Some studies have reported changes in cellular localization as a result of αB-crystallin phosphorylation [11]. Other studies have reported increased anti-apoptotic activity and chaperone function using phospho-mimics at serine 19, 45, and 59 [5]. Moreover, known cataract mutations are reported to affect these activities suggesting the importance of phosphorylation in αB-crystallin function in vivo [12], [13]. While phosphorylation of αB-crystallin has been characterized by a number of studies, other PTMs have only been limited. One amino acid that is a target of PTM in multiple proteins including αB-crystallin is lysine. Previous studies looking at αB-crystallin lysine modifications have characterized K92 and K166 both of which undergo acetylation [14], [15]. Moreover, acetylation at K92 is reported to have improved chaperone activity and anti-apoptotic activity indicating the importance of this lysine modification. However, eight additional lysines are present in αB-crystallin, but have not been characterized. Since studies with mutant forms of αB-crystallin still detect changes in its ubiquitination state, suggesting that other lysines may be modified [16]. Herein, we set out to determine how alanine substitutions of the ten lysines effect αB-crystallin oligomeric complex formation, chaperone activity, its ability to modulate actin polymerization, and if these changes impact antiapoptotic activity.
2. Materials and methods
2.1. Construction of αB-crystallin substitution mutant plasmids
The wildtype αB-crystallin (WT-αB) construct has been previously reported [17]. The αB-crystallin substitution mutants were designed for cloning into the pET23d vector for expression in E. coli. For lysines 72, 82, 90,92, 103, 121,150 and 166 sequence overlap extension PCR (SOE PCR)was performed. In the first step of PCR the αB forward primer was mixed with reverse (rev) primers for each of the eight alanine substitution mutants (Table 1). Additionally, αB reverse primer was mixed with forward (for) primers for each of the eight alanine substitution mutants (Table 1). The wildtype αB-crystallin plasmid was used as a template. PCR was performed by 5 min at 95 °C for Taq polymerase activation followed by 30 cycles of 30 s at 95 °C for denaturing, 30 s at 55 °C for annealing, and 30 s at 72 °C amplification. PCR products were separated on a 1% Tris-acetate-EDTA (TAE) gel and DNA fragments were extracted using Qiaquick gel extraction kit (Qiagen, location). Second step SOE PCR was performed under similar conditions, except using first step PCR DNA fragments as templates. Purified PCR products were gel purified as before, digested with NcoI and HindIII and cloned into the same sites in pET23d using the quick ligation kit (New England Biolabs, Ipswich, MA). Plasmids were confirmed by DNA sequencing and transformed into BL-21 E. coli cells for expression. For amino acid substitution of K174 and K175 with alanine, a single reverse primer K174/175A (Table 1) was used with αB Forward and amplified using the same conditions as first step SOE PCR. The DNA fragment was separated on a 1% TAE agarose gel, gel purified as other DNA fragments, digested with NcoI and HindIII and cloned and sequenced as above.
Table 1.
Oligonucleotides sequences used for PCR and cloning.
| Name | Sequence (5′–3′)a |
|---|---|
| αB Forward | TTTCCATGGACATCGCCATC |
| αB Reverse | AACAAGCTTTCATTTCTTGGGGGCTGC |
| K72A For | CTGGAGGCGGACAGGTTCTCTGTC |
| K72A Rev | CCTGTCCGCCTCCAGGCGCATCTC |
| K82A For | GATGTGGCGCACTTCTCCCCAGAG |
| K82A Rev | GAAGTGCGCCACATCCAGGTTGAC |
| K90A For | GAACTCGCAGTTAAGGTGTTGGGAG |
| K90A Rev | CTTAACTGCGAGTTCCTCTGGGGAG |
| K92A For | AAAGTTGCGGTGTTGGGAGATGTG |
| K92A Rev | CAACACCGCAACTTTGAGTTCCTC |
| K103A For | CATGGAGCACATGAAGAGCGCCAG |
| K103A Rev | TTCATGTGCTCCATGCACCTCAATC |
| K121A For | CACAGGGCATACCGGATCCCAGCTG |
| K121A Rev | CCGGTATGCCCTGTGGAACTCCCTG |
| K150A For | CCAAGGGCACAGGTCTCTGGCCCTG |
| K150A Rev | GACCTGTGCCCTTGGTCCATTCAC |
| K166A For | AAGAGGCGCCTGCTGTCACCGCAG |
| K166 Rev | AGCAGGCGCCTCTTCACGGGTGATG |
| K174/175A Reverse | TATAAGCTTCTATGCCGCGGGGGCTGCGGTGAC |
Restriction endonuclease recognition sites are underlined.
