Abstract
Background
Exacerbations of asthma and chronic obstructive pulmonary disease (COPD) are heterogeneous.
Objective
We sought to investigate the sputum cellular, mediator, and microbiome profiles of both asthma and COPD exacerbations.
Methods
Patients with severe asthma or moderate-to-severe COPD were recruited prospectively to a single center. Sputum mediators were available in 32 asthmatic patients and 73 patients with COPD assessed at exacerbation. Biologic clusters were determined by using factor and cluster analyses on a panel of sputum mediators. Patterns of clinical parameters, sputum mediators, and microbiome communities were assessed across the identified clusters.
Results
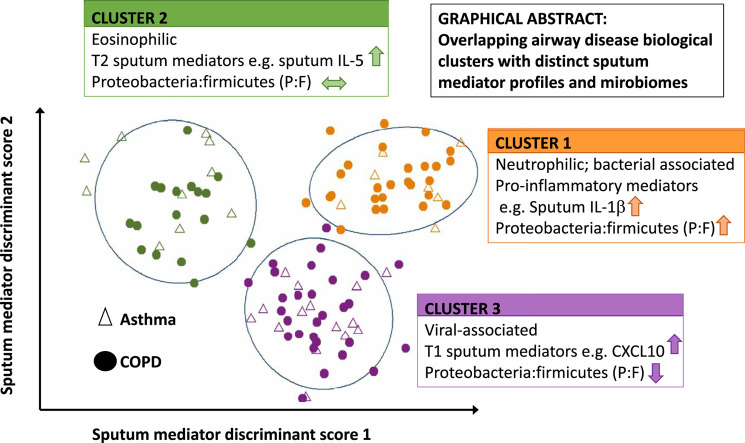
The asthmatic patients and patients with COPD had different clinical characteristics and inflammatory profiles but similar microbial ecology. Three exacerbation biologic clusters were identified. Cluster 1 was COPD predominant, with 27 patients with COPD and 7 asthmatic patients exhibiting increased blood and sputum neutrophil counts, proinflammatory mediators (IL-1β, IL-6, IL-6 receptor, TNF-α, TNF receptors 1 and 2, and vascular endothelial growth factor), and proportions of the bacterial phylum Proteobacteria. Cluster 2 had 10 asthmatic patients and 17 patients with COPD with increased blood and sputum eosinophil counts, type 2 mediators (IL-5, IL-13, CCL13, CCL17, and CCL26), and proportions of the bacterial phylum Bacteroidetes. Cluster 3 had 15 asthmatic patients and 29 patients with COPD with increased type 1 mediators (CXCL10, CXCL11, and IFN-γ) and proportions of the phyla Actinobacteria and Firmicutes.
Conclusions
A biologic clustering approach revealed 3 subgroups of asthma and COPD exacerbations, each with different percentages of patients with overlapping asthma and COPD. The sputum mediator and microbiome profiles were distinct between clusters.
Key words: Asthma, chronic obstructive pulmonary disease, asthma and chronic obstructive pulmonary disease heterogeneity, inflammatory profiles, microbiome abundances, phylum and genus levels, factor and cluster analyses
Abbreviation used: COPD, Chronic obstructive pulmonary disease; IL-6R, IL-6 receptor; P/F ratio, Proteobacteria/Firmicutes ratio; T1, Type 1; T2, Type 2; TNF-R, TNF receptor; VAS, Visual analog scale; VEGF, Vascular endothelial growth factor
Graphical abstract
The prevalences of asthma and chronic obstructive pulmonary disease (COPD) continue to increase, exceeding 358 million and 174 million worldwide, respectively.1 Asthma and COPD are heterogeneous for clinical characteristics, cellular sources of inflammation, causes of exacerbations, and responses to therapies.2, 3, 4, 5, 6, 7 They share similar features, such as symptoms, airflow limitation, bronchial hyperresponsiveness, and inflammatory profiles.8 A previous examination of asthma and COPD biologic clusters during stable disease demonstrated an overlap of sputum inflammatory profiles.7 However, understanding the distinctive and common heterogeneities of both diseases at exacerbation remains elusive.
Despite current guidelines on management strategies for asthma and COPD, many patients still experience exacerbations. Asthma exacerbations impair health-related quality of life, result in lost productivity, and increase health care resource use. Moreover, COPD exacerbations are associated with accelerated loss of lung function, poorer health-related quality of life, comorbidities, significant mortality, and increased health care costs.9, 10 Standard treatment for asthma and COPD exacerbations include use of bronchodilators, corticosteroids, and antibiotics, with little attention paid to the underlying heterogeneity of these exacerbations. Biologic heterogeneity of COPD exacerbations has been demonstrated previously,4 with sputum IL-1β levels, serum CXCL10 levels, and peripheral blood eosinophil counts best identifying bacteria-, virus-, or eosinophil-associated exacerbations, respectively. However, whether this biologic heterogeneity is similar between asthma and COPD exacerbations is unknown.
The main objective of this study was to identify the degree of overlap in biologic clusters of asthma and COPD exacerbations derived from sputum mediator profiling as a measure of airway inflammation and to determine the airway bacterial ecology in each of these clusters.
Methods
Study population
Patients with severe asthma or moderate-to-severe COPD were recruited from a single center at Glenfield Hospital, Leicester, United Kingdom. Assignment to the asthma or COPD group was made by the patients' physicians consistent with definitions of asthma or COPD based on the Global Initiative for Asthma9 or the Global Initiative for Chronic Obstructive Lung Disease10 guidelines, respectively. The asthmatic patients had participated in a published stable study,7, 11 and the patients with COPD had participated in a published exacerbation study.4, 12 All patients were assessed at stable state at least 6 weeks from an exacerbation. They had assessment at exacerbation, which was defined as an increase in symptoms necessitating a course of oral corticosteroids, antibiotic therapy, or both. All patients provided written informed consent. The studies were approved by the local Leicestershire, Northamptonshire, and Rutland Ethics Committee.
Measurements
Demographics and clinical and lung function data, including prebronchodilator and postbronchodilator FEV1 and forced vital capacity, were collected. Patients were asked to score the severity of their dyspnea and cough by using the visual analog scale (VAS). The VAS is a horizontal line that is 100 mm in length and anchored by word descriptors at both ends. It uses absence of breathlessness or cough on one end and maximum breathlessness or cough on the other end. It is scored by measuring the distance from the left end to the mark indicated by the patient. A bacterially associated exacerbation was defined as colony-forming units greater than 107/mL sputum or a positive culture result. Microbiome communities were obtained from 16S rRNA sequencing of bacterial genomic DNA extracted from the sputum samples by using the Qiagen DNA Mini kit (Qiagen, Valencia, Calif), as described previously.4 The sequencing reads were processed by using the QIIME pipeline.13 RNA was extracted from selected sputum plugs, and an RT-PCR panel for all common respiratory viruses (rhinoviruses, other picornaviruses, respiratory syncytial virus, human parainfluenza viruses 1-3, adenoviruses, influenza viruses A and B, coronavirus 229E and OC43, human metapneumovirus, and human bocavirus) was undertaken for the COPD samples, as described previously.4 For the asthma samples, the same methods were used, but virus detection was limited to rhinoviruses, other picornaviruses, respiratory syncytial virus, and influenza viruses A and B. A virus-associated exacerbation was defined as those exacerbations in which a virus was detected.
Inflammatory mediators were measured in sputum supernatants and serum by using the Meso Scale Discovery Platform (MSD, Gaithersburg, Md). The mediators measured were selected to reflect cytokines, chemokines, and proinflammatory mediators implicated in airway disease. The performance of the MSD platform for recovery of spiked exogenous recombinant proteins has been described previously.8 Sputum and serum inflammatory mediator levels of less than the detectable range were replaced with their corresponding halves of the lower limits of quantification. In addition, mediator levels less than the limit of quantification for more than 60% of the patients were excluded from further analysis.
Statistical methods
A 2-stage (factor and cluster analyses) approach was performed to identify the common and distinctive biologic subgroups of asthma and COPD. First, factor analysis was applied to the panel of sputum inflammatory mediators and reduced to small independent factors. Sampling adequacy for factor analysis was assessed by using the Kaiser-Meyer-Olkin test. Optimal factors were retained based on scree plots (factors above the break in the curve) and eigenvalue of greater than 1. Subsequently, corresponding factor scores representing each patient were generated and used as input variables into the k-means clustering algorithm to identify the clusters. The optimal number of clusters was chosen based on a scree plot (clusters above the break in the curve) by plotting within the cluster sum of the squares against a series of a sequential number of clusters.
In addition, linear discriminant analysis was performed on the sputum mediators across clusters to validate how the identified clusters from the factor scores can be predicted by using the actual mediators' measurements and to identify the contribution of each mediator in discriminating the clusters (data not shown). Discriminant scores for individual patients were calculated and used to represent the patients' biologic cluster membership graphically.
Microbiome measurements from 16S rRNA sequencing at both the phylum and genus levels were performed. Thirty species at the phylum level and 400 species at the genus level were screened. The relative abundance of each species was calculated, and the alpha (within-patient) and beta (between-patient) diversities at both the phylum and genus levels were estimated by using Shannon-Weiner and Sorensen indices, respectively (Vegan R-package, version 2.3). The patterns were compared between diseases and the identified biologic clusters. In addition, patterns of those most abundant species (median relative abundance greater than 2%) and/or those known to be major airway pathogens in asthmatic patients and patients with COPD at both the phylum and genus levels were presented graphically across the diseases and the identified biologic clusters.
