Abstract
Prostaglandin E2 (PGE2) is a lipid signaling molecule important for brain development and function. Various genetic and environmental factors can influence the level of PGE2 and increase the risk of developing Autism Spectrum Disorder (ASD). We have previously shown that in neuronal cell lines and mouse brain, PGE2 can interfere with the Wnt canonical pathway, which is essential during early brain development. Higher levels of PGE2 increased Wnt-dependent motility and proliferation of neuroectodermal stem cells, and modified the expression of Wnt genes previously linked to autism disorders. We also recently established a cross-talk between these two pathways in the prenatal mouse brain lacking PGE2 producing enzyme (COX-/-). The current study complements the published data and reveals that PGE2 signaling also converges with the Wnt canonical pathway in the developing mouse brain after maternal exposure to PGE2 at the onset of neurogenesis. We found significant changes in the expression level of Wnt-target genes, Mmp7, Wnt2, and Wnt3a, during prenatal and early postnatal stages. Interestingly, we observed variability in the expression level of these genes between genetically-identical pups within the same pregnancy. Furthermore, we found that all the affected genes have been previously associated with disorders of the central nervous system, including autism. We determined that prenatal exposure to PGE2 affects the Wnt pathway at the level of β-catenin, the major downstream regulator of Wnt-dependent gene transcription. We discuss how these results add new knowledge into the molecular mechanisms by which PGE2 may interfere with neuronal development during critical periods.
Keywords: Lipids, Prostaglandin E2, Gene expression, Wnt signaling pathway, β-catenin, Autism Spectrum Disorder
Highlights
-
•
Maternal PGE2 affects expression level of Wnt genes in the mouse brain of offspring.
-
•
PGE2 interferes with the Wnt pathway in the prenatal and early postnatal brain.
-
•
PGE2 affects genetically identical individuals from the same pregnancy differently.
1. Introduction
The brain contains high lipid content and its healthy development relies on the supply and function of these molecules [15]. Prostaglandin E2 (PGE2) is one of the major lipid metabolites released from phospholipid membranes through the action of phospholipase A2 (PLA2) and cyclooxygenase-1 or − 2 (COX-1 or −2) [25]. PGE2 exerts its physiological function through four G-protein coupled E-prostanoid receptors (EP1–4) [31], [36], [57]. Recent research shows that PGE2 can play an important role in brain development including dendritic spine formation [14], [2] and neuronal plasticity [16], [34]. Increased levels of COX-2 during embryogenesis [52], and higher expression levels of EP receptors during neurogenesis [61], further strengthen the importance of PGE2 in development.
We previously described that abnormalities in the PGE2 signaling pathway due to genetic defects or exposure to environmental factors, during critical periods of brain development, have been associated with the etiology of neurodevelopmental disorders such as Autism Spectrum Disorder (ASD) [61], [76]. The overwhelming evidence shows that various environmental risk factors, including deficient dietary supplementation, infections, exposure to drugs such as misoprostol (prostaglandin E analogue), air pollutants, chemicals found in food and personal care products like cosmetics, disrupt the level of PGE2 and are independently linked to ASD [61], [76]. These exogenous chemicals can be shuttled through the Blood Brain Barrier during a critical time in development and impact signaling of the PGE2 pathway [76]. The molecular mechanisms by which changes in PGE2 levels influence brain development are still not well known.
Thus far, in vitro research in our lab has shown that increased levels of PGE2 can augment proliferation and migration of neuroectodermal stem cells [74], accelerate neuronal differentiation [75], retract neuronal extensions [62] and elevate calcium level in the cytosol and growth cones [22], [60]. More interestingly, we showed for the first time in neuronal cell lines that PGE2 signaling can interact with the major developmental pathway, Wingless-Type MMTV Integration Site Family (Wnt) canonical pathway [74]. In neuroectodermal (NE-4C) stem cells PGE2 can modulate the Wnt canonical pathway via protein kinase A (PKA), phosphoinositide 3-kinase (PI-3K) and β-catenin, the key regulator of the Wnt pathway [74], [75]. In addition, higher level of PGE2 modified the expression of many downstream Wnt-regulated genes important in development and previously implicated in ASD. We also found cross-talk between the PGE2 and Wnt signaling pathways for the first time in the brain of the COX-2-/- knockout mouse [47]. These findings are interesting because signaling of the Wnt signaling pathway is crucial during early brain development [19] and its abnormal regulation have also been associated with ASD [32], [83].
In this study, we use mouse as the experimental model system to determine the effects of maternal exposure to PGE2 at the onset of neurogenesis (embryonic day 11) on expression of various developmental genes in the mouse brain. We identified differential expression of Wnt-regulated genes, including Mmp7, Wnt2, and Wnt3a, in offspring from the same pregnancy. We found that the affected genes were previously linked to neurodevelopmental disorders. PGE2 greatly affected the normal expression level of these genes across three developmental stages tested: embryonic day 16 (E16), embryonic day 19 (E19) and early postnatal day 8 (P8). Moreover, we detected variations in expression level between offsprings with the same genetic composition that persisted through E16, E19, and P8. We also show that PGE2 altered the levels of PKA-activated phospho-β-catenin-Ser-552 and non-phospho (active) β-catenin (Ser33/37/Thr41), the major downstream regulator of gene transcription in the Wnt canonical signaling pathway. These findings provide novel in vivo data that maternal exposure to exogenous PGE2 during critical periods can affect the expression level of important developmental genes in the brain of offspring.
2. Materials and methods
2.1. Animals
Male and female mice (C57BL/6) were obtained from Charles River Laboratories. C57BL/6 colonies are inbred and thus are considered as genetically identical based on testing of single nucleotide polymorphisms (SNPs) by the distributor. The animals were maintained at the animal facility at York University, kept at a 12 h light/dark cycle, and provided with unlimited food and water. All protocols for animal procedures used in this study were approved by the Animal Care Committee (ACC).
2.2. Maternal injections
Male and female mice were mated overnight, and the females were checked every morning until a vaginal plug was observed. This day was considered as gestation day 1, and the females were housed separately for the remaining time. On gestation day 11, the pregnant females were weighed, and injected subcutaneously with 0.2 mg/kg concentration of 16,16-dimethyl prostaglandin E2 (dmPGE2; Cayman Chemical) in saline as in previous studies [18], [65]. The compound dmPGE2 was used as it has a slower metabolism rate than PGE2, and therefore remains active for a longer period of time [44], [58]. Control animals were injected with saline only. Three pregnant females were administered with a single dose of saline or the PGE2 compound, resulting in three litters for each developmental stage tested. Embryonic day 11 (E11) was chosen as the day of PGE2 exposure to the embryo as it is the onset of neurogenesis in the embryonic mouse brain, and also equivalent to the time during which the analogous drug misoprostol was consumed in human clinical studies that resulted in manifestation of Moebius syndrome and ASD [45], [7].
2.3. Sample collection
We obtained three litters from independent females treated with PGE2 at stages embryonic day 16 (E16; n = 21: 6, 8 and 7 pups from each litter), embryonic day 19 (E19; n = 20: 6, 8 and 6 pups from each litter), and postnatal day 8 (P8; n = 19: 3, 9 and 7 pups from each litter). Three litters from each stage were also collected from control females injected with saline at E16 (n = 16: 5, 4 and 7 pups from each litter), E19 (n = 20: 4, 7 and 9 pups from each litter) and P8 (n = 20: 6, 6 and 8 pups from each litter). Total RNA and protein from the whole brain were extracted at the same time from each brain sample using the trizol (Sigma) method. The samples were collected from three stages of neuronal development: E16 at the peak of neurogenesis, E19 the pre-birth brain [49], [50] and P8 [53], [54] during which the prefrontal cortex, hippocampus, and cerebellum still continue developing post-birth [50]. Tail samples were also collected from each pup for genomic DNA extraction for genotyping analysis to distinguish between males and females.
2.4. Genotyping – polymerase chain reaction (PCR)
Tail samples were boiled in alkaline lysis reagent (25 mM NaOH), and neutralized with Tris-HCL buffer. The resulting genomic DNA was used for male/female genotyping using primers for the sex determining region Y (SRY) gene (Table 1), which is only present in males. Standard PCR with the SRY gene is commonly used for genotyping [46] and was performed on the samples (annealing temperature = 62 °C), which were subsequently loaded onto an agarose gel to determine the presence (male) or absence (female) of the SRY gene.
Table 1.