2.2. Expression and purification of recombinant αB-crystallin proteins
Expression and purification of αB-crystallin mutant proteins was performed similar to wildtype αB-crystallin over a Macro-S column (Bio-Rad, Hercules, CA) followed by gel filtration on Sephacryl S400-HR as previously reported [17], [18], [19], [20]. All purified αB-crystallins were stored at − 80 °C.
2.3. Analysis of oligomeric complexes (OC)
Similar to previous analyses, purified modified αB-crystallins were injected onto a Superose 6 gel filtration column using an AKTA FPLC (GE Healthcare Bio-Sciences, Pittsburgh, PA) [17]. αB-Crystallin proteins were eluted in PBS into 1 mL fractions. The elution chromatograms of αB-crystallins mutants were detected by absorbance (280 nm) and plotted against wildtype αB-crystallin.
2.4. Individual substrate chaperone activity assays
Chemical and thermal chaperone activity assays were performed as previously described with either 1:1 or 1:2 M ratio of client substrate to αB-crystallin [17], [21]. Briefly, for thermal chaperone assays, 125 µg/mL of γD-crystallin was incubated in the presence or absence of 6.25 µg/mL αB-crystallin proteins. Protein samples were incubated in 50 mM Phosphate buffer pH [7.4] for 1 h at 65 °C in a Cary 1E UV/vis spectrophotometer fitted with a Peltier controlled sample carrier. Samples were constantly monitored for light scattering at 360 nm. Similarly, for chemical chaperone assays, 10 μM lysozyme (EMD Millipore, Philadelphia, PA) was mixed with 2 mM DTT in the presence or absence of 1 μM αB-crystallin protein. Reactions were performed in PBS as a total volume of 1 mL PBS. Samples were monitored as above for 1 h at 37 °C.
2.5. Actin Polymerization assays
Actin Polymerization Assays were performed using a modified assay with the actin polymerization kit from Cytoskeleton Inc. (Denver, CO). The modified assay used G-buffer by combining 10 mL of General Actin Buffer with 40 µL ATP. Actin buffer (AB) was prepared by adding 50 µL of 20 µg/µL actin with 2.25 mL of G-buffer. Actin oligomers were depolymerized by incubating AB on ice for 60 min and centrifuged at 20,000×g for 30 min at 4 °C. Reactions were setup in black 96-well plates (corning) using 65 µLG-buffer, 10 µL AB, and 25 µL of 1 µM αB-crystallin protein or PBS control. Assays were started by adding 12 µL of actin polymerization buffer (500 mM KCl, 20 mM MgCl2, 50 mM guanidine carbonate, and 10 mM ATP). Wells were monitored for 60 min at excitation (λ 350 nm) and emission (λ 407 nm) on a Synergy 4 Multi-Mode Microplate Reader and Gen5 Reader Control and Data Analysis Software (BioTek, Winooski, VT). Data were plotted and analyzed statistically by ANOVA on repeated measure with Tukey's multiple comparison with GraphPad Prism (La Jolla, CA).