The statistical summary of all the available characteristics is presented across the diseases and identified biologic clusters and within each cluster by disease subgroup. The clusters were interpreted according to the patterns of these characteristics. In addition, the change in clinical characteristics and mediators between stable and exacerbation states within each cluster was assessed. Parametric data were presented as means with SEMs, and log-transformed data were presented as geometric means with 95% CIs. The χ2 or Fisher exact test and 1-way ANOVA were used to compare percentages and means across groups. Nonparametric data were presented as median values with first and third quartiles, and the Kruskal-Wallis test was used to compare these data between clusters. All statistical analyses were performed with SPSS software (version 24; IBM, Armonk, NY), STATA/IC (version 14.0) software for Windows (StataCorp, College Station, Tex), and R software (version 3.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Thirty-two asthmatic patients and 73 patients with COPD with sputum mediator records at exacerbation were included in this study. Their demographics, clinical characteristics, and sputum mediators were summarized across the diseases in Table E1 in this article's Online Repository at www.jacionline.org. Inhaled corticosteroid dose was not different between asthmatic patients and patients with COPD. All asthmatic patients were receiving long-acting β-agonist treatment, and all but 2 patients with COPD were receiving a long-acting β-agonist and/or a long-acting muscarinic antagonist. Asthmatic patients were younger and more obese and had better lung function than patients with COPD. VAS scores, cellular profiles, and bacteria-associated exacerbations were not significantly different between the 2 diseases.
Levels of several sputum mediators (IL-5, IL-6 receptor [IL-6R], CXCL10, CXCL11, CCL5, and CCL26) were significantly increased in asthmatic patients versus patients with COPD, whereas levels of IL-6, CCL3, CCL4, and TNF receptor (TNF-R) 1 were significantly increased in patients with COPD versus asthmatic patients. However, levels of the majority of mediators, such as IL-1β, IL-8, IL-10, IL-13, CCL2, CCL13, CCL17, TNF-α, TNF-R2, vascular endothelial growth factor (VEGF), and IFN-γ, were not significantly different between asthmatic patients and patients with COPD (see Table E1). Similarly, levels of the serum mediators IL-5, IL-8, CXCL10, CXCL11, CCL17, CCL26, TNF-α were increased in asthmatic patients versus levels of IL-1β, IL-6, CCL4, and TNF-R1, which were increased in patients with COPD, and others (CCL2, CCL13, TNF-R2, and VEGF) were not different between groups (see Table E1).
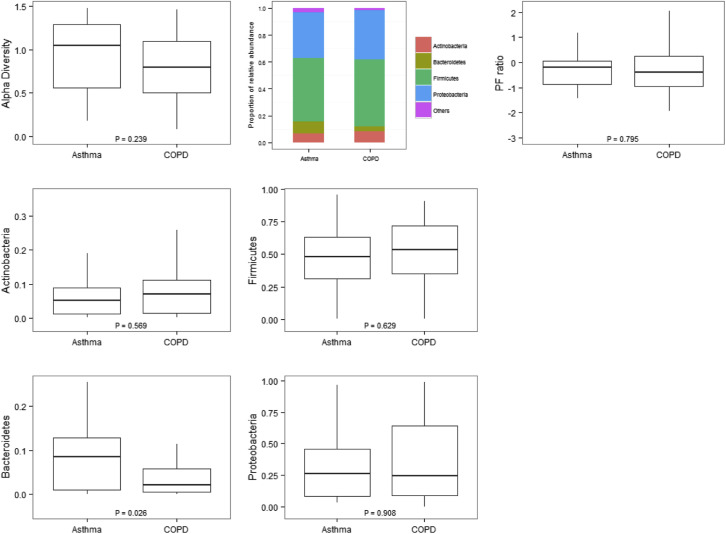
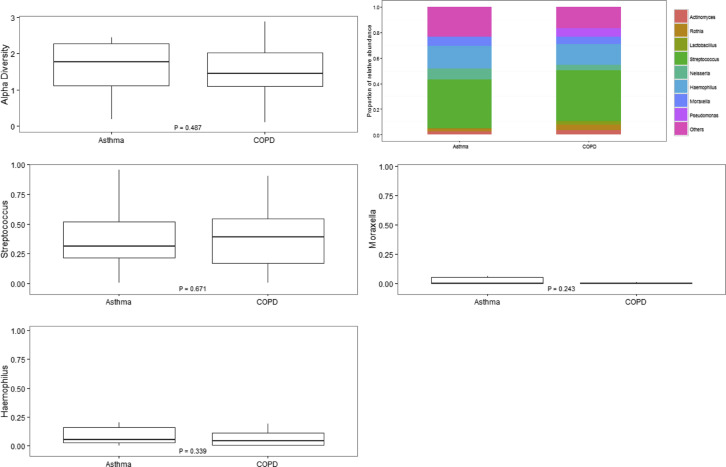
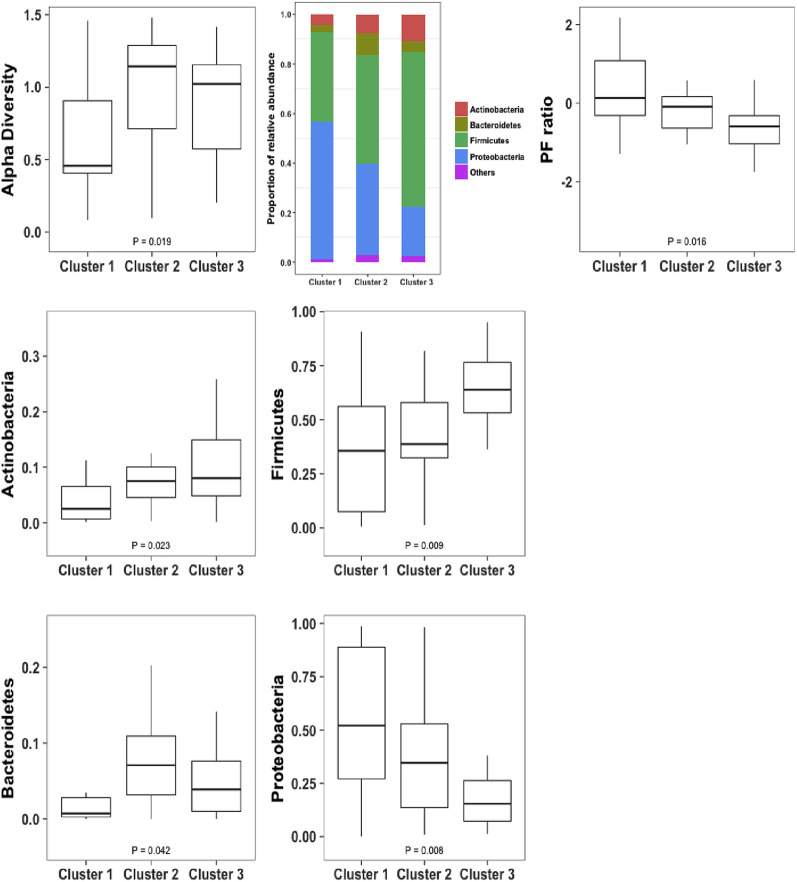
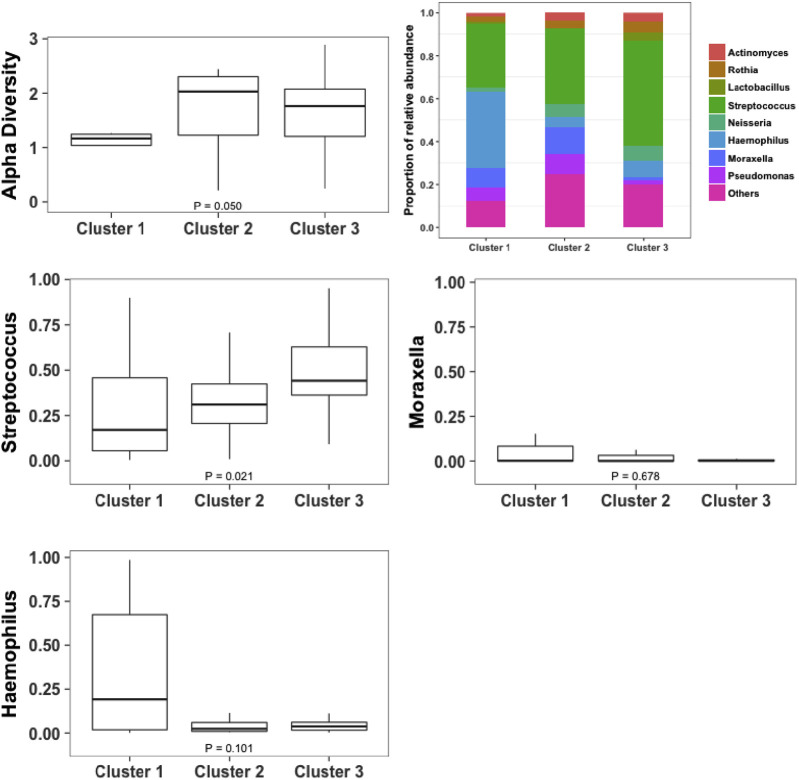
Fourteen asthmatic patients and 40 patients with COPD who had sputum mediators at exacerbation also provided sufficient sputum for further microbiome analysis. Alpha diversities at the phylum and genus levels were not different between the asthma and COPD groups, as shown in Figs E1 and E2 in this article's Online Repository at www.jacionline.org. Beta diversities were 0.25 for asthmatic patients and 0.20 for patients with COPD at the phylum level. At the genus level, beta diversities were 0.52 for asthmatic patients and 0.49 for patients with COPD. Phyla and genera with median relative abundance of greater than 2% were Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria at the phylum level and Actinomyces and Rothia species (both phylum Actinobacteria), Lactobacillus and Streptococcus species (both phylum Firmicutes), and Neisseria, Haemophilus, Moraxella, and Pseudomonas species (all phylum Proteobacteria) at the genus level. The relative abundance of the most abundant phyla and/or those known to be major airway pathogens across asthmatic patients and patients with COPD and the Proteobacteria/Firmicutes ratio (P/F ratio) for asthmatic patients versus patients with COPD are presented (see Fig E1). At the phylum level, the airway ecology was similar between asthmatic patients and patients with COPD with only Bacteroidetes significantly increased for asthmatic patients compared with patients with COPD (see Fig E1). The relative abundance of the most abundant and clinically relevant genera (Streptococcus, Haemophilus, and Moraxella) was also not significantly different between asthmatic patients and patients with COPD (see Fig E2).