Forward and reverse primer sequences for PCR and real-time PCR.
| Gene | Primer Sequences (5′ → 3′) | Amplicon size (base pairs) |
|---|---|---|
| SRY | F: TCCCAGCATGCAAAATACAGAGATCAGC | 297 |
| R: TTGGAGTACAGGTGTGCAGCTCTAC | ||
| Hprt | F: TCCATTCCTATGACTGTAGATTTTATCAG | 75 |
| R: AACTTTTATGTCCCCCGT TGACT | ||
| Pgk1 | F: CAGTTGCTGCTGAACTCAAATCTC | 65 |
| R: GCCCACACAATCCTTCAAGAA | ||
| Mmp7 | F: TTGACAAGGATGAGTACTGGACTGAT | 73 |
| R: AATTCATGGGTGGCAGCAAA | ||
| Wnt2 | F: GCCCTGATGAACCTTCACAAC | 77 |
| R: TGACACTTGCATTCTTGTTTCAA | ||
| Wnt3a | F: GCACCACCGTCAGCAACAG | 57 |
| R: GGGTGGCTTTGTCCAGAACA |
2.5. Quantitative Real-Time PCR (qRT-PCR)
mRNA was converted to cDNA using reverse transcriptase (MMuLV; New England Biolabs), and the cDNA was used for qRT-PCR. The normal expression of EP receptors was tested in the brain tissue across all developmental stages, using primers (Table 1) as previously described [61]. We used Custom Taqman plates (Applied Biosystems) as a preliminary screening tool to test 44 commercially available developmental genes (Apc, Axin1, Bmp2, Bmp3, Bmp4, Bmp7, Ccnd1, Cldn1, Creb1, Crebbp, Csnk1a1, Ctnnb1, Dvl1, Enc1, Fosl1, Fzd1, Gli1, Gsk3b, Hnf1a, Jun, Lef1, Lrp5, Lrp6, Mmp7, Myc, Nrcam, Ppard, Ptch2, Ptgs2, Pygo1, Smarca4, Tcf3, Tcf4, Tcf7l2, Wnt1, Wnt2, Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt7a, Wnt8a, Wnt8b). Each plate also contained 4 standard housekeeping control genes [18 S ribosomal RNA (18 s), glyceraldehyde-3-phosphate dehydrogenase (Gapdh), hypoxanthine phosphoribosyl transferase (Hprt), and β-glucuronidase (Gusb)]. The screen test was initially performed on pooled RNA samples from saline- and PGE2-exposed litters. The expression levels of only 3 genes reached at least 1.5-fold change (of Mmp7, Wnt2, and Wnt3) and were further validated and quantified in each individual offspring with gene-specific forward and reverse primers (Table 1) using the 7500 Fast Real-Time PCR System with SYBR Green reagent (Applied Biosystems) as we previously described [61], [73].
Quantitative values were obtained from the threshold cycle (CT) number. For the real-time PCR we used two housekeeping genes, Pgk1 (phosphoglycerate kinase) and Hprt. We verified that Pgk1 and Hprt can be used as they have stable expression across all developmental stages with geNorm values of less than 0.5 as described in [66] (data not shown). The raw CT values from experimental (PGE2-exposed pups) and control (saline-exposed pups) samples were normalized using the geometric mean of the housekeeping genes Pgk1 and Hprt to obtain the ∆CT values. The ∆CT values of the samples (each individual animal) were compared with a calibrator (average of all saline-exposed pups from three different litters) to generate the relative quantity (RQ) value which represents the fold change expression of each target sample compared to the reference sample, known as the comparative CT method. Each experimental sample was run in triplicates on a 96-well plate which was considered as one real-time PCR experiment, and was then replicated three times. To determine whether there were sex differences in gene expression of Mmp7, Wnt2, and Wnt3, the RQ values were compared between male and female pups. All RQ values obtained are shown in the Supplementary Table 1.
2.6. Western blot
Protein samples were loaded equally based on concentration, fractionated using polyacrylamide gel electrophoresis (PAGE), and transferred onto a nitrocellulose membrane. EP receptors’ protein expression was tested using the primary rabbit polyclonal antibodies anti-EP1 (Santa Cruz Biotechnology), anti-EP2 (Santa Cruz Biotechnology), anti-EP3 (Santa Cruz Biotechnology), and anti-EP4 (Santa Cruz Biotechnology). The anti-EP3 antiody recognizes all three isoforms (α, β, γ) of the EP3 protein. The levels of β-catenin were tested using the primary antibodies against phospho-β-catenin (Ser552) and non-phospho β-catenin (Ser33/37/Thr41) (Cell Signaling), respectively. Rabbit polyclonal anti-phospho-β-catenin (Ser552) antibody recognizes the active form phosphorylated by protein kinase A (PKA), and rabbit monoclonal anti-nonphospho-β-catenin (Ser33/37/Thr41) antibody recognizes the stabilized form of β-catenin that is functionally active. For comparative analysis, anti-GAPDH (Abcam) was used on the same blots. Horseradish peroxidase-conjugated secondary antibodies used were α-rabbit (Abcam), α-goat (Santa Cruz Biotechnology), or α-mouse (Abcam). Clarity Western ECL Substrate reagent (Bio-rad) was used for detection by Perkin Elmer Geliance 600 Imaging System.
2.7. Statistical analysis
All data presented in this study was expressed as mean ± SEM. The statistical analysis used was one-way ANOVA, followed by student t-test. Results were considered significant if the p value was less than 0.05.
3. Results
3.1. Expression of E-prostanoid (EP) receptors in the normally developing mouse brain
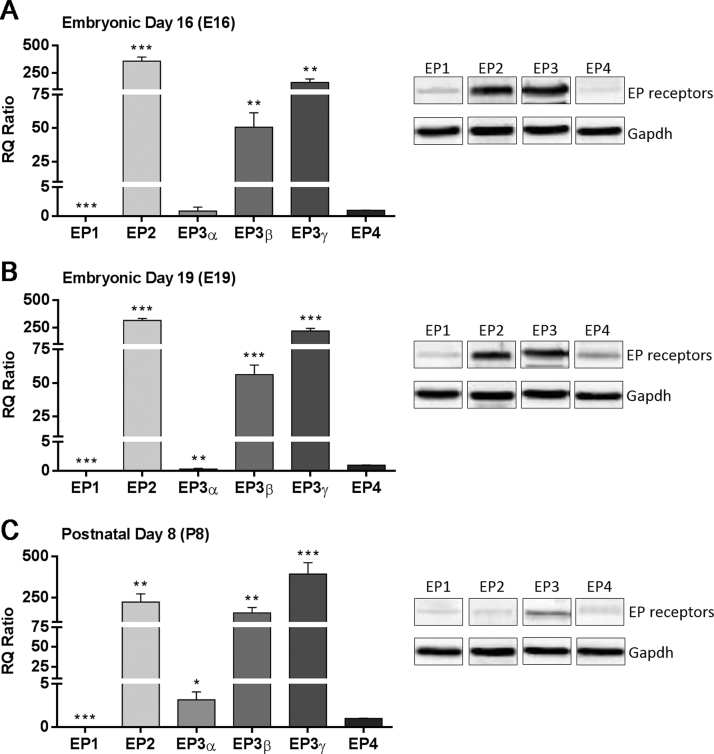
In this study, in order to establish whether our wild type mouse model is suitable to for prenatal exposure to PGE2 we examined for the first time the expression profile of the EP receptors (EP1, EP2, EP3α, EP3β, EP3γ, and EP4) in the brain at the E16, E19, and P8 stages, using real-time PCR assay. The expression level of all receptors for each sample was first normalized to the geometric mean of the housekeeping genes Pgk1 and Hprt (see methods) and further shown as relative quantity (RQ) values compared to the EP4 receptor (RQ = 1), which was the same across all developmental stages tested (Fig. 1, left panels). The expression pattern of all EP receptors at E16 shows that EP2 was the most highly expressed (RQ = 355.8; p < 0.0005), followed by EP3γ (RQ = 161.4; p = 0.001), EP3β (RQ = 50.7; p = 0.0013), EP3α (RQ = 0.9, p = 0.41), EP4 (RQ = 1), and EP1 (RQ = 0.01; p < 0.0005) (Fig. 1A). At E19, EP2 was again the most highly expressed (RQ = 316.3; p < 0.0005), followed by EP3γ (RQ = 220.1; p < 0.0005), EP3β (RQ = 56.2; p < 0.0005), EP3α (RQ = 0.3; p = 0.001), and EP1 (RQ = 0.002; p < 0.0005) (Fig. 1B). At P8, EP3γ shows the highest expression (RQ = 392.0; p = 0.0005), followed by EP2 (RQ = 221.7; p = 0.002), EP3β (RQ = 155.0; p = 0.001), EP3α (RQ = 3.1; p = 0.02), and EP1 (RQ = 0.04; p < 0.0005) (Fig. 1C). Overall, EP2, EP3γ, and EP3β are predominantly expressed in the mouse brain at all stages. Western blot analysis confirmed the expression of all EP receptors in each developmental stage at the protein level (Fig. 1, right panels). In summary, we established the expression profile of all EP receptors in the mouse brain across developmental stages making it a good model for subsequent PGE2 injections performed in this study.
Fig. 1.
Expression of EP receptors during mouse brain development. RQ values for EP1, EP2, EP3α, EP3β, and EP3γ were normalized to RQ values of the EP4 receptor. (A) The EP2, EP3β, and EP3γ receptors were the most predominantly expressed at Embryonic day 16 (E16). (B-C) Similar pattern was seen at Embryonic day 19 (E19), and Postnatal day 8 (P8). **p < 0.005, ***p < 0.0005. (A-C) Western blot confirmed protein expression of EP1, EP2, EP3 (all isoforms), and EP4 receptors in the developing brain (right panel). Each value was averaged from three separate experiments (n = 3).
