Abstract
Members of NADPH oxidase (Nox) enzyme family are important sources of reactive oxygen species (ROS) and are known to be involved in several physiological functions in response to various stimuli including UV irradiation. UVB-induced ROS have been associated with inflammation, cytotoxicity, cell death, or DNA damage in human keratinocytes. However, the source and the role of UVB-induced ROS remain undefined.
Here, we show that Nox1 is involved in UVB-induced p38/MAPK activation and cytotoxicity via ROS generation in keratinocytes. Nox1 knockdown or inhibitor decreased UVB-induced ROS production in human keratinocytes. Nox1 knockdown impaired UVB-induced p38 activation, accompanied by reduced IL-6 levels and attenuated cell toxicity. Treatment of cells with N-acetyl-L-cysteine (NAC), a potent ROS scavenger, suppressed p38 activation as well as consequent IL-6 production and cytotoxicity in response to UVB exposure. p38 inhibitor also suppressed UVB-induced IL-6 production and cytotoxicity. Furthermore, the blockade of IL-6 production by IL-6 neutralizing antibody reduced UVB-induced cell toxicity.
In vivo assay using wild-type mice, the intradermal injection of lysates from UVB-irradiated control cells, but not from UVB-irradiated Nox1 knockdown cells, induced inflammatory swelling and IL-6 production in the skin of ears. Moreover, administration of Nox1 inhibitor suppressed UVB-induced increase in IL-6 mRNA expression in mice skin.
Collectively, these data suggest that Nox1-mediated ROS production is required for UVB-induced cytotoxicity and inflammation through p38 activation and inflammatory cytokine production, such as IL-6. Thus, our findings suggest Nox1 as a therapeutic target for cytotoxicity and inflammation in response to UVB exposure.
Abbreviations: UV, Ultraviolet; ROS, Reactive Oxygen Species; O2-, Superoxide; OH, Hydroxyl radical; H2O2, Hydrogen peroxide; NOX, NADPH oxidase; PBS, Phosphate-buffered saline; DNA, Deoxyribonucleic acid; RNA, Ribonucleic acid; DPI, Diphenyleneiodonium; NAC, N-acetyl cysteine; H2DCFDA, Fluorescent 2',7'-dichlorofluorescein diacetate; LDH, Lactate dehydrogenase; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-alpha; GM-CSF, Granulocyte-macrophage colony-stimulating factor; NF-κB, Nuclear factor kappa B;; STAT3, Signal transducer and activator of transcription 3; MAPK, Mitogen-activated protein kinase; JNK, Jun N-terminal kinases;; Erk, Extracellular Signal-regulated kinase; MKK, MAP Kinase; MKP, MAPK phosphatase; ASK1, Apoptosis signal-regulating kinase 1; Bax, BCL2-associated X protein
Keywords: UVB, NADPH oxidase 1, Reactive oxygen species, Keratinocyte, P38/MAP kinase, Cytotoxicity
Highlights
-
•
Nox1 knockdown decreased UVB-increased cellular ROS in keratinocytes.
-
•
Nox1 knockdown suppressed UVB-induced p38 activation, accompanied by reduced in IL-6 levels and attenuated cell toxicity.
-
•
UVB-induced cytotoxicity is involved in p38/MAPK pathway and IL-6 production, which is partially dependent on Nox1-generated ROS.
1. Introduction
Solar ultraviolet (UV) radiation is a primary risk factor for skin carcinogenesis, such as basal and squamous cells carcinomas [1], [2], [3]. Among three classified types of UV radiation (UVA, UVB, and UVC), UVB (280–320 nm) is the most energetic and leads to biological harm, including direct DNA damage, the activation of receptor-mediated signaling pathways, and the production of reactive oxygen species in high amounts (ROS; O2−, OH, and H2O2) [3], [4]. The adverse effect of UVB-induced ROS in keratinocytes has been associated with the initiation of inflammation and cytotoxicity, followed by carcinogenesis, in which various molecular pathways are involved [1], [5], [6]. Previous studies have shown that UVB radiation activates several signaling pathways including nuclear factor-κB (NF-κB), the signal transducer and activator of transcription 3 (STAT3), p38/MAPK, or c-Jun N-terminal kinase (JNK) signaling, which affect apoptosis, cytotoxicity, or inflammation in human keratinocytes [1], [3], [6]. Among these pathways, the UVB-induced activation of p38 and JNK signaling has been reported to be partially ROS dependent in keratinocytes. However, mechanisms underlying the UVB-induced activation of these pathways remain undefined. In addition, the source of ROS generation in keratinocytes in response to UVB radiation has not been completely determined [1], [7], [8].
Enzyme NADPH oxidase (NOX) is one of the most important sources of intracellular ROS in mammalian cells. NOX is activated by various cellular stresses, such as chemical factors, cellular stimuli, or UV exposure [9], [10]. Members of NOX family (Nox1–5, Duox1, and 2) are involved in several physiological functions, including innate immunity, signal transduction, and biochemical reactions, and they show different tissue distribution with discrete activation mechanisms through ROS production [9], [11]. Among the members of NOX family, Nox1 and 2 have been observed to generate ROS in response to UVA or UVB radiation in human keratinocytes [12], [13], [14], [15], [16], [17], [18], [19]. In response to UVA, ROS production has been shown to be dependent on Nox1 and/or Nox2 in keratinocytes [13], [14], [17]. In contrast, though Ryu et al., have suggested the involvement of Nox1 in UVB-induced ROS production and apoptosis in keratinocytes [12], the source and the role of UVB-induced ROS are still undefined. Because ROS act as signaling molecules and regulate a variety of cellular responses [20], [21], we postulated that Nox1-generated ROS might be implicated in some specific cell signaling pathways, leading to cell death or inflammation in response to UVB irradiation in keratinocytes.
