Abstract
Introduction
The follow-up schedule for colorectal cancer patients after curative surgery is inconsistent among the guidelines. Evaluation of time to recurrence (TTR) and survival after recurrence (SAR) may provide evidence for appropriate follow-up.
Methods
We assessed 3039 colon cancer (CC) and 1953 rectal cancer (RC) patients who underwent curative surgery between 2007 and 2008. We evaluated the pre- and post-recurrent clinicopathological factors associated with TTR and SAR in each stage of CC and RC.
Results
The recurrence rates of stages I, II, and III were 1.2%, 13.1%, and 26.3%, respectively, for CC, and 8.4%, 20.0%, and 30.4%, respectively, for RC. In CC patients, high carcinoembryonic antigen (CEA) level and lymphovascular invasion were independent predictors of short TTR. In RC patients, metastatic factors (liver metastasis in stage III) and venous invasion (stage III) were independent predictors of short TTR. The prognostic factors of SAR were age (stage II CC and stage III RC), female gender (stage III RC), high CEA level (stage II RC), histological type (stage III CRC), nodal status (stage III CC), recurrence within 1 year (stage III RC), M1b recurrence (stage II CRC), local recurrence (stage II CC), and no surgical resection after recurrence (stage II and III CRC).
Conclusions
The follow-up schedule for stage I should be different from that for the other stages. We recommend that intensive follow-up is appropriate in stage III CC patients with undifferentiated adenocarcinoma or N2 nodal status, stage II RC patients with high preoperative CEA level, and stage III RC patients.
Keywords: colorectal cancer, curative surgery, follow-up, recurrence, survival
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies worldwide [1]. CRC has been increasing annually in Japan, and was estimated to be the most common malignancy in 2016 and the second most common cause of cancer-related deaths in 2014 [2].
Recurrence rates vary among patients who receive curative surgery, with recurrence reported in about 30% of stage III patients, 15% of stage II patients, and <5% of stage I patients in Japan [3]. Adequate surveillance is therefore required to both reduce costs and improve survival. However, surveillance is generally provided for patients without any recurrence.
The usefulness of intensive surveillance with routine carcinoembryonic antigen (CEA) monitoring and computed tomography (CT) scans remains controversial. Although early detection of recurrence has been shown to improve survival, early detection using intensive surveillance did not always result in improved overall survival [4–9]. The guidelines of the Japanese Society for Cancer of the Colon and Rectum propose intensive surveillance, including CEA checks every 3 months and CT every 6 months for 3 years, regardless of clinical stage and risk of recurrence [10]. This reflects the intensive surveillance schedules recommended by the European Society for Medical Oncology, American Society of Clinical Oncology, and National Comprehensive Cancer Network (NCCN) guidelines, which recommended CEA checks every 3–6 months and CT every 6–12 months for 2–3 years, depending on the risk of the patient [11–15].
However, these surveillances are not strictly categorized by risk factors other than clinical stage. Information on time to recurrence (TTR) and survival after recurrence (SAR) may help to determine the appropriate follow-up schedule, and has previously been used to evaluate the usefulness of intensive follow-up in clinical trials and meta-analysis [3, 5, 7, 8].
In this study, we evaluated the clinicopathological factors predicting TTR and SAR in patients with colon cancer (CC) or rectal cancer (RC) during intensive follow-up after curative resection, to determine the factors influencing the appropriate follow-up schedule.