2.6. Cell lysate aggregation assay
Assays were performed similar to those previously described [22]. Briefly, Human embryonic kidney (HEK293) cells from were grown in DMEM (4.5 g/L glucose) with 10% fetal calf serum plus Penicillin/Streptomycin (standard media). At 90% confluency, cells were washed in PBS, scraped from the plate and centrifuged down in PBS containing 1 mM DTT and protease inhibitor cocktail (ThermoFisher). Cell membranes were disrupted by passage through a 27 gauge needle and sonicated before pelleting insoluble debris. The soluble fraction was incubated with 20 U/mL of T4 kinase (New England Biosciences) and 2 mM MgCl2 for 30 min at 37 °C. Lysated were subsequently quantified by BCA (ThermoFisher) and frozen in aliquots at − 80 °C. Cell lysates (1 mg/mL) were mixed with a range of concentrations (0–8 μM) of wildtype or mutant αB-crystallin or control human aldose reductase and incubated at 45 °C for 90 min. Following incubation samples were pelleted, washed in PBS and pellets were suspended in SDS-PAGE loading buffer with 1% 2-mercaptoethanol. Samples were heated at 95 °C for 5 min, loaded and run onto 4–20% mini protean TGX stain free gels (Bio-Rad) at 200 V for 30 min. Gels were imaged using ChemiDoc XRS+ system (Bio- Rad). Densitometry was determined using ImageJ software with background subtracted from each lane. Each assay was performed at least three times. The IC50 values were determined as concentration of protein at which half-maximum aggregation was suppressed, normalized to the concentration of total cell lysate protein and significance determined using Graphpad Prism software.
2.7. Apoptosis assays
HEK293 cells were plated at a density of 8 × 105 cells per well in a 6-well plate overnight in standard media. Cells were transfected with 5 μg of αB-crystallin, mutant α-crystallin, or β-galactosidsase as a control using the Xfect protein transfection reagent (Clontech, Mountain View, CA) following manufacturer's protocol. After washing twice with standard media cells were treated with or without 100 nM staurosporine (STS) for 16 h to induce apoptosis. Cells were collected by trypsinization and analyzed for apoptosis by detecting increased levels of caspase-3 using the fluorometric caspase-3 assay (Abcam, Cambridge, MA) according to manufacturer's protocol. Samples were analyzed in a black 96-well plate using a Synergy 4 (BioTek, Winooski, VT) plate reader with excitation at 400 nm and emission at 505 nm. Experiments were performed at least three times and data was analyzed by Graphpad Prism.
3. Results
3.1. Formation of OC by purified αB-crystallin lysine-to-alanine substitution mutants
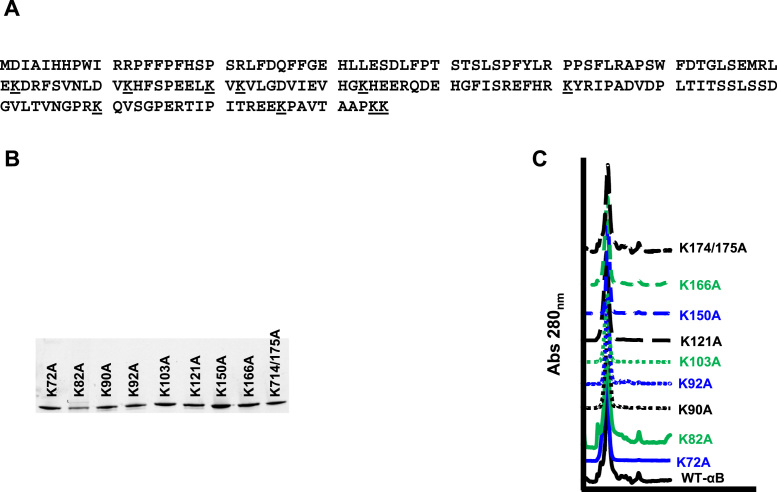
In the present study we constructed, nine lysine-to-alanine mutant αB-crystallin recombinant proteins in which individual (8) or double substitutions (1) were made in order to mutate all ten lysines in αB-crystallin (Fig. 1A). Recombinant αB-crystallin proteins were expressed in BL-21 E. coli and purified to near homogeneity as determined by SDS-PAGE (Fig. 1B). We assessed the ability of these αB-crystallin mutants to form OCs by passing the proteins over a Superose 6 size exclusion column. All nine αB-crystallin mutants formed OCs similar to WT-αB (Fig. 1C). Together, these data indicate that substitution of lysine with alanine does not hinder the formation of OCs.
Fig. 1.
Identified lysine-to-alanine mutations of recombinant αB-crystallin do not alter expression of formation of OCs. (A) The amino acid sequence of αB-crystallin with lysines that were substituted with alanine underlined. (B) SDS-PAGE showing soluble purified recombinant αB-crystallin substitution mutants. (C) Gel filtration analysis of wildtype and αB-crystallin substitution mutants showing the formation of similar OCs.
