Abstract
Developmental and epileptic encephalopathies (DEEs) represent a large clinical and genetic heterogeneous group of neurodevelopmental diseases. The identification of pathogenic genetic variants in DEEs remains crucial for deciphering this complex group and for accurately caring for affected individuals (clinical diagnosis, genetic counseling, impacting medical, precision therapy, clinical trials, etc.). Whole-exome sequencing and intensive data sharing identified a recurrent de novo PACS2 heterozygous missense variant in 14 unrelated individuals. Their phenotype was characterized by epilepsy, global developmental delay with or without autism, common cerebellar dysgenesis, and facial dysmorphism. Mixed focal and generalized epilepsy occurred in the neonatal period, controlled with difficulty in the first year, but many improved in early childhood. PACS2 is an important PACS1 paralog and encodes a multifunctional sorting protein involved in nuclear gene expression and pathway traffic regulation. Both proteins harbor cargo(furin)-binding regions (FBRs) that bind cargo proteins, sorting adaptors, and cellular kinase. Compared to the defined PACS1 recurrent variant series, individuals with PACS2 variant have more consistently neonatal/early-infantile-onset epilepsy that can be challenging to control. Cerebellar abnormalities may be similar but PACS2 individuals exhibit a pattern of clear dysgenesis ranging from mild to severe. Functional studies demonstrated that the PACS2 recurrent variant reduces the ability of the predicted autoregulatory domain to modulate the interaction between the PACS2 FBR and client proteins, which may disturb cellular function. These findings support the causality of this recurrent de novo PACS2 heterozygous missense in DEEs with facial dysmorphim and cerebellar dysgenesis.
Keywords: intellectual disability, PACS2, PACS-2, epilepsy, cerebellar dysgenesis
Main Text
Epilepsy is a common neurologic disorder of childhood, affecting approximately 7 in 10,000 children before 2 years of age and often associated with developmental delay/intellectual disability (ID).1 The developmental and epileptic encephalopathies (DEEs) are a group of severe infantile- and childhood-onset epilepsies characterized by developmental slowing or regression in the context of recurrent seizures and frequent interictal epileptiform discharges, often on a background of developmental delay.2 The DEEs have a wide range of etiologies, including both acquired and genetic causes. These include specific congenital or acquired structural brain lesions, metabolic disorders, chromosomal anomalies, copy-number variants (CNV), or single gene defects.3, 4, 5 Variants in hundreds of genes have been associated with epilepsy to date, with a variety of inheritance patterns or arising de novo.6 Although genes encoding ion channels correspond to one third of the epilepsy-associated genes, others can affect diverse molecular pathways involved in membrane excitability, synaptic plasticity, presynaptic neurotransmitter release, postsynaptic receptors, transporters, cell metabolism, and many processes important in early brain development.7, 8
The identification of pathogenic genetic variants related to the epileptic disorders, including the DEEs, remains crucial, providing more precise definition of the clinical diagnosis, allowing accurate genetic counseling, impacting medical management including precision therapy in some cases, linking families to appropriate support/family groups, and opening options for gene-specific clinical trials.9 Currently available next-generation sequencing strategies exhibit a diagnostic yield of 20%–50% in epilepsy broadly.10, 11, 12, 13, 14, 15 The yield is higher (∼60%–83%) in individuals with onset <2 months of age and in certain groups such as Ohtahara syndrome.16, 17 Whole-exome sequencing (WES) studies demonstrated the importance of de novo single-nucleotide variations (SNVs) in DEEs.18, 19, 20 WES has also become a powerful approach for identifying new genes that underlie Mendelian disorders when previous approaches, including chromosomal microarray analysis and epilepsy gene panel testing, have failed.21, 22, 23, 24, 25, 26, 27, 28, 29
Previously, WES identified a de novo missense variant, GenBank: NM_018026.2 (c.607C>T), in PACS1 (MIM: 607492) in two unrelated individuals with unexplained ID and strikingly similar facial dysmorphisms.30 This variant is highly recurrent and is now reported in 19 unrelated individuals with ID.30, 31, 32 PACS1 encodes a trans-Golgi-membrane traffic regulator that directs protein cargo and several viral-envelope proteins, with high expression during human embryonic brain development and downregulation after birth.33, 34, 35, 36 The p.Arg203Trp substitution triggers cytoplasmic aggregates from altered PACS1, leads to protein-trafficking defects, and most likely abrogates the ability of the protein to perform its normal function. This was the first report of variants in a phosphofurin acid cluster sorting protein leading to human disease. Mutant pacs1 zebrafish embryos showed craniofacial defects driven by aberrant specification and migration of cranial neural-crest cells, most likely due to a dominant-negative effect.30
We identified a recurrent de novo missense variant in PACS2, in individuals with neonatal/early-infantile-onset DEEs, with or without extra-neurological features. Using trio WES, we first identified (Supplemental Data) a heterozygous missense variant (chr14:g.105834449G>A; GenBank: NM_001100913.2; c.625G>A) (ClinVar SUB3731210) in PACS2 (MIM: 610423), predicted to result in a glutamate-to-lysine substitution (p.Glu209Lys) (Figure 1, Table 1; individuals 1 and 2) in two unrelated individuals with DEE and facial dysmorphism. WES had been performed according to standard procedures using the Agilent CRE Capture kit on an Illumina HiSeq 2000. Raw data had been processed as previously describe.37 Sanger sequencing (polymerase chain reaction, PCR) in both individuals and their parents confirmed the presence of the variant in the individuals and absence in the parents, consistent with de novo occurrence. This variant, absent from the gnomAD and EVS databases (see Web Resources), involves a highly conserved amino acid located in an acid hydrophobic domain of the PACS2 protein that leads to polarity and protein conformation changes and is predicted to be damaging by PolyPhen-2 and SIFT (see Web Resources). By data sharing through GeneMatcher,38 GeneDx (see Web Resources), and French AnDDI-Rares network (see Web Resources), we ascertained 12 additional individuals harboring the same de novo heterozygous missense variant, GenBank: NM_018026.2; c.607C>T (p.Glu209Lys) (Figure 1). Three of the individuals had been detected through a targeted method to identify genes with significant clustering patterns of de novo variants in a dataset of 4,061 de novo missense mutations from published trio WES studies of 5,302 individuals with ID and developmental anomalies.29 All individuals’ variants were identified by research or clinical diagnostic WES, initially as candidate gene variants, and there were not alternative genetic or non-genetic diagnoses.