RESULTS
Differences in clinicopathological factors between CC and RC
We compared the clinicopathological factors between CC and RC (Table 1). Age group, gender, preoperative CEA level, tumor depth, nodal status, venous invasion, application of adjuvant therapy, and clinical stage all differed significantly between the two locations.
Table 1. Clinicopathological factors of patients by tumor location.
| Location | CC | RC | P value | CC | RC | P value | ||
|---|---|---|---|---|---|---|---|---|
| Factors | (N=3039) Number (%) |
(N=1953) Number (%) |
CC vs RC | Recurrence rate |
P value | Recurrence rate |
P value | CC vs RC |
| Age | ||||||||
| ≤64 | 1041 (34) | 998 (51) | <0.0001 | 15.4% | NS | 19.3% | NS | 0.018 |
| 65–74 | 1090 (36) | 629 (32) | 13.1% | 21.6% | <0.0001 | |||
| 75≤ | 908 (30) | 326 (17) | 13.4% | 18.4% | 0.03 | |||
| Gender | ||||||||
| Male | 1678 (55) | 1192 (61) | <0.0001 | 14.8% | NS | 21.4% | 0.041 | <0.0001 |
| Female | 1361 (45) | 761 (39) | 13.0% | 17.6% | 0.0044 | |||
| Preoperative CEA | ||||||||
| High | 831 (27) | 561 (29) | 0.043 | 23.9% | <0.0001 | 30.5% | <0.0001 | 0.0068 |
| Normal | 2115 (70) | 1310 (67) | 10.3% | 15.3% | <0.0001 | |||
| Unknown | 93 ( 3) | 82 (4) | 9.7% | 20.7% | 0.04 | |||
| Histological Type | ||||||||
| Well/Moderately | 2867 (94) | 1881 (96) | 0.0069 | 13.6% | NS | 19.2% | 0.0002 | <0.0001 |
| Poorly | 74 (2) | 31 (2) | 21.6% | 41.9% | 0.034 | |||
| Mucinous | 98 (3) | 41 (2) | 18.4% | 36.6% | 0.021 | |||
| Tumor depth | ||||||||
| T1 | 592 (19) | 355 (18) | <0.0001 | 1.0% | <0.0001 | 4.8% | <0.0001 | 0.0003 |
| T2 | 447 (15) | 438 (22) | 3.6% | 11.9% | <0.0001 | |||
| T3 | 1411 (46) | 971 (50) | 14.0% | 25.4% | <0.0001 | |||
| T4 | 589 (19) | 189 (10) | 35.0% | 38.6% | NS | |||
| Nodal status | ||||||||
| N0 | 2039 (67) | 1256 (64) | <0.0001 | 7.9% | <0.0001 | 14.1% | <0.0001 | <0.0001 |
| N1 | 786 (26) | 483 (25) | 22.3% | 26.3% | NS | |||
| N2 | 214 (7) | 214 (119) | 41.1% | 39.7% | NS | |||
| Lymphatic invasion | ||||||||
| ly0 | 1323 (44) | 793 (41) | NS | 8.9% | <0.0001 | 13.1% | <0.0001 | 0.0023 |
| ly1 | 1261 (41) | 853 (44) | 14.9% | 21.3% | 0.0001 | |||
| ly2 | 378 (12) | 262 (13) | 23.5% | 32.4% | 0.013 | |||
| ly3 | 58 (2) | 37 (2) | 48.3% | 45.9% | NS | |||
| Unknown | 19 (1) | 8 (0) | 10.5% | 12.5% | NS | |||
| Venous invasion | ||||||||
| v0 | 1193 (39) | 610 (31) | <0.0001 | 6.4% | <0.0001 | 10.5% | <0.0001 | 0.0025 |
| v1 | 1188 (39) | 765 (39) | 15.3% | 19.3% | 0.021 | |||
| v2 | 493 (16) | 436 (22) | 23.5% | 30.0% | 0.025 | |||
| v3 | 140 (5) | 125 (6) | 32.1% | 35.2% | NS | |||
| Unknown | 25 (1) | 17 (1) | 20% | 11.8% | NS | |||
| Stage | ||||||||
| I | 890 (29) | 641 (33) | <0.0001 | 1.2% | <0.0001 | 8.4% | <0.0001 | <0.0001 |
| II | 1149 (38) | 614 (31) | 13.1% | 20.0% | 0.0002 | |||
| III | 1000 (33) | 697 (36) | 26.3% | 30.4% | NS | |||
| Adjuvant therapy | ||||||||
| Stage I | ||||||||
| Yes | 18 (2) | 26 (4) | 0.019 | 5.6% | NS | 7.7% | NS | NS |
| No | 872(98) | 615 (96) | 1.1% | 8.5% | <0.0001 | |||
| Stage II | ||||||||
| Yes | 184 (16) | 126 (20) | 0.019 | 17.9% | 0.036 | 25.4% | NS | NS |
| No | 965 (84) | 489 (80) | 12.2% | 18.6% | 0.001 | |||
| Stage III | ||||||||
| Yes | 643 (64) | 509 (73) | 0.0002 | 25.8% | NS | 31.0% | NS | NS |
| No | 357 (36) | 188 (27) | 27.2% | 28.7% | NS | |||
CEA: Carcinoembryonic antigen, CC: colon cancer, NS: Not significant, Poorly: poorly differentiated adenocarcinoma, Mucinous: mucinous adenocarcinoma.
RC: rectal cancer, Well/Moderately: well differentiated/moderately differentiated adenocarcinoma.
Factors associated with recurrence