3.2. Chaperone activity of αB-crystallin substitution mutants
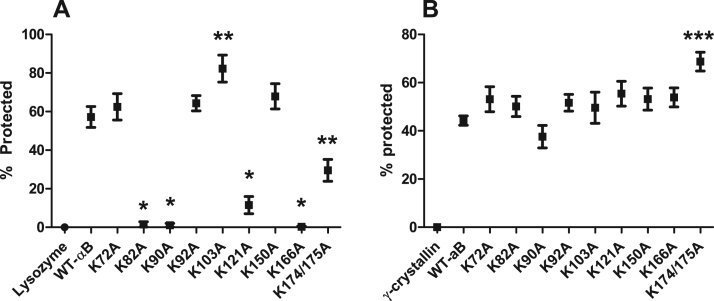
Both chemical and thermal protein aggregation assays were used to assess the chaperone activity of lysine-to-alanine substitution mutants. The ability of individual αB-crystallin mutants to prevent protein aggregation of lysozyme by chemical denaturation varied greatly (Fig. 2A). Three mutants, K72A, K92A, and K150A all retained wildtype levels of chaperone activity. Mutants K121A and K174/175A had significantly reduced activity, while K82A, K90A, and K166A all showed no protection. In contrast, K103A had significantly enhanced protection against chemical aggregation of lysozyme. Analysis of thermally induced protein aggregation of γ-crystallin indicated most αB-crystallin mutants retained chaperone activity similar to WT-αB; however, K174/175A had significantly higher protection (Fig. 2B). These data indicate that lysine substitutions of αB-crystallin do not display similar protection in both chemical and thermal protein aggregation assays.
Fig. 2.
Lysine-to-alanine substitution differentially alters chaperone function in αB-crystallin. Wildtype and αB-crystallin substitution mutants were examined for chaperone activity using chemical (A) and thermal (B) aggregation assays. (A) DTT induced lysozyme aggregation was measured for 1 h at 360 nm. Reduction in aggregation of substrate protein alone (lysozyme) by the addition of αB-crystallin was determined to be the percent of protection. (B). Heat induced γ-crystallin aggregation was measured for 1 h at 360 nm. Reduction in γ-crystallin aggregation by the addition of αB-crystallin was determined to be the percent of protection as compared to γ-crystallin alone (γ-crystallin). Statistical changes in rates were determined by ANOVA. p-value > 0.0015 denoted by *, p-values > 0.0001 denoted by ** and p-values > 0.05 denoted by ***.
3.3. Levels of actin polymerization in the presence of αB-crystallin substitution mutants
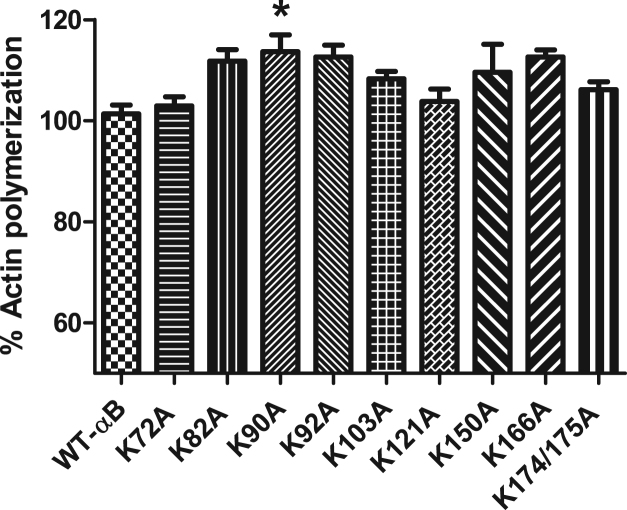
Previous studies with αB-crystallin have indicated that it can modify rates of actin polymerization [23], [24]. Therefore, we analyzed the ability of substitution mutants to affect rates of actin polymerization in vitro. Addition of 0.25 μM αB-crystallin substitution mutant to actin polymerization assay indicated that only K90A had a significant increase in actin polymerization as compared to WT-αB control (Fig. 3).