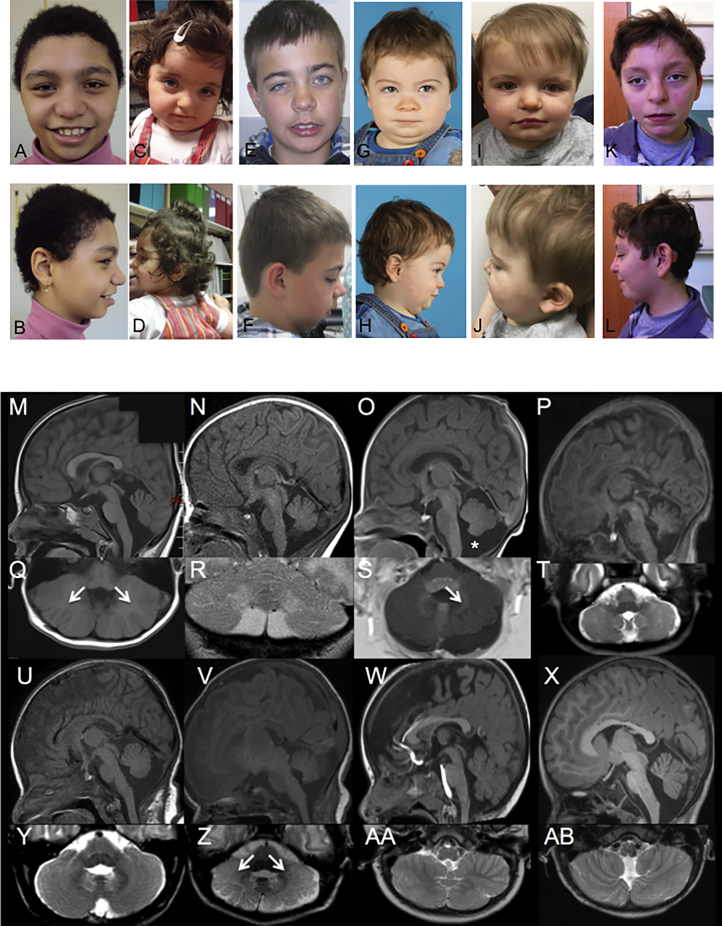
Figure 1.
Clinical and Imaging Features
(A) Pictures of individuals 1 (a, b), 2 (c, d), 3 (e, f), 5 (g, h), 7 (i, j), and 11 (k, l): variable facial dysmorphism.
(B) Spectrum of posterior fossa abnormalities in the PACS2 cohort. Sagittal T1 weighted (m–p, u–x), axial T2 weighted (t, y–ab), axial T1 weighted (q, s), and coronal T2 weighted (r) imaging for subject 2 at 5 years of age (m, q), subject 4 at 3 weeks of age (n, r), subject 5 at 7 days of age (o, s), subject 9 at 1 week of age (p, t), subject 10 at 1 month of age (u, y), subject 12 at 3 months of age (v, z), subject 13 at 23 months of age (w, aa), and subject 14 at 2.5 years of age (x, ab). Of 8 subjects with centrally reviewed imaging, there was prominence of the cisterna magna (asterisk in o) in all but subject 13 (w) and widening of the foramen Magendie in all subjects except subject 12 (v). Mild inferior vermian hypoplasia was also evident in subjects 2 (m), 4 (n), 5 (o), and 14 (x). Cerebellar hemisphere dysplasia was present in subjects 2 (q), 4 (r), 5 (s), 12 (z), and 13 (aa) manifest as unusual centrifugal orientation of the folia bilaterally in subjects 2 (q, arrows) and 12 (z, arrows) and on the left side only in subject 5 (s, arrow). Distortion of the foliar pattern was present without centrifugal orientation in subjects 4 (r) and 13 (aa). Subtler foliar distortion was visible in subjects 9 (t) and 14 (ab).
Table 1.
Detailed Extra-neurological Phenotype of Individuals with PACS2 p.Glu209Lys
| Individual 1 | Individual 2 | Individual 3 | Individual 4 | Individual 5 | Individual 6 | Individual 7 | Individual 8 | Individual 9 | Individual 10 | Individual 11 | Individual 12 | Individual 13 | Individual 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | F | F | M | F | M | M | M | F | F | M | M | F | F | F |
| Gestational age (WG) | 38 | 37 | 38 | 35 | 37 | 40 | at term | 37 | at term | at term | 39.5 | 34 | 33 | 39 |
| Birth Parameters | ||||||||||||||
| Weight | −0.3 SD | median | +2 SD | +1.8 SD | −1.7 SD | +2.5 SD | +1 SD | −3 SD | median | NA | median | +0.5 SD | −0.5 SD | +0.6 SD |
| Length | +0.6 SD | NA | NA | NA | −0.9 SD | +2 SD | +1 SD | −1.5 SD | −2 SD | NA | −1 SD | −1.5 SD | median | +0.9 SD |
| OFC | +0.4 SD | NA | NA | NA | −2.3 SD | >3 SD | median | −2.3 SD | median | NA | median | NA | median | −0.1 SD |
| Growth Parameters | ||||||||||||||
| Age at last follow-up | 16 year | 4 year | 15 year | 8 year | 19 mo | 8 year | 16 mo | 5 year | 3 year | 7 year | 12.5 year | 9 mo | 3.5 year | 5.5 year |
| Weight | +1 SD | median | +1.6 SD | +1.5 SD | −1.0 SD | +0.5 SD | +1.5 SD | −1 SD | median | +1.3 SD | +2.5 SD | +1 SD | median | −2 SD |
| Height | +2 SD | median | +0.7 SD | −0.8 SD | −1.8 SD | +2 SD | −1 SD | −0.5 SD | −0.7 SD | +1.8 SD | −1 SD | - | median | −0.4 SD |
| OFC | median | +1 SD | −0.7 SD | +0.4 SD | −1.9 SD | +2 SD | −1 SD | −1 SD | −1.0 SD | +0.4 SD | median | −1.5 SD | +1 SD | −1.4 SD |
| Facial Dysmorphism | ||||||||||||||
| Synophris | + | + | + | + | − | − | − | − | + | “mildly dysmorphic”∗ | − | − | − | − |
| Hypertelorism | + | + | + | + | − | − | − | + | − | − | + | + | − | |
| Down-slanting palpebral fissures | + | + | + | + | − | + | + | + | − | − | − | + | − | |
| Broad nasal root | − | + | − | + | + | − | + | + | + | + | + | + | + | |
| Thin vermillon of upper lip | + | + | + | + | + | + | − | + | + | + | + | + | + | |
| Wide mouth with downturned corners | + | + | + | + | +/− | − | − | + | − | + | + | + | − | |
| Prominent incisors | + | − | + | + | NA | − | NA | − | − | − | NA | NA | − | |
| Widely spaced teeth | + | − | + | − | NA | − | NA | − | − | + | NA | NA | − | |
| Everted vermillon of lower lip | + | − | + | − | − | + | − | − | − | − | − | + | + | |
| Distal limb anomalies | slender fingers | − | 2/3 syndactyly of toes | − | V finger clinodactyly, variant transverse palmar crease | finger pads | − | − | − | bilateral palmar crease | broad and tapering short fingers, V finger brachyclino-dactyly | V finger clinodactyly | - | right transverse palmar crease, V finger clinodactyly |
| Hematological Anomalies | ||||||||||||||
| Neutropenia | moderate | − | − | − | − | NA | − | − | − | − | − | − | − | − |
| Anemia | − | + | − | + | + | NA | mild | − | − | − | − | − | − | − |
| Eye/hearing features | moderate myopia | − | − | strabismus | strabismus | strabismus astigmatism, myopia, anisocoria | − | hypermetropic astigmatism | myopia, astigmatism, cortical visual impairment | − | hypermetropic astigmatism, frequent otitis | − | − | − |