Fig E1.
Alpha diversity at the phylum level (using the Shannon-Weiner index): relative abundances of the most abundant phyla and P/F ratio in log format (base 10) across asthmatic patients and patients with COPD.
Fig E2.
Alpha diversity at the genus level (using the Shannon-Weiner index): relative abundances of the most abundant genera and known major airway pathogens across asthmatic patients and patients with COPD.
Asthma and COPD biologic factors at exacerbation
Factor analysis with varimax rotation was performed by using sputum mediators at exacerbation for all 32 asthmatic patients and 73 patients with COPD, and 3 factors were retained. IL-13 levels were less than the limit of detection for most of the patients, and CCL3 levels were missing for several patients. Therefore those values were excluded from factor and cluster analyses. However, their patterns were assessed for the identified biologic subgroups. The rotated factor loadings are depicted in Table E2 in this article's Online Repository at www.jacionline.org, indicating the relationship between the factors and mediators. Proinflammatory mediators appeared to load together in factor 1, type 1 (T1) mediators in factor 2, and type 2 (T2) mediators in factor 3.
Biologic exacerbation clusters
Three biologic clusters were identified by using factor scores (derived from sputum mediators) as input into the k-means clustering algorithm. The patterns of clinical parameters and sputum mediators are presented across the identified clusters in Tables I and II , respectively. The serum mediators across the clusters are as shown in Table E3 in this article's Online Repository at www.jacionline.org.
Table I.
Summary statistics across the 3 identified exacerbation biologic clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
P value |
||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients, n = 7; patients with COPD, n = 27 | Asthmatic patients, n = 10; patients with COPD, n = 17 | Asthmatic patients, n = 15; patients with COPD, n = 29 | Cluster 1 vs cluster 2 | Cluster 1 vs cluster 3 | Cluster 2 vs cluster 3 | ANOVA | |
| Male sex, no. (%) | 20 (58.8) | 17 (63.0) | 30 (68.2) | .74 | .39 | .65 | .69 |
| Current or ex-smokers, no. (%) | 24 (70.6) | 21 (77.8) | 32 (72.7) | .53 | .84 | .64 | .81 |
| Pack-year history∗‡ | 45.5 (22.0-51.0) | 37.0 (25.8-48.0) | 37.5 (22.9-62.7) | .84 | .80 | .66 | .90 |
| Age (y)† | 67.2 (1.7) | 62.9 (2.2) | 68.0 (5.2) | .49 | .90 | .39 | .67 |
| BMI (kg/m2)† | 26.9 (1.0) | 27.9 (1.2) | 27.9 (0.9) | .53 | .47 | .99 | .74 |
| Exacerbations in last year∗ | 3.0 (1-6) | 4.5 (2-6) | 3.0 (2-5) | .35 | .42 | .65 | .58 |
| Maintenance prednisolone, no. (%) | 10 (29.4) | 7 (25.9) | 11 (25.0) | .76 | .66 | .93 | .90 |
| Daily prednisolone dose (mg)∗§ | 5 (5-15) | 10 (7.5-10) | 10 (7.5-15) | .44 | .26 | .47 | .44 |
| Daily ICS dose (μg/d)∗‖ | 2000 (800-2000) | 1600 (800-2000) | 1300 (800-2000) | .74 | .47 | .61 | .73 |
| Pre-FEV1 (L)† | 1.21 (0.10) | 1.35 (0.20) | 1.37 (0.11) | .49 | .29 | .91 | .62 |
| Post-FEV1 (L)† | 1.30 (0.09) | 1.43 (0.24) | 1.33 (0.11) | .52 | .82 | .62 | .81 |
| Pre-FEV1 (% predicted)† | 49.98 (4.12) | 45.97 (5.05) | 44.96 (4.59) | .57 | .42 | .88 | .70 |
| Post-FEV1 (% predicted)† | 53.54 (4.01) | 48.12 (6.06) | 44.02 (4.81) | .48 | .14 | .58 | .33 |
| Pre-FEV1/FVC ratio (%)† | 56.06 (2.88) | 54.91 (2.99) | 55.72 (2.25) | .78 | .93 | .84 | .96 |
| VAS, cough (mm)† | 69.36 (2.88) | 61.23 (4.51) | 64.27 (3.36) | .14 | .27 | .56 | .31 |
| VAS, dyspnea (mm)† | 68.76 (3.77) | 69.31 (4.67) | 66.73 (2.98) | .92 | .67 | .63 | .86 |
| Blood neutrophils (× 109/L)† | 7.98 (0.58) | 5.91 (0.49) | 6.54 (0.43) | .008 | .044 | .39 | .02 |
| Blood eosinophils (× 109/L) | 0.13 (0.10-0.17) | 0.30 (0.20-0.45) | 0.12 (0.09-0.16) | .002 | .59 | <.0001 | .0004 |
| TCC (× 106 cells/g sputum) | 16.48 (11.19-24.29) | 3.00 (1.92-4.69) | 3.06 (1.95-4.81) | <.0001 | <.0001 | .94 | <.0001 |
| Sputum neutrophil count (%)† | 89.17 (2.71) | 58.44 (4.22) | 64.78 (3.59) | <.0001 | <.0001 | .21 | <.0001 |
| Sputum eosinophil count (%) | 0.34 (0.27-0.43) | 6.35 (3.13-12.88) | 0.83 (0.49-1.39) | <.0001 | .004 | <.0001 | <.0001 |
| Bacterial load (log10 CFU/mL) | 7.0 (1.0) | 5.7 (0.9) | 6.3 (1.0) | .0007 | .047 | .147 | .001 |
| Bacteria-associated exacerbation (%) | 78.8 | 23.1 | 21.1 | <.0001 | <.0001 | .85 | <.0001 |
| Viral (%) | 29.0 | 30.0 | 47.1 | .94 | .14 | .22 | .25 |
Data are presented as geometric means with 95% CIs, unless stated otherwise. Values in boldface indicate statistical significance.
CFU, Colony-forming unit; BMI, body mass index; ICS, inhaled corticosteroid; FVC, forced vital capacity; TCC, total sputum cell count.
Median (first and third quartiles).
Mean (SEM).
Pack-year history of current smokers and ex-smokers.
Dose for only those patients prescribed daily prednisolone.
Beclomethasone dipropionate equivalent.
Table II.
Summary statistics across the 3 identified exacerbation biologic clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
P value |
||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients, n = 7; patients with COPD, n = 27 | Asthmatic patients, n = 10; patients with COPD, n = 17 | Asthmatic patients, n = 15; patients with COPD, n = 29 | Cluster 1 vs cluster 2 | Cluster 1 vs cluster 3 | Cluster 2 vs cluster 3 | ANOVA | |
| IL-1β (pg/mL) | 2,167.3 (1,441.1-3,259.2) | 42.6 (22.4-81.2) | 72.1 (42.1-123.5) | <.0001 | <.0001 | .17 | <.0001 |
| IL-5 (pg/mL) | 0.6 (0.4-0.9) | 8.4 (5.7-12.4) | 1.2 (0.8-1.9) | <.0001 | .03 | <.0001 | <.0001 |
| IL-6 (pg/mL) | 620.2 (374.8-1,026.3) | 171.5 (96-306.3) | 374 (201.1-695.7) | .005 | .22 | .068 | .018 |
| IL-6R (pg/mL) | 828.0 (596.4-1,149.5) | 213.8 (154.5-295.8) | 169.9 (119.1-242.3) | <.0001 | <.0001 | .36 | <.0001 |
| IL-8 (pg/mL) | 13,195.3 (10,356.0-16,813.1) | 3,192.5 (2,252.4-4,524.9) | 2,967.8 (2,010.5-4,380.8) | <.0001 | <.0001 | .77 | <.0001 |
| IL-10 (pg/mL) | 17.1 (10.1-29.0) | 1.9 (1.6-2.2) | 7.7 (4.2-14.2) | <.0001 | .057 | <.0001 | <.0001 |
| IL-13 (pg/mL) | 8.7 (7.7-9.7) | 12.8 (9.6-17.1) | 9.8 (8.5-11.4) | .005 | .20 | .043 | .017 |
| CXCL10 (pg/mL) | 163.0 (94.6-280.7) | 406.3 (253.0-652.4) | 1,542.4 (820.1-2,901.1) | .042 | <.0001 | .002 | <.0001 |
| CXCL11 (pg/mL) | 4.2 (2.5-7.0) | 24.8 (13.1-47.0) | 149.6 (58.5-382.6) | .004 | <.0001 | .002 | <.0001 |
| CCL2 (pg/mL) | 312.3 (221.0-441.4) | 383.4 (262.0-561.1) | 695.5 (454.3-1,064.8) | .50 | .006 | .041 | .009 |
| CCL3 (pg/mL) | 90.0 (55.1-147.1) | 49.1 (29.4-81.9) | 61.6 (36.6-103.8) | .12 | .30 | .54 | .28 |
| CCL4 (pg/mL) | 1,087.3 (656.7-1,800.2) | 1,366.7 (910.5-2,051.5) | 898.9 (542.2-1,490.5) | .54 | .60 | .24 | .50 |
| CCL5 (pg/mL) | 14.2 (9.5-21.0) | 4.6 (2.9-7.3) | 10.8 (6.5-17.7) | .002 | .41 | .013 | .006 |
| CCL13 (pg/mL) | 10.8 (8.9-13.1) | 30.4 (22.3-41.4) | 17.6 (12.9-24) | <.0001 | .015 | .008 | <.0001 |
| CCL17 (pg/mL) | 4.8 (3.0-7.6) | 71.0 (47.1-107.0) | 10.7 (7.8-14.5) | <.0001 | .003 | <.0001 | <.0001 |
| CCL26 (pg/mL) | 2.3 (1.8-3.0) | 26.1 (18.3-37.2) | 4.0 (2.8-5.7) | <.0001 | .02 | <.0001 | <.0001 |
| TNF-α (pg/mL) | 133.4 (84.3-211.2) | 2.5 (1.5-4.4) | 12.7 (6.2-26.1) | <.0001 | <.0001 | .001 | <.0001 |
| TNF-R1 (pg/mL) | 5,598.7 (4,499.7-6,966.1) | 770.5 (566.6-1,047.8) | 760.7 (557.9-1,037.2) | <.0001 | <.0001 | .95 | <.0001 |
| TNF-R2 (pg/mL) | 1,950.9 (1,439.8-2,643.4) | 345.2 (246.3-483.9) | 418.6 (258.7-677.3) | <.0001 | <.0001 | .52 | <.0001 |
| VEGF (pg/mL) | 2,428.1 (1,947.3-3,027.6) | 1,177.2 (946.9-1,463.7) | 1,071.2 (909.7-1,261.4) | <.0001 | <.0001 | .50 | <.0001 |
| IFN-γ (pg/mL) | 1.0 (0.6-1.8) | 0.3 (0.3-0.4) | 3.3 (1.4-7.7) | .034 | .03 | <.0001 | <.0001 |
Data are presented as geometric means with 95% CIs. Values in boldface indicate statistical significance.