The aim of this study was to determine the role of Nox1 in UVB-induced cell signaling, inflammation, or cytotoxicity via ROS production in keratinocytes. We utilized human keratinocyte cell lines HaCaT with Nox1 knockdown by siRNA. Here, we report that Nox1 is required for UVB-induced inflammation and cytotoxicity via a mechanism involving UVB-induced ROS production by Nox1 in p38/MAPK cell signaling pathway.
2. Materials and methods
2.1. Cell line and UVB irradiation
The human keratinocyte HaCaT cells (a kind gift of Dr Fusenig, German Cancer Research Center, Heidelberg, Germany) were cultured in Dulbecco's Modified Eagle's medium (DMEM, WAKO) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (5% CO2, 37 °C). Cells were transfected with Nox1 or non-targeting-siRNA (ON-TARGET plus SMART pool, GE Healthcare) using Lipofectamine 2000™ (Invitrogen Life Technologies) according to the manufacturer's protocol [22]. After the cultures reached 90% confluency, the culture medium was replaced to phosphate-buffered saline (PBS), and cells were exposed to UVB radiation (15–100 mJ/cm2, 302 nm peak, UV Bench Lamp, UVP) monitoring with a UV Light Meter. Cells were harvested at different time point as described later.
2.2. Cellular ROS analysis
Cellular ROS level was assayed using the CM-H2DCFDA reagent (Invitrogen Life Technologies) according to manufacturer's instructions using a microplate reader. Briefly, cells seeded in a 96-well plate were stained with CM-H2DCFDA reagent (20 μM in PBS, 10 min), and washed by PBS. The cellular fluorescence was assessed by a fluorescent microplate reader (SpectraMax Paradigm, Molecular Devices, Ex 492 nm, Em 525 nm). In some setting, cells were incubated with ML171 (10 μM), GKT137831 (100 μM), diphenyleneiodonium (DPI, 50 μM), NAC (5 mM) or vehicle (Veh) for 1 h, and followed by UVB irradiation.
To assess the intracellular H2O2 level, cells were transfected with H2O2 sensitive probe pHyPer cDNA plasmid (Evorgen) with Lipofectamine 2000 (Invitrogen) according to a manufacture's instruction [23], [24]. G418 resistant transfectants were isolated as pHyPer stably transfected cells. Cellular fluorescence was assessed by a fluorescent microplate reader (SpectraMax Paradigm, Molecular Devices, YFP: Ex 497 nm, Em 525 nm, CFP: Ex 434 nm, Em 525 nm). YFP to CFP excited (497/434) ratio was calculated as intracellular H2O2 level [23], [25].
2.3. viability and cytotoxicity
The culture medium of HaCaT cells seeded in a 96-well plate was replaced to PBS, and cells were exposed to UVB radiation (15 or 30 mJ). Cells were cultured in DMEM medium containing 10% FBS for 24 h. To assess cell viability, cells were estimated with a Cell Count Reagent SF kit (Nacalai Tesque). For cytotoxicity assay, culture medium (DMEM) was replaced to PBS, and collected PBS after incubation for 2 h. Cytotoxicity was determined by Cytotoxicity LDH assay kit according to manufacturer's instructions (WAKO).
2.4. Real-time quantitative RT-PCR
HaCaT cells in a 24-well plate were irradiated with UVB (30 mJ/cm2) and extracted mRNA at 6 h. Total RNA was extracted using TRIZOL (Invitrogen Life Technologies). The cDNA was reverse transcribed from total RNA using the Prime Script RT reagent kit (Takara Bio). Quantitative RT-PCR was performed using SYBR Green I (Takara Bio) and real-time PCR apparatus Step One Plus (Applied Biosystems).
2.5. Immunoblotting, ELISA assay, Immunofluorescence
Cells seeded in 60 mm culture dish were lysed with RIPA buffer (Cell Signaling Technology). The supernatant (10,000 g, 10 min, 4 °C) was performed for immunoblotting with antibodies against phospho-Erk, Erk, phosphor-p38, p38, phospho-Akt and Akt (Cell Signaling Technology, 1:1000), or β-actin (Sigma, 1:2000).
IL-6 amount in the culture supernatant (96-well plate) was quantified with IL-6 human ELISA Kit (Thermo Fisher Scientific) according to a manufacture's instruction.
For Nox1 immunostaining, cells were fixed with 10% formalin, and permeabilized with 0.2% saponin, and immunostained with anti-Nox1 (Santa Cruz), followed by anti-rabbit secondary antibody (FITC, Sigma).
For immunostaining with γH2AX, cells were fixed with 4% formalin, and permeabilized with 0.3% Triton X-100. Cells were stained with anti-phospho-Histone H2A.X (ser139, Cell Signaling Technology, 1:200), followed by anti-rabbit secondary antibody (cy3, Sigma). Cells were also stained with Alexa488-conjugated phalloidin (Invitrogen, Carlsbad, CA). Fluorescent images were obtained with a confocal microscopy LSM710 (Carl Zeiss).
2.6. Skin inflammation in vivo
C57BL/6 mice (8 weeks, female, JAPAN SLC) were administered intradermal ear injections of GKT137831 (200 μM in PBS, 50μl/ear) or vehicle (PBS, 50μl/ear). After 1 h, the mice were irradiated with UVB (100 mJ). The total RNA was extracted from harvested skin tissue at 24 h after UVB irradiation using TRIZOL (Invitrogen Life Technologies), and the mRNA level was analyzed by real-time PCR as described above.