| Additional clinical features | metatarsus varus | dextrocardia | cryptorchidism | none | none | small ventricular septal defect, testis ectopia | cryptorchidism, 1 cm café au lait birthmark, mild conductive hearing loss, poor feeding, frequent ear and respiratory infections | none | dysphasia, accessory caudally placed nipples | none | micropenis, unilateral cryptorchidism, infantile hypertrophic pyloric stenosis, velopharyngeal hypotonia, central precocious puberty | brachycephaly, inverted nipples | atrial septal defect, sacral pit | features consistent with Chinese heritage |
Abbreviations: F, female; M, male; m, median; mo, months; NA, not available; SD, standard deviation; year, years; ∗ no details available.
This recurrence strongly supported the implication of the PACS2 c.607C>T (p.Glu209Lys) missense variant in human disease, supported further by its absence in gnomAD and EVS. However, because WES frequently identifies de novo variants in individuals with ID and epilepsy and more than 60% of the variants are missense, interpretation represents a great challenge. Many factors are considered when evaluating the significance of candidate genes, including recurrence, strikingly similar phenotypic outcomes in recurrent variants, previous evidence of overlap with pathogenic copy-number variation, localization of the variant in the protein, variant burden among healthy individuals, and membership of the candidate gene in disease-implicated protein networks.39
Detailed retrospective phenotyping of the 14 individuals with the recurrent PACS2 p.Glu209Lys variant demonstrated a consistent phenotype characterized by neonatal- to early-infantile-onset epilepsy, global developmental delay with variable autistic features, facial dysmorphism, and cerebellar dysgenesis (Tables 1 and 2, Figure 1; see Supplemental Note). All individuals presented with early epilepsy, the majority with onset in the first 2 weeks of life (13/14 case subjects), with one individual presenting in the 2nd month. The predominant seizure types were focal motor, and some had accompanying autonomic, tonic, and generalized tonic-clonic seizures (GTCs). One individual had myoclonic seizures. Neonatal seizures captured on EEG had either clear focal onset (often multifocal) or at times diffuse attenuation with later focal features. Most often neonatal seizures were focal and over time GTCs and tonic seizures were seen, including at times episodes of status epilepticus. Data from older individuals suggest that the epilepsy may resolve in early childhood for at least a subset. Early EEGs often showed excess sharp waves with or without mild excess discontinuity but more severe encephalopathy patterns such as burst suppression or hypsarrhythmia were not seen in this cohort. Over time, focal, multifocal, or generalized epileptiform activity were reported, and electrical status epilepticus of sleep was seen in a single individual in correlation with developmental regression. While late follow-up EEG data are limited, it has shown (when available) generalized or focal slowing in some and normalization of the background in others, with or without epileptiform activity. EEG and evolution for this cohort of individuals are summarized in Table 2. Overall, the epilepsy appears to start as focal neonatal and evolve to mixed focal and generalized epilepsy with status epilepticus in many individuals. Epilepsy appears most difficult to control in infancy with improvement after the first year of life. While larger cohorts and longer follow-up are needed for further epilepsy phenotypic characterization, there are clear patterns seen even in this initial cohort of 14 individuals. Variable degrees of global developmental delay were observed in all case subjects and behavioral disturbances in a subset (8/14 case subjects). Neurological examination evidenced hypotonia (7/11 case subjects), hand stereotypies (6/14 case subjects), nystagmus (3/14 case subjects), increased reflexes or pyramidal syndrome (2/14 case subjects), and wide-based gait (2/14 case subjects). Aside from wide-based gait, no definitive cerebellar features were noted, but the individuals were not systematically screened prospectively and many individuals have severe motor impairment. Facial dysmorphisms were variable including coarse features, hypertelorism, broad nasal root, and thin superior lip (Figure 1). Additional features included variable minor distal limb features (8/14 case subjects) and hematological disturbances (5/13 case subjects) (Table 1). Brain MRI demonstrated dysgenesis of the cerebellar folia in at least 9/14 case subjects including 7/8 case subjects available for review of original data by a board-certified pediatric neuroradiologist (E.Y.) (Figure 1B, Table 2). It was at times subtle and not mentioned on the clinical reports. Mega cisterna magna and inferior vermian hypoplasia were additionally seen in 8/14 and 6/14 case subjects, respectively (Figure 1B, Table 2). Three individuals had a hypothalamic fusion anomaly (Figure 1B, Table 2).
Table 2.