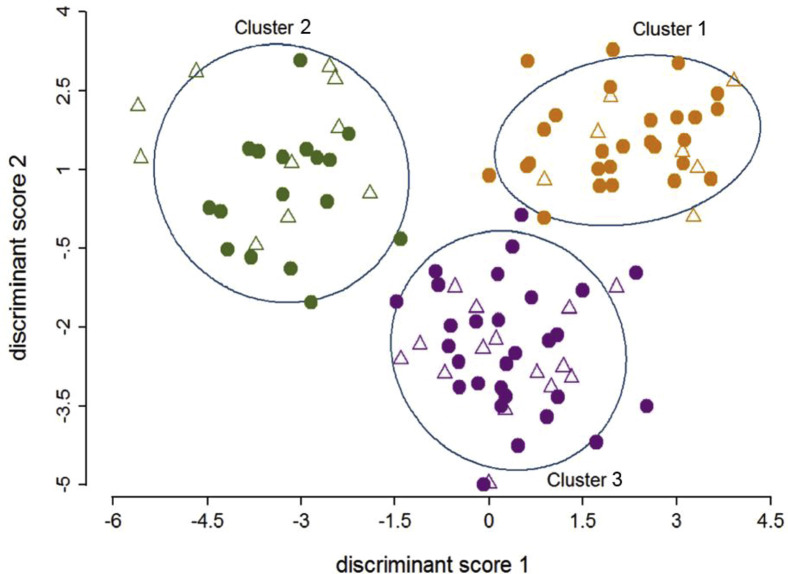
Summary data (clinical parameters, sputum, and serum mediators) for asthmatic patients and patients with COPD within each cluster are presented in Table E4, Table E5, Table E6 in this article's Online Repository at www.jacionline.org, respectively. There were no significant differences in age, sex, smoking status, pack-year history, body mass index, frequency of exacerbations, corticosteroid dosage, symptom scores, and lung function between the clusters. The clusters are presented graphically across the first 2 discriminant scores in Fig 1 . Microbiome data were available for 19 patients (asthmatic patients, 4; patients with COPD, 15) in cluster 1, 11 patients (asthmatic patients, 4; patients with COPD, 7) in cluster 2, and 24 patients (asthmatic patients, 6; patients with COPD, 18) in cluster 3. Alpha diversity, the proportions and patterns of relative abundance of the most abundant phyla, and P/F ratios are presented for each cluster in Fig 2 . Similarly, both alpha diversity and the proportions and patterns of the relative abundance of the most abundant genera are presented in Fig 3 . The change in characteristics between stable and exacerbation states within each cluster are reported (clinical parameters and sputum mediators in Table III and serum mediators in Table E7 in this article's Online Repository at www.jacionline.org), and further breakdown of these characteristics by disease within each cluster is reported in Tables E8 and E9 in this article's Online Repository at www.jacionline.org, respectively. At exacerbation, both prebronchodilator and postbronchodilator FEV1 decreased, whereas the VAS scores of cough and dyspnea increased significantly for all 3 clusters, with no significant differences between clusters.
Fig 1.
The 3 identified exacerbation biologic clusters presented using the subjects' discriminant scores. Open triangles indicate asthmatic patients, and solid circles indicate patients with COPD. Orange, green, and purple colors represent clusters 1, 2, and 3, respectively.
Fig 2.
Alpha diversity at the phylum level (using the Shannon-Weiner index): proportion and patterns of relative abundance of the most abundant phyla and P/F ratio in log format (base 10) across the identified exacerbation biologic clusters.
Fig 3.
Alpha diversity at the genus level (using the Shannon-Weiner index): proportion and patterns of relative abundance of the most abundant genera or those known to be important airway pathogens across the identified exacerbation biologic clusters.
Table III.
Change in clinical characteristics and mediators between stable and exacerbation states within each identified exacerbation biologic cluster
| Cluster 1 (n = 34) |
Cluster 2 (n = 27) |
Cluster 3 (n = 44) |
||||
|---|---|---|---|---|---|---|
| P value | P value | P value | ||||
| Pre-FEV1 (L)∗ | −0.14 (0.04) | .004↓ | −0.19 (0.06) | .006↓ | −0.12 (0.04) | .003↓ |
| Post-FEV1 (L)∗ | −0.14 (0.05) | .004↓ | −0.10 (0.04) | .038↓ | −0.24 (0.04) | <.0001↓ |
| Pre-FEV1 (% predicted)∗ | −6.22 (1.81) | .002↓ | −7.80 (2.41) | .004↓ | −4.37 (1.39) | .003↓ |
| Post-FEV1 (% predicted)∗ | −6.15 (1.84) | .002↓ | −3.49 (1.45) | .028↓ | −8.24 (1.36) | <.0001↓ |
| Pre-FEV1/FVC ratio (%)∗ | 1.22 (1.86) | .52 | 3.59 (1.47) | .024↑ | 1.80 (1.83) | .33 |
| VAS score, cough (mm)∗ | 17.38 (5.00) | .002↑ | 33.92 (5.97) | <.0001↑ | 27.19 (4.14) | <.0001↑ |
| VAS score, dyspnea (mm)∗ | 14.52 (5.35) | .011↑ | 37.15 (6.12) | <.0001↑ | 23.65 (3.63) | <.0001↑ |
| Blood neutrophils (× 109/L)∗ | 2.03 (0.52) | .0004↑ | 0.77 (0.40) | .062 | 0.60 (0.37) | .11 |
| Blood eosinophils (× 109/L) | 0.69 (0.47-1.02) | .06 | 1.10 (0.77-1.58) | .58 | 0.60 (0.42-0.88) | .009↓ |
| TCC (× 106 cells/g sputum) | 3.49 (2.10-5.79) | <.0001↑ | 1.27 (0.74-2.16) | .37 | 1.67 (0.94-2.99) | .08 |
| Sputum neutrophil count (%)∗ | 15.22 (5.29) | .008↑ | −2.77 (4.63) | .56 | −3.18 (3.92) | .42 |
| Sputum eosinophil count (%) | 0.51 (0.33-0.79) | .004↓ | 2.14 (0.93-4.94) | .074 | 0.68 (0.40-1.16) | .15 |
| IL-1β (pg/mL) | 10.4 (5.2-20.9) | <.0001↑ | 1.42 (0.59-3.38) | .42 | 1.20 (0.72-2.01) | .47 |
| IL-5 (pg/mL) | 0.40 (0.23-0.69) | .002↓ | 2.41 (1.33-4.37) | .006↑ | 0.92 (0.56-1.52) | .74 |
| IL-6 (pg/mL) | 1.48 (0.90-2.42) | .12 | 1.06 (0.59-1.90) | .83 | 2.16 (1.11-4.19) | .025↑ |
| IL-6R (pg/mL) | 2.98 (1.90-4.68) | <.0001↑ | 1.60 (1.09-2.34) | .02↑ | 1.06 (0.72-1.56) | .77 |
| IL-8 (pg/mL) | 1.67 (1.19-2.32) | .004↑ | 1.34 (0.80-2.26) | .25 | 0.82 (0.55-1.23) | .33 |
| IL-10 (pg/mL) | 3.34 (1.96-5.70) | .0001↑ | 0.86 (0.59-1.26) | .43 | 4.09 (2.23-7.49) | <.0001↑ |
| IL-13 (pg/mL) | 0.82 (0.64-1.07) | .14 | 1.05 (0.76-1.44) | .76 | 1.23 (1.06-1.42) | .008↑ |
| CXCL10 (pg/mL) | 0.45 (0.24-0.87) | .019↓ | 1.00 (0.53-1.90) | .99 | 3.78 (2.01-7.10) | .0001↑ |
| CXCL11 (pg/mL) | 0.23 (0.10-0.51) | .0008↓ | 0.89 (0.39-2.06) | .78 | 7.60 (2.68-21.56) | .0003↑ |
| CCL2 (pg/mL) | 0.46 (0.28-0.77) | .004↓ | 0.87 (0.58-1.32) | .49 | 1.71 (1.07-2.74) | .025↑ |
| CCL3 (pg/mL) | 1.07 (0.65-1.77) | .78 | 0.89 (0.42-1.92) | .77 | 1.52 (0.88-2.62) | .13 |
| CCL4 (pg/mL) | 1.06 (0.69-1.64) | .77 | 1.84 (1.04-3.26) | .037↑ | 1.56 (0.89-2.73) | .12 |
| CCL5 (pg/mL) | 1.96 (1.30-2.94) | .002↑ | 1.15 (0.67-1.99) | .59 | 3.01 (1.88-4.82) | <.0001↑ |
| CCL13 (pg/mL) | 0.36 (0.26-0.51) | <.0001↓ | 0.85 (0.57-1.26) | .40 | 0.65 (0.48-0.88) | .007↓ |
| CCL17 (pg/mL) | 0.23 (0.13-0.41) | <.0001↓ | 1.80 (1.08-3.01) | .026↑ | 0.58 (0.40-0.85) | .007↓ |
| CCL26 (pg/mL) | 0.54 (0.34-0.85) | .01↓ | 2.48 (1.57-3.92) | .0004↑ | 1.01 (0.68-1.52) | .95 |
| TNF-α (pg/mL) | 7.92 (4.01-15.62) | <.0001↑ | 1.22 (0.56-2.66) | .60 | 3.92 (1.82-8.48) | .0009↑ |
| TNF-R1 (pg/mL) | 2.95 (2.02-4.30) | <.0001↑ | 1.26 (0.85-1.87) | .24 | 0.99 (0.70-1.39) | .93 |
| TNF-R2 (pg/mL) | 2.93 (1.94-4.41) | <.0001↑ | 1.61 (1.00-2.59) | .05 | 1.54 (0.89-2.67) | .12 |
| VEGF (pg/mL) | 1.44 (1.10-1.88) | .01↑ | 1.03 (0.76-1.40) | .85 | 0.84 (0.67-1.04) | .10 |
| IFN-γ (pg/mL) | 1.76 (1.02-3.03) | .042↑ | 0.70 (0.39-1.25) | .22 | 9.33 (3.85-22.60) | <.0001↑ |
Data are presented as geometric means with 95% CIs, unless otherwise stated. Fold changes in mediators are shown. Values in boldface indicate statistical significance.