HaCaT cells with control- or Nox1-siRNA were irradiated with UVB (30 mJ/cm2). Cells were collected at 1 h and sonicated in PBS. C57BL/6 mice (8 weeks, female, JAPAN SLC) were administered intradermal ear injections of the cell lysate (50μl, 1 × 105 cells/ear). The ear thickness was measured using a micrometer at 24 h after injection. The mRNA level of harvested skin tissue at 24 h after injection was analyzed by real-time PCR.
All animal experiments were approved by the Committee on Animal Research of Keio University.
2.7. Statistical analysis
Unless specifically stated, statistical analysis was performed with the unpaired two-tailed Student's t-test.
3. Results
3.1. UVB-induced ROS production via Nox1 in keratinocytes
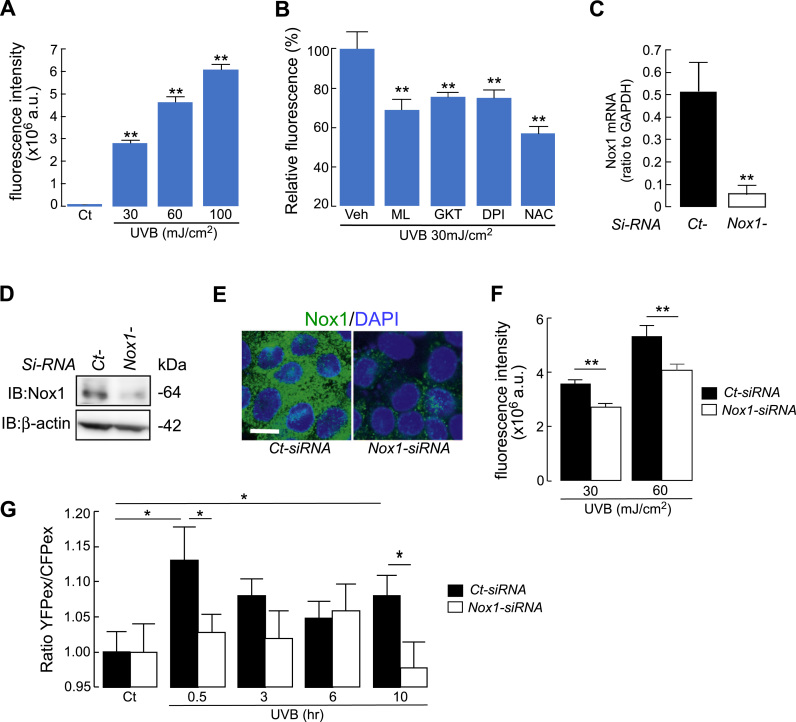
Human keratinocytes cell lines HaCaT were irradiated with UVB (30–100 mJ/cm2) and intracellular ROS levels were measured using the ROS-reactive fluorescent dye 2′,7′-dichlorodihydrofluorescin diacetate (H2DCFDA) [24], [26], [27], [28]. Cellular fluorescence intensity, which is an indicative of intracellular ROS levels, increased after UVB exposure in a dose-dependent manner (Fig. 1A). To confirm the involvement of Nox1 in cellular ROS production by UVB irradiation, keratinocytes were incubated with Nox1 specific inhibitor ML171 [29]. The increase in cellular fluorescence intensity in response to UVB irradiation was decreased by ML171, suggesting the involvement of Nox1 in UVB-induced ROS production (Fig. 1B). The Nox1/4 dual inhibitor GKT137831 also reduced the fluorescence intensity as cellular ROS level [30], [31] (Fig. 1B). Moreover, treating the cells with general Nox inhibitor diphenyleneiodonium (DPI) or antioxidant N-acetyl-L-cysteine (NAC) attenuated UVB-induced cellular ROS production (Fig. 1B).
Fig. 1.
A. Cellular ROS level in HaCaT cells. Cells were irradiated with UVB (30, 60 or 100 mJ/cm2), and cellular ROS was detected at 3 min after UVB irradiation using CM-H2DCFDA fluorescence (SE; n = 12, **p < 0.01; control v.s. UVB irradiation). B. Cells were incubated with ML171 (10 μM), GKT137831 (100 μM), DPI (50 μM), NAC (5 mM) or vehicle (Veh) for 1 h, and followed by UVB irradiation (30 mJ/cm2). Intracellular ROS level at 3 min after UVB irradiation (relative fluorescence compared to vehicle) (SE; n = 6, **p < 0.01; vehicle v.s. inhibitor treated cells). C-F. HaCaT cells were transfected with Nox1- or non-targeting control (Ct)-siRNA. C. Relative mRNA expression of Nox1/GAPDH (SE, n = 4, **p < 0.01). D. Immunoblot with anti-Nox1 and β-actin. E. Immunostaining with anti-Nox1 (FITC, green) and DAPI (blue). Bar, 10 µm. F. Cellular ROS level with UVB irradiation (30 or 60 mJ/cm2) in Ct- or Nox1-siRNA transfected cells by CM-H2DCFDA fluorescence (SE; n = 6, **p < 0.01). G. Cellular H2O2 level by Hyper fluorescence at indicated time point in response to UVB irradiation (30 mJ/cm2, SE; n =11, *p < 0.05). HyPer positive cells were transfected with Nox1- or non-targeting control (Ct)-siRNA. The ratio of cellular fluorescence in YFP to CFP excited (497/434) was calculated as intracellular H2O2 level.
To investigate the role of Nox1 in UVB-induced ROS production, Nox1 was knocked down using Nox1-siRNA in HaCaT cells. Nox1-RNAi introduction into HaCaT cells successfully decreased Nox1 mRNA and protein expression (Fig. 1C, D). Immunofluorescence staining confirmed suppressed Nox1 expression in Nox1-siRNA-transfected cells (Fig. 1E). In this setting, intracellular ROS levels at 3 min after UVB irradiation were significantly reduced by Nox1 knockdown (Fig. 1F).