The recurrence rates of stages I, II, and III CC were 1.2% (11/890), 13.1% (151/1149), and 26.3% (263/1000), and of RC were 8.4% (54/641), 20.0% (123/614), and 30.4% (212/697), respectively (Table 1). High preoperative CEA level, tumor depth, nodal status, lymphatic invasion, venous invasion, and clinical stage were significantly associated with recurrence, regardless of tumor location (Table 1). Gender and histological type were significantly associated with recurrence in RC, but not in CC.
We also assessed the influence of tumor location on recurrence in relation to each clinicopathological factor (Table 1). There were significant differences in recurrence rates between CC and RC for patients in each age group, with each CEA level, each histological type, and with tumor depth of T1-3, N0 nodal status, lymphatic invasion of ly0-2, venous invasion of v0-2, stage I-II and stage I-II without adjuvant therapy. However, there were no significant differences in recurrence rates by tumor location in relation to clinically-advanced factors including: depth of T4, N1 and N2 nodal status, lymphatic invasion of ly3, venous invasion of v3, and stage III.
Factors associated with RFS and OS
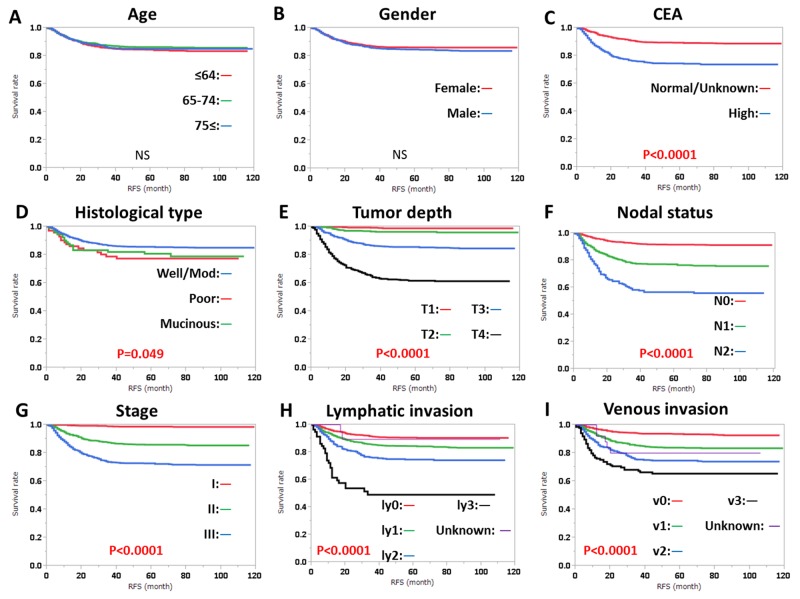
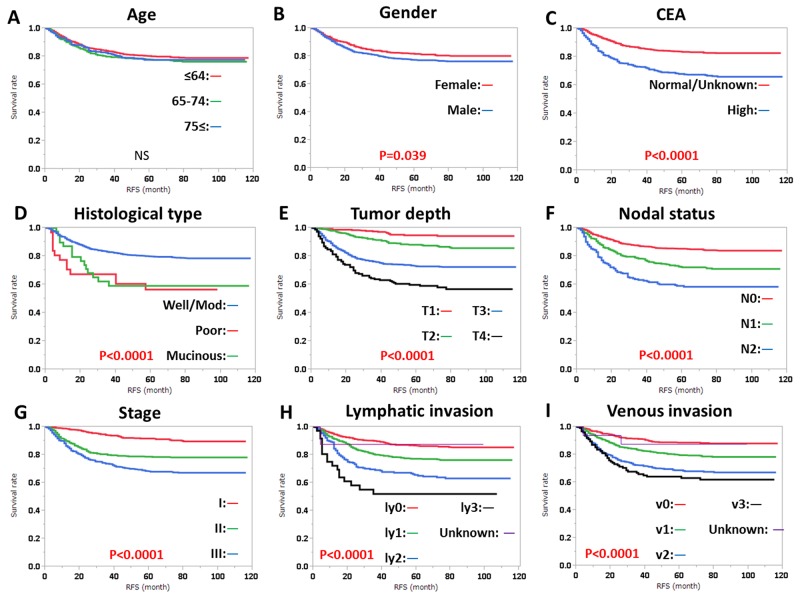
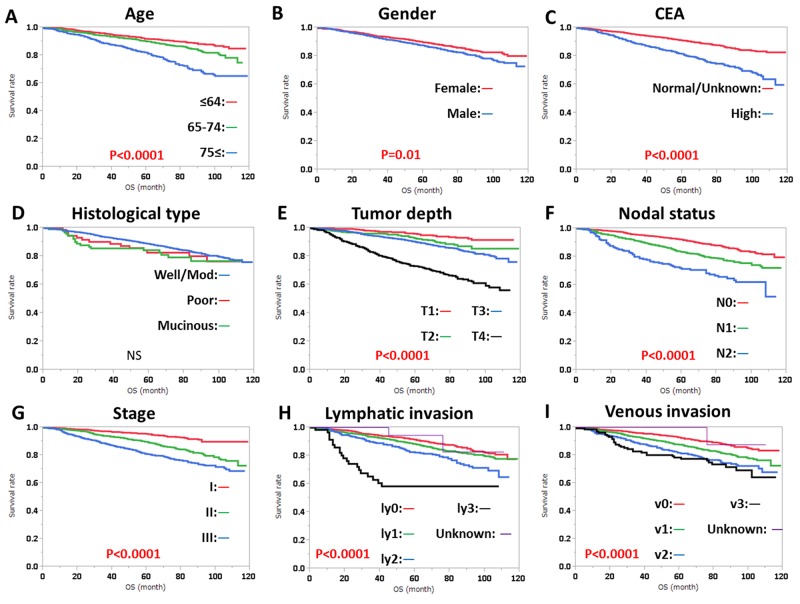
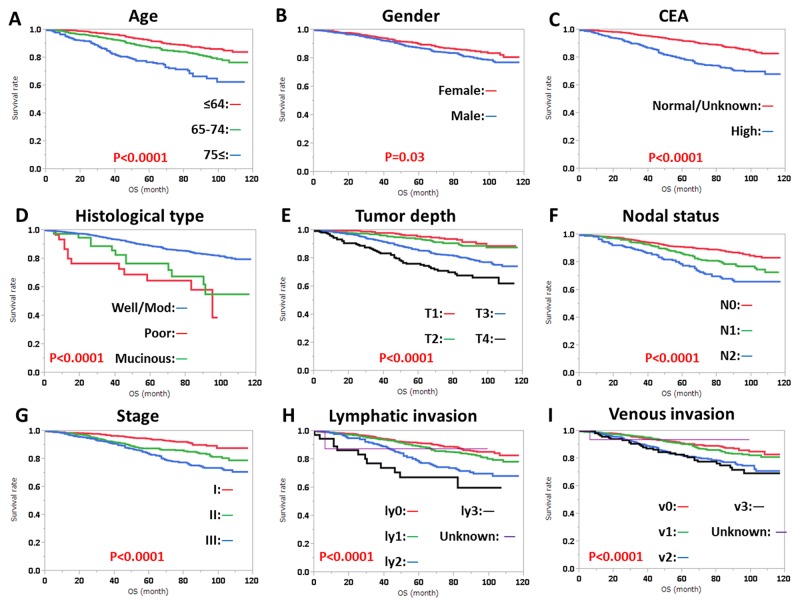
RFS was significantly influenced by preoperative CEA level, histological type, tumor depth, nodal status, stage, lymphatic invasion, and venous invasion, regardless of tumor location (Figures 1, 2). RFS was also significantly influenced by gender in patients with RC (Figure 2). OS was significantly influenced by age, gender, preoperative CEA level, tumor depth, nodal status, stage, lymphatic invasion, and venous invasion, regardless of tumor location (Figures 3, 4). OS was also significantly influenced by histological type in RC alone (Figure 4).
Figure 1.
Relapse-free survival (RFS) in colon cancer according to (A) age, (B) gender, (C) CEA level, (D) histological type, (E) tumor depth, (F) nodal status, (G) clinical stage, (H) lymphatic invasion, and (I) venous invasion. Subgroups were compared with log-rank test. P values were provided when differences were significant (P<0.05) and as NS when differences were not significant.
Figure 2.
Relapse-free survival (RFS) in rectal cancer according to (A) age, (B) gender, (C) CEA level, (D) histological type, (E) tumor depth, (F) nodal status, (G) clinical stage, (H) lymphatic invasion, and (I) venous invasion. Subgroups were compared with log-rank test. P values were provided when differences were significant (P<0.05) and as NS when differences were not significant.
Figure 3.
Overall survival (OS) in colon cancer according to (A) age, (B) gender, (C) CEA level, (D) histological type, (E) tumor depth, (F) nodal status, (G) clinical stage, (H) lymphatic invasion, and (I) venous invasion. Subgroups were compared with log-rank test. P values were provided when differences were significant (P<0.05) and as NS when differences were not significant.
Figure 4.
Overall survival (OS) in rectal cancer according to (A) age, (B) gender, (C) CEA level, (D) histological type, (E) tumor depth, (F) nodal status, (G) clinical stage, (H) lymphatic invasion, and (I) venous invasion. Subgroups were compared with log-rank test. P values were provided when differences were significant (P<0.05).
Clinicopathological factors in patients with recurrence
Age group, gender, tumor depth, nodal status, stage, TTR, type of recurrence, and sites of recurrence differed significantly between CC and RC in patients with recurrence (Table 2). The liver was the most common metastatic site in CC patients, followed by the lungs, peritoneum, and distant lymph nodes. In contrast, the lungs were the most common metastatic site in RC patients, followed by the liver, local recurrence, and distant lymph nodes. There was no significant difference in preoperative CEA, histological type, or treatment after recurrence according to tumor location.