FVC, Forced vital capacity; TCC, total sputum cell count.
Mean (SEM).
Cluster 1
Cluster 1 was a COPD-predominant group of 34 patients (asthmatic patients, 7; patients with COPD, 27). Of these, 59% were men, the group's mean age was 67 years, and 71% were current smokers or ex-smokers. Patients in this cluster had increased blood and sputum neutrophil counts and sputum proinflammatory mediator levels (IL-1β, IL-6, IL-6R, IL-8, TNF-α, TNF-R1, TNF-R2, and VEGF) and greater proportions of bacteria-associated exacerbations compared with those in clusters 2 and 3. In addition, this group had greater Proteobacteria and P/F ratios (Fig 2), with beta diversities of 0.16 and 0.46 at the phylum and genus levels, respectively. When compared with the stable state, blood and sputum neutrophil counts, sputum total cell counts, and concentrations of IL-1β, IL-6R, IL-8, IL-10, CCL5, TNF-α, TNF-R1, TNF-R2, VEGF, and IFN-γ were increased significantly. Sputum eosinophil and macrophage counts and IL-5, CXCL10, CXCL11, CCL2, CCL13, CCL17, and CCL26 levels were significantly lower (Table III). Serum IL-6, TNF-R1, and TNF-R2 levels increased and CCL2, CCL13, CCL17, and CCL26 levels decreased at exacerbation compared with stable visits (see Table E7).
Cluster 2
Cluster 2 consisted of 27 patients (asthmatic patients, 10; patients with COPD, 17). Some 63% were men with a mean age of 63 years, and 78% were current smokers or ex-smokers. Patients in this cluster had increased sputum and blood eosinophil counts and increased levels of sputum IL-5, IL-13, CCL13, CCL17, and CCL26 and serum IL-5 and CCL26 T2 mediators (Table I and see Table E3). This cluster exhibited significantly greater alpha diversity of the microbiome and a greater proportion of Bacteroidetes at the phylum level compared with cluster 1 (Fig 2), with beta diversities of 0.24 and 0.57 at the phylum and genus levels, respectively. In the paired comparison between stable and exacerbation states, sputum IL-5, IL-6R, CCL4, CCL17, and CCL26 levels were significantly increased at exacerbation, whereas in contrast, serum IL-8 and TNF-α levels were decreased (Table III and see Table E7).
Cluster 3
Cluster 3 consisted of 44 patients (asthmatic patients, 15; patients with COPD, 29). Some 68% were men with a mean age of 68 years, and 73% were current smokers or ex-smokers. Patients in this cluster had increased levels of the T1 mediators CXCL10, CXCL11, and IFN-γ in sputum (Table I) and serum CXCL10 levels (see Table E3). There were greater proportions of Actinobacteria and Firmicutes at the phylum level and Streptococcus (phylum Firmicutes) at the genus level, with a lesser proportion of Proteobacteria and P/F ratio (Figs 2 and 3). The beta diversities were 0.29 at the phylum and 0.62 at the genus level. Blood eosinophil counts and sputum CCL13 and CCL17 concentrations were lower and IL-6, IL-10, IL-13, CXCL10, CXCL11, CCL2, CCL5, TNF-α, and IFN-γ concentrations were greater at exacerbation compared with stable state (Table III). Serum CXCL10 levels increased at exacerbation versus the stable state, and serum CCL13 and CCL17 levels decreased (see Table E7).
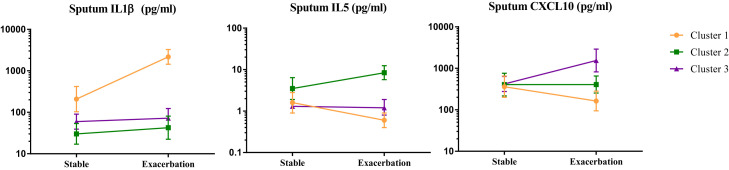
Changes within each cluster for the highest loading factors of sputum IL-1β, IL-5, and CXCL10 between the stable and exacerbation states are as shown in Fig E3 in this article's Online Repository at www.jacionline.org. Sputum IL-1β and IL-5 levels increased significantly in clusters 1 and 2, respectively, with concentrations for these mediators also increased in the stable state. Sputum CXCL10 concentrations increased at exacerbation in cluster 3, but in contrast, levels of this mediator were not different between the groups in the stable state. Changes between the stable state and exacerbations for both asthmatic patients and patients with COPD within each cluster are as shown in Tables E8 and E9.
Fig E3.
Sputum IL-1β, IL-5, and CXCL10 levels in each cluster at stable state and exacerbation.
Discussion
In this study 3 exacerbation biologic clusters were identified by using a combination of factor and cluster analyses. Each cluster had different percentages of asthmatic patients and patients with COPD. Interestingly, the clusters seemed to demonstrate 3 distinct and biologically plausible inflammatory profiles. Cluster 1 was a COPD-predominant group, with evidence of neutrophilic inflammation, increased proinflammatory mediators, bacteria-associated exacerbations, and proportions of Proteobacteria and P/F ratio at the phylum level. Patients in cluster 2 exhibited evidence of eosinophilic inflammation, with increased levels of T2 mediators and proportion of Bacteroidetes. Patients in cluster 3 had greater T1 mediator levels and greater proportions of Actinobacteria and Firmicutes at the phylum level. Importantly, comparisons with assessments performed while the patients were stable demonstrated that levels of the proinflammatory, T2, and T1 mediators were increased at exacerbation in clusters 1, 2, and 3, respectively, whereas eosinophilic inflammation and T2 mediator levels were decreased in clusters 1 and 3 at exacerbation. In clusters 1 and 2 proinflammatory and T2 mediator levels were also increased in the stable state, respectively. Therefore our findings indicate that 3 exacerbation biologic clusters are shared between asthma and COPD. In addition, the inflammatory profiles in these clusters are increased compared with those in the stable state and are associated with distinct airway bacterial ecologies.
The strength of our study was our ability to use statistical techniques that were applied previously to asthma and COPD independently2, 4 to characterize the biologic heterogeneity of asthma and COPD combined by using sputum mediators assessed with the same protocols and the same analytic platform. Similarities and differences of asthma and COPD, which were identified by comparing their characteristics at the disease level, have been published.8 This simple separation approach might not reflect the underlying biologic heterogeneity and does not provide insight into the multidimensional characteristics of the diseases.
Cluster analysis has uncovered biological clusters within diseases and can uncover the common and distinctive meaningful subgroups of both diseases that are not evident at disease level. We have extended the examination of such derived biologic clusters to include their associations with airway bacterial ecology to aid the understanding of the pathophysiologic connection with outcomes of airway diseases toward the realization of potentially new biomarkers and targeted therapies.
