Abstract
The chloroplast membranes of all higher plants contain very high proportions of trienoic fatty acids. To investigate how these lipid structures are important in photosynthesis, we have generated a triple mutant line of Arabidopsis that contains negligible levels of trienoic fatty acids. For mutant plants grown at 22°C, photosynthetic fluorescence parameters were indistinguishable from wild type at 25°C. Lowering the measurement temperature led to a small decrease in photosynthetic quantum yield, ΦII, in the mutant relative to wild-type controls. These and other results indicate that low temperature has only a small effect on photosynthesis in the short term. However, long-term growth of plants at 4°C resulted in decreases in fluorescence parameters, chlorophyll content, and thylakoid membrane content in triple-mutant plants relative to wild type. Comparisons among different mutant lines indicated that these detrimental effects of growth at 4°C are strongly correlated with trienoic fatty acid content with levels of 16:3 + 18:3, approximately one-third of wild type being sufficient to sustain normal photosynthetic function. In total, our results indicate that trienoic fatty acids are important to ensure the correct biogenesis and maintenance of chloroplasts during growth of plants at low temperatures.
The biophysical reactions of light harvesting and electron transport during photosynthesis take place in a uniquely constructed bilayer, the thylakoid. In all photosynthetic eukaryotes, the complement of atypical glycerolipid molecules that form the foundation of this membrane are characterized by sugar headgroups and a very high level of unsaturation in the fatty acids that occupy the central portion of the thylakoid bilayer. α-Linolenic (18:3) or a combination of 18:3 and hexadecatrienoic (16:3) acids typically account for approximately two-thirds of all the thylakoid membrane fatty acids and over 90% of the fatty acids of monogalactosyldiacylglycerol, the major thylakoid lipid (Douce and Joyard, 1982; Harwood, 1982). Hence, these trienoic fatty acids might have some crucial role in photosynthetic membranes to be of such universal occurrence.
To investigate the role of the trienoic fatty acids in the photosynthetic membrane, a triple mutant line of Arabidopsis has been produced that is completely deficient in 18:3 and 16:3 fatty acids (McConn and Browse, 1996). There are two distinct pathways in plant cells for the biosynthesis of glycerolipids and the associated production of polyunsaturated fatty acids (Browse and Somerville, 1991). Both pathways are initiated by the synthesis of a 16:0-acyl carrier protein (ACP) in the plastid by the fatty acid synthase. This 16:0-ACP may be elongated to 18:0-ACP and then desaturated to 18:1-ACP by a soluble desaturase so that 16:0-ACP and 18:1-ACP are the primary products of plastid fatty acid synthesis. These products are either used directly in the prokaryotic pathway located in the chloroplast inner envelope for the synthesis of the glycerolipid components of the chloroplast membranes or exported from the chloroplast as CoA thioesters and incorporated into phosphatidylcholine and other lipids in the endoplasmic reticulum by the eukaryotic pathway. In addition, the diacylglycerol moiety of phosphatidylcholine can be returned to the chloroplast envelope and used as a second source of precursors for the synthesis of chloroplast glycerolipids. In each pathway, further desaturation of 16:0 and 18:1 occurs only after these fatty acids have been incorporated into the major membrane lipids.
In Arabidopsis, three gene products, FAD3, FAD7, and FAD8, mediate the synthesis of trienoic fatty acids from 18:2 and 16:2. The FAD3 gene product is the endoplasmic reticulum desaturase. The FAD7 and FAD8 genes encode two chloroplast isozymes that recognize as a substrate either 18:2 or 16:2 attached to any of the chloroplast lipids. A mutation in one of these three genes results in no more than a partial reduction of the trienoic fatty acid content. On its own, the fad3 mutation reduces the desaturation level of the thylakoid galactolipids only marginally. The fad7 mutation results in a temperature-dependent reduction in the 18:3 and 16:3 content in thylakoid-specific leaf lipids (Browse et al., 1986), whereas the fatty acid composition of fad8 is indistinguishable from wild type (McConn et al., 1994). To obtain a more pronounced alteration in the trienoic fatty acid content, it has been necessary to generate multiple mutant lines (McConn and Browse, 1996). Leaves of the double mutant fad7-2 fad8 contain 17% trienoic fatty acids. The triple mutant fad3-2 fad7-2 fad8 has essentially no 18:3 or 16:3 either in the thylakoid or any other membrane of the cell.