Table 2. Clinicopathological factors of patients with recurrence by tumor location.
| Factors / Location | CC (N=425) Number (%) |
RC (N=389) Number (%) |
P value CC vs RC |
|---|---|---|---|
| Age | |||
| ≤64 / 65–74/ 75≤ | 160/143/122 (38/33/29) |
193/136/60 (50/35/15) |
<0.0001 |
| Gender | |||
| Male / Female | 248/177 (58/42) |
255/134 (66/34) |
0.035 |
| Preoperative CEA | |||
| High / Normal / Unknown | 199/217/9 (47/51/2) |
171/201/17 (44/52/4) |
NS |
| Histological Type | |||
| Well/Moderately / Poorly /Mucinous | 391/16/18 (92/2/2) |
361/13/15 (93/3/4) |
NS |
| Tumor depth | |||
| T1 / T2 / T3 / T4 | 6/16/197/206 (1/4/46/48) |
17/52/247/73 (4/13/63/19) |
<0.0001 |
| Nodal status | |||
| N0 / N1 / N2 | 162/175/88 (38/41/21) |
177/127/85 (46/33/22) |
0.034 |
| Lymphatic invasion | |||
| ly0 / ly1 / ly2 / ly3/Unknown | 118/188/89/28/2 (28/44/21/7/1) |
104/182/85/17/1 (27/47/22/4/0) |
NS |
| Venous invasion | |||
| v0 / v1 / v2 / v3 / Unknown | 77/182/116/45/5 (18/43/27/11/1) |
64/148/131/44/2 (16/38/34/11/1) |
NS |
| Stage | |||
| I / II / III | 11/151/263 (3/36/62) |
54/123/212 (14/32/54) |
<0.0001 |
| Time to recurrence (year) | |||
| < 1 / 1–2/ 2–3 / 3≤ | 190/136/64/35 (45/32/15/8) |
142/122/57/68 (37/31/15/17) |
0.0007 |
| Type of recurrence | |||
| Local alone /M1a / M1b | 29/251/145 (7/59/34) |
77/243/69 (20/62/18) |
<0.0001 |
| Liver metastasis | |||
| (+) / (-) | 203/222 (48/52) |
128/261 (33/67) |
<0.0001 |
| Lung metastasis | |||
| (+) / (-) | 130/295 (31/69) |
148/241 (38/62) |
0.025 |
| Peritoneal metastasis | |||
| (+) / (-) | 83/342 (20/80) |
28/361 (7/93) |
<0.0001 |
| Local metastasis | |||
| (+) / (-) | 41/384 (10/90) |
112/277 (29/71) |
<0.0001 |
| Distant lymph node metastasis | |||
| (+) / (-) | 63/362 (15/85) |
45/344 (12/88) |
NS |
| Adjuvant therapy | |||
| (+) / (-) | 200/225 (47/53) |
192/197 (49/51) |
NS |
| Treatment after recurrence | |||
| Best supportive care / Surgery (-) / Surgery (+) | 38/188/199 (9/44/47) |
30/167/192 (8/43/49) |
NS |
CEA: Carcinoembryonic antigen, CC: colon cancer, Mucinous: mucinous adenocarcinoma, NS: Not significant, Poorly: poorly differentiated adenocarcinoma, RC: rectal cancer, Well/Moderately: well differentiated/moderately differentiated adenocarcinoma
Time to recurrence (TTR)
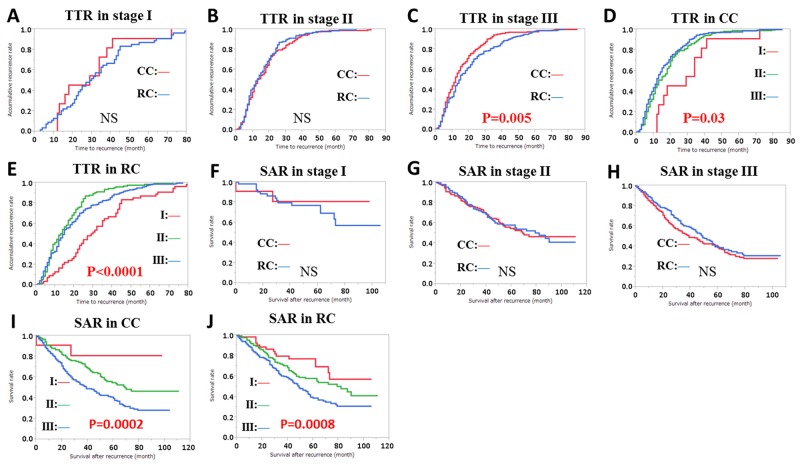
TTR was significantly longer in stage III patients with RC compared with CC patients, but not in stage I and II patients (Figure 5A-5C). The 1-, 2-, 3-, and 5-year accumulative recurrence rates in CC patients were 18%, 46%, 73%, and 91% for stage I; 46%, 78%, 90% and 99% for stage II; 53%, 80%, 94% and 97% for stage III, respectively (Figure 5D). The equivalent recurrence rates in RC patients were 17%, 41%, 65%, and 87% for stage I; 49%, 81%, 94%, and 98% for stage II; 44%, 71%, 83%, and 98% for stage III respectively (Figure 5E).
Figure 5.
Time to recurrence (TTR) and accumulative recurrence rate (A-E), and survival after recurrence (SAR) and survival rate (F-J), by clinicopathological factors. TTR and accumulative recurrence rate in: (A) stage I patients by colon cancer (CC) and rectal cancer (RC), (B) stage II patients by CC and RC, (C) stage III patients by CC and RC, (D) CC by stage, (E) RC by stage. SAR and survival rate in: (F) stage I patients by CC and RC, (G) stage II patients by CC and RC, (H) stage III patients by CC and RC, (I) CC by stage, (J) RC by stage. Subgroups were compared with log-rank test. P values were provided when differences were significant (P<0.05) and NS was used when differences were not significant.
We further assessed TTR for CC and RC patients depending on stage, except for stage I, because there were fewer patients and longer TTR than for the other stages.
TTR in stage II CC was significantly associated with gender, preoperative CEA level, lymphatic invasion, and distant lymph node metastasis (Table 3). TTR in stage III CC was significantly associated with preoperative CEA level, lymphatic invasion, and venous invasion (Table 3). Multivariate analysis showed that high preoperative CEA level and lymphatic invasion were independent predictors for short TTR in both stage II and stage III CC (Table 4).