Detailed Neurological Features of Individuals with PACS2 p.Glu209Lys
| Individual 1 | Individual 2 | Individual 3 | Individual 4 | Individual 5 | Individual 6 | Individual 7 | Individual 8 | Individual 9 | Individual 10 | Individual 11 | Individual 12 | Individual 13 | Individual 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developmental Features | ||||||||||||||
| Sitting age | 8 mo | 20 mo | 6 mo | NA | 16 mo | 16 mo | NA | 10 mo | 18 mo | 12 mo | 9 mo | 7 mo | 12 mo | 11 mo |
| Walking age | 22 mo | NA | 18 mo | 18 mo | 18 mo | 22 mo | not walking | 27 mo | not walking | 24 mo | 24 mo | NA | 36 mo | 36 mo |
| Speech delay | + | + | + | + | + | + | + | + | + | + | + | NA | few single words | + |
| DD/ID | + | + | + | + | + | + | mild | + | + | + | + | + | mild | mild |
| Neurological Features | ||||||||||||||
| Hypotonia | + | NA | NA | NA | − | − | axial | − | diffuse | diffuse | − | + | axial | diffuse |
| Nystagmus | − | + | − | − | + | − | − | − | − | − | + (transient) | − | − | − |
| Stereotypies | − | + (transient) | − | − | − | + | − | − | + | + | − | + (transient) | + | − |
| Others | increased tendon reflexes | wide-based gait | − | − | visual problems | − | slightly increased tone in hands | − | − | − | − | − | increased tendon reflexes | wide-based gait |
| Psychiatric/behavioral features | − | sleeping and behavioral disturbances | − | mild autistic disorder | − | obsessive compulsive disorder | − | atypical social and behavioral features | atypical social and behavioral features | autism spectrum disorder | autism spectrum disorder | − | − | selective mutism |
| Epilepsy Details | ||||||||||||||
| Age of onset | 6 days | 4 days | 4 days | 7 days | 2 days | 2 days | 2 days | 2 weeks | 2 days | 1–2 mo | 1 day | 3 days | 2 weeks | 3 days |
| Seizure types | focal | GTCs | NA | GTCs | clonic and GTC | NA | focal with tonic stiffening and autonomic features, later clonic | focal, later tonic | focal tonic,tonic-clonic, and myoclonic, later GTCs and generalized tonic | clonic seizure with eye deviation, later GTC | focal (stopped at 2 months), later GTCs | focal tonic-clonic and tonic; status epilepticus | focal tonic-clonic, later focal or generalized | tonic, later tonic or GTCs |
| Longest seizure-free interval or age at last seizure | NA | 6 mo | NA | 2 years, immediate recurrence after withdrawal of valproate | 9 mo | status epilepticus 3 mo, 3.5 years; status epilepticus without fever: 3.5 year: | NA | 2 years, off AEDs since 3.5 year | 2 years | 2 years | NA | NA | NA | NA |
| EEG features | NA | neonatal to 3.5 mo: focal spikes, normal background; 1 year: normal | NA | neonatal: excess discontinuity, excessive multifocal sharp waves → generalized bursts of epileptic activity + MF sharp waves | neonatal: normal; 4 months: generalized slowing with MF sharp waves and frequent focal seizures | neonatal: left temporal spikes; 3.5 year (awake only): left paroxysmal temporal rolandic spikes, generalized slowing. | 6–7 wk: MF epileptiform activity; 9 mo: normal | 6 wk: subtle aberration R frontocentral and L temporal | neonatal: excess discontinuity, excess MF sharp waves; 2 year: intermittent generalized slowing, intermittent L temporal slowing | 4 mo: normal; 17 mo: rare generalized spikes | neonatal: epileptic discharges, Lrolandic region | neonatal: excess MF spikes and sharp waves, especially bilateral temporal regions; 2 mo: background poorly organized high amplitude background, lack of state change, MF spikes | 6 d: normal; neonatal/infantile: MF epileptiform activity, high amplitude slow spikes bilateral temporal; 17 mo: diphasic spikes at vertex, field to right frontocentral region, enhanced in sleep 3 year: ESES |
neonatal: excessive L and R central and temporal sharp waves; 10 mo: frequent L frontocentral region spikes; 22 mo: diffuse, frontally predominant 2-3 Hz spike or polyspike and wave, up to 500uV. 3 year: mild generalized slowing, frequent Left temporal epileptiform discharges |
| Brain MRI (age) | mild foliar distortion of the left cerebellar hemisphere, mega cisterna magna∗ (5 yr) | inferior vermian hypoplasia with prominent foramen Magendie and cisterna magna, severe foliar distortion of cerebellar hemispheres with centrifugal orientation, hypothalamic fusion anomaly (5 yr) | increased subarachnoid spaces∗ (NA) | mild inferior vermian hypoplasia with a patulous foramen Magendie and mega cisterna magna, mild distortion of the cerebellar folia (3 wk) | retrocerebellar arachnoidal cyst, inferior vermian hypoplasia with prominent foramen Magendie and a mega cisterna magna, severe foliar distortion of the left cerebellar hemisphere with centrifugal orientation, hypothalamic fusion anomaly (7 d) | normal∗ (10 d); normal∗ (4 mo) | inferior vermian hypoplasia, left retrocerebellar cyst, causing distortion of the smaller left cerebellar hemisphere and thinning of the overlying bones∗ (2 mo) | normal∗ (neonatal) | mega cisterna magna and patulous foramen Magendie, subtle cerebellar foliar distortion, hypothalamic fusion anomaly (1 wk) | mega cisterna magna, patulous foramen Magendie (1 mo) | thick corpus callosum, inferior vermian hypoplasia∗ (12.5 yr) | mega cisterna magna, severe foliar distortion with centrifugal orientation (3 mo) | moderate cerebellar foliar distortion (23 mo) | mild scattered subarachnoid hemorrhage structurally normal (2 mo); prominent cisterna magna and patulous foramen magendie with subtle foilar distortion (left side predominant) (31 mo) |
| Treatment | carbamazepine | phenobarbital, valproate | carbamazepine | phenobarbital, P5P, pyridoxine, valproate | levetiracetam, phenobarbital carbamazepine | topiramate | phenobarbital | phenobarbital sodium valproate | levetiracetam, phenobarbital | levetiracetam | valproate | levetiracetam, phenobarbital, oxcarbazepine | vigabatrin, levetiracetam, pyridoxalphosphate, pyridoxine, lamotrigine, valproate, clobazam | phenobarbital, pyridoxine, levetiracetam, lacosamide |
Abbreviations: d, day; DD/ID, developmental delay or intellectual disability; ESES, electrical status epilepticus of sleep; GTC, generalized tonic clonic seizure; L, left; m, months; MF, multifocal; mo, month; NA, not available; P5P, pyridoxal-5-phosphate; R, right; unsup., unsupported; ∗ brain MRI not reviewed by neurologist E.Y. from Boston Children’s hospital.