This triple mutant remarkably has morphological, growth, and developmental characteristics similar to those of wild-type Arabidopsis for most of its life cycle when plants are grown at 25°C. The quantum efficiencies of photosystem II (PSII) and steady-state, whole-chain electron transport in leaves of the mutant grown at room temperature, as measured by noninvasive chlorophyll a fluorescence techniques, were very similar to those of the wild type. Trienoic fatty acids obviously are not absolutely necessary for growth and photosynthesis in Arabidopsis (McConn and Browse, 1996). Cyanobacterial cells resemble the chloroplasts of higher plants in their membrane structure and glycerolipid composition. In line with the results obtained with the Arabidopsis triple mutant, no changes have been found in the photosynthetic characteristics, growth, or development of a cyanobacterium Synechocystis PCC6803 mutant, Fad6, which is substantially deficient in trienoic fatty acids (Murata and Wada, 1995). Furthermore, when shifted to low temperature (from normal 33°C to low 22°C temperature) the Synechocystis Fad6 mutant grew as rapidly as the wild type and the photosynthesis rates of Fad6, and wild-type cells were indistinguishable. In cyanobacteria, photosynthesis and growth are affected only when dienoic fatty acids as well as trienoic fatty acids are substantially eliminated from the organism (Tasaka et al., 1996).
Notwithstanding these observations, there remains a widespread belief that high levels of unsaturation are particularly important for membrane function at low temperatures. For this reason we set out to characterize photosynthesis in the triple mutant and wild-type Arabidopsis. In the work reported here, we show that photosynthetic processes in the Arabidopsis fad3-2 fad7-2 fad8 mutants are only subtly affected by low temperatures in the short term. Instead, altered membrane composition affects the biogenesis and maintenance of chloroplasts and results in gradual deterioration of photosynthetic function over several weeks. Even after 30 d at 4°C, mutant plants recover rapidly following return to 22°C.
RESULTS
Short-Term Measurements of Photosynthesis at Different Temperatures
When the fad3-2 fad7-2 fad8 mutant was grown under standard conditions (100–150 μmol quanta m−2 s−1, 22°C, 50%–70% relative humidity) the developing rosette plants were phenotypically similar to the wild type. Metabolic processes, and in particular photosynthesis, did not appear significantly compromised by the trienoic fatty acid deficiency (McConn and Browse, 1996). To better assess the extent of the similarity between the mutant and the wild type, and to lay the basis for investigating photosynthesis at low temperatures, we first measured the chlorophyll, lipid, and protein contents of the leaves (Table I). The fad3-2 fad7-2 fad8 mutant plants exhibited slight chlorosis due to a 19% decrease in total chlorophyll per unit of fresh weight. Chlorophylls a and b were reduced by 17% and 25%, respectively. The chlorophyll a/b ratio was 3.13 for wild type and 3.49 for the fad3-2 fad7-2 fad8 mutant. There was no significant difference in either the membrane lipid content (measured as fatty acids per gram fresh weight) or the protein content of mutant leaves compared with the wild type.
Table I.
Comparison of the relative amounts of chlorophyll, fatty acid, and protein together with fluorescence parameters in wild-type and mutant leaves
| Wild Type | fad3-2 fad7-2 fad8 | |
|---|---|---|
| Chlorophyll a/b ratio | 3.13 ± 0.07 | 3.49 ± 0.08 |
| Total chlorophyll (mg g−1 fresh wt) | 1.64 ± 0.10 | 1.33 ± 0.09 |
| Protein (mg g−1 fresh wt) | 24.55 ± 1.40 | 22.15 ± 1.80 |
| Fatty acids (μg g−1 fresh wt) | 6.29 ± 0.65 | 6.26 ± 0.28 |
| Fv/Fm | 0.759 ± 0.005 | 0.769 ± 0.005 |
| ΦII | 0.575 ± 0.007 | 0.578 ± 0.007 |
Plants were grown at 22°C and under continuous illumination of 125 μmol m−2 s−1. Fluorescence measurements were made at 25°C. Values are means ± se of 25 leaves from five independent experiments.