Table 3. Summary of associations between clinicopathological factors and TTR.
| Location | Colon cancer | Rectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | Stage II (N=151) | Stage III (N=263) | Stage II (N=123) | Stage III (N=212) | ||||
| Factors | Mean (months) |
P | Mean (months) | P | Mean (months) |
P | Mean (months) |
P |
| Age | ||||||||
| ≤74 | 17.5 | NS | 16.6 | NS | 16.2 | NS | 20.0 | NS |
| 75≤ | 18.0 | 13.9 | 17.4 | 19 | ||||
| Gender | ||||||||
| Male | 19.6 | 0.04 | 16.5 | NS | 16.9 | NS | 20.5 | NS |
| Female | 15.3 | 14.8 | 15.3 | 18.7 | ||||
| Preoperative CEA | ||||||||
| High | 14.7 | 0.02 | 14.1 | 0.04 | 16 | NS | 18.8 | NS |
| Normal/Unknown | 20.3 | 17.5 | 16.7 | 20.9 | ||||
| Histological Type | ||||||||
| Well/Moderately | 17.5 | NS | 16.0 | NS | 16.3 | NS | 20.2 | NS |
| Poorly/Mucinous | 20.5 | 14.1 | 18 | 16.7 | ||||
| Tumor depth | ||||||||
| T1 | - | NS | 15.3 | NS | NS | 28.5 | NS | |
| T2 | - | 14.9 | 30.7 | |||||
| T3 | 19.3 | 13.9 | 15.7 | 19.2 | ||||
| T4 | 15.6 | 15.8 | 19.2 | 18.7 | ||||
| Nodal status | ||||||||
| N0 | 17.7 | - | NS | 16.4 | - | 0.03 | ||
| N1 | 16.3 | 21.8 | ||||||
| N2 | 14.9 | 16.9 | ||||||
| Lymphatic invasion | ||||||||
| ly0/1 | 18.5 | 0.008 | 16.7 | NS | 15.8 | NS | 16.7 | NS |
| ly2/3 | 11.4 | 15.1 | 20.4 | 22.0 | ||||
| ly0-2 | 17.8 | NS | 16.4 | 0.008 | 16.4 | - | 20.6 | 0.009 |
| ly3 | 13 | 10.3 | - | 11.9 | ||||
| Venous invasion | ||||||||
| v0/1 | 18.7 | NS | 17.2 | NS | 15.3 | NS | 22.4 | 0.02 |
| v2/3 | 15.7 | 14.0 | 17.5 | 17.1 | ||||
| v0-2 | 17.9 | NS | 16.6 | 0.001 | 16.3 | NS | 20.0 | NS |
| v3 | 15.2 | 9.7 | 16.9 | 18.9 | ||||
| Adjuvant therapy | ||||||||
| Yes | 16.3 | NS | 16.9 | NS | 13.9 | NS | 19.7 | NS |
| No | 18.1 | 14.0 | 17.3 | 20.3 | ||||
| Type of recurrence | ||||||||
| M1a | 18.8 | NS | 15.5 | NS | 17.4 | NS | 21.0 | 0.02 |
| M1b | 15.0 | 15.2 | 13 | 14.8 | ||||
| Local alone | 20.5 | 23.4 | 15.7 | 22.9 | ||||
| Liver metastasis | ||||||||
| (-) | 18.6 | NS | 16.9 | NS | 18.6 | 0.01 | 23.0 | <0.0001 |
| (+) | 16.8 | 14.5 | 13 | 12.8 | ||||
| Lung metastasis | ||||||||
| (-) | 17.2 | NS | 15.6 | NS | 15.8 | NS | 19.4 | NS |
| (+) | 18.5 | 16.2 | 17.6 | 20.4 | ||||
| Peritoneal metastasis | ||||||||
| (-) | 17.8 | NS | 16.2 | NS | 16.4 | NS | 20.2 | NS |
| (+) | 16.9 | 14.3 | 16 | 17.3 | ||||
| Local recurrence | ||||||||
| (-) | 17.6 | NS | 15.5 | NS | 16.8 | NS | 19.4 | NS |
| (+) | 18.1 | 18.9 | 15.4 | 21.1 | ||||
| Distant LN metastasis | ||||||||
| (-) | 18.3 | 0.04 | 15.2 | NS | 27.7 | 0.02 | 19.8 | NS |
| (+) | 11.9 | 18.4 | 15.5 | 20.4 | ||||
CEA: Carcinoembryonic antigen, LN: lymph node, Mucinous: mucinous adenocarcinoma, NS: Not significant, Poorly: poorly differentiated adenocarcinoma, TTR: time to recurrence, Well/Moderately: well differentiated/moderately differentiated adenocarcinoma.
Table 4. Multivariate analysis of predictors for short TTR.
| Location | Colon cancer | Rectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | Stage II | Stage III | Stage II | Stage III | ||||
| Factors | HR CI |
P | HR CI |
P | HR CI |
P | HR CI |
P |
| Gender: Male | 0.73 0.53-1.02 |
NS | ||||||
| CEA: High | 1.47 1.06-2.03 |
0.02 | 1.34 1.04-1.73 |
0.02 | ||||
| Nodal status: N2 | 1.23 0.92-1.64 |
NS | ||||||
| Lymphatic invasion | ||||||||
| ly2/3 | 1.83 1.03-3.05 |
0.04 | ||||||
| ly3 | 1.73 1.09-2.64 |
0.02 | 1.5 0.86-2.45 |
NS | ||||
| Venous invasion | ||||||||
| v2/3 | 1.42 1.07-1.88 |
0.01 | ||||||
| v3 | 1.63 1.09-2.36 |
0.02 | ||||||
| Type of recurrence: | ||||||||
| M1b | 1.25 0.88-1.74 |
NS | ||||||
| Recurrence sites | ||||||||
| Liver (+) | 1.44 0.99-2.09 |
NS | 1.92 1.41-2.61 |
<0.0001 | ||||
| Distant LN: (+) | 1.53 0.84-2.61 |
NS | 0.52 0.23-1.03 |
NS | ||||
CEA: Carcinoembryonic antigen, CI: confidence interval, HR: hazard ratio, LN: lymph node, NS: Not significant, TTR: time to recurrence.
TTR in stage II RC was significantly associated with metastatic factors (liver metastasis and distant lymph node metastasis), but not pathological factors. TTR in stage III RC was significantly associated with both pathological factors (lymphatic invasion and vascular invasion) and metastatic factors (M1b recurrence and liver metastasis) (Table 3). Multivariate analysis showed that venous invasion and liver metastasis were independent predictors for short TTR in stage III RC (Table 4).
Survival after recurrence (SAR)
The 5-year survival rates after recurrence for CC and RC were 81% and 77% in stage I; 55% and 58% in stage II; 40% and 39% in stage III, respectively (Figure 5F–5H). There were significant differences in the 5-year SAR rates among stages, but not between CC and RC (Figure 5F–5J).