For instance, cluster 1, with its neutrophilic inflammation, increased proinflammatory mediator levels, and bacteria-associated exacerbations, represents the group that would most likely respond to antibiotics. There is some role of antibiotics for the prevention of COPD exacerbations.14, 15 Antibiotics for patients admitted to the intensive care unit for COPD exacerbations also appeared to be beneficial.16, 17 There is still continued uncertainty in asthmatic patients, with macrolide antibiotics providing some benefit in patients with stable disease,18, 19 whereas in patients with acute exacerbations, some benefit was demonstrated with telithromycin20 but not azithromycin.21 In the latter study almost half of those screened had already received antibiotics in primary care, and the study was underpowered. The lack of response observed might have been a consequence of failure to include those most likely to respond to antibiotics.
With advances in culture-independent techniques, we can now study the role of the lung microbiome in respiratory diseases to a better extent. Of interest, Wang et al22 have found that lung microbiome dynamics were associated with COPD exacerbations. Therefore identification of biomarkers and a biologic cluster with evidence of microbial dysbiosis might enable future targeted antibiotic trials. The P/F ratio is also emerging as a possible simple measure that reflects bacterial composition, and whether this can be applied in future intervention studies to guide therapies needs to be tested.23, 24
Cluster 2 would seem likely to respond to therapies targeting eosinophilic and T2 inflammation. Targeting eosinophilic inflammation in both asthmatic patients and patients with COPD with corticosteroids reduces exacerbation frequency.12, 25, 26 Likewise, mAb therapies targeting the IL-5 cytokine and its receptor are effective for decreasing the risk of exacerbation in patients with eosinophilic asthma,27, 28, 29, 30, 31, 32, 33, 34 with evidence of some benefit, although less consistent, in patients with eosinophilic COPD.35, 36 Findings from 2 pivotal phase III studies evaluating the efficacy and safety of mepolizumab, an anti–IL-5 mAb, in addition to standard of care in patients with COPD demonstrated reductions in the frequency of moderate-to-severe exacerbations in those with higher blood eosinophil counts.37 Our study underscores the importance of an eosinophilic phenotype in asthmatic patients and patients with COPD, but future studies need to consider whether the underlying mechanisms are common in asthmatic patients and patients with COPD. One limitation was that IgE levels were not assessed at exacerbation, and the role of allergy could be explored further in future studies.
The interferon-inducible chemokines CXCL10 and CXCL11 in sputum and serum were not only increased in cluster 3 but were also increased significantly at exacerbation compared with those in the stable state. This biologic cluster with high levels of interferon-inducible chemokines was not observed previously in patients with stable asthma and those with COPD.7 Because these chemokines have been identified as biomarkers of virus-associated exacerbations,4, 38, 39 it would seem likely that viral infections are possible triggers and that this cluster would be most amenable to future antiviral interventions. Consistently, the proportion of virus-associated exacerbations was greater in cluster 3, but this did not reach statistical significance between clusters with asthma and COPD combined or independently. Viral identification is challenging, especially because sputum viral load can peak before the peak of lower respiratory symptoms.40 Therefore our findings likely underrepresented the proportion of virus-associated exacerbations.
One major limitation of this study is that the number of patients with asthma was small and the percentage of both asthmatic patients and patients with COPD who provided sufficient samples to study the microbial ecology was approximately half of the patients. The remaining patients were unable to produce sufficient sputum for analysis. The clinical characteristics between those who did versus those who did not provide samples for microbiological assessment were similar, suggesting these groups were comparable. We cannot exclude the possibility of an acquisition bias toward a microbial ecology associated with more sputum production. Notwithstanding this limitation, we did find consistent differences in the microbial ecology between the clusters.
Second, we cannot demonstrate the stability of these exacerbation biologic clusters based on assessment of a single exacerbation. Therefore these findings need to be explored in larger multicenter studies. Another limitation of the size of the study is that we could not analyze the patients according to the severities of their asthma or COPD or the severities of exacerbations. We did not control for baseline therapy between asthmatic patients and patients with COPD; however, the corticosteroid dosages were very similar between the groups, which indicates this was unlikely to have had a differential effect on the underlying inflammatory or microbial profile. A limitation of cluster analysis is that it is specific to the data set studied, and therefore neither the asthma and COPD groups nor the stable visits can be analyzed independently to generate the proportions of the same clusters. This means cluster stability cannot be simply determined. However, we found that the patterns of the inflammatory profiles were similar for those asthmatic patients or patients with COPD between clusters; the patterns were similar between the stable and exacerbation state for the proinflammatory (cluster 1) and eosinophilic (cluster 2) phenotypes, with amplification of an underlying inflammatory profile at the exacerbation event. However, the T1 mediators that were increased at exacerbation in cluster 3 were not increased in the stable state. Whether the stable state mediator profiles can identify subjects most likely to respond to specific anti-inflammatory or antibiotic therapy to reduce future exacerbation risk requires further study. We included a large number of cytokines and chemokines in the analysis, but this still only reflects a minority of the number of mediators present in the sputum and serum samples. Because of limitations of the available sample, we could not extend the study to include other important mediators, such as eicosanoids and β-interferons. Metabolomic approaches, such as somologics, might provide more insight into the inflammatory mediator network and should be considered in future studies.
In conclusion, we used a biologic clustering approach to look at asthma and COPD exacerbations. We identified a COPD-predominant cluster with evidence of neutrophilic inflammation, increased proinflammatory mediator levels, and bacteria-associated exacerbations, a cluster with evidence of eosinophilic inflammation and increased T2 mediator levels and a cluster with increased T1 mediator levels. Our study aids in the understanding of the heterogeneity of asthma and COPD exacerbations and suggests that endotype might be more important than an asthma or COPD diagnosis. It highlights the need for further research in developing novel biomarkers to predict disease outcome and guide targeted therapies.
Clinical implications.
Sputum mediator and microbiome profiling can determine the distinct and overlapping asthma and COPD biologic exacerbation clusters, highlighting the heterogeneity of these exacerbations.
Footnotes
Supported by MedImmune and a Wellcome Trust Senior Fellowship (to C.E.B.). The research was performed in laboratories funded in part by the European Regional Development Fund (ERDF 05567). This study was also part supported by the Medical Research Council; the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, United Kingdom; and AirPROM (FP7-270194). S.L.J. was supported by the Asthma UK Clinical Chair (CH11SJ) and an MRC Centre grant (no. G1000758). The views expressed are those of the author or authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Disclosure of potential conflict of interest: P. H. Pang reports personal fees from Teva UK Limited outside the submitted work. D. Desai reports personal fees from AstraZeneca, Boehringer Ingelheim, and Chiesi outside the submitted work. M. Bafadhel reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer outside the submitted work. S. Cohen, P. Newbold, L. Rapley, J. Woods, and P. Rugman are employees of MedImmune, which supported the study. I. D. Pavord reports personal fees and nonfinancial support from AstraZeneca and Boehringer Ingelheim and personal fees from Aerocrine, Almirall, Novartis, GlaxoSmithKline, Genentech, Regeneron, Merck & Co, Schering-Plough, Mylan Specialty (Dey Pharma), Napp Pharmaceuticals and Respivert outside the submitted work. S. L. Johnston reports grants/personal fees from Apollo Therapeutics, AstraZeneca, Boehringer Ingelheim, Bioforce, Chiesi, Genentech, GlaxoSmithKline, Merck, Novartis, Sanofi/Regeneron, Synairgen, and Therapeutic Frontiers; is a director of Therapeutic Frontiers; and holds patents on the use of inhaled interferons as treatments for exacerbations of airway disease outside the submitted work. R. D. May is a former employee of MedImmune, which supported the study. C. E. Brightling has received grants and personal fees paid to his institution from MedImmune (AstraZeneca), GlaxoSmithKline, Roche/Genentech, Novartis, Chiesi, Pfizer, Teva, Sanofi/Regeneron, Glenmark, Mologic, PreP, and Vectura, outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Summary statistics across asthmatic patients and patients with COPD