In the literature, 16 individuals have been described with microdeletion of chromosome 14q32.33, encompassing PACS2.40 The common phenotype of these individuals includes varying degrees of developmental delay or ID and facial dysmorphism, but less consistently epilepsy (4/12 case subjects) or febrile seizures with normal EEG (1/12 case subjects). Indeed, although seizures seem prevalent in individuals with a ring chromosome 14,41 their relationship with terminal chromosome 14q deletions remains unclear, especially since seizures in the 4-year-old boy with the smallest deletion (0.31 Mb) were possibly related to an independent family susceptibility, since his mother and maternal grandmother also had epilepsy.40 The relationship between PACS2 haploinsufficiency has been less clearly demonstrated and may be less penetrant than in this series of individuals with a recurrent missense variant in PACS2.
The present findings are consistent with prior reports of developmental delay/ID, facial dysmorphism, and variably epilepsy in individuals with a recurrent missense variant in the related gene PACS1. Indeed, 19 unrelated individuals affected with ID have been reported with a recurrent causal de novo variant (p.Arg203Trp) in PACS1.30, 31, 32 Despite the important PACS1/PACS2 homology, these hotspot variants do not occur at homologous positions but rather appear to affect specifically each protein function, resulting in disease (Figure S1). Moreover, individuals with PACS1 p.Arg203Trp or PACS2 p.Glu209Lys variants present with some clinical similarities such as constant ID, speech delay, dysmorphic facial appearance (arched eyebrows, hypertelorism with downslanting palpebral fissures, bulbous nasal tip, wide mouth with downturned corners, and thin upper lip), as well as frequent hypotonia, behavioral disturbances, cryptorchidism, and cerebellar abnormalities. Cerebellar dysgenesis is now well defined in both cohorts of individuals with PACS1 p.Arg203Trp and PACS2 p.Glu209Lys, making a strong argument to consider this feature as a genetically determined abnormality. While neonatal seizures appear a consistent feature in individuals with the recurrent PACS2 variant, febrile or afebrile seizures occurred in 12/19 individuals with the PACS1 recurrent variant, being well controlled with anti-epileptic drugs.30, 31, 32 Details of the epilepsy in this cohort are limited. The epilepsy appears to have earlier onset and be more often refractory in infancy for the individuals with the recurrent PACS2 variant. While cardiac malformations occur commonly in individuals with the recurrent PACS1 variant, only one individual with the recurrent PACS2 variant present with dextrocardia.31 These differences may reflect differences between the role of PACS1 and PACS2 proteins, or alternately may reflect differential impacts of the variants on protein function.
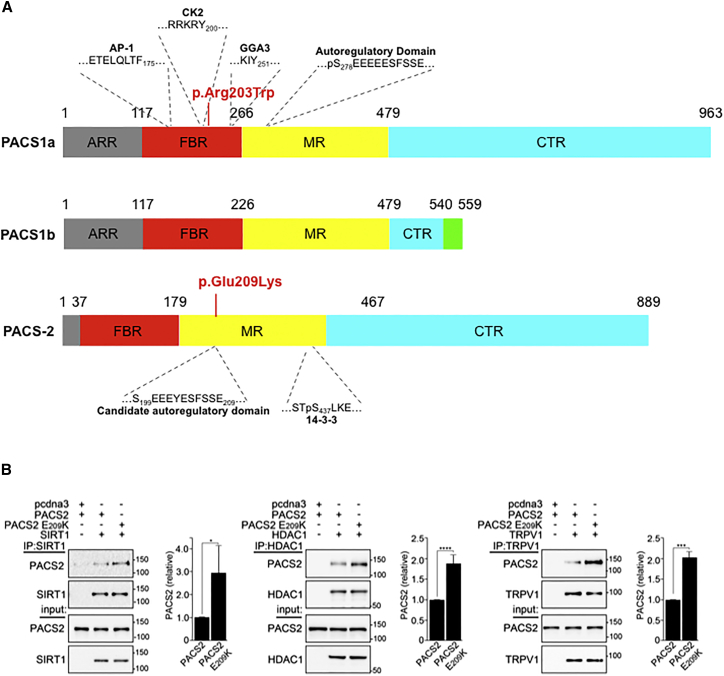
The PACS1 and PACS2 genes are broadly expressed, with selective enrichment in peripheral blood lymphocytes and spinal cord, respectively (GTEx data). Although they are both transcribed in brain tissue, PACS1 and PACS2 protein levels are differentially distributed at the cellular level, the former being enriched in neuronal centers while the latter is enriched in glial cells-enriched white matter. PACS2 encodes a multifunctional sorting protein involved in nuclear gene expression and pathway traffic regulation, whereas PACS1 is a trans-Golgi-membrane regulator that directs phosphorylated cargo molecules.36, 42 Both proteins harbor cargo(furin)-binding regions (FBRs) that bind cargo proteins, sorting adaptors, and cellular kinase.36 PACS1 and PACS2 appear highly localized during human prenatal brain development (see Allen Brain Atlas).33, 35 The canonical 963-amino acid PACS1 and 889-amino acid PACS2 proteins are important paralogs, sharing overall 54% sequence identity and nearly 80% sequence identity in the ∼150 aa cargo (furin) binding regions (FBRs) which binds client proteins at acidic clusters that can be phosphorylated by casein kinase 2 (CK2), as well as at α helices.43 The ID-associated PACS1 p.Arg203Trp mutation is located in the PACS1 FBR and, consequently, reduces the interaction of PACS1 with client proteins. However, the PACS2 p.Glu209Lys mutation reported here is located C-terminal to the FBR in the disordered middle region (MR) (Figure 2A). In PACS1, the MR contains an autoregulatory domain, which includes a CK2 phosphorylatable acidic cluster that reversibly binds the FBR to modulate access to client proteins.44 Because the PACS2 p.Glu209Lys mutation is located in the corresponding acidic cluster in the PACS2 MR, it suggests that mutation of this segment may also alter the ability of the nearby PACS2 FBR to interact with client proteins. We therefore compared the ability of HA-tagged PACS2 or PACS2 p.Glu209Lys to interact with Flag-tagged client proteins, including the histone deacetylases SIRT1 and HDAC1 as well as the ion channel TRPV1. Co-immunoprecipitation analysis showed that each of the client proteins interacted, to a greater extent with PACS2 p.Glu209Lys than with WT PACS2 (Figure 2B), suggesting that the PACS2 mutation reduces the ability of the predicted autoregulatory domain to modulate the interaction between the PACS2 FBR and client proteins, which may disturb cellular function.