SAR was significantly associated with age (all groups), gender (stage III RC), CEA level (stage I/II RC), histological type (stage III CRC), tumor depth (CC), nodal status (stage III CC), lymphatic invasion (stage III CRC), adjuvant therapy (stage III RC), recurrence within 1 year (stage III RC), M1b recurrence (stage II/III CRC), liver metastasis (stage II RC), peritoneal metastasis (CC and stage III RC), local recurrence (stage II CC), and treatment after recurrence (all groups) (Table 5).
Table 5. Summary of associations between clinicopathological factors and SAR.
| Location | Colon cancer | Rectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | Stage II (N=151) | Stage III (N=263) | Stage II (N=123) | Stage III (N=212) | ||||
| Factors | Mean (months) |
P | Mean (months) | P | Mean (months) |
P | Mean (months) |
P |
| Age | ||||||||
| ≤74 | 59.1 | 0.0001 | 46.9 | 0.005 | 64.2 | 0.01 | 51.1 | <0.0001 |
| 75≤ | 33.3 | 34.1 | 35.0 | 24.2 | ||||
| Gender | ||||||||
| Male | 51.9 | NS | 44.9 | NS | 59.5 | NS | 51.6 | 0.004 |
| Female | 51.9 | 41.5 | 64.7 | 39.6 | ||||
| Preoperative CEA | ||||||||
| High | 48.8 | NS | 44.9 | NS | 46.8 | 0.049 | 45.6 | NS |
| Normal/Unknown | 55.4 | 41.6 | 67.9 | 50.0 | ||||
| Histological Type | ||||||||
| Well/Moderately | 52.6 | NS | 45.8 | 0.003 | 61.8 | NS | 50.3 | <0.0001 |
| Poorly/Mucinous | 44.5 | 18.4 | 17.4 | 23.8 | ||||
| Tumor depth | ||||||||
| T1 | 0.009 | 16 | 0.002 | NS | 55 | NS | ||
| T2 | 34.7 | 42.8 | ||||||
| T3 | 49.3 | 53.4 | 62.8 | 49.7 | ||||
| T4 | 48.4 | 35.8 | 55.9 | 48.0 | ||||
| Nodal status | ||||||||
| N1 | 47.1 | 0.02 | 50.3 | NS | ||||
| N2 | 36.4 | 41.5 | ||||||
| Lymphatic invasion | ||||||||
| ly0/1 | 51.4 | NS | 46.5 | NS | 63.2 | NS | 50.7 | NS |
| ly2/3 | 49.6 | 38.7 | 37.2 | 40.7 | ||||
| ly0-2 | 53.4 | NS | 45.5 | 0.004 | 61.6 | - | 49.2 | 0.005 |
| ly3 | 14.7 | 16.3 | - | 29.6 | ||||
| Venous invasion | ||||||||
| v0/1 | 51.5 | NS | 44.0 | NS | 65.8 | NS | 48.9 | NS |
| v2/3 | 51.3 | 43.3 | 54.3 | 45.6 | ||||
| v0-2 | 50.6 | NS | 44.3 | NS | 60.7 | NS | 48.3 | NS |
| v3 | 58.1 | 38.8 | 53.7 | 44.2 | ||||
| Adjuvant therapy | ||||||||
| Yes | 57.0 | NS | 45.0 | NS | 65.5 | NS | 50.9 | 0.02 |
| No | 50.9 | 41.1 | 58.8 | 37.7 | ||||
| Time to recurrence | ||||||||
| less than 1 year | 48.9 | NS | 37.8 | NS | 58.1 | NS | 41.2 | 0.004 |
| more than 1 year | 53.0 | 48.1 | 62.9 | 50.4 | ||||
| Type of recurrence | ||||||||
| M1a | 59.0 | 0.0002 | 49.8 | 0.0001 | 61.2 | 0.001 | 53.6 | 0.0002 |
| M1b | 43.8 | 33.6 | 35.2 | 29.6 | ||||
| Local alone | 42.7 | 46.8 | 64.9 | 43.2 | ||||
| Liver metastasis | ||||||||
| (-) | 46.7 | 0.01 | 41.3 | NS | 60.4 | NS | 47.4 | NS |
| (+) | 57.8 | 46.3 | 48.9 | 48.4 | ||||
| Lung metastasis | ||||||||
| (-) | 56.0 | NS | 42.8 | NS | 65.9 | 0.02 | 46.0 | NS |
| (+) | 42.7 | 45.0 | 46.3 | 49.0 | ||||
| Peritoneal metastasis | ||||||||
| (-) | 54.2 | 0.03 | 47.8 | <0.0001 | 62.5 | NS | 50.0 | 0.001 |
| (+) | 45.2 | 29.3 | 15.4 | 21.8 | ||||
| Local recurrence | ||||||||
| (-) | 54.1 | 0.04 | 44.1 | NS | 59.6 | NS | 48.3 | NS |
| (+) | 42.6 | 41.2 | 57.0 | 46.0 | ||||
| Distant LN metastasis | ||||||||
| (-) | 53.7 | NS | 44.5 | NS | 61.2 | NS | 49.4 | NS |
| (+) | 43.1 | 37.4 | 66.8 | 28.9 | ||||
| Therapy after recurrence | ||||||||
| Surgical resection (-) | 34.2 | <0.0001 | 32.9 | <0.0001 | 44.0 | <0.0001 | 35.1 | <0.0001 |
| Surgical resection (+) | 65.8 | 57.5 | 74.4 | 62.2 | ||||
CEA: Carcinoembryonic antigen, LN: lymph node, NS: Not significant, Mucinous: mucinous adenocarcinoma, Poorly: poorly differentiated adenocarcinoma, SAR: survival after recurrence, Well/Moderately: well differentiated/moderately differentiated adenocarcinoma.
Multivariate analysis identified the following independent prognostic factors: age (stage II CC and stage III RC), female gender (stage III RC), high CEA level (stage II RC), histological type (stage III CRC), nodal status (stage III CC), recurrence within 1 year (stage III RC), M1b recurrence (stage II CRC), local recurrence (stage II CC), and no surgical resection after recurrence (stage II and III CRC) (Table 6).