| Asthmatic patients (n = 32) | Patients with COPD (n = 73) | P value | |
|---|---|---|---|
| Male sex, no. (%) | 14 (43.8) | 53 (72.6) | .005 |
| Current or ex-smokers, no. (%) | 7 (21.9) | 70 (95.9) | <.0001 |
| Pack-year history∗‡ | 10 (2.5-22.5) | 45.4 (26-60.0) | .045 |
| Age (y)† | 55.47 (2.01) | 71.22 (3.05) | .001 |
| BMI (kg/m2)† | 32.14 (1.2) | 25.59 (0.53) | <.0001 |
| Exacerbations in last year∗ | 3 (2-5) | 4 (2-6) | .31 |
| Maintenance prednisolone, no. (%) | 22 (68.8) | 6 (8) | <.0001 |
| Daily prednisolone dose (mg)∗§ | 10 (7.5-15) | 5 (5-5) | .001 |
| Daily ICS dose (μg/d)∗‖ | 1600 (900-2000) | 1200 (800-2000) | .5 |
| Pre-FEV1 (L)† | 1.91 (0.19) | 1.13 (0.06) | <.0001 |
| Post-FEV1 (L)† | 2.05 (0.22) | 1.16 (0.06) | <.0001 |
| Pre-FEV1 (% predicted)† | 68.29 (5.47) | 40.76 (2.61) | <.0001 |
| Post-FEV1 (% predicted)† | 74.16 (6.13) | 41.87 (2.65) | <.0001 |
| Pre-FEV1/FVC ratio (%)† | 70.61 (2.81) | 51.49 (1.46) | <.0001 |
| VAS, cough (mm)† | 66.87 (3.3) | 64.39 (2.58) | .58 |
| VAS, dyspnea (mm)† | 64.23 (3.32) | 69.67 (2.63) | .24 |
| Blood neutrophils (× 109/L)† | 6.81 (0.48) | 6.85 (0.37) | .95 |
| Blood eosinophils (× 109/L) | 0.14 (0.09-0.23) | 0.16 (0.13-0.2) | .56 |
| TCC (× 106 cells/g sputum) | 3.39 (1.87-6.14) | 6.22 (4.47-8.67) | .06 |
| Sputum neutrophil count (%)† | 63.73 (4.97) | 74.1 (2.7) | .05 |
| Sputum eosinophil count (%) | 1.07 (0.48-2.38) | 1.09 (0.72-1.65) | .96 |
| Bacterial load (log10 CFU/mL) | 6.1 (1.0) | 6.5 (1.1) | .10 |
| Bacteria-associated exacerbation (%) | 29.2 | 35.2 | .17 |
| Viral (%) | 47.1 | 33.8 | .31 |
| Sputum IL-1β | 152.4 (68.8-337.5) | 208.7 (120.5-361.4) | .52 |
| Sputum IL-5 | 2.7 (1.4-5.1) | 1.3 (0.9-1.8) | .026 |
| Sputum IL-6 | 102.2 (56-186.3) | 626.6 (438-896.4) | <.0001 |
| Sputum IL-6R | 442.1 (296.1-660.1) | 254.3 (189.9-340.5) | .034 |
| Sputum IL-8 | 4941.6 (3111.4-7848.3) | 4885.2 (3685.6-6475.4) | .97 |
| Sputum IL-10 | 10.5 (4.7-23.3) | 5.8 (4.1-8.3) | .11 |
| Sputum IL-13 | 11.8 (9.1-15.3) | 9.4 (8.5-10.4) | .05 |
| Sputum CXCL10 | 1405.4 (754.7-2617.2) | 344.4 (219.9-539.4) | .001 |
| Sputum CXCL11 | 72.4 (26.4-198.9) | 19.9 (10.7-37.3) | .027 |
| Sputum CCL2 | 439.9 (278.2-695.7) | 469.8 (354.8-622) | .8 |
| Sputum CCL3 | 35.6 (19.9-63.9) | 80.6 (57.7-112.5) | .016 |
| Sputum CCL4 | 668.4 (362.1-1233.8) | 1305.9 (968-1761.9) | .028 |
| Sputum CCL5 | 20.1 (11.5-35) | 6.8 (5.1-9.1) | <.0001 |
| Sputum CCL13 | 15.5 (11.3-21.1) | 18.1 (14.6-22.6) | .42 |
| Sputum CCL17 | 20.2 (12.5-32.6) | 11.2 (7.7-16.2) | .07 |
| Sputum CCL26 | 8.7 (4.8-15.8) | 4.4 (3.3-5.8) | .02 |
| Sputum TNF-α | 13.5 (5.4-33.6) | 20.4 (11.8-35.1) | .42 |
| Sputum TNF-R1 | 953.2 (574.1-1582.5) | 1754.2 (1341.9-2293.1) | .021 |
| Sputum TNF-R2 | 670.2 (382.1-1175.4) | 649.5 (470.9-895.9) | .92 |
| Sputum VEGF | 1454 (1111.2-1902.6) | 1420.3 (1220.3-1653.1) | .87 |
| Sputum IFN-γ | 2.3 (0.9-6.1) | 1 (0.6-1.5) | .06 |
| Serum IL-1β | 3.28 (2.21-4.86) | 7.31 (6.49-8.24) | .0003 |
| Serum IL-5 | 4.19 (3.00-5.84) | 2.46 (2.26-2.68) | .004 |
| Serum IL-6 | 2.8 (2.0-4.1) | 21.5 (16.8-27.6) | <.0001 |
| Serum IL-8 | 11.4 (8.7-14.9) | 6.3 (5.3-7.4) | .0002 |
| Serum CXCL10 | 175.1 (138.7-221.2) | 58.9 (50.1-69.3) | <.0001 |
| Serum CXCL11 | 205.6 (155.4-271.9) | 64.6 (56.4-74.0) | <.0001 |
| Serum CCL2 | 571.6 (486.4-671.6) | 599.5 (537.2-669.1) | .632 |
| Serum CCL4 | 180.6 (142.1-229.4) | 389.3 (351.1-431.7) | <.0001 |
| Serum CCL13 | 575.2 (488.3-677.5) | 576.0 (498.3-665.9) | .990 |
| Serum CCL17 | 652.1 (459.3-926.0) | 368.0 (307.4-440.4) | .002 |
| Serum CCL26 | 20.9 (10.9-40.0) | 9.9 (8.9-11.0) | .027 |
| Serum TNF-α | 6.31 (4.16-9.56) | 3.48 (3.17-3.82) | .008 |
| Serum TNF-R1 | 4136.0 (3682.4-4645.5) | 8828.2 (8016.7-9721.9) | <.0001 |
| Serum TNF-R2 | 5493.2 (4725.0-6386.3) | 5345.3 (4924.3-5802.2) | .733 |
| Serum VEGF | 889.3 (658.1-1201.7) | 801.6 (725.5-885.7) | .508 |
Data are presented as geometric means with 95% CIs, unless otherwise stated. Values in boldface indicate statistical significance.
BMI, Body mass index; CFU, colony-forming unit; ICS, inhaled corticosteroid; FVC, forced vital capacity; TCC, total sputum cell count.
Median (first and third quartiles).
Mean (SEM).
Pack-year history of current smokers and ex-smokers.
Dose for only those patients prescribed daily prednisolone.
Beclomethasone dipropionate equivalent.
Table E2.
Factor loading matrix for sputum mediators after orthogonal varimax rotation
| Variable | Factor loadings |
C1 | U2 | |||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |||
| IL-β | 0.91 | 0.01 | −0.24 | −0.08 | 0.89 | 0.11 |
| IL-5 | 0.03 | 0.24 | 0.89 | 0.04 | 0.85 | 0.15 |
| IL-6 | 0.54 | 0.17 | −0.01 | 0.52 | 0.59 | 0.41 |
| IL-6R | 0.90 | 0.08 | 0.16 | −0.15 | 0.86 | 0.14 |
| IL-8 | 0.81 | 0.03 | 0.00 | 0.09 | 0.67 | 0.33 |
| IL-10 | 0.69 | 0.46 | −0.08 | −0.05 | 0.69 | 0.31 |
| CXCL10 | 0.00 | 0.88 | 0.26 | 0.07 | 0.84 | 0.16 |
| CXCL11 | −0.14 | 0.78 | 0.28 | 0.10 | 0.72 | 0.28 |
| CCL2 | 0.09 | 0.57 | 0.20 | 0.49 | 0.61 | 0.39 |
| CCL4 | 0.44 | 0.23 | 0.37 | 0.25 | 0.45 | 0.55 |
| CCL5 | 0.58 | 0.66 | 0.05 | −0.08 | 0.78 | 0.22 |
| CCL13 | −0.10 | 0.14 | 0.53 | 0.54 | 0.61 | 0.39 |
| CCL17 | −0.25 | 0.04 | 0.76 | 0.24 | 0.70 | 0.30 |
| CCL26 | −0.09 | 0.16 | 0.85 | −0.09 | 0.76 | 0.24 |
| TNF-α | 0.85 | 0.30 | −0.24 | 0.06 | 0.86 | 0.14 |
| TNF-R1 | 0.94 | −0.12 | −0.12 | 0.15 | 0.93 | 0.07 |
| TNF-R2 | 0.92 | 0.22 | 0.04 | 0.09 | 0.91 | 0.09 |
| VEGF | 0.67 | −0.22 | 0.05 | 0.17 | 0.53 | 0.47 |
| IFN-γ | 0.34 | 0.79 | 0.03 | 0.04 | 0.74 | 0.26 |
Values in boldface indicate statistical significance.
C1, Proportion of total variation accounted for by the common factors (common variance); U2, proportion of total variation not accounted for by the common factors (unique variance).
Table E3.
Summary statistics for serum mediators across the 3 identified biologic exacerbation clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
P value |
||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients, n = 7; patients with COPD, n = 27 | Asthmatic patients, n = 10; patients with COPD, n = 17 | Asthmatic patients, n = 15; patients with COPD, n = 29 | Cluster 1 vs cluster 2 | Cluster 1 vs cluster 3 | Cluster 2 vs cluster 3 | ANOVA | |
| IL-1β (pg/mL) | 5.5 (4.3-6.9) | 5.9 (4.2-8.4) | 5.9 (4.4-7.7) | .920 | .907 | 1.000 | .896 |
| IL-5 (pg/mL) | 2.3 (1.9-2.8) | 3.3 (2.8-4.0) | 3.0 (2.5-3.6) | .037 | .087 | .779 | .028 |
| IL-6 (pg/mL) | 17.2 (10.1-29.2) | 8.1 (4.8-13.8) | 11.2 (7.5-16.6) | .104 | .361 | .630 | .116 |
| IL-8 (pg/mL) | 7.6 (5.8-10.1) | 6.6 (5.1-8.5) | 7.9 (6.2-10.1) | .738 | .978 | .597 | .613 |
| CXCL10 (pg/mL) | 61.7 (49.5-76.8) | 68.8 (51.4-92.0) | 109.6 (82.2-146.2) | .864 | .006 | .055 | .005 |
| CXCL11 (pg/mL) | 68.9 (53.9-88.1) | 90.8 (63.8-129.3) | 100.8 (78.0-130.4) | .388 | .095 | .862 | .108 |
| CCL2 (pg/mL) | 554 (493-623) | 553 (445-689) | 644 (556-747) | 1.000 | .320 | .377 | .252 |
| CCL4 (pg/mL) | 286 (229-358) | 294 (227-381) | 340 (284-408) | .985 | .444 | .612 | .420 |
| CCL13 (pg/mL) | 531 (411-684) | 724 (601-871) | 538 (466-620) | .095 | .994 | .089 | .066 |
| CCL17 (pg/mL) | 397 (299-527) | 451 (334-609) | 457 (340-614) | .843 | .759 | .998 | .757 |
| CCL26 (pg/mL) | 7.3 (5.9-9.2) | 17.1 (11.6-25.2) | 15.0 (10.3-21.9) | .006 | .007 | .864 | .002 |
| TNF-α (pg/mL) | 3.5 (3.0-4.0) | 4.0 (3.1-5.1) | 4.9 (3.6-6.5) | .762 | .117 | .519 | .133 |
| TNF-R1 (pg/mL) | 7650 (6506-8995) | 5917 (4852-7217) | 7358 (6200-8731) | .146 | .941 | .210 | .135 |
| TNF-R2 (pg/mL) | 5315 (4793-5894) | 4756 (4282-5283) | 5840 (5111-6673) | .473 | .492 | .060 | .073 |
| VEGF (pg/mL) | 918 (783-1076) | 771 (568-1046) | 795 (678-932) | .474 | .508 | .975 | .421 |
Data are presented as geometric means with 95% CIs. Values in boldface indicate statistical significance.