Figure 2.
PACS2 Variant Location
(A) Schematic of PACS1 and PACS2 illustrating the proposed domains (ARR, atrophin-1-related region; FBR, furin (cargo)-binding region; MR, middle region; CTR, C-terminal region) and residues important for partner protein binding (AP-1, adaptor protein complex 1; CK2, protein kinase CK2; GGA, Golgi-associated γ-adaptin ear homology domain ARF-interacting protein), with location of PACS1 and PACS2 missense variants responsible for intellectual disability.
(B) HCT116 cells, which can be efficiently transfected with plasmids, expressing the indicated proteins were harvested in mRIPA (50 mM Tris-HCl [pH 8.0] plus 1% NP-40, 1% deoxycholate, and 150 mM NaCl) containing proteinase inhibitors (0.5 mM PMSF, 0.1 μM each of aprotinin, E-64, and leupeptin) and phosphatase inhibitors (1 mM Na3VO4 and 20 mM NaF). The FLAG-tagged cargo proteins SIRT1, HDAC1, or TRPV1 were immunoprecipitated with anti-Flag antibody (Sigma #F7425) and co-precipitating HA-tagged PACS2 or PACS2 p.Glu209Lys was detected by western blot using anti-HA antibody (Cell Signaling Technology #3724S) and developed with the Pierce ECL Western Blotting Substrate (ThermoFisher) using a FluorChem E image acquisition system (ProteinSimple). Signals were quantified using the AlphaView image analysis software package (ProteinSimple) and normalized to wild-type PACS2. Data are mean ± standard deviation, n = 4.
PACS2 has roles in both the nucleus and cytoplasm.43 In the nucleus, PACS2 controls the SIRT1-p53-21 axis to promote cell cycle arrest following DNA damage response by directly inhibiting SIRT1-dependent deacetylation of p53.45 In the cytoplasm, PACS2 regulates endoplasmic reticulum (ER) homeostasis, ER-mitochondria communication, autophagy, and endosomal trafficking of ion channels, receptors, and enzymes.42, 46 In response to death ligands, PACS2 switches to a pro-apoptotic effector that coordinates trafficking steps leading to Bim- and Bid-dependent lysosomal and mitochrondria outer membrane permeabilization, respectively, to trigger activation of executioner caspases and cell death.46, 47 Phosphorylation of PACS2 Ser437 by mTORC2/Akt promotes binding to 14-3-3 proteins. The phosphorylation state of PACS2 Ser437 acts like a molecular switch that separates PACS2’s broad anabolic (survival) and catabolic (apoptotic) roles.43
The multi-functional roles for PACS2 in cell and tissue homeostasis suggest that the p.Glu209Lys mutation may alter a putative regulatory domain that alters binding of PACS2 to one or more client proteins critical for neuron communication, neurogenesis, or cerebellar development. The increased interaction between PACS2 p.Glu209Lys and SIRT1 or HDAC1 suggest the mutation may alter deacetylase functions, such as the control of p53, that impact epilepsy or cerebellar development.48, 49, 50 Similarly, the increased interaction between PACS2 p.Glu209Lys and TrpV1 suggest an altered function of one or more ion channels, contributing indirectly to channelopathies associated with excitability disorders.51 Finally, the p.Glu209Lys mutation may affect important roles for mTORC2/Akt and 14-3-3 in neuronal migration and dendritic arborization.52, 53
In conclusion, the recurrent de novo missense variant resulting in PACS2 p.Glu209Lys in 14 unrelated individuals with a well-defined phenotype supports causality of PACS2 variant in DEE with facial dysmorphism and cerebellar dysgenesis. The pathogenic p.Glu209Lys variant disturbs the interaction between PACS2 and its related proteins, which may alter one or more cellular functions that underlie this neurodevelopmental disease.
Consortia
The C4RCD Research Group includes the clinical team and laboratory research team involved in individual enrollment, sample processing, exome sequencing, data processing, preparation of variant annotation files, data analysis, validation of data, and return of research data to families. Candidate genes are identified and discussed at data analysis meetings of the entire group. The following members of the group (listed in alphabetical order) have contributed significantly to this work: Chris Balak, Newell Belnap, Ana Claasen, Amanda Courtright, David W. Craig, Matt de Both, Matthew J. Huentelman, Madison LaFleur, Sampathkumar Rangasamy, Ryan Richholt, Isabelle Schrauwen, Ashley L. Siniard, and Szabolcs Szelinger.
Acknowledgments
We thank the affected individuals and their families involved in the study and the University of Burgundy Centre de Calcul (CCuB, see Web Resources) for technical support and management of the informatics platform. The authors also thank the Genome Aggregation Database (gnomAD) and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about. We thank the Integragen society and CNG for exome analysis. pTRPV1/f was a gift from D. Julius and pHDAC1/f was a gift from E. Verdin (Addgene #13820). We also thank P. Narvakar and S. Luan for assistance. The authors acknowledge the contributions of all members (current and past) of The C4RCD Research Group. This work was funded by the Regional Council of Burgundy / Dijon University hospital (PARI 2013), the French Ministry of Health (PHRC N° 2013-A00103-42), and NIH (grants R01 CA151564, DK112844, and DK114855).
Published: April 12, 2018
Footnotes
Supplemental Data include one figure and supplemental notes and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.03.005.