Recent study showed that UVB irradiation to keratinocytes induced biphasic activation of Nox, rapid burst (less than 1 h) and later sustained (8–12 h) [32], which predicted the involvement of Nox1 in UVB-induced H2O2 production not only in short time but also in longer time period after UVB exposure. To this end, we transfected HaCaT cells with HyPer, a genetically encoded ratiometric sensor that is selective to H2O2 and allows dynamic monitoring of intracellular H2O2 concentration [23], [25]. Fig. 1G showed that Nox1 knockdown suppressed UVB-induced rapid and late increase in cellular H2O2 level. Collectively, these results suggest the involvement of Nox1 in UVB-induced cellular ROS production in human keratinocytes.
3.2. Nox1 knockdown attenuated UVB-increased cytotoxicity and inflammatory cytokine levels
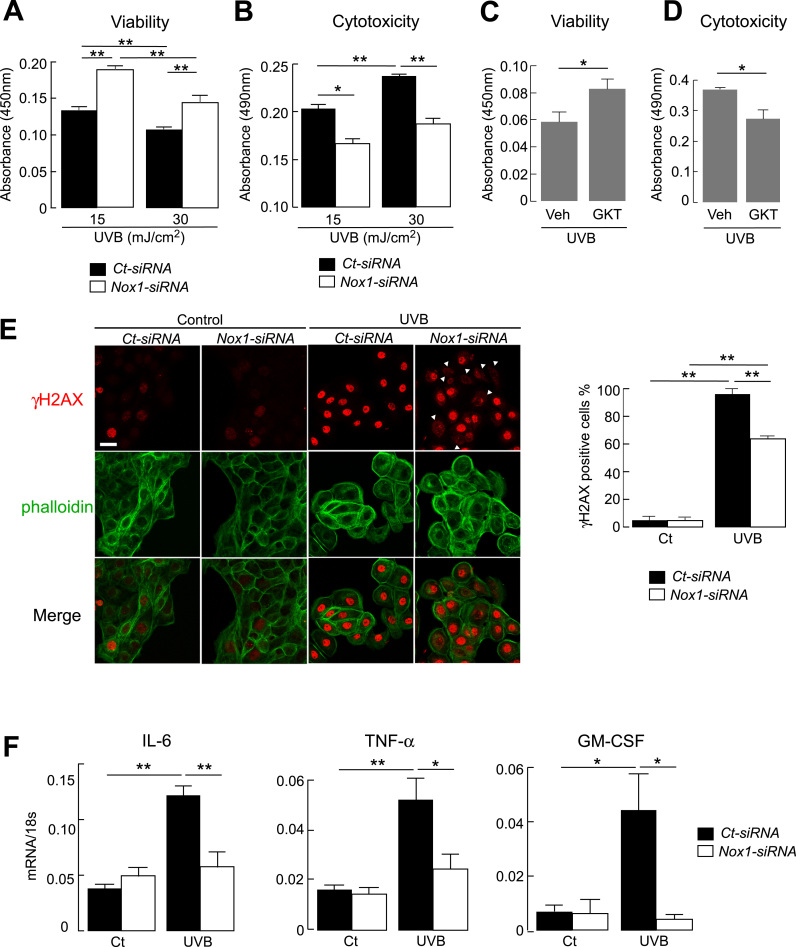
Previous studies have demonstrated that UVB irradiation induced cell death, such as necrosis and apoptosis, and cellular cytotoxicity partially via elevated ROS levels [1], [5]. Cell viability after UVB exposure was significantly greater in Nox1 knockdown than that in control cells (Fig. 2A). Coincidentally, UVB-induced cytotoxicity, measured by lactate dehydrogenase (LDH) leakage, was suppressed in Nox1 knockdown cells compared with that in control cells (Fig. 2B). Moreover, pretreatment cells with Nox1 inhibitor GKT137831 before exposure to UVB increased cell viability with reduced cytotoxicity compared to vehicle treated cells (Fig. 2C, D). These results suggest the involvement of Nox1 in UVB-induced cell toxicity.
Fig. 2.
A. B. HaCaT cells with Nox1- or non-targeting control (Ct)-siRNA were irradiated with UVB (15 or 30 mJ/cm2), and evaluated at 24 h later. A. Cell viability was assessed by Cell Count Reagent SF kit (SE, n = 6, **p < 0.01). B. Cytotoxicity was assessed by LDH leakage into medium (SE, n = 6, *p < 0.05, **p < 0.01). C. D. HaCaT cells were incubated with GKT137831 (100 μM, 1 h) and irradiated with UVB (30 mJ/cm2). C. Cell viability was assessed at 24 h later by Cell Count Reagent SF kit (SE, n = 5, *p < 0.05). D. Cytotoxicity was assessed by LDH leakage into medium (SE, n = 5, *p < 0.05). E. (left) Representative immunofluorescence of γH2AX (cy3, red) and phalloidin (FITC, green) in Ct- or Nox1-siRNA transfected HaCaT cells. Cells were irradiated with UVB (30 mJ), and fixed at 18 h later. Bar, 20 µm. Arrow head; γH2AX negative cells. (right) The ratio of γH2AX positive cells in the nucleus (SE, over 100 cells from 4 different fields, **p < 0.01). F. mRNA expression in Ct- or Nox1-siRNA transfected cells by real-time RT-PCR. Cells were irradiated with UVB (30 mJ/cm2) and extracted mRNA at 6 h. Data are expressed as the ratio to 18 s (SE, n = 4–6, *p < 0.05, **p < 0.01).