Chlorophyll fluorescence analysis is a noninvasive technique for investigating photosynthetic function that is particularly well suited for assessing changes in electron transport reactions within the thylakoid (Schreiber, 1983; Havaux and Lannoye, 1984; Krause and Weis, 1991). Several parameters calculated from chlorophyll a fluorescence have proven useful in comparing the photosynthetic capabilities of plants. In particular, Fv/Fm is an estimate of the maximal quantum yield of PSII photochemistry in dark-adapted leaves (Kitajima and Butler, 1975). This parameter describes the efficiency of the electron transfer within PSII. ΦII is the quantum yield of linear electron transfer (Genty et al., 1989) measured at steady state under ambient light levels. This parameter measures PSII quantum yield but reflects the efficiency of the whole photosynthetic process because PSII is coupled to downstream processes (including PSI and CO2 assimilation) in the light. At 25°C, there were no major differences between the wild type and fad3-2 fad7-2 fad8 mutant in either of these fluorescence parameters (Table I). At 22°C, the CO2 and light-saturated rates of CO2 fixation by wild-type and mutant plants were indistinguishable at 32.1 ± 1.9 and 32.6 ± 2.1 μmol CO2 m−2 s−1, respectively (M.E. Poulson, G.E. Edwards, J. Browse, unpublished data). Taken as a whole, these and other data (McConn and Browse, 1996) demonstrate that trienoic fatty acids are substantially dispensable for photosynthesis at normal temperatures and modest light levels.
At measuring temperatures lower than 25°C, temperature treatment strongly affects the pattern of the fluorescence induction curve and the steady-state values of the fluorescence parameters (Havaux and Lannoye, 1984; Havaux, 1987). To compare the in vivo functioning of the wild-type and fad3-2 fad7-2 fad8 photosynthetic membranes at low temperatures, we recorded the fluorescence characteristics of detached leaves during exposure to a range of temperatures from 25°C to 5°C. As illustrated by wild-type Arabidopsis in Figure 1, typically the values of both Fv/Fm and ΦII are decreased when the temperature is lowered (Havaux, 1987; Georgieva and Yordanov, 1994). The more rapid decline of ΦII compared with Fv/Fm shows that plant leaves exposed to below-normal temperatures exhibited a progressive decrease in their photosynthetic electron transfer capabilities. When the electron transfer is limited, the photosystem components become more completely reduced and ΦII declines. In the fad3-2 fad7-2 fad8 mutant, this cold response was qualitatively similar but quantitatively stronger compared with the wild type. For instance, 1.5 h at 5°C caused a 46% inhibition of ΦII in mutant (compared with the same measurement at 25°C), whereas the same temperature treatment induced a more limited inhibition of 39% in wild type. Although this differential effect of low temperature on ΦII of the mutant was consistently reproducible in several series of experiments, it was not possible to demonstrate a consistent difference in CO2 gas exchange rates between wild-type and fad3-2 fad7-2 fad8 plants measured at 10°C (data not shown). Thylakoid preparations from wild-type and mutant plants assayed for whole chain electron transport (McCourt et al., 1987) at 10°C also failed to demonstrate a significant effect (data not shown). Both these techniques are relatively insensitive at low temperatures but together with the data from fluorescence analysis, they demonstrate that temperatures as low as 5°C have only subtle effects on photosynthetic processes of the mutant relative to wild type. Any consequences of the lack of trienoic fatty acids reduce photosynthesis by less than 10% even at temperatures as low as 5°C.
Figure 1.
Effects of temperature on the maximum quantum yield of PSII, Fv/Fm (▪, □), and on the quantum yield of linear electron transfer, ΦII (●, ○), of wild-type (black symbols) and fad3-2 fad7-2 fad8 mutant (white symbols) Arabidopsis leaves. ΦII was recorded at temperatures from 25°C to 5°C on detached leaves under 100 μmol m−2 s−1 photosynthetically active radiation. Values are means ± se of five leaves.