3.2. Mmp7 expression in brain of individual pups at developmental stages E16, E19, and P8, following maternal exposure to PGE2
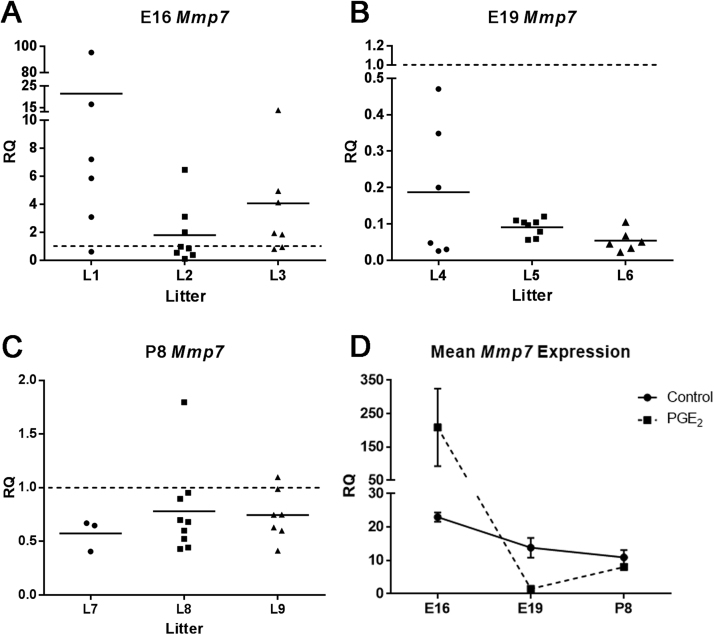
Using Custom Taqman plates we screened 44 various developmental genes (see methods) in offsprings from all developmental stages (E16, E19 and P8). We detected that three of these genes that belong to the Wnt canonical pathway were differentially expressed at all developmental stages (E16, E19, P8): Mmp7, Wnt2, and Wnt3a. We confirmed the expression level of these genes using real-time PCR in each individual offspring from three independent litters following maternal exposure to PGE2 and compared to control animals represented as RQ value of 1 across all stages (Fig. 2, Fig. 3, Fig. 4; Supplementary Table 1). At E16, there was increased expression of Mmp7 in all three litters (Fig. 2A). Interestingly, the expression considerably varied between the pups of any given litter, suggesting that genetically identical pups of the same pregnancy are affected differently by the exogenous PGE2 compound. Litter 1 (L1) had a total of six pups with RQ values of 95.2 (p = 0.014), 16.5 (p = 0.017), 7.2 (p = 0.026), 5.8 (p = 0.0081), 3.1 (p = 0.27) and 0.60 (p = 0.12). Eight pups in L2 had RQ values of 6.5 (p = 0.0058), 3.1 (p = 0.017), 2.0 (p = 0.0072), 1.0 (p = 0.75), 0.84 (p = 0.24), 0.53 (p = 0.012), and 0.38 (p = 0.0048), 0.10 (p < 0.0005). In L3, there were seven pups with RQ values of 13.8 (p = 0.044), 4.9 (p = 0.015), 4.1 (p = 0.022), 1.9 (p = 0.16), 1.8 (p = 0.18), 0.93 (p = 0.84), and 0.80 (p = 0.057). The average expression of Mmp7 at E16 in PGE2-exposed animals (L1, L2, and L3) had RQ value of 8.2 (p = 0.31) above the level in control animals. This mean value did not reach significance because of the great variation between individual pups.
Fig. 2.
Expression of Mmp7 mRNA in developing mouse brain following maternal exposure to PGE2. (A) The expression of Mmp7 increased at E16 in all three litters (L1-L3) following PGE2 exposure. There were individual differences between pups. (B) At E19, the expression of Mmp7 decreased in all three litters L4-L6. (C) There was continued decrease in expression of Mmp7 at P8 in pups of all three litters L7-L9. Horizontal lines represent the mean value within each litter. (D) In the control mouse brain, Mmp7 expression is highest at E16 (p < 0.0005), and decreases throughout development in E19 (p = 0.012) and P8 (p = 0.010). Following PGE2 exposure, mean Mmp7 expression from all pups is greater at E16 but not statistically significant (p = 0.31), and decreases at E19 (p < 0.0005) and P8 (p = 0.0022), from the normal expression in controls. Each value was averaged from animals from three litters and three independent experiments.
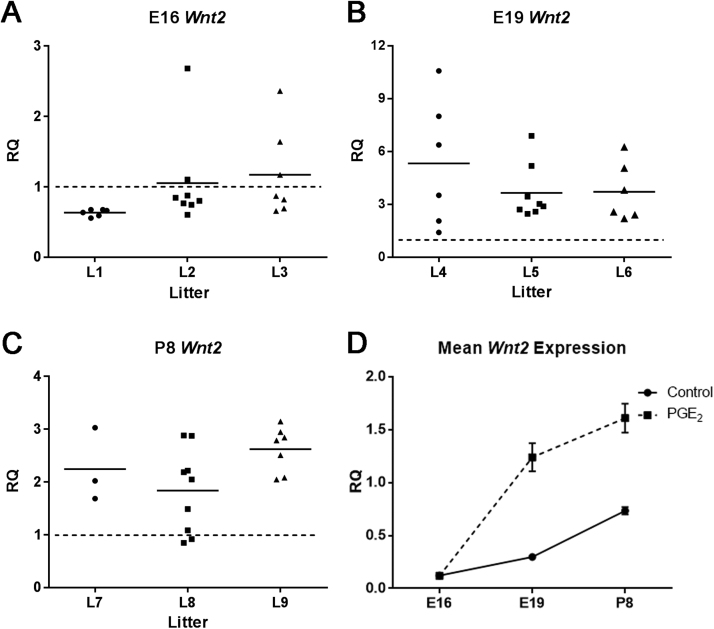
Fig. 3.
Expression of Wnt2 mRNA in developing mouse brain following maternal exposure to PGE2. (A) The expression of Wnt2 decreased in most pups at E16 (L1–3), with the exception of a few pups in which levels were increased. (B) At E19, the expression of Wnt2 increased in all three litters (L4-L6). (C) There was continued increase in expression of Wnt2 at P8 in pups of all three litters (L7-L9). Horizontal lines represent the mean value within each litter. (D) In the control mouse brain, Wnt2 expression is lowest at E16 (p = 0.00084), and increases throughout development in E19 (p = 0.0020) and P8 (p = 0.033). Following PGE2 exposure, mean Wnt2 expression from all pups is similar at E16 (p = 0.82), and significantly increases at E19 (p < 0.0005) and P8 (p < 0.0005), from the normal expression in controls. Each value was averaged from animals from three litters and three separate experiments.
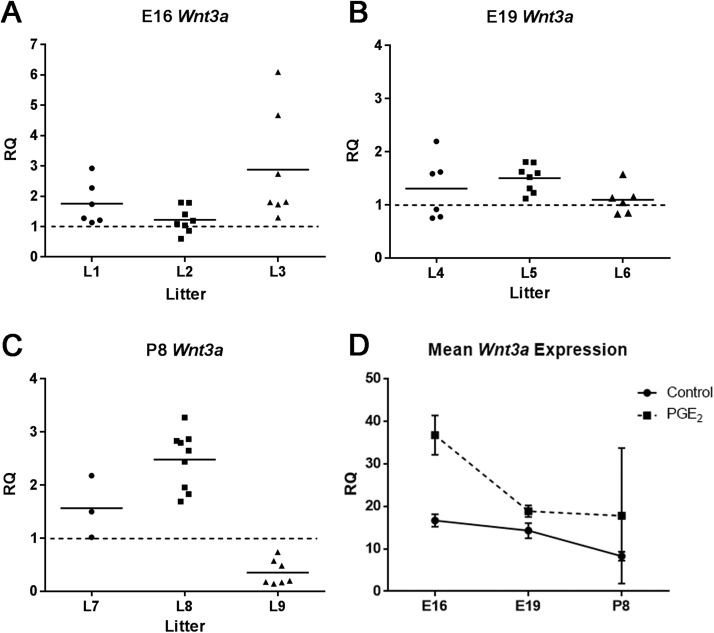
Fig. 4.
Expression of Wnt3a mRNA in developing mouse brain following maternal exposure to PGE2. (A) The expression of Wnt3a was generally increased in most pups at E16 (L1-L3). (B) At E19, the expression of Wnt3a was increased in all three litters (L4-L6). (C) Two litters L7 and L8 had increased Wnt3a expression at P8, while L9 had decreased expression. Horizontal lines represent the mean value within each litter. (D) In the control mouse brain, Wnt3a expression is highest at E16 (p = 0.00092), and decreases throughout development in E19 (p = 0.0035) and P8 (p = 0.0050). Following PGE2 exposure, mean Wnt3a expression from all pups is significantly greater at E16 (p = 0.0040), and remains elevated at E19 (p = 0.0022) and P8 (p = 0.040), from the normal expression in controls. Each value was averaged from animals from three litters and three separate experiments.
In contrast, at developmental stage of E19, the expression of Mmp7 from the three litters was considerably decreased when compared to control animals (Fig. 2B). In L4, there was a significant decrease of Mmp7 expression in all six pups (p < 0.0005) with RQ values of 0.47, 0.34, 0.20, 0.048, 0.030, and 0.026. In L5, all eight pups showed a significant decrease of Mmp7 expression (p < 0.0005) with RQ values of 0.12, 0.11, 0.10, 0.10, 0.10, 0.08, 0.058 and 0.056. Significant reduction in Mmp7 expression was also observed in all six pups of L6 (p < 0.0005) with RQ values of 0.11, 0.067, 0.050, 0.045, 0.033, and 0.022. The overall expression of Mmp7 at E19 in PGE2-exposed animals (L4, L5, and L6), decreased with an RQ value of 0.11 (p < 0.0005) or 9.1 times below the level from the controls.