Table 6. Multivariate analysis of prognostic factors for SAR.
| Location | Colon cancer | Rectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | Stage II | Stage III | Stage II | Stage III | ||||
| Factors | HR CI |
P | HR CI |
P | HR CI |
P | HR CI |
P |
| Age: 75≤ |
2.71 1.56-4.67 |
0.0005 | 1.33 0.93-1.9 |
NS | 1.77 0.81-3.56 |
NS | 1.78 1.03-2.96 |
0.04 |
| Gender: Female |
1.69 1.14-2.47 |
0.009 | ||||||
| CEA: High |
2.33 1.34-4.11 |
0.003 | ||||||
| Histological type: Poorly/Mucinous |
2.04 1.15-3.41 |
0.02 | 2.54 1.34-4.54 |
0.005 | ||||
| Tumor depth: T4 |
1.42 0.84-2.43 |
NS | 1.32 0.93-1.87 |
NS | ||||
| Nodal status: N2 |
1.5 1.05-2.12 |
0.03 | ||||||
| Lymphatic invasion: ly3 |
1.23 0.66-2.15 |
NS | ||||||
| Recurrence: within 1 year |
1.64 1.12-2.4 |
0.01 | ||||||
| Type of recurrence: M1b |
2.50 1.25-4.83 |
0.01 | 1.39 0.84-2.18 |
NS | 2.69 1.31-5.25 |
0.008 | 1.68 0.97-2.78 |
NS |
| Recurrence sites | ||||||||
| Liver (+) | 1.04 0.58-1.84 |
NS | ||||||
| Lung: (+) | 1.25 0.68-2.24 |
NS | ||||||
| Peritoneal: (+) | 0.74 0.35-1.61 |
NS | 1.37 0.82-2.36 |
NS | 1.11 0.53-2.27 |
NS | ||
| Local: (+) | 3.54 1.5-7.69 |
0.005 | ||||||
| No adjuvant therapy | 1.00 0.64-1.53 |
NS | ||||||
| No surgical resection after recurrence | 5.0 2.73-9.45 |
<0.0001 | 2.36 1.64-3.43 |
<0.0001 | 3.41 1.93-6.18 |
<0.0001 | 4.03 2.65-6.25 |
<0.0001 |
CEA: Carcinoembryonic antigen, CI: confidence interval, HR: hazard ratio, Mucinous: mucinous adenocarcinoma, NS: Not significant, Poorly: poorly differentiated adenocarcinoma, SAR: survival after recurrence.
DISCUSSION
We evaluated the clinicopathological factors associated with TTR and SAR in patients with CC and RC, as critical factors for guiding appropriate follow-up after curative surgery [3–5]. Appropriate follow-up after curative surgery has been proposed by TTR or the accumulative recurrence rate, but not SAR.
The recurrence rate in this study (16.3%) was similar to those in previous studies [3, 7]. The recurrence rate in stage I CC patients was only 1.2%, so we excluded these patients from analysis of TTR and SAR. The recurrence rate in stage I RC patients was 8.4%, and the accumulative recurrence rates were 16.7% and 64.8% at 1 and 3 years, respectively. Therefore, stage I RC patients may require different surveillance from stage II and III patients. We also excluded stage I RC patients for further analysis of TTR and SAR.
Among stage II and III patients, the accumulative recurrence rate was about 50% at 1 year, which was higher than in some previous reports, but similar to that in the FACS trial involving intensive surveillance, including CT [3, 7]. The 2- and 3-year recurrence rates in patients with stages II and III were 70%–80% and 80%–90%, respectively. These are similar to the previous data by Kobayashi et al and suggest that intensive surveillance with CEA checks every 3 months are necessary for at least 3 years, in contrast to the NCCN recommendation of 2 years [3, 14, 15].
TTR differed significantly between CC and RC in stage III patients. Predictors for TTR were different between CC and RC. In CC, patients with high preoperative CEA level and lymphovascular invasion could be followed-up as candidates for early recurrence. On the other hand, predictors in RC were venous invasion and liver metastasis. Therefore, follow-up in RC should include CT to evaluate liver metastasis.
Our data showed that predictors for TTR were not prognostic factors for SAR. Short TTR has been previously reported to influence survival in small cases of study [16, 17]. In this study, recurrence within 1 year was an independent prognostic factor for poor SAR in stage III RC patients. Age (≥75), type of recurrence (M1b), and treatment after recurrence (surgical resection) were also identified as independent prognostic factors for SAR in the current study. Surgical resection was considered a strong prognostic factor for advanced CRC [18, 19]. Recent improvement of treatments against metastatic CRC may provide better SAR than that in current study [20, 21].
Therefore, we proposed that patients with prognostic factors for poor SAR should receive intensive treatment and follow-up after curative surgery. These patients include stage III CC patients with undifferentiated adenocarcinoma or N2 nodal status, stage II RC patients with high preoperative CEA level, and stage III RC patients.
Our study had several limitations. First, it was a retrospective study, and we had no genetic information, such as RAS mutation and microsatellite instability statuses, which are critical genetic markers for prognosis and treatment. KRAS mutation analysis has been available for clinical use in Japan since 2010. Moreover, universal screening for Lynch syndrome was performed in <10% of the hospitals specializing in CRC treatment in Japan [22]. Genetic information was therefore not considered for CRC treatment at the time of surgery in this study. Second, this study also lacked information on the usage of molecular-targeted drugs. Vascular endothelial growth factor and epidermal growth factor antibodies have been used clinically in Japan since 2007 and 2008, respectively. Patients with recurrence should thus have received these drugs for treatment after recurrence. Finally, this was a retrospective study with no rules about follow-up or treatment after surgery, or treatment after recurrence.
Although it seems advisable to detect recurrence as soon as possible to improve the chances of curative resection, the usefulness of intensive follow-up remains controversial. Furthermore, it is unclear if early detection of recurrence could increase the rate of curative treatment and improve survival. Our data demonstrated that short TTR was an independent prognostic factor for SAR in stage III RC patients, who should have received intensive follow-up. However, further prospective studies are needed to confirm our results. Genetic assessment is indispensable in the era of molecular-targeted therapy.