Effects of Prolonged Exposure to 4°C on Photosynthesis and Chloroplast Ultrastructure
Although low temperatures had little effect on photosynthesis of fad3-2 fad7-2 fad8 plants in the short-term, prolonged incubation of mutant plants at 4°C revealed a very different mutant phenotype. After as little as 10 d at 4°C, newly developed leaf tissue of mutant plants exhibited chlorosis that was not evident in wild-type controls (Fig. 2, A and B). The degree and extent of chlorosis became progressively more pronounced in mutant plants as the low-temperature treatment continued (Fig. 2, C and D). After 30 d at 4°C, most leaves on the mutant plants were pale green and the plants were noticeably smaller than wild-type controls. Measurements of chlorophyll content (Fig. 2E) showed that both mutant and wild-type plants lost chlorophyll at the beginning of the 4°C treatment so that plants of both genotypes contained 30% less chlorophyll after 10 d. After this initial loss, the chlorophyll content of the wild type increased again while the chlorophyll content of the mutant continued to decline throughout the cold treatment.
Figure 2.
Changes in appearance and chlorophyll content of wild-type and fad3-2 fad7-2 fad8 mutant plants during low-temperature treatment and recovery. A through D, Wild type (left) and fad3-2 fad7-2 fad8 mutant (right) were grown for 15 d at 22°C (A) and then transferred to 4°C for 10 (B), 20 (C), or 30 d (D). E, Changes in chlorophyll content of wild type (▪) and fad3-2 fad7-2 fad8 mutant (□) during 4°C treatment and subsequent recovery at 22°C. Results are expressed as percentage of pretreatment values and are the means of five plants. (Initial chlorophyll contents were 2.1 and 1.7 mg g−1 fresh weight for wild type and fad3-2 fad7-2 fad8, respectively.) F, Wild-type (left) and fad3-2 fad7-2 fad8 mutant (right) were kept at 4°C for 30 d and then transferred to 22°C for 6 d.
These changes in photosynthetic performance and chlorophyll content were accompanied by extensive changes in chloroplast ultrastructure in the mutant. Before transfer to low temperature, the thylakoid structure and organization of mutant chloroplasts were substantially similar to wild type (Fig. 3, A and B). Wild-type chloroplasts retained this structure even after 30 d at 4°C. However, the same treatment resulted in extensive loss of thylakoids from fad3-2 fad7-2 fad8 chloroplasts and a marked reduction in stacked membranes (Fig. 3, C and D).
Figure 3.
Chloroplast ultrastructure in wild-type and fad3-2 fad7-2 fad8 mutant leaves. Wild-type (A) and mutant chloroplasts (B) in leaves from plants grown at 22°C for 13 d. Wild-type (C) and mutant (D) chloroplasts in leaves that developed during 30 d at 4°C. Bar = 0.2 μm.
Despite their chlorotic appearance and altered chloroplast ultrastructure, fad3-2 fad7-2 fad8 plants, after 30 d at 4°C, retained a substantial capacity for recovery. Four days after being returned to 22°C, mutant plants had chlorophyll contents that were 75% of pretreatment values and more than 90% of wild-type controls (Fig. 2E). After 6 d, plants had bolted and were beginning to flower (Fig. 2F). We have successfully maintained mutant plants for 3 months at 4°C. At the end of this time, the fresh weight of the mutants averaged less than 35% that of wild-type controls.
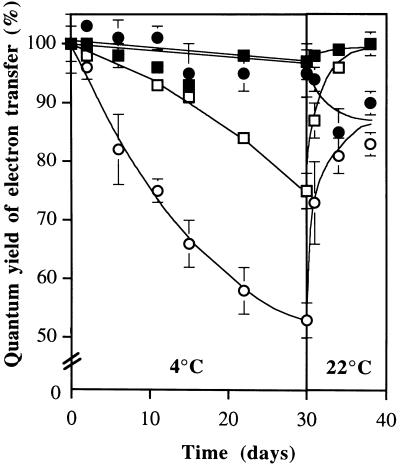
We investigated the photosynthetic characteristics of mutant and wild-type plants during growth at 4°C. To do this, we harvested leaves at different times during the experiment described in Figure 2, warmed them to 25°C for 1 h in the dark, and then determined their fluorescence characteristics. During low-temperature treatment, both Fv/Fm and ΦII in wild-type leaves remained substantially unchanged (Fig. 4). In the mutant, ΦII and Fv/Fm declined gradually from the start of the cold treatment. After 30 d, the mutant plants exhibited a 25% inhibition of Fv/Fm and a 47% inhibition of ΦII, relative to the wild type (Fig. 4). When the plants were subsequently brought back to 22°C, the fluorescence parameters were similar for the wild type and the fad3-2 fad7-2 fad8 mutants after 4 d recovery.