Web Resources
Allen Brain Atlas, http://www.brain-map.org/
AnDDI-Rares network, http://www.anddi-rares.org/
ExAC Browser, http://exac.broadinstitute.org/
GeneDx, https://www.genedx.com
GeneMatcher, https://genematcher.org/
gnomAD Browser, http://gnomad.broadinstitute.org/
GTEx Portal, https://www.gtexportal.org/home/
Human Gene Mutation Database (HGMD), http://www.biobase-international.com/product/hgmd
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
Primer3, http://bioinfo.ut.ee/primer3
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SeattleSeq Annotation 131, http://snp.gs.washington.edu/SeattleSeqAnnotation131/
Supplemental Data
References
- 1.Eltze C.M., Chong W.K., Cox T., Whitney A., Cortina-Borja M., Chin R.F., Scott R.C., Cross J.H. A population-based study of newly diagnosed epilepsy in infants. Epilepsia. 2013;54:437–445. doi: 10.1111/epi.12046. [DOI] [PubMed] [Google Scholar]
- 2.von Deimling M., Helbig I., Marsh E.D. Epileptic encephalopathies-clinical syndromes and pathophysiological concepts. Curr. Neurol. Neurosci. Rep. 2017;17:10. doi: 10.1007/s11910-017-0720-7. [DOI] [PubMed] [Google Scholar]
- 3.Tavyev Asher Y.J., Scaglia F. Molecular bases and clinical spectrum of early infantile epileptic encephalopathies. Eur. J. Med. Genet. 2012;55:299–306. doi: 10.1016/j.ejmg.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Olson H., Shen Y., Avallone J., Sheidley B.R., Pinsky R., Bergin A.M., Berry G.T., Duffy F.H., Eksioglu Y., Harris D.J. Copy number variation plays an important role in clinical epilepsy. Ann. Neurol. 2014;75:943–958. doi: 10.1002/ana.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McTague A., Howell K.B., Cross J.H., Kurian M.A., Scheffer I.E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15:304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Lin Z.J., Liu L., Xu H.Q., Shi Y.W., Yi Y.H., He N., Liao W.P. Epilepsy-associated genes. Seizure. 2017;44:11–20. doi: 10.1016/j.seizure.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Myers C.T., Mefford H.C. Genetic investigations of the epileptic encephalopathies: Recent advances. Prog. Brain Res. 2016;226:35–60. doi: 10.1016/bs.pbr.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Mei D., Parrini E., Marini C., Guerrini R. The impact of next-generation sequencing on the diagnosis and treatment of epilepsy in paediatric patients. Mol. Diagn. Ther. 2017;21:357–373. doi: 10.1007/s40291-017-0257-0. [DOI] [PubMed] [Google Scholar]
- 9.Sheidley B.R., Poduri A. Genetics in clinical epilepsy: Issues in genetic testing and counseling. J. Pediatr. Epilepsy. 2012;1:135–142. [Google Scholar]
- 10.Lemke J.R., Riesch E., Scheurenbrand T., Schubach M., Wilhelm C., Steiner I., Hansen J., Courage C., Gallati S., Bürki S. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–1398. doi: 10.1111/j.1528-1167.2012.03516.x. [DOI] [PubMed] [Google Scholar]
- 11.Mercimek-Mahmutoglu S., Patel J., Cordeiro D., Hewson S., Callen D., Donner E.J., Hahn C.D., Kannu P., Kobayashi J., Minassian B.A. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia. 2015;56:707–716. doi: 10.1111/epi.12954. [DOI] [PubMed] [Google Scholar]
- 12.Trump N., McTague A., Brittain H., Papandreou A., Meyer E., Ngoh A., Palmer R., Morrogh D., Boustred C., Hurst J.A. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J. Med. Genet. 2016;53:310–317. doi: 10.1136/jmedgenet-2015-103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen N.M., Conroy J., Shahwan A., Lynch B., Correa R.G., Pena S.D., McCreary D., Magalhães T.R., Ennis S., Lynch S.A., King M.D. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia. 2016;57:e12–e17. doi: 10.1111/epi.13250. [DOI] [PubMed] [Google Scholar]
- 14.Thevenon J., Duffourd Y., Masurel-Paulet A., Lefebvre M., Feillet F., El Chehadeh-Djebbar S., St-Onge J., Steinmetz A., Huet F., Chouchane M. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole-exome sequencing as a first-line diagnostic test. Clin. Genet. 2016;89:700–707. doi: 10.1111/cge.12732. [DOI] [PubMed] [Google Scholar]
- 15.EpiPM Consortium A roadmap for precision medicine in the epilepsies. Lancet Neurol. 2015;14:1219–1228. doi: 10.1016/S1474-4422(15)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson H.E., Kelly M., LaCoursiere C.M., Pinsky R., Tambunan D., Shain C., Ramgopal S., Takeoka M., Libenson M.H., Julich K. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann. Neurol. 2017;81:419–429. doi: 10.1002/ana.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg A.T., Coryell J., Saneto R.P., Grinspan Z.M., Alexander J.J., Kekis M., Sullivan J.E., Wirrell E.C., Shellhaas R.A., Mytinger J.R. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr. 2017;171:863–871. doi: 10.1001/jamapediatrics.2017.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vissers L.E., de Ligt J., Gilissen C., Janssen I., Steehouwer M., de Vries P., van Lier B., Arts P., Wieskamp N., del Rosario M. A de novo paradigm for mental retardation. Nat. Genet. 2010;42:1109–1112. doi: 10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
- 19.Veeramah K.R., Johnstone L., Karafet T.M., Wolf D., Sprissler R., Salogiannis J., Barth-Maron A., Greenberg M.E., Stuhlmann T., Weinert S. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia. 2013;54:1270–1281. doi: 10.1111/epi.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lelieveld S.H., Wiel L., Venselaar H., Pfundt R., Vriend G., Veltman J.A., Brunner H.G., Vissers L.E.L.M., Gilissen C. Spatial clustering of de novo missense mutations identifies candidate neurodevelopmental disorder-associated genes. Am. J. Hum. Genet. 2017;101:478–484. doi: 10.1016/j.ajhg.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biesecker L.G. Exome sequencing makes medical genomics a reality. Nat. Genet. 2010;42:13–14. doi: 10.1038/ng0110-13. [DOI] [PubMed] [Google Scholar]
- 22.Bamshad M.J., Ng S.B., Bigham A.W., Tabor H.K., Emond M.J., Nickerson D.A., Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 23.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H., Deignan J.L., Dorrani N., Strom S.P., Kantarci S., Quintero-Rivera F., Das K., Toy T., Harry B., Yourshaw M. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312:1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Møller R.S., Dahl H.A., Helbig I. The contribution of next generation sequencing to epilepsy genetics. Expert Rev. Mol. Diagn. 2015;15:1531–1538. doi: 10.1586/14737159.2015.1113132. [DOI] [PubMed] [Google Scholar]