MATERIALS AND METHODS
Patients and data collection
This retrospective multicenter study was conducted by the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer (JFUP-CRC). Clinical data were collected for CRC patients who underwent curative surgery at 22 hospitals in Japan between 2007 and 2008. The study was approved by the institutional review board or ethics committee at each hospital and was performed according to the Declaration of Helsinki and Ethical Guidelines for Clinical Research. All patients provided written informed consent. The JFUP-CRC office pooled and prepared the data available for clinical study, as described in the 7th edition of the Japanese Classification of Colorectal Carcinoma [23]. Lymphatic (ly) or venous (v) invasion was classified according to the degree of invasion, as follows: no invasion (ly0/v0), minimal invasion (ly1/v1), moderate invasion (ly2/v2), or severe invasion (ly3/v3). A total of 4992 CRC patients who underwent curative resection in 2007 and 2008, without preoperative chemotherapy/radiotherapy, hereditary CRC, lateral lymph node metastasis, or colitic cancer, were considered suitable for assessment. These patients usually received follow-up with CEA tests every 3 months and CT every 6 months for 3 years, and then CEA every 6 months and CT every 12 months for 3–5 years. The median follow-up time was 72 months in all patients and 60 months in patients with recurrence.
Data analysis
Differences in clinicopathological factors according to tumor location (CC or RC) were analyzed for all patients and for patients with recurrence. Factors associated with recurrence were assessed using χ2 tests.
The influences of clinicopathological factors on relapse-free survival (RFS) and overall survival (OS) in all patients, and TTR and SAR in patients with recurrence were assessed using log-rank tests. Prognostic factors associated with TTR and SAR were subjected to multivariate analyses using a Cox proportional hazard model or logistic analysis. A P value of <0.05 was considered significant for all analyses.
CONCLUSIONS
We recommend that intensive follow-up after surgery is appropriate in stage III CC patients with undifferentiated adenocarcinoma or N2 nodal status, stage II RC patients with high preoperative CEA level, and stage III RC patients.
Acknowledgments
We thank the members of JFUP-CRC for collecting the clinical data: I. Takemasa (Sapporo Medical University), K. Hakamada (Hirosaki University), H. Kameyama (Niigata University), Y. Takii (Niigata Cancer Center Hospital), K. Hase (National Defense Medical College), H. Ozawa (Tochigi Cancer Center), H. Nozawa (Tokyo University), K. Takahashi (Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital), Y. Kanemitsu (National Cancer Center Hospital), M. Itabashi (Tokyo Women’s Medical University), H. Yano (National Center for Global Health and Medicine), Y. Kinugasa (Tokyo Medical and Dental University), H. Hasegawa (Keio University), Y. Hashiguchi (Teikyo University), T. Masaki (Kyorin University), M. Watanabe (Kitasato University), T. Hanai (Fujita Health University), K. Komori (Aichi Cancer Center Hospital), Y. Sakai (Kyoto University), M. Ohue (Osaka Medical Center for Cancer and Cardiovascular Diseases), S. Noura (Osaka Rosai Hospital), and Y. Akagi (Kurume University).
We also thank Editage for editing the English text of a draft of this manuscript.
Abbreviations
- CC
Colon Cancer
- CEA
Carcinoembryonic antigen
- CRC
Colorectal cancer
- CT
Computed tomography
- JFUP-CRC
Japanese study group for postoperative follow-up of colorectal cancer
- NCCN
National Comprehensive Cancer Network
- OS
Overall survival
- RC
Rectal cancer
- RFS
Relapse free survival
- SAR
Survival after recurrence
- TTR
Time to recurrence
Author contributions
Conception and study design: TY. Data analysis: TY, SY. Acquisition of data: TY, SY, KT, MN, MK, MH, AB, KK, CS, AI, ST, MI, NT, KS. Manuscript writing: TY. All authors read and approved the final version of the manuscript.
DECLARATIONS
Ethics approval and consent to participate
This study was approved by the Central Institutional Review board (Tokyo Medical and Dental University) and local ethical committee. Written informed consent was obtained from all patients.
Availability of data and material
The datasets used and analyzed during this study are available from the corresponding author on reasonable request under permission of JFUP-CRC.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer information service in Japan Available: http://ganjoho.jp/public/index.html.
- 3.Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, Maeda K, Hirai T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141:67–75. doi: 10.1016/j.surg.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Verberne CJ, Zhan Z, van den Heuvel E, Grossmann I, Doornbos PM, Havenga K, Manusama E, Klaase J, van der Mijle HC, Lamme B, Bosscha K, Baas P, van Ooijen B, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41:1188–96. doi: 10.1016/j.ejso.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–56. doi: 10.1093/annonc/mdu543. [DOI] [PubMed] [Google Scholar]
- 6.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–99. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 7.Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D, FACS Trial Investigators Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–70. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 8.Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, Takkenberg JJ, Treasure T. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103:1259–68. doi: 10.1002/bjs.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. doi: 10.1002/14651858.CD002200.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, et al. Japanese Society for Cancer of the Colon and Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 12.Glimelius B, Tiret E, Cervantes A, Arnold D, ESMO Guidelines Working Group Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi81–88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, Benson AB, 3rd, American Society of Clinical Oncology Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–70. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology Colon cancer. doi: 10.6004/jnccn.2009.0056. Available: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed]
- 15.NCCN Clinical Practice Guidelines in Oncology Rectal cancer. doi: 10.6004/jnccn.2009.0057. Available: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [DOI] [PubMed]
- 16.Huh JW, Kim CH, Lim SW, Kim HR, Kim YJ. Early recurrence in patients undergoing curative surgery for colorectal cancer: is it a predictor for poor overall survival? Int J Colorectal Dis. 2013;28:1143–49. doi: 10.1007/s00384-013-1675-z. [DOI] [PubMed] [Google Scholar]
- 17.Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, Jun SH, Kwon YC. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014;86:143–51. doi: 10.4174/astr.2014.86.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadden WJ, de Reuver PR, Brown K, Mittal A, Samra JS, Hugh TJ. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB. 2016;18:209–20. doi: 10.1016/j.hpb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, D’Angelica MI. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg. 2017;265:158–65. doi: 10.1097/SLA.0000000000001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loree JM, Kopetz S. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2017;9:551–64. doi: 10.1177/1758834017714997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017;3:e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamano T, Hamanaka M, Babaya A, Kimura K, Kobayashi M, Fukumoto M, Tsukamoto K, Noda M, Matsubara N, Tomita N, Sugihara K. Management strategies in Lynch syndrome and familial adenomatous polyposis: a national healthcare survey in Japan. Cancer Sci. 2017;108:243–49. doi: 10.1111/cas.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Japanese Classification of Colorectal Carcinoma. 7th ed. Tokyo: Kanehara Shuppan; 2009. [in Japanese] [Google Scholar]