Figure 4.
Effect of long-term exposure to 4°C and subsequent recovery at 22°C on photosynthesis of wild-type (black symbols) and fad3-2 fad7-2 fad8 mutant (white symbols) Arabidopsis. Data shown are for Fv/Fm (▪, □) and ΦII (●, ○). Plants were grown at 22°C for 13 d prior to transfer to 4°C. During low-temperature treatment, detached leaves were rewarmed to 25°C for 1 h in the dark and then Fv/Fm and ΦII were measured at 25°C. Results are expressed as percentage of pretreatment values. Values are means ± se of five leaves. Pretreatment values for wild type were Fv/Fm = 0.761 ± 0.004; ΦII = 0.553 ± 002; and for fad3-2 fad7-2 fad8 were Fv/Fm = 0.768 ± 001; ΦII = 0.551 ± 001.
Low Levels of Trienoic Fatty Acids Protect Photosynthesis from Low-Temperature Damage
We have previously used a leaky allele of fad7 (fad7-1) to generate plants with between 1% and 6% trienoic acids (McConn et al., 1994). We again took advantage of the fad7-1 allele to quantitatively asses how the inhibition of photosynthesis was related to the trienoic fatty acid deficiency, we measured Fv/Fm and ΦII in a series of different Arabidopsis mutants including plants that were segregating for the fad7-1and fad7-2 alleles. The mutants contained from 0.3% to 65% 16:3 + 18:3 fatty acids in their leaf lipids after exposure to 4°C for 30 d. In this experiment, both of the fluorescence parameters Fv/Fm and ΦII were strongly correlated with the level of these fatty acids (Fig. 5). Trienoic fatty acids represent more than 60% of the total fatty acids in leaves of wild-type plants. However, we observed that only much lower levels of trienoic fatty acids (approximately 20%) were needed to support wild-type values of Fv/Fm and ΦII throughout a 30-d cold treatment. It is noteworthy that low temperature did not affect photosynthesis in either the fad3-2 mutant or the double mutant fad7-2 fad8. Neither of these mutants showed any visible signs of chlorosis after 30 d at 4°C.
Figure 5.
Relationship between the quantum yield of photosynthesis measured after 30 d treatment at 4°C and trienoic fatty acid content in leaves of different Arabidopsis mutant lines. Plants were grown at 22°C for 13 d prior to transfer to 4°C. After 30 d at 4°C, detached leaves were rewarmed at 25°C for 1 h in the dark and then Fv/Fm and ΦII were measured at 25°C. Values are means ± se of six leaves. The numbered points represent: fad3-2 fad7-2 fad8 (1); individual plants of an F2 population from a cross of fad3-2 fad7-1 fad8 and fad3-2 fad7-2 fad8 (fad7-1 is a leaky allele) (2–5); fad7-2 fad8 (6); fad3-2 (7); and wild type (8).
These observations again indicate that the biochemical defect in trienoic fatty acid synthesis is the direct cause of the chilling phenotypes reported here. Therefore, even though the trienoic fatty acids are largely irrelevant for the growth of the fad3-2 fad7-2 fad8 mutant at normal temperatures, our results make it clear that trienoic fatty acids have an essential role in maintaining photosynthetic activity at low temperatures.
DISCUSSION
A surprising finding during the initial isolation and characterization of the fad3-2 fad7-2 fad8 line was that triple mutant plants lacking trienoic fatty acids were indistinguishable from wild type in vegetative growth and development at 22°C (McConn and Browse, 1996). The more detailed analyses reported here of the fluorescence and electron transport characteristics of mutant plants confirm that 18:3 and 16:3 fatty acids are essentially dispensable for photosynthetic processes at 25°C under our growth and measurement conditions. Measurements of fluorescence parameters at lower temperatures revealed a small differential effect of temperature on mutant plants such that ΦII in mutant leaves decreased approximately 10% relative to wild-type controls in measurements made at 5° (Fig. 1).