Similar to E19, Mmp7 expression in P8 remained decreased in three PGE2-exposed pups of L7 (Fig. 2C). The RQ values for each pup were 0.67 (p = 0.040), 0.65 (p = 0.022), and 0.40 (p = 0.0012). In L8, there were nine pups with RQ values of 1.8 (p = 0.35), 0.95 (p = 0.56), 0.90 (p = 0.45), 0.70 (p = 0.24), 0.68 (p = 0.030), 0.59 (p < 0.0005), 0.52 (p = 0.0032), 0.44 (p < 0.0005), and 0.43 (p < 0.0005). In L9, there were seven pups with RQ values of 1.09 (p = 0.52), 0.99 (p = 0.89), 0.75 (p = 0.055), 0.74 (p = 0.23), 0.63 (p = 0.0039), 0.60 (p < 0.0005), and 0.41 (p < 0.0005). The overall expression of Mmp7 in all PGE2-exposed animals (L7, L8, and L9) continued to be suppressed at P8 with an RQ value of 0.73 (p = 0.0022), which represents 1.4 times reduction from the normal level seen in controls. In addition, there was no significant difference in expression of Mmp7 observed between males and females at E16 (p = 0.69), E19 (p = 0.49) or P8 (p = 0.69) (data not shown).
The actual RQ values of normal expression level of Mmp7 at E16, E19 and P8 in the control animals (represented as the RQ value of 1 in Fig. 2A-C) are plotted in Fig. 2D. The expression level of Mmp7 appears highest at E16, and continued to decline throughout development (E19 and P8) with RQ values of 23.0 (p < 0.0005), 13.8 (p = 0.012), and 10.9 (p = 0.010), respectively. As indicated above, the expression of Mmp7 was greatly influenced by exposure to PGE2 in all developmental stages. Fig. 2D shows that, at E16, the overall expression level of Mmp7 in PGE2-exposed animals was increased by 8.2 fold change. The expression level at E19 was overall decreased with PGE2 by 9.1 and continued to be suppressed at P8 by 1.4.
3.3. Wnt2 expression in brain of individual pups at developmental stages E16, E19, and P8, following maternal exposure to PGE2
The expression of Wnt2 in the brain was overall reduced at developmental stage of E16 and was elevated in E19 and P8 as compared to control animals (Fig. 3). At E16, we detected a significant decrease in expression of Wnt2 in all six pups in L1 with RQ values of 0.68 (p < 0.0005), 0.67 (p = 0.041), 0.66 (p < 0.0005), 0.64 (p = 0.0022), 0.59 (p = 0.00052), and 0.55 (p < 0.0005). In L2, there were eight pups with RQ values of 2.7 (p < 0.0005), 1.1 (p = 0.37), 0.87 (p = 0.31), 0.84 (p = 0.16), 0.80 (p = 0.14), 0.76 (p = 0.028), 0.74 (p = 0.013), and 0.60 (p = 0.0046). More variation was found in the expression level of Wnt2 within the seven pups of L3 with RQ values of 2.4 (p = 0.0059), 1.6 (p = 0.036), 1.2 (p = 0.54), 0.87 (p = 0.67), 0.82 (p = 0.45), 0.69 (p = 0.11), and 0.66 (p = 0.0023). Although there are significant changes in Wnt2 expression at E16 in individual PGE2-exposed animals, the overall average across three litters was not significantly different from the controls with an average RQ value of 0.97 (p = 0.82).
Exposure to PGE2 largely resulted in elevated Wnt2 expression in the brain across the pups of three independent litters at developmental stage of E19 (Fig. 3B). In L4, there were six pups with RQ values of 10.6 (p = 0.00082), 8.0 (p = 0.0013), 6.4 (p < 0.0005), 3.5 (p = 0.0031), 2.1 (p = 0.034), and 1.4 (p = 0.33). In L5, all eight pups show increased Wnt2 expression with RQ values of 6.9 (p < 0.0005), and 5.2 (p < 0.0005), 3.4 (p = 0.015), 3.0 (p = 0.0045), 2.9 (p = 0.014), 2.7 (p = 0.030), 2.6 (p = 0.023), 2.5 (p = 0.021). Similarly, the expression of Wnt2 was also increased in all six pups of L6 with RQ values of 6.3 (p < 0.0005), 5.1 (p < 0.0005), 3.8 (p = 0.0026), 2.6 (p = 0.031), 2.4 (p = 0.049), and 2.2 (p = 0.045). The overall expression of Wnt2 in PGE2-exposed animals at E19 (litter L4, L5, and L6), was 4.2 times greater than the control (p < 0.0005).
At developmental stage of P8, the expression of Wnt2 continues to be increased (Fig. 3C). In L7, there were three pups with RQ values of 3.0 (p = 0.027), 2.0 (p = 0.032), and 1.7 (p = 0.20). In L8, there were nine pups with RQ values of 2.9 (p < 0.0005), 2.9 (p = 0.043), 2.2 (p = 0.0043), 2.2 (p < 0.0005), 2.0 (p = 0.0012), 1.5 (p = 0.16), 1.1 (p = 0.80), 0.92 (p = 0.82), and 0.85 (p = 0.62). In L9, all seven pups had significantly increased expression of Wnt2 with RQ values of 3.1 (p < 0.0005), 2.9 (p = 0.00085), 2.8 (p < 0.0005), 2.8 (p = 0.00057), 2.5 (p = 0.0079), 2.1 (p = 0.0085), 2.0 (p = 0.035). The overall expression of Wnt2 in PGE2-exposed animals at P8 (L7, L8, and L9) was 2.2 times greater than the controls (p < 0.0005). In addition, there was no significant difference in expression of Wnt2 observed between males and females at E16 (p = 0.29), E19 (p = 0.80) or P8 (p = 0.33) (data not shown).
In the control animals, the normal expression level of Wnt2 in the brain (represented as the RQ value of 1 in Fig. 3A-C) was lowest at E16, and continued to increase throughout development (E19 and P8) with RQ values of 0.1 (p = 0.00084), 0.3 (p = 0.0020), and 0.7 (p = 0.033) respectively (Fig. 3D). Maternal exposure to PGE2 did not have a significant effect on the overall expression of Wnt2 at developmental stage E16 with the average fold change decrease of 1.03. However, PGE2 increased the overall Wnt2 expression level at E19 by a fold change of 4.2, and remained elevated at P8 with a fold change of 2.2.
3.4. Wnt3a expression in brain of individual pups at developmental stages E16, E19, and P8, following maternal exposure to PGE2
Exposure to maternal PGE2 at E11 resulted in overall increased Wnt3a expression level in the brain at developmental stage of E16, E19, and P8 as compared to control animals (Fig. 4). At E16, all six pups from L1 had elevated Wnt3a expression compared to control with RQ values of 2.9 (p = 0.024), 2.3 (p = 0.19), 1.7 (p = 0.69), 1.3(p = 0.99), 1.2 (p = 0.99), 1.1 (p = 0.99). In L2, there were eight pups with RQ values of 1.8 (p = 0.42), 1.8 (p = 0.43), 1.4 (p = 0.93), 1.2 (p = 0.99), 1.1 (p = 0.99), 1.0 (p = 1.0), 0.86 (p = 0.99), 0.59 (p = 0.93). Wnt3a expression was also increased in all of the seven pups of L3 with RQ values of 6.1 (p = 0.047), 4.7 (p = 0.016), 2.7 (p = 0.082), 1.8 (p = 0.13), 1.8 (p = 0.20), 1.7 (p = 0.16), 1.3 (p = 0.23). The overall mean expression of Wnt3a at E16 (L1, L2, L3) in PGE2-exposed animals is RQ value of 1.9, which is higher than the controls (p = 0.0040).
Wnt3a expression was generally elevated in the brain across the pups of three independent litters at developmental stage of E19 (Fig. 4B). In L4, there were six pups with RQ values of 2.2 (p = 0.032), 1.6 (p = 0.031), 1.6 (p = 0.048), 0.91 (p = 0.46), 0.78 (p = 0.17), 0.75 (p = 0.0016). In L5, all eight pups show increased Wnt3a expression with RQ values of 1.8 (p = 0.0050), 1.8 (p = 0.071), 1.6 (p = 0.032), 1.6 (p = 0.0012), 1.5 (p = 0.087), 1.3 (p = 0.15), 1.2 (p = 0.16), 1.1 (p = 0.0099). Wnt3a expression in the six pups of L6 had RQ values of 1.6 (p = 0.85), 1.2 (p = 0.99), 1.1 (p = 0.99), 1.0 (p = 1.0), 0.9 (p = 0.99), 0.8 (p = 0.99). The average expression of Wnt3a at E19 (L4, L5, and L6) in PGE2-exposed animals has the RQ value of 1.3 which is above the level of the controls (p = 0.0022).