Fig. 3.
Actin polymerization rate show minimal change with lysine-to-alanine substitution. Wildtype and αB-crystallin substitution mutants were examined for effects on actin polymerization rate. Actin polymerization reactions were performed with αB-crystallin proteins for 1 h. Wildtype αB-crystallin was set to 100%. Levels of polymerization by mutants were compared to wildtype proteins. Statistical changes in rates were determined by ANOVA. p-values > 0.05 denoted by *.
3.4. K90A and K103A αB-crystallin substitution mutants on cell lysate aggregation
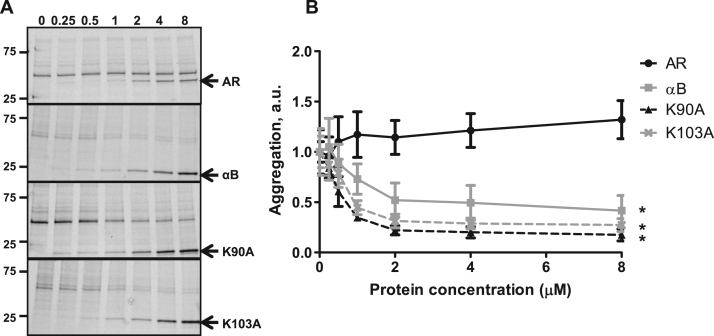
Since lysine-to-alanine mutants showed wide variability in individual substrate assays, we selected K90A and K103A for further analysis. These mutants were selected because K90A showed no activity in lysozyme assay, lower levels in the γ-crystallin assay, and had the best activity in the actin polymerization assay. In contrast, K103 had good activity in both chaperone assays, and near wildtype αB-crystallin levels on actin polymerization. Using a cell lysate model to determine broad spectrum of chaperone activity, we found that both lysine-to-alanine mutants prevented protein aggregation similar to wildtype αB-crystallin that was significantly better than human aldose reductase control (Fig. 4).
Fig. 4.
Cell lysate aggregations assays indicate αB-crystallin mutants prevent protein aggregation. Soluble cell lysates were mixed with varying concentrations (0–8 μM) of human aldose reductase (AR), wildtype or mutant αB-crystallin proteins. Samples were incubated at 45 °C for 90 min to induce protein aggregation. (A) Changes in the insoluble protein levels were detected on 4–20% SDS-PAGE. Micromolar concentration of recombinant protein is noted at the top of the gel. Molecular weight markers of 75 and 25 kDa are indicated on the left. Increased αB-crystallin levels showed reduced insoluble protein, while AR had no effect. Arrows indicate recombinant protein added to sample. (B) Densitometry analysis of insoluble protein levels was plotted over the concentration of recombinant protein to determine aggregation (arbitrary units, a.u.). Assays were performed in triplicate and the mean with S.E. plotted. * = p-value ≤ 0.05.
3.5. K90A and K103A αB-crystallin substitution mutants on prevention of cell death
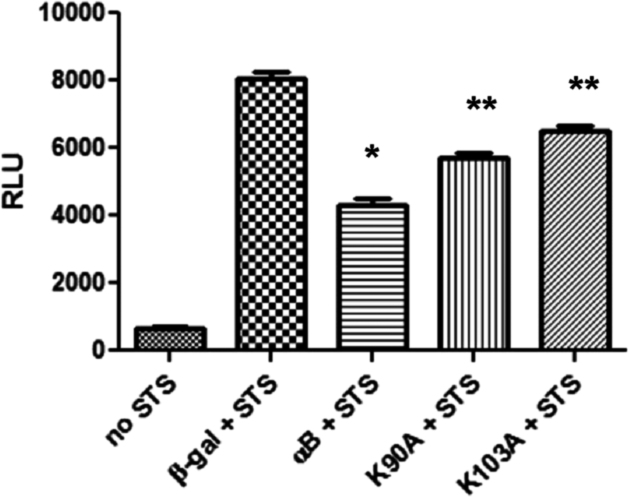
αB-Crystallin is also reported to prevent apoptosis [25], [26]. To determine the effects of lysine-to-alanine substitution might have on αB-crystallin anti-apoptotic activity, HEK293 cells were treated with wildtype, K90A or K103A protein or β-galactosidase protein, exposed to STS and analyzed for caspase-3 activity indicative of cell death. At 16 h post-STS exposure, β-galactosidase treated cells showed high levels of cell death, in contrast wildtype and mutant αB-crystallin treated cells had significantly reduced cell death (Fig. 5). However, both mutants showed significantly reduced anti-apoptotic activity as compared to wildtype αB-crystallin (Fig. 5).