- 27.Kambouris M., Thevenon J., Soldatos A., Cox A., Stephen J., Ben-Omran T., Al-Sarraj Y., Boulos H., Bone W., Mullikin J.C., NISC Comparative Sequencing Program Biallelic SCN10A mutations in neuromuscular disease and epileptic encephalopathy. Ann. Clin. Transl. Neurol. 2016;4:26–35. doi: 10.1002/acn3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assoum M., Philippe C., Isidor B., Perrin L., Makrythanasis P., Sondheimer N., Paris C., Douglas J., Lesca G., Antonarakis S. Autosomal-recessive mutations in AP3B2, adaptor-related protein complex 3 beta 2 subunit, cause an early-onset epileptic encephalopathy with optic atrophy. Am. J. Hum. Genet. 2016;99:1368–1376. doi: 10.1016/j.ajhg.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardies K., Weckhuysen S., De Jonghe P., Suls A. Lessons learned from gene identification studies in Mendelian epilepsy disorders. Eur. J. Hum. Genet. 2016;24:961–967. doi: 10.1038/ejhg.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuurs-Hoeijmakers J.H., Oh E.C., Vissers L.E., Swinkels M.E., Gilissen C., Willemsen M.A., Holvoet M., Steehouwer M., Veltman J.A., de Vries B.B. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012;91:1122–1127. doi: 10.1016/j.ajhg.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuurs-Hoeijmakers J.H., Landsverk M.L., Foulds N., Kukolich M.K., Gavrilova R.H., Greville-Heygate S., Hanson-Kahn A., Bernstein J.A., Glass J., Chitayat D., DDD study Clinical delineation of the PACS1-related syndrome–Report on 19 patients. Am. J. Med. Genet. A. 2016;170:670–675. doi: 10.1002/ajmg.a.37476. [DOI] [PubMed] [Google Scholar]
- 32.Gadzicki D., Döcker D., Schubach M., Menzel M., Schmorl B., Stellmer F., Biskup S., Bartholdi D. Expanding the phenotype of a recurrent de novo variant in PACS1 causing intellectual disability. Clin. Genet. 2015;88:300–302. doi: 10.1111/cge.12544. [DOI] [PubMed] [Google Scholar]
- 33.Wan L., Molloy S.S., Thomas L., Liu G., Xiang Y., Rybak S.L., Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 34.Schermer B., Höpker K., Omran H., Ghenoiu C., Fliegauf M., Fekete A., Horvath J., Köttgen M., Hackl M., Zschiedrich S. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J. 2005;24:4415–4424. doi: 10.1038/sj.emboj.7600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molloy S.S., Thomas L., Kamibayashi C., Mumby M.C., Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J. Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youker R.T., Shinde U., Day R., Thomas G. At the crossroads of homoeostasis and disease: roles of the PACS proteins in membrane traffic and apoptosis. Biochem. J. 2009;421:1–15. doi: 10.1042/BJ20081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thauvin-Robinet C., Lee J.S., Lopez E., Herranz-Pérez V., Shida T., Franco B., Jego L., Ye F., Pasquier L., Loget P. The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat. Genet. 2014;46:905–911. doi: 10.1038/ng.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoischen A., Krumm N., Eichler E.E. Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nat. Neurosci. 2014;17:764–772. doi: 10.1038/nn.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holder J.L., Jr., Lotze T.E., Bacino C., Cheung S.W. A child with an inherited 0.31cMb microdeletion of chromosome 14q32.33: further delineation of a critical region for the 14q32 deletion syndrome. Am. J. Med. Genet. A. 2012;158A:1962–1966. doi: 10.1002/ajmg.a.35289. [DOI] [PubMed] [Google Scholar]
- 41.Zollino M., Seminara L., Orteschi D., Gobbi G., Giovannini S., Della Giustina E., Frattini D., Scarano A., Neri G. The ring 14 syndrome: clinical and molecular definition. Am. J. Med. Genet. A. 2009;149A:1116–1124. doi: 10.1002/ajmg.a.32831. [DOI] [PubMed] [Google Scholar]
- 42.Simmen T., Aslan J.E., Blagoveshchenskaya A.D., Thomas L., Wan L., Xiang Y., Feliciangeli S.F., Hung C.H., Crump C.M., Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas G., Aslan J.E., Thomas L., Shinde P., Shinde U., Simmen T. Caught in the act - protein adaptation and the expanding roles of the PACS proteins in tissue homeostasis and disease. J. Cell Sci. 2017;130:1865–1876. doi: 10.1242/jcs.199463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott G.K., Gu F., Crump C.M., Thomas L., Wan L., Xiang Y., Thomas G. The phosphorylation state of an autoregulatory domain controls PACS-1-directed protein traffic. EMBO J. 2003;22:6234–6244. doi: 10.1093/emboj/cdg596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkins K.M., Thomas L.L., Barroso-González J., Thomas L., Auclair S., Yin J., Kang H., Chung J.H., Dikeakos J.D., Thomas G. The multifunctional sorting protein PACS-2 regulates SIRT1-mediated deacetylation of p53 to modulate p21-dependent cell-cycle arrest. Cell Rep. 2014;8:1545–1557. doi: 10.1016/j.celrep.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werneburg N.W., Bronk S.F., Guicciardi M.E., Thomas L., Dikeakos J.D., Thomas G., Gores G.J. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2) J. Biol. Chem. 2012;287:24427–24437. doi: 10.1074/jbc.M112.342238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aslan J.E., You H., Williamson D.M., Endig J., Youker R.T., Thomas L., Shu H., Du Y., Milewski R.L., Brush M.H. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol. Cell. 2009;34:497–509. doi: 10.1016/j.molcel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henshall D.C., Kobow K. Epigenetics and epilepsy. Cold Spring Harb. Perspect. Med. 2015;5:12. doi: 10.1101/cshperspect.a022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barthelery N.J., Manfredi J.J. Cerebellum development and tumorigenesis: a p53-centric perspective. Trends Mol. Med. 2016;22:404–413. doi: 10.1016/j.molmed.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marzban H., Del Bigio M.R., Alizadeh J., Ghavami S., Zachariah R.M., Rastegar M. Cellular commitment in the developing cerebellum. Front. Cell. Neurosci. 2015;8:450. doi: 10.3389/fncel.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catterall W.A., Dib-Hajj S., Meisler M.H., Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J. Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbanska M., Gozdz A., Swiech L.J., Jaworski J. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J. Biol. Chem. 2012;287:30240–30256. doi: 10.1074/jbc.M112.374405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyo-oka K., Wachi T., Hunt R.F., Baraban S.C., Taya S., Ramshaw H., Kaibuchi K., Schwarz Q.P., Lopez A.F., Wynshaw-Boris A. 14-3-3ε and ζ regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J. Neurosci. 2014;34:12168–12181. doi: 10.1523/JNEUROSCI.2513-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.