To evaluate UVB-induced DNA damage, we next examined the phosphorylation of histone H2AX (γH2AX at Ser 139), which is one of the major markers for DNA double-strand breaks, by immunofluorescence. γH2AX positive cells was substantially increased in response to UVB irradiation (Fig. 2E, left). The ratio of γH2AX positive cells was less in Nox1 knockdown cells than in control cells (Fig. 2E, right), indicating that Nox1 may be involved in the DNA damage response to UVB irradiation.
Further, to investigate the role of Nox1 in UVB-induced cell viability and cytotoxicity, the expression of mRNA genes related to inflammation and apoptosis was examined using quantitative RT-PCR. UVB irradiation increased mRNA levels of IL-6, TNF-α, and GM-CSF, which are related to inflammation, with markedly lesser increases in Nox1 knockdown cells (Fig. 2F). In contrast, mRNA levels of apoptosis-related genes, including a key enzyme in the apoptotic pathway caspase-3, an apoptosis promoter Bax, or an apoptosis inhibitor gene Bcl-2, were comparable between control- and Nox1-siRNA-transfected cells (Supplementary Figure 1). These results indicate that Nox1 is required for the UVB-induced production of inflammatory cytokines.
3.3. UVB-induced p38 activation was dependent on Nox1 and ROS
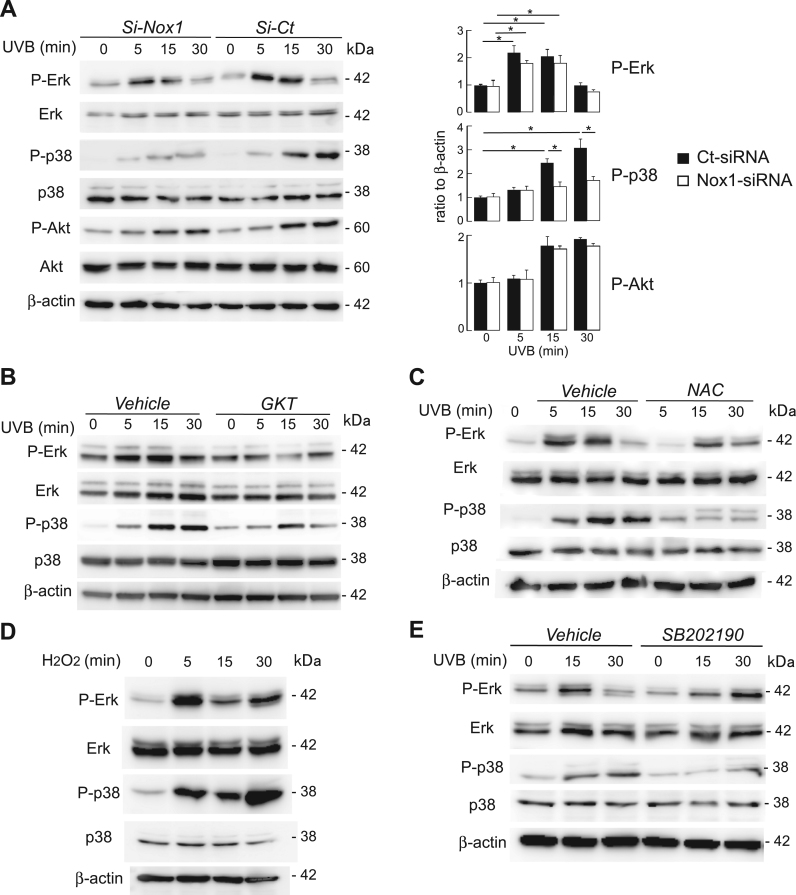
Previous studies have shown that UV exposure activates Erk, p38, or Akt cell signaling, leading to the production of inflammatory cytokines and inflammation [1], [33]. The involvement of Nox1 in UVB-induced cell signaling pathways was determined. Immunoblot analysis revealed that UVB irradiation induced the phosphorylation of Erk, p38, and Akt in control cells (Fig. 3A, left). UVB-induced phosphorylation of p38 was significantly suppressed in Nox1 knockdown cells, whereas Erk and Akt activation remained unaffected by Nox1 knockdown (Fig. 3A, right). Similarly, pretreatment of cells with GKT137831 also attenuated UVB-induced p38 phosphorylation (Fig. 3B).
Fig. 3.
A. HaCaT cells with control- or Nox1-siRNA were irradiated with UVB (30 mJ/cm2) for indicated time point. (left) Representative immunoblot using antibodies against phospho-Erk, Erk, phospho-p38, p38, phospho-Akt, Akt and β-actin. (right) Quantification of immunoblots. P-Erk, P-p38, and P-Akt were normalized to β-actin detected, from data as in Fig. 3A (SE, n = 3, *p < 0.05). B. HaCaT cells were incubated with vehicle (PBS) or GKT137831 (20 μM, 6 h), and irradiated with UVB (30 mJ/cm2). Representative immunoblot for indicated time point and antibodies. C. HaCaT cells were incubated with vehicle (PBS) or NAC (5 mM), and irradiated with UVB (30 mJ/cm2). Representative immunoblot for indicated time point and antibodies. D. HaCaT cells were incubated with H2O2 (500 μM) for 5–30 min. Representative immunoblot using indicated antibodies. E. HaCaT cells were incubated with vehicle (PBS) or P38 inhibitor SB202190 (25uM), then irradiated with UVB (30 mJ/cm2). Representative immunoblot for indicated time point and antibodies.