Fig. 5.
Wildtype and substitution mutant αB-crystallin prevent STS induced cell death. HEK293 cells were transfected with 5 μg of protein (wildtype or mutant αB-crystallin, or β-galactosidase) and cell death induced with 100 nM STS. 16 h later cells were collected and caspase-3 determined using the fluorometric assay. Experiments were performed in triplicate and results analyzed by Graphpad Prism. Results are reported in relative light units (RLU) as an average with SEM. * = p-value ≤ 0.0001 to β-galactosidase. ** = p-value ≤ 0.0001 to wildtype αB-crystallin.
4. Discussion
In these studies we analyzed the effects of lysine-to-alanine substitution on αB-crystallin in vitro and in vivo activities. All nine mutant proteins expressed in E. coli and could be purified using a standard αB-crystallin protocol (Fig. 1). Analysis of these purified recombinant proteins indicated that all were able to form OC that were similar in size to WT-αB protein (Fig. 2). Moreover, these proteins had similar protection in thermal aggregation assays with only K174/175A having an increased chaperone activity (Fig. 2). However, the chaperone activities of these proteins varied significantly with some having little to no activity, while other had increased protection in chemical protein aggregation assays. Actin polymerization assays also indicated that lysine-to-alanine substitution resulted in similar activity to WT-αB, with the exception K90A enhanced the level of actin polymerization. Based on these findings we selected the K90A and K103A mutants for further analysis to determine if these in vitro assays were indicative of other activities. Cell lysate analysis indicated that individual protein assays were not representative of activity on a broad spectrum of proteins (Fig. 4). Whereas, apoptosis assays of these mutants indicated both prevent cell death, but to a lesser extent than wildtype αB-crystallin (Fig. 5.). Together, these data suggest that all lysine residues appear dispensable in lysozyme thermal chaperone and actin assays as substitution had no detrimental effects. In contrast, lysine residues K82, K90, K121, K166 K174 and K175 are required for efficient chaperone activity of γ-crystallin under chemical denaturation conditions, while modification to other lysines (72, 92, 103, 150) are amenable to substitution. The analysis of two of these the lysine mutants, K90A and K103A, suggest that the γ-crystallin may better predict how αB-crystallin mutants are affected in whole cell lysate assays. In contrast, none of these in vitro assays were able to predict the outcome of apoptosis assays.
Previous studies have looked at K92 and acetylation [27]. Characterization of single client protein chaperone and anti-apoptotic protective properties was shown to increase when the lysine was modified to acetyllysine. In our studies, we found there was no increase in chaperone activity when the lysine was substituted to an alanine (Fig. 2). Together, these studies suggest that K92 is amenable to substitution, but the addition of a negatively charged or a bulkier R-group may promote increased protective activity in αB-crystallin.
The increased protective effects of the K174/175A mutant in thermal aggregation of γ-crystallin are similar to those found with porcine αB-crystallin [28]. Alignment of human and porcine αB-crystallin proteins resulted in a highly conserved 97% homology. Therefore, not surprisingly the previously reported mutation of K174 and K175 to alanine in porcine αB-crystallin showed an improved chaperone activity (Fig. 2B) [28]. These conserved findings in chaperone activity suggest that studies on porcine αB-crystallin correlate with the human protein as well. It may also suggest that K174/175A mutant would have improve activity in cell lysate assays, however further studies are needed to confirm this prediction.