The elimination of 18:3 and 16:3 fatty acids from fad3-2 fad7-2 fad8 plants has more easily visible consequences for long-term growth and photosynthesis at low temperature. After 30 d at 4°C, the chlorophyll content of mutant plants was reduced by 70%, the plants were noticeably smaller than wild-type controls (Fig. 2), and the ultrastructure of mutant chloroplasts showed a marked reduction in thylakoid stacking compared with wild-type controls (Fig. 4). Measurements of Fv/Fm and ΦII made on leaves sampled from 4°C-grown plants and assayed at 25°C (Fig. 5) showed that the chlorophyll loss was accompanied by a decline in photosynthetic efficiency. In particular, ΦII fell steadily throughout the experiment to reach 53% of the starting value after 30 d. By contrast, Fv/Fm showed relatively little change during the first 5 to 10 d at 4°C and had declined by less than 25% by 30 d. The decline was associated with marked changes in chloroplast ultrastructure that included an overall reduction in thylakoid membranes and a large decrease in the extent of thylakoid stacking.
The characterization of lipid mutants in Arabidopsis has now provided five examples of mutants that grow well at 22°C but not at low temperatures (2°C°–6°C). The mutant lines are fab1 (Wu et al., 1997), fad2 (Miquel et al., 1993), fad5 (previously fadB), fad6 (previously fadC) (Hugly and Somerville, 1992), and now the fad3-2 fad7-2 fad8 triple mutant. Each of these mutants shows a distinct lipid phenotype and a distinct pattern of symptoms that develop at low temperature. For this reason, we believe different mechanisms of low-temperature damage are operating in the mutants even though they all share a common general feature, which is a net decrease in the overall extent of fatty acid unsaturation.
When fab1 plants were transferred to 2°C, their fluorescence characteristics remained indistinguishable from wild type for the first 8 to 10 d but, if the cold treatment was extended, both Fv/Fm and ΦII declined to very low values over the subsequent 10 to 14 d, chloroplasts within the leaf were broken down, and the plants eventually died (Wu et al., 1997). These observations were interpreted as a primary disruption of PSII center function that triggers an autophagic response. The death of fad2 plants incubated at 4°C occurred with a timetable similar to that described for fab1 and general symptoms, including a cessation of growth and loss of chlorophyll, were comparable for the two mutants (Miquel et al., 1993). However, the values of Fv/Fm and ΦII remained high throughout cold treatment of fad2 plants, and after 30 d the values were within 10% of the values measured for wild-type controls (J.-M. Routaboul, P. Vijayan, unpublished data). Despite the severe chlorosis of fad2 plants in the cold, it appears that the residual chlorophyll remains part of competent photosystems. The fad2 mutation has little or no effect on chloroplast fatty acid composition (Miquel and Browse, 1992) so these observations suggest that loss of chloroplasts in this mutant is a secondary consequence of biochemical lesions outside the chloroplast or elsewhere in the plant.
In comparison with these severe phenotypes, fad5 and fad6 plants are much less affected by low-temperature treatments. In these mutants, chlorosis affects only the tissue that develops in the cold. Existing leaves do not become chlorotic and chloroplasts in them retain a normal ultrastructure (Hugly and Somerville, 1992). Growth of the plants is slowed at 5°C, relative to wild-type controls, but they flower and produce seed. When 13-d-old fad5 and fad6 plants (grown at 22°C) were transferred to 4°C for 30 d, we observed relatively slight chlorosis of new tissue that developed in the cold and measurements of Fv/Fm and ΦII made on both mutants produced values that were the same as those for wild-type controls (J.-M. Routaboul, unpublished data).
Our analyses of the fad3-2 fad7-2 fad8 mutant line provide evidence for a fourth distinct class of low-temperature phenotype associated with membrane lipid changes. In overall appearance, the triple mutant is most similar to fad6 (Fig. 2) (Hugly and Somerville, 1992) and the ultrastructure of chloroplasts that developed at low temperature is also similar. However, the loss of chlorophyll is more extensive in the triple mutant and involves both developing and mature tissues. Also, both ΦII and Fv/Fm are decreased indicating a reduction in efficiency of the photosystems, which does not occur in fad6 plants subject to the same low-temperature regime.