Further, effects of intracellular ROS levels on UVB-induced cell signaling were investigated. The NAC-treated HaCaT cells, with decreased cellular ROS levels, as shown in Fig. 1B, evidently suppressed the UVB-induced phosphorylation of p38 in HaCaT cells (Fig. 3C). In contrast, the exogenous addition of H2O2 at high concentration could modulate Erk and p38 in HaCaT cells (Fig. 3D). The pretreatment of the cells with p38 inhibitor SB202190 completely suppressed UVB-induced p38 activation, but not Erk activation, in HaCaT cells (Fig. 3E). These results suggest that Nox1 is involved in UVB-induced p38 activation, probably due to Nox1-generated ROS.
3.4. Involvement of p38 in UVB-induced IL-6 production and cytotoxicity
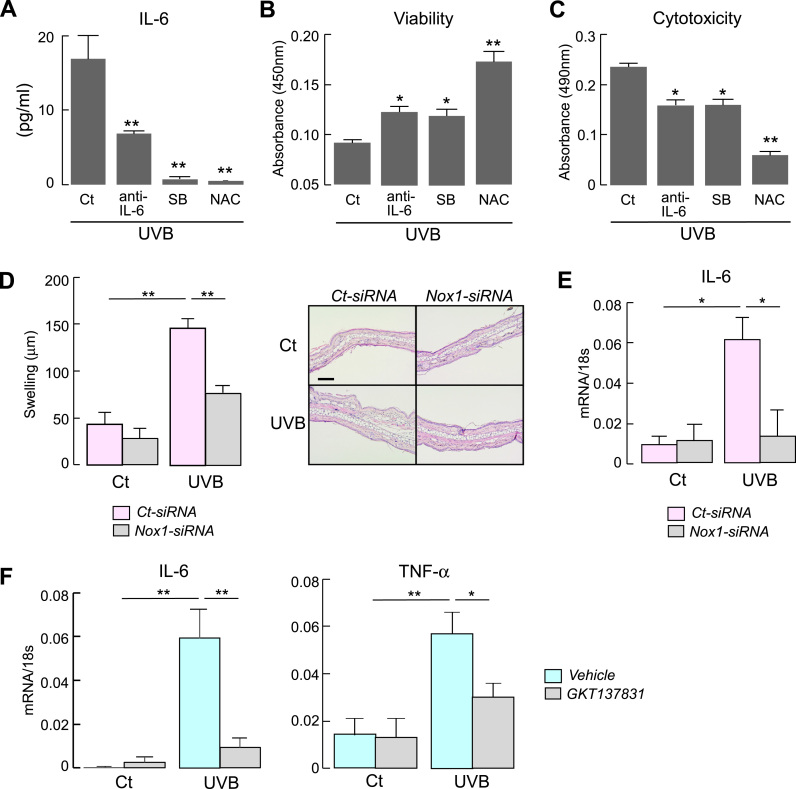
The involvement of p38/MAPK cell signaling in UVB-induced IL-6 production and its mediated cytotoxicity were examined. HaCaT cells were incubated with anti-IL-6 neutralizing monoclonal antibody, p38 inhibitor SB202190, or NAC, followed by UVB irradiation. Fig. 4A shows that the treatment of the cell with anti-IL-6 antibody, SB202190, or NAC suppressed UVB-increased IL-6 production, indicating that UVB-induced IL-6 production was dependent on p38 cell signaling and ROS levels. Furthermore, the cell viability after UVB exposure was increased by anti-IL-6 antibody, SB202190, or NAC treatment (Fig. 4B). Coincidently, cytotoxicity levels after UVB exposure was ameliorated by anti-IL-6 antibody, SB202190, or NAC treatment (Fig. 4C). These findings support that UVB-induced cytotoxicity depends on p38/MAPK pathway and ROS levels via IL-6 production.
Fig. 4.
A-C. HaCaT cells were incubated with anti-IL-6 antibody (10 μg/ml), SB202190 (25 μM), NAC (5 mM) or vehicle (PBS) for 1 h, and irradiated with UVB (15 mJ/cm2). The culture medium and cells were assessed at 24 h. A. IL-6 amount in culture medium assessed by IL-6 Elisa kit (SE, n = 5, **p < 0.01; v.s. control). B. Cell viability assessed by cell Count Reagent SF kit (SE, n = 5, *p < 0.05, **p < 0.01; v.s. control). C. Cytotoxicity assessed by LDH assay (SE, n = 5, *p < 0.05, **p < 0.01; v.s. control). D-E. HaCaT cells with control- or Nox-siRNA were irradiated with UVB (30 mJ/cm2). Mice were administered intradermal ear injections of collected cell lysate (1 × 105 cells/ear) at 1 h after UVB irradiation. Evaluations were done at 24 h after injection. D. (left) The swelling of the ear skin (SE, n = 5, **p < 0.01). (right) Representative hematoxylin and eosin staining of ears. Bar, 100 µm. E. qPCR measurements of IL-6 mRNA in tissue extracts of ear skin (SE, n = 5, *p < 0.05). F. GKT137831 (200 μM, 50 μl) or vehicle (PBS, 50 μl) was intradermally injected into the ear. The skin was irradiated by UVB (100 mJ) at 1 h after injection, and skin samples were excised at 24 h after UVB irradiation. mRNA expression of IL-6 and TNF-α in skin tissues determined by real-time RT-PCR (SE, n = 4–6,*p < 0.05, **p < 0.01). Data are expressed as the ratio to 18 s.
Next, UVB-induced inflammation in keratinocytes was investigated in vivo. Ears of C57BL/6 mice were intradermally injected with lysates from UVB-irradiated or non-irradiated HaCaT cells, as previously described [34]. Lysate injections from control-siRNA-transfected cells with UVB irradiation induced ear swelling (Fig. 4D) and increased IL-6 mRNA level in the skin (Fig. 4E), while UVB irradiated-Nox1 knockdown lysate suppressed the ear swelling and reduced IL-6 expression (Fig. 4D, E).