A 20 amino acid peptide fragment of αB-crystallin has been reported to display both chaperone and anti-apoptotic activity indicative of full-length protein [29]. Within this peptide are K82, K90 and K92 suggestive of their importance in protein function. Previous studies looking at these lysines include the above-mentioned studies on K92, and work looking at mutation of K90 to cysteine [30]. The K90C mutation resulted in modest changes in chaperone activity, while chemical modification of it by N-(2-bromoethyl)-3-oxidopyridinium hydrobromide resulted in a greater loss of activity. Our findings with thermal aggregation of γ-crystallin showed lower but not significant decrease in chaperone activity similar to previous findings; however, we found complete loss of activity in chemical aggregation of lysozyme with it or K82A. These findings indicate the important role of these lysines in chaperone activity.
The ocular lens consists mainly of denucleated fiber cells that remain for decades. These fiber cells require not only soluble crystallin protein for transparency, but also a rigid cell structure that can withstand tension forces extruded by lens accommodation. Previous studies have indicated that α-crystallin proteins mediate increased rate of actin polymerization, likely by slowing the rate of actin depolymerization [23], [24]. Analysis of lysine-to-alanine mutation of αB-crystallin indicated that all mutants retained increased levels of actin polymerization with K90A having even higher levels of polymerized actin (Fig. 3). Our data suggest that the mechanism by which αB-crystallin mediates reduction in actin depolymerization does not require lysine resides.
One type of PTM is ubiquitination; a protein moiety that can either change protein function or target it for proteasome mediated degradation. Previous work has reported genetic mutations in αB-crystallin that affect its solubility and function due to higher levels of ubiquitination [13], [31]. Since protein ubiquitination occurs on lysine, alanine substitution of these residues has the potential to increase protein half-life of exogenously added αB-crystallin protein if used as a therapeutic similar to our previous studies [17], [18], [19]. However, further work is needed to determine which residues are ubiquitinated in αB-crystallin.
In conclusion, substitutions of lysines with alanines in αB-crystallin affect some protein functions. While some of these modifications improve function activity in assays, none show improvement in all assays. Analysis of two of these mutants suggest that γ-crystallin thermal aggregation assay may better predict results with similar cell lysate assays, but none of these assays predict the effects of amino acid substitution on anti-apoptotic activity. Additionally, mutations that improved activity in one assay appeared to lose activity in other assays suggesting a change in protein structure that favored one activity over another. Further studies are needed to determine the effects of lysine-to-alanine substitution have on αB-crystallin structure and whether the net charge is needed for this.
Acknowledgments
We would like to thank David A. Ammar and Rooban Nahomi for critical review of the manuscript. This work was supported in part by a Challenge Grant to the Department of Ophthalmology from Research to Prevent.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.03.001.
Appendix A. Transparency document
Supplementary material
References
- 1.Bloemendal H., de Jong W., Jaenicke R., Lubsen N.H., Slingsby C., Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J.P., Schlosser R., Ma W.Y., Dong Z., Feng H., Lui L., Huang X.Q., Liu Y., Li D.W. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp. Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- 4.Pasupuleti N., Matsuyama S., Voss O., Doseff A.I., Song K., Danielpour D., Nagaraj R.H. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Disease. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecroyd H., Meehan S., Horwitz J., Aquilina J.A., Benesch J.L.P., Robinson C.V., Macphee C.E., Carver J.A. Mimicking phosphorylation of +›B-crystallin affects its chaperone activity. Biochem. J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer H., Chamrad D.C., Herrmann M., Stuwe J., Becker G., Klose J., Blueggel M., Meyer H.E., Marcus K. Study of posttranslational modifications in lenticular alphaA-crystallin of mice using proteomic analysis techniques. Biochim. Biophys. Acta. 2006;1764:1948–1962. doi: 10.1016/j.bbapap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer H., Marcus K., Sickmann A., Herrmann M., Klose J., Meyer H.E. Identification of phosphorylation and acetylation sites in alphaA-crystallin of the eye lens (Mus musculus) after two-dimensional gel electrophoresis. Anal. Bioanal. Chem. 2003;376:966–972. doi: 10.1007/s00216-003-1983-1. [DOI] [PubMed] [Google Scholar]