At developmental stage of P8, the expression of Wnt3a was increased in two independent litters, while a third independent litter had decreased expression (Fig. 4C). In L7, there were three pups with RQ values of 2.2 (p < 0.0005), 1.5 (p = 0.10), 1.0 (p = 0.67). In L8, all nine pups had increased Wnt3a expression with RQ values of 3.3 (p = 0.0028), 2.9 (p < 0.0005), 2.8 (p < 0.0005), 2.8 (p = 0.020), 2.6 (p = 0.00024), 2.4 (p = 0.022), 2.0 (p = 0.011), 1.8 (p = 0.0029), 1.7 (p = 0.048). In contrast, L9 had seven pups with decreased expression of Wnt3a with RQ values of 0.74 (p = 0.26), 0.57 (p = 0.099), 0.48 (p = 0.0069), 0.20 (p = 0.0013), 0.17 (p < 0.0005), 0.16 (p < 0.0005), 0.14 (p < 0.0005). The overall expression of Wnt3a at P8 (L7, L8, and L9) in PGE2-exposed animals has the RQ value of 1.6 which was greater than the controls (p = 0.040). As for the previous genes there was no significant difference in expression of Wnt3a between males and females at E16 (p = 0.27), E19 (p = 0.82) or P8 (p = 0.91) (data not shown).
The normal expression level of Wnt3a in the brain of control animals (represented as the RQ value of 1 in Fig. 4A-C) was highest at E16, and decreased at E19 and P8, with RQ values of 16.6 (p = 0.00092), 14.3 (p = 0.0035), and 8.3 (p = 0.0050), respectively (Fig. 4D). Following maternal exposure to PGE2 we observed significantly increased expression of Wnt3a in the brain of offsprings at developmental stage E16, E19, and P8, above the normal level by 1.9-fold, 1.3-fold, and 1.6-fold, respectively. These results show that maternal exposure to PGE2 at the onset of neurogenesis, can cause changes in expression of critical developmental genes of the Wnt signaling pathway, that may last throughout brain development.
3.5. Expression of β-catenin protein in mouse brain at developmental stages E16, E19, and P8, following maternal exposure to PGE2
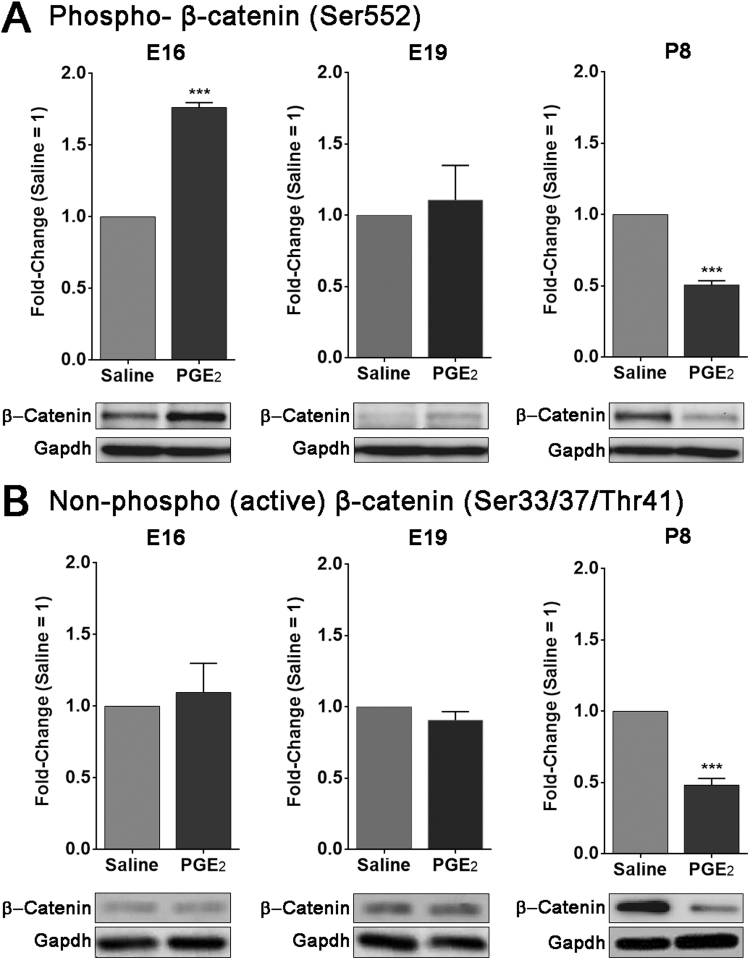
To investigate the convergence of the PGE2 and Wnt pathways in the brain at each developmental stage we tested the protein levels of β-catenin, a key modulator of the Wnt canonical pathway. Activation and degradation of β-catenin can be regulated by a complex multiple phosphorylation sites. We used antibodies against β-catenin activated through phosphorylation of the Serine-552 site (phospho-β-catenin-Ser552) by PKA [63], [64], [67]. We also tested the levels of non-phospho (active) β-catenin (Ser33/37/Thr41), which is the most stable form of β-catenin [26]. Phosphorylation at Ser33, Ser37, and Thr41 by GSK-3β destabilizes β-catenin and marks it for degradation [33], [38], [78]. Our Western blot analysis shows that at E16 there was a significant 1.8 fold increase in the level of phospho-β-catenin (Ser552) in animals exposed to PGE2 (p < 0.0005) (Fig. 5A). At E19, we did not observe any significant change (p = 0.72). However, at P8 we detected a significant decrease in the level of phospho-β-catenin (Ser552) in PGE2 exposed pups, with a fold change of 0.51 (p < 0.0005). Interestingly, the shift in the phosphorylation pattern of phospho-β-catenin (Ser552) and the change in expression levels of Wnt genes (Fig. 2, Fig. 3, Fig. 4) both occur at E19. These results indicate that after prenatal exposure PGE2 may interfere with the Wnt pathway via PKA and that this could be specific to different developmental stage.
Fig. 5.
The level of phosphorylated β-catenin (Ser-552) and non-phosphorylated β-catenin (Ser33/37/Thr41) in developing mouse brain following maternal exposure to PGE2. The protein expression of two active forms of β-catenin was confirmed by western blot from whole brain following maternal exposure to saline and PGE2 at E16, E19 and P8. (A; left panel) There was a significant increase in phosphorylation of β-catenin (Ser-552) at E16, (A; middle panel) no change at E19, and (A; left panel) significant decrease at P8. (B; left and middle panels) There was no change in expression of nonphosphorylated β-catenin at Ser33/37/Thr41 sites at E16 and E19, (B; right panel) and significant decrease at P8. The results represent data from three litters and three independent experiments. * ** p < 0 0.0005.
The expression of non-phospho β-catenin (Ser33/37Thr41) was not affected at E16 and E19 with fold-change values of 1.1 (p = 0.34) and 0.91 (p = 0.20), respectively (Fig. 5B). At P8, there was a significant decrease in PGE2-exposed animals with a fold-change of 0.48 (p < 0.0005). These results indicate that the exposure to PGE2 during prenatal stages result in phosphorylation of the Wnt pathway mediator β-catenin by PKA at Ser-552 as well as the stabilization of non-phospho β-catenin at Ser33/37Thr41. Moreover, we observe that these effects might be developmentally regulated.
4. Discussion
Previous studies in the postnatal brain show that PGE2 signaling can influence formation of dendritic spines and neuronal plasticity [14], [16], [2]. This study provides novel data for the role of PGE2 signaling in the prenatal brain. Using an in vivo mouse model, we showed the effect of maternal exposure to the exogenous lipid mediator PGE2 on the offspring during brain development.
First, we confirmed that all EP receptors are expressed in the brain throughout development. Second, we showed that PGE2 can influence the expression level of genes from the canonical Wnt signaling pathway, including Mmp7, Wnt2, and Wnt3a, all of which have been previously linked to neurodevelopmental disorders such as ASD. Lastly, we determined that the convergence of PGE2 with the Wnt canonical pathway was also apparent by higher level of PKA-activated phosphorylated β-catenin (Ser552) and active non-phospho β-catenin (Ser33/37/Thr41). These finding are important because current research from human genetics and animal studies also show the involvement of the canonical Wnt pathway in the pathology of ASD [61], [76], [83].
We found that maternal exposure to PGE2 at the onset of neurogenesis resulted in differential expression of Wnt-regulated genes, including Mmp7, Wnt2, and Wnt3a. There was a significant PGE2–dependent increase in expression of Mmp7 above the normal level at E16 and a noticeable decline in the expression below the basal level at E19 that persisted to the P8 stage. Mmp7 belongs to a family of matrix metalloproteinases that are involved in many functions such as breakdown of the extracellular matrix, development, inflammation, and synaptic plasticity [24]. The observed changes in the Mmp7 level due to exposure to PGE2 may have potential consequences at various stages of the developing brain. Mmp7 can be involved in the release of neurotransmitters from the presynaptic membrane [59] and regulate the structure of dendritic spines [9]. Increased levels of Mmp7 were found in the animal model [12] and human brain samples [21], [4] of multiple sclerosis. Interestingly, Mmp7 also activates Mmp9 [69], [70], which has been implicated in multiple sclerosis [41], epilepsy [35], fragile-X syndrome [55], and ASD [1]. We previously found elevated levels of Mmp9 expression in neuroectodermal stem cells exposed to exogenous PGE2 [74]
We also show that following maternal exposure to PGE2, the mean expression of Wnt2 was not greatly affected at E16 but significantly increased above the normal level at E19 and P8. Considering the importance of Wnt2 in the brain, these PGE2-triggered changes in its expression might have an effect on the course of development. Normally, Wnt2 is expressed in the rat and mouse ventral midbrain during and after neurogenesis [48], [56], and is shown to play a role in the proliferation of dopaminergic neurons [56]. Wnt2 is also located on the widely known autism susceptibility locus on the chromosome 7q31–33 [6]. Two mutations of Wnt2 were found in two families with ASD [72], SNP mutations were detected in patients with autism [39], and strong associations were reported within a Chinese population of individuals with ASD [17]. A more recent study tested polymorphisms of the Wnt2 gene in individuals on the autism spectrum, and found that it may play a potential role in delayed speech [37].