Finally, we verified the involvement of Nox1 in UVB-induced pro-inflammatory response in vivo assay. GKT137831 was injected into the skin of the ear of C57BL/6 wild-type mice prior to UVB irradiation. Fig. 4F showed that administration of GKT137831 significantly suppressed UVB-induced increase in IL-6 and TNF-α mRNA expression in the skin.
Together, these findings provide evidence for the involvement of Nox1 in UVB-induced cytotoxicity and inflammation in human keratinocytes.
4. Discussion
In this study, the involvement of Nox1 in UVB-induced p38/MAPK activation, cytotoxicity, and inflammation via ROS production was demonstrated in human keratinocytes. Nox1 knockdown or inhibitor decreased UVB-induced ROS production in human keratinocytes HaCaT. UVB-mediated p38 activation was impaired by Nox1 knockdown, accompanied by reduced IL-6 levels and attenuated cell toxicity. Treatment of cells with NAC, a potent ROS scavenger, suppressed p38 activation as well as consequent IL-6 production and cytotoxicity in response to UVB irradiation. p38 inhibitor also suppressed UVB-induced IL-6 production and cytotoxicity. In addition, the blockade of IL-6 production by IL-6 neutralizing antibody reduced UVB-induced cell toxicity. Together, these findings support the involvement of Nox1 in UVB-induced cell toxicity and inflammatory response by a mechanism involving ROS production and processes downstream of p38-dependent cell signaling.
p38 is a member of the mitogen-activated protein kinase (MAPK) family of proteins and plays an essential role in regulating many cellular processes, including inflammation, cell growth, or cell death. p38 is activated in response to extracellular stimuli, such as cytokines or oxidative stress [35], [36]. Previous studies have suggested that UVB exposure activated the p38 pathway by ROS-dependent [37], [38] and independent mechanisms [39], [40], [41], [42]. ROS-dependent p38 activation has been associated with several signaling molecules, including MAP kinases (MKK) 3/6, MAPK phosphatase (MKP), or apoptosis signal-regulating kinase 1 (ASK1) [1]. The findings of this study indicate that UVB-induced p38 activation is ROS-dependent via Nox1. In addition, we demonstrated that p38 activation was involved in inflammatory cytokine IL-6 production and cytotoxicity in response to UVB irradiation. Although pathophysiological effects of UVB-induced p38 activation in keratinocytes remains controversial [1], Nox1 may be required for UVB-induced p38 cell signaling, which may lead to cytotoxicity and inflammation. Further research is required to determine the role of p38 signaling pathway in UV-induced cytotoxicity and inflammation.
Most parts of the mammalian body are covered by skin, which protects the inner organs from environmental stresses, including UV radiation. Several lines of evidence suggest that UVR-induced oxidative stress, defined as unbalanced ROS production and antioxidant defense, has been associated with multiple skin conditions, including aging and carcinogenesis [1], [21]. UVR exposure has been reported to induce ROS production at high levels, greatly exceeding the antioxidative defenses, leading to inflammation, DNA damage, and mutations [4], [43], [44]. Previous in vitro experiments using keratinocytes have demonstrated that the depletion of antioxidant glutathione increased UVR-induced cellular ROS levels and increased UVR sensitivity [45]. Correspondingly, glutathione deactivation by glutathione peroxidase deficiency predisposed mice to UVR-induced cutaneous squamous cell carcinoma, which is a common malignancy of keratinocytes in response to excessive UV exposure [46]. In addition, a more recent study has demonstrated that Nox1 inhibitor administration reduced UVB-induced carcinogenesis in both XPC-proficient and -deficient mice, in which Nox1 inhibition increased nucleotide excision repair and reduced apoptosis [32]. In this study, Nox1 knockdown reduced UVB-induced cytotoxicity and inflammatory response, supporting the involvement of Nox1 in UVB-induced keratinocytes damage. Taken together, Nox1 inhibition may be a potential target for preventing UV-induced initial skin damage, protecting the skin against carcinogenesis or cellular senescence.
In summary, our findings suggest that Nox1 is required for UVB-induced p38 cell signaling in human keratinocytes. Nox1-mediated ROS production may be associated with cytotoxicity and inflammation in response to UVB exposure. Therefore, our findings suggest Nox1 as a therapeutic target for UVB-induced cytotoxicity and inflammation in keratinocytes.
Acknowledgements
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (16K10125, M.H-C).
Acknowledgments
Competing financial interests
The authors declare no competing financial interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.03.004.