- 8.MacCoss M.J., McDonald W.H., Saraf A., Sadygov R., Clark J.M., Tasto J.J., Gould K.L., Wolters D., Washburn M., Weiss A., Clark J.I., Yates J.R., 3rd Shotgun identification of protein modifications from protein complexes and lens tissue. Proc. Natl. Acad. Sci. USA. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiels A., Hejtmancik J.F. Molecular genetics of cataract. Prog. Mol. Biol. Transl. Sci. 2015;134:203–218. doi: 10.1016/bs.pmbts.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiels A., Hejtmancik J.F. Genetics of human cataract. Clin. Genet. 2013;84:120–127. doi: 10.1111/cge.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Engelsman J., Gerrits D., de Jong W.W., Robbins J., Kato K., Boelens W.C. Nuclear import of {alpha}B-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J. Biol. Chem. 2005;280:37139–37148. doi: 10.1074/jbc.M504106200. [DOI] [PubMed] [Google Scholar]
- 12.Bova M.P., Yaron O., Huang Q., Ding L., Haley D.A., Stewart P.L., Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin- related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Nal. Acad. Sci. USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar L.V., Ramakrishna T., Rao C.M. Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins. J. Biol. Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- 14.Nahomi R.B., Oya-Ito T., Nagaraj R.H. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin. Biochim. Biophys. Acta. 2013;1832:195–203. doi: 10.1016/j.bbadis.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahomi R.B., Oya-Ito T., Nagaraj R.H. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbadis.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Rajasekaran N.S., Orosz A., Xiao X., Rechsteiner M., Benjamin I.J. Selective degradation of aggregate-prone CryAB mutants by HSPB1 is mediated by ubiquitin-proteasome pathways. J. Mol. Cell. Cardiol. 2010;49:918–930. doi: 10.1016/j.yjmcc.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller N.H., Ammar D.A., Petrash J.M. Cell penetration peptides for enhanced entry of alphaB-crystallin into lens cells. Investig. Ophthalmol. Vis. Sci. 2013;54:2–8. doi: 10.1167/iovs.12-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller N.H., Fogueri U., Pedler M.G., Montana K., Petrash J.M., Ammar D.A. Impact of subunit composition on the uptake of alpha-crystallin by lens and retina. PLoS One. 2015;10:e0137659. doi: 10.1371/journal.pone.0137659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christopher K.L., Pedler M.G., Shieh B., Ammar D.A., Petrash J.M., Mueller N.H. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim. Biophys. Acta. 2014;1843:309–315. doi: 10.1016/j.bbamcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andley U.P., Mathur S., Griest T.A., Petrash J.M. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J. Biol. Chem. 1996;271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 21.Nahomi R.B., Wang B., Raghavan C.T., Voss O., Doseff A.I., Santhoshkumar P., Nagaraj R.H. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J. Biol. Chem. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mymrikov E.V., Daake M., Richter B., Haslbeck M., Buchner J. The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem. 2017;292:672–684. doi: 10.1074/jbc.M116.760413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh J.G., Houck S.A., Clark J.I. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int. J. Biochem. Cell Biol. 2007;39:1804–1815. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., Spector A. alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur. J. Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehlen P., Kretz-Remy C., Preville X., Arrigo A.P. Humanhsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 26.Mehlen P., Schulze-Osthoff K., Arrigo A.P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 27.Nagaraj R.H., Nahomi R.B., Shanthakumar S., Linetsky M., Padmanabha S., Pasupuleti N., Wang B., Santhoshkumar P., Panda A.K., Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim. Biophys. Acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao J.H., Lee J.S., Wu S.H., Chiou S.H. COOH-terminal truncations and site-directed mutations enhance thermostability and chaperone-like activity of porcine alphaB-crystallin. Mol. Vis. 2009;15:1429–1444. [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya J., Padmanabha Udupa E.G., Wang J., Sharma K.K. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharyya J., Shipova E.V., Santhoshkumar P., Sharma K.K., Ortwerth B.J. Effect of a single AGE modification on the structure and chaperone activity of human alphaB-crystallin. Biochemistry. 2007;46:14682–14692. doi: 10.1021/bi701326b. [DOI] [PubMed] [Google Scholar]
- 31.Cobb B.A., Petrash J.M. Structural and functional changes in the alpha A-crystallin R116C mutant in hereditary cataracts. Biochemistry. 2000;39:15791–15798. doi: 10.1021/bi001453j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material