Furthermore, we found that PGE2 significantly increased Wnt3a expression above the normal level in all developmental stages (E16, E19, and P8). As many Wnt-regulated genes, Wnt3a plays an important role in brain development [40], in specific events such as neurite outgrowth, neuronal polarization, intracellular calcium modulation, and neurotransmission [29], [5], [8]. Previous literature indicates that Wnt3a is also involved in the proliferation and differentiation of neural stem cells into neurons [3], [79], [80]. Interestingly, the brain regions that have been shown to be highly impacted by Wnt3a signaling during development–thalamus, cerebellum, and hippocampus—have also been implicated in ASD [11], [68], [71].
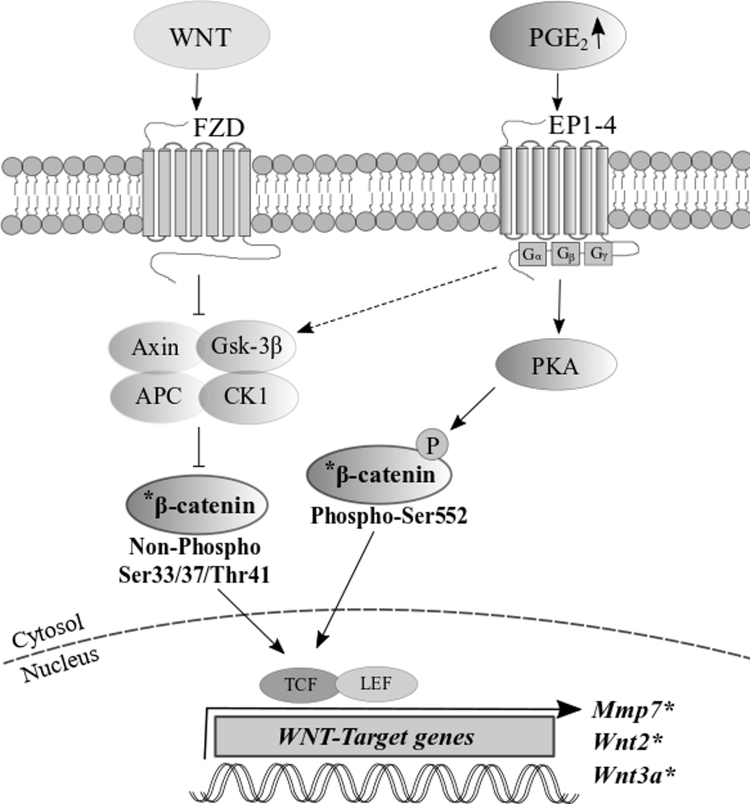
In this study, we provide evidence in an in vivo model that PGE2 may interact with the Wnt pathway during brain development following maternal exposure to PGE2 likely through activation of β-catenin, a key modulator of Wnt signaling. It is apparent through the differential expression of three Wnt-target genes, changes in the level of PKA-phosphorylated β-catenin (Ser552) [63], [64], and non-phosphorylated β-catenin (Ser33/37/Thr41) (Fig. 6). PKA signaling is one of the major downstream components of the PGE2 signaling pathway [74], [77]. PKA-induced activation at the Serine-552 phosphorylation site has been previously linked to increased transcriptional activity of the Wnt canonical pathway [63], [84]. The Ser33/37/Thr41 sites can be phosphorylated by GSK-3β, which destabilizes β-catenin and marks it for degradation [33], [38], [78]. Non-phosphorylation at these sites leads to increased levels of the active form of β-catenin, which can translocate to the nucleus to act on transcription factors and gene expression [26]. Previous literature has indicated that PGE2 treatment may act on GSK-3β to alter the phosphorylation processing of β-catenin [23], [82].
Fig. 6.
A proposed model of the interaction between PGE2 and Wnt pathways in mouse brain. Elevated level of PGE2 due to exposure to exogenous risk factors could affect phosphorylation of β-catenin by PKA at the Serine-552 site, and stabilization of active β-catenin (Non-phospho Ser33/37/Thr41) and its subsequent translocation to the nucleus to act on transcription factors to initiate transcription of Wnt-target genes. The Wnt genes and β-catenin affected by the exposure to PGE2 in this study are shown in bold with an asterisk. The broken line represents proposed cross-talk.
The current findings are in line with previous literature. An interaction between PGE2 and the Wnt pathway has been established previously in non-neuronal cell types [30], [74], [81], and shown in vivo in a zebrafish model [43]. Our lab previously showed that PGE2 signaling can also cross-talk with the Wnt canonical pathway in neuronal cell cultures and another in vivo model system. The addition of PGE2 to neuronal cells, led to changes in the expression of Wnt genes and activation of non-phospho β-catenin (Ser33/Ser37/Thr41) [74], [75]. Interestingly, our recent microarray analysis of the prenatal brain of gene knockout mouse lacking the PGE2-producing enzymes (COX-1-/- and COX-2-/-) showed differential regulation of 8 Wnt-regulated genes and significant changes in expression of both active forms of β-catenin, non-phospho β-catenin (Ser33/Ser37/Thr41) and PKA-activated phospho-β-catenin (Ser-552) [47]. Our previous and current findings suggest that abnormal PGE2 levels in the mouse brain during development may interfere with the Wnt pathway via phosphorylation of β-catenin at multiple sites, which can lead to differential expression of crucial neurodevelopmental genes.
This study also shows that there is differential regulation of gene expression between pups of the same genetic composition within a given pregnancy. As indicated by previous research, the observed differences in gene expression between offsprings could be attributed to various factors such as dissimilar metabolism of exogenous PGE2 by the mother, unequal distribution of PGE2 to each pup due to separate placentas, or inconsistent epigenetic changes between the pups [13], [27], [28]. A previous study showed that monozygotic twins that have separate placentas (dichorionic) show more variability in epigenetic changes compared to monozygotic twins that share a placenta (monochorionic) [13]. Another study found variable levels of ethanol metabolites in human and guinea pig placentas following identical maternal exposure to ethanol [27]. These results provide some insight into the mechanisms by which environmental factors that modulate PGE2 levels in the brain [61], [74], [76] may influence brain development via gene expression. This study might also help with a better understanding of how the external environment can influence the behavioural differences observed between human monozygotic twins affected with autism [20], [42]. Monozygotic-monochorionic twins are more likely to have higher concordance rates compared to monozygotic-dichorionic twins that do not share the same placenta [10]. This can potentially explain why the concordance in monozygotic twins has been reported between 36% and 88% [51].
In conclusion, this study shows that exposure to maternal PGE2 at a critical time can have an effect on expression of Wnt-target genes during prenatal (E16 and E19) brain development that can continue postnatally at P8. It also adds further molecular evidence that the convergence between the PGE2 and Wnt pathways occur in the critical periods of the developing brain. Further ongoing in vivo studies in our lab aim to determine the involvement of the PGE2-Wnt interaction in brain pathology.
Acknowledgements
This research work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) [grant no. is RGPIN/341493-2012]. The authors declare no competing financial interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.03.012.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.03.012.