Although the two mutant lines are easily distinguished on these criteria, it remains possible that the same basic mechanism of cold damage is operating with different degrees of severity. It has been suggested that the fatty acid changes in fad6 (and fad5) plants primarily affect processes in chloroplast biogenesis because tissue that had developed at 22°C before transfer to low temperature did not become chlorotic or show changes in chloroplast ultrastructure (Hugly and Somerville, 1992). In principle, a partial defect in transport of proteins through the chloroplast envelope, or into or through the thylakoid membrane, could explain such a phenotype. During chloroplast biogenesis, when protein transport is maximal, even a partial defect could have severe consequences. Transport processes remain important for maintenance of the mature chloroplast, but the quantitatively lower demands on the transport machinery might be met despite the defect. Under such a scenario, a more severe defect would produce large effects on new tissue and also begin to compromise maintenance processes in the mature chloroplasts of older tissue. This is the essence of the comparative symptomology in the fad3-2 fad7-2 fad8 mutant line relative to fad6. It is also noteworthy that the chloroplast ultrastructure and leaf chlorosis seen in fad6 and the triple mutant at 4°C are also observed in the Arabidopsis ppi1 mutant, which is deficient in the plastid general import apparatus (Jarvis et al., 1998). Testing this hypothesis will require measurements of not only chloroplast protein import but also thylakoid transport for preparations of wild-type and mutant Arabidopsis assayed at low temperatures. We are currently developing appropriate assays for these processes.
The fad7-1 allele contains a leaky mutation that, on the basis of leaf fatty acid composition, retains a small amount of the FAD7 desaturase activity (McConn et al., 1994). The availability of this allele has allowed us to produce plants with very low levels of 16:3 and 18:3 fatty acids (McConn and Browse, 1996). When a collection of these plants and other mutants were incubated at 4°C for 30 d, there was a clear correlation between the severity of chlorosis and the proportion of 16:3 plus 18:3 fatty acids in leaves of the different plants. When leaves were detached, warmed to 25°C, and subject to fluorescence analysis, this correlation was extended to both ΦII and Fv/Fm measurements (Fig. 5). Our data suggest that ΦII (as well as the visual appearance of plants growing at 4°C) can be maintained at wild-type levels by 15% to 20% trienoic fatty acids in the leaf membranes even though leaves of wild-type plants contain more than 60% of these fatty acids.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The lines of Arabidopsis used in this study were descended from the ecotype Columbia wild type. We have previously described (McConn and Browse, 1996) the derivation and the biochemical properties of the mutant lines. Plants were grown at 22°C (70% humidity) in continuous fluorescent illumination (100–150 μmol m−2 s−1) in soil irrigated with mineral nutrient solution. After 13 d, the seedlings were transferred to the experimental growth conditions. None of the single mutant lines, fad3, fad7, or fad8, is measurably affected in growth rate or chlorophyll content at low temperature (J.-M. Routaboul, unpublished data). For this reason, we conclude that the phenotypes described in this paper for the triple mutant are caused by the deficiency in trienoic fatty acids, resulting from the combining of these three mutations and do not involve any additional mutation.
Extraction and Analysis of Chlorophyll and Lipids
Total chlorophyll and chlorophyll a/b ratio were measured in 80% (v/v) acetone. Fatty acid methyl esters were made from leaves or from extracts by transesterification in hot methanolic-HCl and quantitated by gas chromatography and flame ionization detection (Browse and Somerville, 1991).
Fluorescence Measurements
Fluorescence measurements were made with a Pulse Amplitude Modulation fluorometer driven by the DA-100 Data Acquisition System software (Walz model 101, Effeltrich, Germany). The fluorometer was set up in a growth chamber and the probe was positioned above a thermoregulated cuvette at an angle so as not interrupt the incident illumination. Prior to experiments, leaves or plants were dark adapted for 1 h and then the weak pulse measuring beam (0.02 μmol m−2 s−1) modulated at 1.6 kHz was switched on to determine Fo. A 1-s flash of saturating white light (2,000 μmol m−2 s−1) gave the Fm. The parameter Fv/Fm = (Fm − Fo)/Fm was calculated from these data. Continuous white light (100 μmol m−2 s−1) was then switched on to record the fluorescence curve. During this period, 1-s flashes of saturating light were applied each 20 s to measure Fm′ (the prime refers to a state that is not dark-adapted). At the end of the light treatment (approximately 10 min), the quantum yield of steady-state PSII electron transport (ΦII) as (Fm′ − Fs)/Fm′ was calculated following the method of Genty et al. (1989).