Appendix A. Transparency document
Supplementary material
References
- 1.Feehan R.P., Shantz L.M. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem. J. 2016;473:2973–2994. doi: 10.1042/BCJ20160471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 3.Bowden G.T. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 4.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int. J. Mol. Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bito T., Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J. Dermatol. Sci. 2012;68:3–8. doi: 10.1016/j.jdermsci.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Assefa Z., Van Laethem A., Garmyn M., Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim. Biophys. Acta. 2005;1755:90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Bode A.M., Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 9.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 11.Lambeth J.D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu H.C., Kim C., Kim J.Y., Chung J.H., Kim J.H. UVB radiation induces apoptosis in keratinocytes by activating a pathway linked to "BLT2-reactive oxygen species'. J. Investig. Dermatol. 2010;130:1095–1106. doi: 10.1038/jid.2009.436. [DOI] [PubMed] [Google Scholar]
- 13.Valencia A., Kochevar I.E. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J. Investig. Dermatol. 2008;128:214–222. doi: 10.1038/sj.jid.5700960. [DOI] [PubMed] [Google Scholar]
- 14.Wu S., Gao J., Dinh Q.T., Chen C., Fimmel S. IL-8 production and AP-1 transactivation induced by UVA in human keratinocytes: roles of D-alpha-tocopherol. Mol. Immunol. 2008;45:2288–2296. doi: 10.1016/j.molimm.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani H.R., Cario-Andre M., Pain C., Ged C., deVerneuil H., Taieb A. Protection of normal human reconstructed epidermis from UV by catalase overexpression. Cancer Gene Ther. 2007;14:174–186. doi: 10.1038/sj.cgt.7701000. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani H.R., Mazurier F., Cario-Andre M., Pain C., Ged C., Taieb A., de Verneuil H. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J. Biol. Chem. 2006;281:17999–18007. doi: 10.1074/jbc.M600536200. [DOI] [PubMed] [Google Scholar]
- 17.He Y.Y., Huang J.L., Block M.L., Hong J.S., Chignell C.F. Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. J. Investig. Dermatol. 2005;125:560–566. doi: 10.1111/j.0022-202X.2005.23851.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Rezvani H.R., Back J.H., Hosseini M., Tang X., Zhu Y., Mahfouf W., Raad H., Ragi G., Athar M., Kim A.L., Bickers D.R. Inhibition of p38 MAPK signaling augments skin tumorigenesis via NOX2 driven ROS generation. PLoS One. 2014;9:e97245. doi: 10.1371/journal.pone.0097245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Kochevar I.E. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Radic. Biol. Med. 2005;38:890–897. doi: 10.1016/j.freeradbiomed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Paulsen C.E., Carroll K.S. Orchestrating redox signaling networks through regulatory cysteine switches. Acs Chem. Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara-Chikuma M., Takahashi K., Chikuma S., Verkman A.S., Miyachi Y. The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch. Dermatol. Res. 2009;301:245–252. doi: 10.1007/s00403-009-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belousov V.V., Fradkov A.F., Lukyanov K.A., Staroverov D.B., Shakhbazov K.S., Terskikh A.V., Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 24.Satooka H., Hara-Chikuma M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol. Cell. Biol. 2016;36:1206–1218. doi: 10.1128/MCB.00971-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiagarajah J.R., Chang J., Goettel J.A., Verkman A.S., Lencer W.I. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA. 2017;114:568–573. doi: 10.1073/pnas.1612921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara-Chikuma M., Watanabe S., Satooka H. Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem. Biophys. Res. Commun. 2016;471:603–609. doi: 10.1016/j.bbrc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S., Moniaga C.S., Nielsen S., Hara-Chikuma M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016;471:191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 28.Hara-Chikuma M., Satooka H., Watanabe S., Honda T., Miyachi Y., Watanabe T., Verkman A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015;6:7454. doi: 10.1038/ncomms8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver J.R., Grzesik W., Taylor-Fishwick D.A. Inhibition of NADPH oxidase-1 preserves beta cell function. Diabetologia. 2015;58:113–121. doi: 10.1007/s00125-014-3398-2. [DOI] [PubMed] [Google Scholar]
- 30.Gaggini F., Laleu B., Orchard M., Fioraso-Cartier L., Cagnon L., Houngninou-Molango S., Gradia A., Duboux G., Merlot C., Heitz F., Szyndralewiez C., Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 2011;19:6989–6999. doi: 10.1016/j.bmc.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Aoyama T., Paik Y.H., Watanabe S., Laleu B., Gaggini F., Fioraso-Cartier L., Molango S., Heitz F., Merlot C., Szyndralewiez C., Page P., Brenner D.A. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raad H., Serrano-Sanchez M., Harfouche G., Mahfouf W., Bortolotto D., Bergeron V., Kasraian Z., Dousset L., Hosseini M., Taieb A., Rezvani H.R. NADPH oxidase-1 plays a key role in keratinocyte responses to uv radiation and uvb-induced skin carcinogenesis. J. Investig. Dermatol. 2017;137:1311–1321. doi: 10.1016/j.jid.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 34.Bernard J.J., Cowing-Zitron C., Nakatsuji T., Muehleisen B., Muto J., Borkowski A.W., Martinez L., Greidinger E.L., Yu B.D., Gallo R.L. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarubin T., Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 36.Ono K., Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 37.Owens D.M., Keyse S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 38.Peus D., Vasa R.A., Meves A., Pott M., Beyerle A., Squillace K., Pittelkow M.R. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J. Investig. Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Chouinard N., Valerie K., Rouabhia M., Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 2002;365:133–145. doi: 10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W., Bowden G.T. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu H., Banno Y., Sumi N., Naganawa T., Kitajima Y., Nozawa Y. Activation of p38 mitogen-activated protein kinase and caspases in UVB-induced apoptosis of human keratinocyte HaCaT cells. J. Investig. Dermatol. 1999;112:769–774. doi: 10.1046/j.1523-1747.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 42.Reinhardt H.C., Aslanian A.S., Lees J.A., Yaffe M.B. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heck D.E., Vetrano A.M., Mariano T.M., Laskin J.D. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J. Biol. Chem. 2003;278:22432–22436. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- 44.Sinha R.P., Hader D.P., UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 45.Tobi S.E., Paul N., McMillan T.J. Glutathione modulates the level of free radicals produced in UVA-irradiated cells. J. Photochem. Photobiol. B. 2000;57:102–112. doi: 10.1016/s1011-1344(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 46.Walshe J., Serewko-Auret M.M., Teakle N., Cameron S., Minto K., Smith L., Burcham P.C., Russell T., Strutton G., Griffin A., Chu F.F., Esworthy S., Reeve V., Saunders N.A. Inactivation of glutathione peroxidase activity contributes to UV-induced squamous cell carcinoma formation. Cancer Res. 2007;67:4751–4758. doi: 10.1158/0008-5472.CAN-06-4192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material