Appendix A. Transparency document
Supplementary material
Appendix B. Supplementary material
Supplementary material
References
- 1.Abdallah M.W., Pearce B.D., Larsen N., Greaves-Lord K., Norgaard-Pedersen B., Hougaard D.M., Mortensen E.L., Grove J. Amniotic fluid MMP-9 and neurotrophins in autism spectrum disorders: an exploratory study. Autism Res. 2012;5:428–433. doi: 10.1002/aur.1254. [DOI] [PubMed] [Google Scholar]
- 2.Amateau S.K., McCarthy M.M. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J. Neurosci.: Off. J. Soc. Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson T., Duckworth J.K., Fritz N., Lewicka M., Sodersten E., Uhlen P., Hermanson O. Noggin and Wnt3a enable BMP4-dependent differentiation of telencephalic stem cells into GluR-agonist responsive neurons. Mol. Cell. Neurosci. 2011;47:10–18. doi: 10.1016/j.mcn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Anthony D.C., Ferguson B., Matyzak M.K., Miller K.M., Esiri M.M., Perry V.H. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol. Appl. Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- 5.Avila M.E., Sepulveda F.J., Burgos C.F., Moraga-Cid G., Parodi J., Moon R.T., Aguayo L.G., Opazo C., De Ferrari G.V. Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J. Biol. Chem. 2010;285:18939–18947. doi: 10.1074/jbc.M110.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badner J.A., Gershon E.S. Regional meta-analysis of published data supports linkage of autism with markers on chromosome 7. Mol. Psychiatry. 2002;7:56–66. doi: 10.1038/sj.mp.4000922. [DOI] [PubMed] [Google Scholar]
- 7.Bandim J.M., Ventura L.O., Miller M.T., Almeida H.C., Costa A.E. Autism and Mobius sequence: an exploratory study of children in northeastern Brazil. Arquivos de Neuro-psiquiatria. 2003;61:181–185. doi: 10.1590/s0004-282x2003000200004. [DOI] [PubMed] [Google Scholar]
- 8.Bernis M.E., Oksdath M., Dupraz S., Nieto Guil A., Fernandez M.M., Malchiodi E.L., Rosso S.B., Quiroga S. Wingless-type family member 3A triggers neuronal polarization via cross-activation of the insulin-like growth factor-1 receptor pathway. Front. Cell. Neurosci. 2013;7:194. doi: 10.3389/fncel.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilousova T.V., Rusakov D.A., Ethell D.W., Ethell I.M. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J. Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohm H.V., Stewart M.G. Brief report: on the concordance percentages for Autistic Spectrum Disorder of twins. J. Autism Dev. Disord. 2009;39:806–808. doi: 10.1007/s10803-008-0683-2. [DOI] [PubMed] [Google Scholar]
- 11.Boucher J., Mayes A., Bigham S. Memory in autistic spectrum disorder. Psychol. Bull. 2012;138:458–496. doi: 10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- 12.Bucan V., Mandel K., Bertram C., Lazaridis A., Reimers K., Park-Simon T.W., Vogt P.M., Hass R. LEF-1 regulates proliferation and MMP-7 transcription in breast cancer cells. Genes Cells. 2012;17:559–567. doi: 10.1111/j.1365-2443.2012.01613.x. [DOI] [PubMed] [Google Scholar]
- 13.Bui M., Benyamin B., Shah S., Henders A.K., Martin N.G., Montgomery G.W., McRae A.F. Sharing a placenta is associated with a greater similarity in dna methylation in monochorionic versus dichorionic twin pars in blood at age 14. Twin Res. Human Gen. 2015;18:680–685. doi: 10.1017/thg.2015.87. [DOI] [PubMed] [Google Scholar]
- 14.Burks S.R., Wright C.L., McCarthy M.M. Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience. 2007;146:1117–1127. doi: 10.1016/j.neuroscience.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderon F., Kim H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen C., Magee J.C., Bazan N.G. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 17.Chien Y.L., Wu Y.Y., Chiu Y.N., Liu S.K., Tsai W.C., Lin P.I., Chen C.H., Gau S.S., Chien W.H. Association study of the CNS patterning genes and autism in Han Chinese in Taiwan. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2011;35:1512–1517. doi: 10.1016/j.pnpbp.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Chung S.W., Gould B., Zhang R., Hu Y., Levy G.A., Gorczynski R.M. Pretreatment of donor stimulator cells by 16,16 dimethyl prostaglandin E2 influences the recipient immune response. Surgery. 1998;123:171–180. [PubMed] [Google Scholar]
- 19.Ciani L., Salinas P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 20.Cook E.H., Jr. Literature review: gastrointestinal symptoms in ASD, brain structure of identical twins with ASD. Autism Res. 2009;2:285–286. doi: 10.1002/aur.95. [DOI] [PubMed] [Google Scholar]
- 21.Cossins J.A., Clements J.M., Ford J., Miller K.M., Pigott R., Vos W., Van der Valk P., De Groot C.J. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997;94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- 22.Davidson J.M., Wong C.T., Rai-Bhogal R., Li H., Crawford D.A. Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells. Mol. Cell. Neurosci. 2016;74:71–77. doi: 10.1016/j.mcn.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Du M., Shi F., Zhang H., Xia S., Zhang M., Ma J., Bai X., Zhang L., Wang Y., Cheng S., Yang Q., Leng J. Prostaglandin E2 promotes human cholangiocarcinoma cell proliferation, migration and invasion through the upregulation of beta-catenin expression via EP3-4 receptor. Oncol. Rep. 2015;34:715–726. doi: 10.3892/or.2015.4043. [DOI] [PubMed] [Google Scholar]
- 24.Ethell I.M., Ethell D.W. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J. Neurosci. Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- 25.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 26.Gao C., Chen G., Romero G., Moschos S., Xu X., Hu J. Induction of Gsk3beta-beta-TrCP interaction is required for late phase stabilization of beta-catenin in canonical Wnt signaling. J. Biol. Chem. 2014;289:7099–7108. doi: 10.1074/jbc.M113.532606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gareri J., Brien J., Reynolds J., Koren G. Potential role of the placenta in fetal alcohol spectrum disorder. Paediatr. Drugs. 2009;11:26–29. doi: 10.2165/0148581-200911010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Gordon L., Joo J.H., Andronikos R., Ollikainen M., Wallace E.M., Umstad M.P., Permezel M., Oshlack A., Morley R., Carlin J.B., Saffery R., Smyth G.K., Craig J.M. Expression discordance of monozygotic twins at birth: effect of intrauterine environment and a possible mechanism for fetal programming. Epigenetics. 2011;6:579–592. doi: 10.4161/epi.6.5.15072. [DOI] [PubMed] [Google Scholar]
- 29.Greer Y.E., Fields A.P., Brown A.M., Rubin J.S. Atypical protein kinase Ciota is required for Wnt3a-dependent neurite outgrowth and binds to phosphorylated dishevelled 2. J. Biol. Chem. 2013;288:9438–9446. doi: 10.1074/jbc.M112.448282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiyama A., Yokoyama K., Nukaga T., Sakai D., Mochida J. Response to tumor necrosis factor-alpha mediated inflammation involving activation of prostaglandin E2 and Wnt signaling in nucleus pulposus cells. J. Orthop. Res. 2015;33:1756–1768. doi: 10.1002/jor.22959. [DOI] [PubMed] [Google Scholar]
- 31.Jones V.C., Birrell M.A., Maher S.A., Griffiths M., Grace M., O'Donnell V.B., Clark S.R., Belvisi M.G. Role of EP2 and EP4 receptors in airway microvascular leak induced by prostaglandin E2. Br. J. Pharmacol. 2016;173:992–1004. doi: 10.1111/bph.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalkman H.O. A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Mol. Autism. 2012;3:10. doi: 10.1186/2040-2392-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimelman D., Xu W. Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 34.Koch H., Huh S.E., Elsen F.P., Carroll M.S., Hodge R.D., Bedogni F., Turner M.S., Hevner R.F., Ramirez J.M. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J. Neurosci. 2010;30:11678–11687. doi: 10.1523/JNEUROSCI.4665-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konopka A., Grajkowska W., Ziemianska K., Roszkowski M., Daszkiewicz P., Rysz A., Marchel A., Koperski L., Wilczynski G.M., Dzwonek J. Matrix metalloproteinase-9 (MMP-9) in human intractable epilepsy caused by focal cortical dysplasia. Epilepsy Res. 2013;104:45–58. doi: 10.1016/j.eplepsyres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc J.L., Lampert A.S., Diller M.A., Dore S. Genetic deletion of the prostaglandin E2 E prostanoid receptor subtype 3 improves anatomical and functional outcomes after intracerebral hemorrhage. Eur. J. Neurosci. 2015;41:1381–1391. doi: 10.1111/ejn.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin P.I., Chien Y.L., Wu Y.Y., Chen C.H., Gau S.S., Huang Y.S., Liu S.K., Tsai W.C., Chiu Y.N. The WNT2 gene polymorphism associated with speech delay inherent to autism. Res. Dev. Disab. 2012;33:1533–1540. doi: 10.1016/j.ridd.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Liu C., Li Y., Semenov M., Han C., Baeg G.H., Tan Y., Zhang Z., Lin X., He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 39.Marui T., Funatogawa I., Koishi S., Yamamoto K., Matsumoto H., Hashimoto O., Jinde S., Nishida H., Sugiyama T., Kasai K., Watanabe K., Kano Y., Kato N. Association between autism and variants in the wingless-type MMTV integration site family member 2 ( WNT2) gene. Int. J. Neuropsychopharmacol. 2010;13:443–449. doi: 10.1017/S1461145709990903. [DOI] [PubMed] [Google Scholar]