Chilling Conditions
Plants grown at 4°C received continuous fluorescence illumination at 100 μmol m−2 s−1. Long-term chilling stresses on photosynthesis were assessed by measurements of Fv/Fm and ΦII on detached leaves rewarmed for 1 h at 25°C. Short-term inhibition of photosynthesis was determined after incubation of leaves in the dark for 90 min at 25°C, 20°C, 15°C, 10°C, or 5°C. Fluorescence parameters were recorded at the incubation temperature.
Electron Microscopy
Small squares, cut from leaves, were fixed in 3% (v/v) glutaraldehyde in 0.1 m PIPES (1,4-piperazinediethane-sulfonic acid) buffer (pH 7.2) overnight at 4°C. After they were washed in 0.1 m PIPES (pH 7.2) four times for 10 min each time, they were put into 2% (w/v) OsO4 overnight at 4°C for secondary fixation. The specimens were washed in 0.1 m PIPES (pH 7.2) again and dehydrated through a graded ethanol series as well as a graded acetone series before being embedded in Spurr's epoxy resin (Sigma, St. Louis). Thin sections were stained with uranyl acetate and lead citrate and examined in an H300 transmission electron microscope (Hitachi, Danbury, CT).
Footnotes
This work was supported by the U.S. National Science Foundation (grant nos. IBN–9407902 and 0084329) and by the Agricultural Research Center, Washington State University. S.F.F. was a student in the Plant Biochemistry Research and Training Center under the U.S. Department of Energy (grant no. DE–FG0694ER20160).
LITERATURE CITED
- Browse J, Somerville C. Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506. [Google Scholar]
- Browse JA, McCourt PJ, Somerville CR. A mutant of Arabidopsis deficient in C18:3 and C16:3 leaf lipids. Plant Physiol. 1986;81:859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Joyard J. Plants galactolipids. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants. New York: Academic Press; 1982. pp. 331–332. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Georgieva K, Yordanov Y. Temperature dependence of photochemical and non-photochemical fluorescence quenching in intact pea leaves. J Plant Physiol. 1994;144:754–759. [Google Scholar]
- Harwood JL. Plant acyl lipids. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants. New York: Academic Press; 1982. pp. 1–55. [Google Scholar]
- Havaux M. Effects of chilling on the redox state of the primary electron acceptor QA of photosystem II in chilling-sensitive and resistant plant species. Plant Physiol Biochem. 1987;25:735–743. [Google Scholar]
- Havaux M, Lannoye R. Effects of chilling temperatures on prompt and delayed chlorophyll fluorescence in maize and barley leaves. Photosynthetica. 1984;18:117–127. [Google Scholar]
- Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992;99:197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Chen LJ, Li H, Peto CA, Fankhauser C, Chory J. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science. 1998;282:100–103. doi: 10.1126/science.282.5386.100. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Butler WL. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothyquinone. Biochim Biophys Acta. 1975;376:105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Phys Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is for pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Hugly S, Somerville C, Browse J. A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol. 1994;106:1609–1614. doi: 10.1104/pp.106.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt PJ, Kunst L, Browse J, Somerville CR. The effects of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol. 1987;84:353–360. doi: 10.1104/pp.84.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Browse J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis: biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem. 1992;267:1502–1509. [PubMed] [Google Scholar]
- Miquel M, James D, Dooner H, Browse J. Arabidopsis requires polyunsaturated lipids for low temperature survival. Proc Natl Acad Sci USA. 1993;90:6208–6212. doi: 10.1073/pnas.90.13.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995;308:1–7. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U. Chlorphyll fluorescence yield changes as a tool in plant physiology: I. The measuring system. Photosynth Res. 1983;4:361–373. doi: 10.1007/BF00054144. [DOI] [PubMed] [Google Scholar]
- Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohba T, Ohki K, Murata N. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lightner J, Warwick N, Browse J. Low temperature damage and subsequent recovery of fab1 mutant Arabidopsis exposed to 2°C. Plant Physiol. 1997;113:347–356. doi: 10.1104/pp.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]