- 40.Mattes B., Weber S., Peres J., Chen Q., Davidson G., Houart C., Scholpp S. Wnt3 and Wnt3a are required for induction of the mid-diencephalic organizer in the caudal forebrain. Neural Dev. 2012;7:12. doi: 10.1186/1749-8104-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirowska-Guzel D., Gromadzka G., Czlonkowski A., Czlonkowska A. Association of MMP1, MMP3, MMP9, and MMP12 polymorphisms with risk and clinical course of multiple sclerosis in a Polish population. J. Neuroimmunol. 2009;214:113–117. doi: 10.1016/j.jneuroim.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell S.R., Reiss A.L., Tatusko D.H., Ikuta I., Kazmerski D.B., Botti J.A., Burnette C.P., Kates W.R. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. Am. J. Psychiatry. 2009;166:917–925. doi: 10.1176/appi.ajp.2009.08101538. [DOI] [PubMed] [Google Scholar]
- 43.North T.E., Babu I.R., Vedder L.M., Lord A.M., Wishnok J.S., Tannenbaum S.R., Zon L.I., Goessling W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA. 2010;107:17315–17320. doi: 10.1073/pnas.1008209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno H., Morikawa Y., Hirata F. Studies on 15-hydroxyprostaglandin dehydrogenase with various prostaglandin analogues. J. Biochem. 1978;84:1485–1494. doi: 10.1093/oxfordjournals.jbchem.a132272. [DOI] [PubMed] [Google Scholar]
- 45.Pastuszak A.L., Schuler L., Speck-Martins C.E., Coelho K.E., Cordello S.M., Vargas F., Brunoni D., Schwarz I.V., Larrandaburu M., Safattle H., Meloni V.F., Koren G. Use of misoprostol during pregnancy and Mobius' syndrome in infants. N. Engl. J. Med. 1998;338:1881–1885. doi: 10.1056/NEJM199806253382604. [DOI] [PubMed] [Google Scholar]
- 46.Patelia E.M., Thakur R., Patel J. Sex determination using polymerase chain reaction. Sci. Rep. 2013;2:1–4. [Google Scholar]
- 47.Rai-Bhogal R., Ahmad E., Li H., Crawford D.A. Microarray analysis of gene expression in the cyclooxygenase knockout mice – a connection to autism spectrum disorder. Eur. J. Neurosci. 2018;47:750–766. doi: 10.1111/ejn.13781. [DOI] [PubMed] [Google Scholar]
- 48.Rawal N., Castelo-Branco G., Sousa K.M., Kele J., Kobayashi K., Okano H., Arenas E. Dynamic temporal and cell type-specific expression of Wnt signaling components in the developing midbrain. Exp. Cell Res. 2006;312:1626–1636. doi: 10.1016/j.yexcr.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 49.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodier P.M. Chronology of neuron development: animal studies and their clinical implications. Dev. Med. Child Neurol. 1980;22:525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 51.Ronald A., Hoekstra R. Progress in understanding the causes of autism spectrum disorders and autistic traits: twin studies from 1977 to the present day. In: Rhee S.H., Ronald A., editors. Vol. 2. Springer; New York: 2014. pp. 33–65. (Behavior Genetics of Psychopathology). [Google Scholar]
- 52.Saint-Dizier M., Guyader-Joly C., Charpigny G., Grimard B., Humblot P., Ponter A.A. Expression of enzymes involved in the synthesis of prostaglandin E2 in bovine in vitro-produced embryos. Zygote (Cambridge, England) 2011;19:277–283. doi: 10.1017/S0967199410000596. [DOI] [PubMed] [Google Scholar]
- 53.Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sengupta P. The laboratory rat: relating its age with human's. Int. J. Prevent. Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 55.Sidhu H., Dansie L.E., Hickmott P.W., Ethell D.W., Ethell I.M. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile x syndrome in a mouse model. J. Neurosci. 2014;34:9867–9879. doi: 10.1523/JNEUROSCI.1162-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa K.M., Villaescusa J.C., Cajanek L., Ondr J.K., Castelo-Branco G., Hofstra W., Bryja V., Palmberg C., Bergman T., Wainwright B., Lang R.A., Arenas E. Wnt2 regulates progenitor proliferation in the developing ventral midbrain. J. Biol. Chem. 2010;285:7246–7253. doi: 10.1074/jbc.M109.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sreeramkumar V., Hons M., Punzon C., Stein J.V., Sancho D., Fresno M., Cuesta N. Efficient T-cell priming and activation requires signaling through prostaglandin E2 (EP) receptors. Immunol. Cell Biol. 2016;94:39–51. doi: 10.1038/icb.2015.62. [DOI] [PubMed] [Google Scholar]
- 58.Steffenrud S. Metabolism of 16, 16-dimethyl-prostaglandin E2 in the human female. Biochem. Med. 1980;24:274–292. doi: 10.1016/0006-2944(80)90022-8. [DOI] [PubMed] [Google Scholar]
- 59.Szklarczyk A., Oyler G., McKay R., Gerfen C., Conant K. Cleavage of neuronal synaptosomal-associated protein of 25 kDa by exogenous matrix metalloproteinase-7. J. Neurochem. 2007;102:1256–1263. doi: 10.1111/j.1471-4159.2007.04625.x. [DOI] [PubMed] [Google Scholar]
- 60.Tamiji J., Crawford D.A. Misoprostol elevates intracellular calcium in Neuro-2a cells via protein kinase A. Biochem. Biophys. Res. Commun. 2010;399:565–570. doi: 10.1016/j.bbrc.2010.07.112. [DOI] [PubMed] [Google Scholar]
- 61.Tamiji J., Crawford D.A. The neurobiology of lipid metabolism in autism spectrum disorders. Neuro-Signals. 2010;18:98–112. doi: 10.1159/000323189. [DOI] [PubMed] [Google Scholar]
- 62.Tamiji J., Crawford D.A. Prostaglandin E(2) and misoprostol induce neurite retraction in Neuro-2a cells. Biocem. Biophys. Res. Commun. 2010;398:450–456. doi: 10.1016/j.bbrc.2010.06.098. [DOI] [PubMed] [Google Scholar]
- 63.Taurin S., Sandbo N., Qin Y., Browning D., Dulin N.O. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biolog. Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 64.Taurin S., Sandbo N., Yau D.M., Sethakorn N., Dulin N.O. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008;294:C1169–C1174. doi: 10.1152/ajpcell.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tessner T.G., Muhale F., Riehl T.E., Anant S., Stenson W.F. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J. Clin. Investig. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Gen. Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verheyen E.M., Gottardi C.J. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev. Dyn. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verhoeven J.S., De Cock P., Lagae L., Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;52:3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- 69.von Bredow D.C., Cress A.E., Howard E.W., Bowden G.T., Nagle R.B. Activation of gelatinase-tissue-inhibitors-of-metalloproteinase complexes by matrilysin. Biochem. J. 1998;331(Pt 3):965–972. doi: 10.1042/bj3310965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F., Reierstad S., Fishman D.A. Matrilysin over-expression in MCF-7 cells enhances cellular invasiveness and pro-gelatinase activation. Cancer Lett. 2006;236:292–301. doi: 10.1016/j.canlet.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 71.Wang S.S., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wassink T.H., Piven J., Vieland V.J., Huang J., Swiderski R.E., Pietila J., Braun T., Beck G., Folstein S.E., Haines J.L., Sheffield V.C. Evidence supporting WNT2 as an autism susceptibility gene. Am. J. Med. Genet. 2001;105:406–413. doi: 10.1002/ajmg.1401. [DOI] [PubMed] [Google Scholar]
- 73.Weingarten L.S., Dave H., Li H., Crawford D.A. Developmental expression of P5 ATPase mRNA in the mouse. Cell. Mol. Biol. Lett. 2012;17:153–170. doi: 10.2478/s11658-011-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong C.T., Ahmad E., Li H., Crawford D.A. Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders. Cell Commun. Signal.: CCS. 2014;12:19. doi: 10.1186/1478-811X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong C.T., Ussyshkin N., Ahmad E., Rai-Bhogal R., Li H., Crawford D.A. Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression. J. Neurosci. Res. 2016;94:759–775. doi: 10.1002/jnr.23759. [DOI] [PubMed] [Google Scholar]
- 76.Wong C.T., Wais J., Crawford D.A. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur. J. Neurosci. 2015;42:2742–2760. doi: 10.1111/ejn.13028. [DOI] [PubMed] [Google Scholar]
- 77.Wright C.L., McCarthy M.M. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J. Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu G., He X. Threonine 41 in beta-catenin serves as a key phosphorylation relay residue in beta-catenin degradation. Biochemistry. 2006;45:5319–5323. doi: 10.1021/bi0601149. [DOI] [PubMed] [Google Scholar]
- 79.Yang X.T., Bi Y.Y., Chen E.T., Feng D.F. Overexpression of Wnt3a facilitates the proliferation and neural differentiation of neural stem cells in vitro and after transplantation into an injured rat retina. J. Neurosci. Res. 2014;92:148–161. doi: 10.1002/jnr.23314. [DOI] [PubMed] [Google Scholar]
- 80.Yin Z.S., Zhang H., Wang W., Hua X.Y., Hu Y., Zhang S.Q., Li G.W. Wnt-3a protein promote neuronal differentiation of neural stem cells derived from adult mouse spinal cord. Neurol. Res. 2007;29:847–854. doi: 10.1179/016164107X223539. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida G.J., Saya H., Zouboulis C.C. Three-dimensional culture of sebaceous gland cells revealing the role of prostaglandin E2-induced activation of canonical Wnt signaling. Biochem. Biophys. Res. Commun. 2013;438:640–646. doi: 10.1016/j.bbrc.2013.07.129. [DOI] [PubMed] [Google Scholar]
- 82.Yu S., Hou Q., Sun H., Liu J., Li J. Upregulation of C-C chemokine receptor type 7 expression by membrane-associated prostaglandin E synthase-1/prostaglandin E2 requires glycogen synthase kinase 3beta-mediated signal transduction in colon cancer cells. Mol. Med. Rep. 2015;12:7169–7175. doi: 10.3892/mmr.2015.4290. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Yuan X., Wang Z., Li R. The canonical Wnt signaling pathway in autism. CNS Neurol. Disord. Drug Targets. 2014;13:765–770. doi: 10.2174/1871527312666131223114149. [DOI] [PubMed] [Google Scholar]
- 84.Zhao J., Yue W., Zhu M.J., Sreejayan N., Du M. AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of beta-catenin at Ser 552. Biochem. Biophys. Res. Commun. 2010;395:146–151. doi: 10.1016/j.bbrc.2010.03.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material