Table E4.
Clinical parameters for asthmatic patients and patients with COPD across the 3 identified biologic exacerbation clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients (n = 7) | Patients with COPD (n = 27) | Asthmatic patients (n = 10) | Patients with COPD (n = 17) | Asthmatic patients (n = 15) | Patients with COPD (n = 29) | ||
| Male sex, no. (%) | 2 (29) | 18 (67) | 5 (50) | 12 (71) | 7 (47) | 23 (79)¶ | |
| Current or ex-smokers, no. (%) | 0 | 24 (89)¶ | 4 (40) | 17 (100)¶ | 3 (20) | 29 (100)¶ | |
| Pack-year history∗‡ | — | 46 (22-51)¶ | 16 (8-24) | 46 (32-54)¶ | 8 (5-9) | 42 (29-65)¶ | |
| Age (y)† | 58.9 (4.3) | 69.4 (1.6)¶ | 54.3 (4.0) | 68.0 (1.7)¶ | 54.7 (2.8) | 68.1 (2.1)¶ | |
| BMI (kg/m2)† | 30.7 (2.9) | 25.9 (0.9) | 33.5 (2.1) | 24.6 (0.8)¶ | 31.9 (1.7) | 25.8 (0.9)¶ | |
| Exacerbations in last year∗ | 2 (2-3) | 3 (1-7) | 2 (2-5) | 5 (2-7) | 4 (2-5) | 3 (2-5)¶ | |
| Maintenance prednisolone, no. (%) | 6 (86) | 5 (19)¶ | 6 (60) | 1 (6)¶ | 10 (67) | 2 (7)¶ | |
| Daily prednisolone dose (mg)∗§ | 12.5 (6.25-18.75) | 5 (5-12.5) | 10 (8.75-10) | 5 | 10 (10-13.75) | 7.5 (6.25-8.75) | |
| Daily ICS dose (μg/d)∗‖ | 2000 (1800-2000) | 1200 (800-2000) | 1600 (1000-1900) | 1600 (800-2000) | 1600 (800-2000) | 1000 (800-2000) | |
| Pre-FEV1 (L)† | 1.73 (0.27) | 1.10 (0.09)¶ | 2.43 (0.52) | 1.03 (0.14) | 1.76 (0.25) | 1.23 (0.10)¶ | |
| Post-FEV1 (L)† | 1.89 (0.22) | 1.17 (0.08)¶ | 2.86 (0.62) | 1.06 (0.16) | 1.77 (0.31) | 1.21 (0.10)¶ | |
| Pre-FEV1 (% predicted)† | 78.60 (12.81) | 43.62 (3.15)¶ | 71.09 (7.27) | 38.58 (4.95)¶ | 60.71 (7.70) | 44.68 (3.33)¶ | |
| Post-FEV1 (% predicted)† | 85.32 (10.50) | 46.47 (2.99)¶ | 83.11 (6.66) | 38.78 (5.27)¶ | 61.31 (9.69) | 44.25 (3.40)¶ | |
| FEV1/FVC ratio (%)† | 77.46 (2.54) | 51.30 (2.73)¶ | 71.05 (3.04) | 50.16 (2.89)¶ | 65.79 (5.45) | 52.49 (2.13)¶ | |
| VAS, cough (mm)† | 75.50 (2.08) | 68.00 (3.44) | 62.30 (4.59) | 60.56 (6.87) | 66.47 (5.94) | 63.14 (4.12) | |
| VAS, dyspnea (mm)† | 66.67 (8.46) | 69.22 (4.27) | 66.80 (4.78) | 70.88 (7.09) | 61.53 (5.28) | 69.41 (3.58) | |
| Blood neutrophils (× 109/L)† | 7.59 (0.69) | 8.07 (0.70) | 6.21 (0.78) | 5.74 (0.65) | 6.90 (0.80) | 6.36 (0.51) | ∗∗ |
| Blood eosinophils (× 109/L) | 0.2 (0.11-0.39) | 0.12 (0.09-0.16) | 0.25 (0.10-0.64) | 0.33 (0.22-0.51) | 0.09 (0.04-0.17) | 0.14 (0.10-0.20) | ∗∗ |
| TCC (× 106 cells/g sputum) | 18.3 (6.2-54.3) | 16.1 (10.3-25.1) | 2.0 (0.8-4.8) | 3.8 (2.3-6.5) | 2.4 (0.9-5.9) | 3.5 (2.0-5.9) | #∗∗ |
| Sputum neutrophil count (%)† | 92.58 (3.22) | 88.39 (3.25) | 53.43 (7.93) | 61.39 (4.88) | 58.34 (6.99) | 68.00 (4.06) | #∗∗ |
| Sputum eosinophil count (%) | 0.27 (0.13-0.57) | 0.36 (0.28-0.46) | 5.89 (1.18-29.35) | 6.63 (3.01-14.61) | 0.55 (0.21-1.40) | 1.02 (0.53-1.95) | #∗∗ |
| Bacterial load (log10 CFU/mL) | 7.3 (0.5) | 6.9 (0.2) | 6.0 (0.3) | 5.4 (0.3) | 5.6 (0.2) | 6.6 (0.2)¶ | #∗∗ |
| Bacteria-associated exacerbation (%) | 84 | 78 | 22 | 24 | 0 | 28 | #∗∗ |
| Viral (%) | 0 | 33 | 40 | 24 | 56 | 38 | |
Data are presented as geometric means with 95% CIs, unless otherwise stated. Values in boldface indicate statistical significance.
BMI, Body mass index; CFU, colony-forming unit; ICS, inhaled corticosteroid; FVC, forced vital capacity; TCC, total sputum cell count.
Median (first and third quartiles).
Mean (SEM).
Pack-year history of current smokers and ex-smokers.
Dose for only those patients prescribed daily prednisolone.
Beclomethasone dipropionate equivalent.
Significant difference between asthmatic patients and patients with COPD within clusters.
Overall significant difference between clusters in asthmatic patients or patients with COPD, respectively.
Table E5.
Sputum mediators for asthmatic patients and patients with COPD across the 3 identified biologic exacerbation clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients (n = 7) | Patients with COPD (n = 27) | Asthmatic patients (n = 10) | Patients with COPD (n = 17) | Asthmatic patients (n = 15) | Patients with COPD (n = 29) | ||
| IL-1β (pg/mL) | 2,914 (1,038-8,179) | 2,007 (1,253-3,215) | 59 (17-206) | 35 (16-79) | 72 (29-179) | 72 (36-146) | †‡ |
| IL-5 (pg/mL) | 1.0 (0.3-3.6) | 0.6 (0.4-0.8) | 10.4 (4.2-25.5) | 7.4 (4.9-11.2) | 1.7 (0.7-4.6) | 1.0 (0.6-1.7) | †‡ |
| IL-6 (pg/mL) | 243 (76-772) | 791 (453-1,381) | 47 (17-127) | 368 (237-571)∗ | 115 (41-319) | 689 (336-1,413)∗ | |
| IL-6R (pg/mL) | 1,327 (598-2,944) | 733 (506-1,061) | 396 (253-620) | 149 (104-214)∗ | 285 (150-542) | 130 (86-197)∗ | †‡ |
| IL-8 (pg/mL) | 16,605 (8,979-30,709) | 12,432 (9,419-16,408) | 3,662 (1,630-8,227) | 2,945 (2,029-4,274) | 3,428 (1,668-7,042) | 2,755 (1,688-4,495) | †‡ |
| IL-10 (pg/mL) | 39.3 (6.2-247.9) | 13.8 (8.2-23.3) | 1.9 (1.5-2.5) | 1.9 (1.6-2.3) | 17.7 (4.7-67.2) | 5.1 (2.7-9.3) | †‡ |
| IL-13 (pg/mL) | 10.0 (5.8-17.5) | 8.3 (7.7-9.1) | 14.3 (7.2-28.5) | 12.0 (8.8-16.2) | 11.2 (8.0-15.7) | 9.2 (7.8-10.8) | ‡ |
| CXCL10 (pg/mL) | 634 (108-3,734) | 115 (69-189)∗ | 663 (272-1,618) | 305 (173-537) | 3,363 (1,408-8,035) | 1,031 (444-2,392)∗ | †‡ |
| CXCL11 (pg/mL) | 13 (2-100) | 3 (2-5) | 34 (11-201) | 21 (9-49) | 269 (47-1,552) | 110 (35-354) | †‡ |
| CCL2 (pg/mL) | 539 (181-1,608) | 271 (189-388) | 210 (141-311) | 547 (329-909)∗ | 656 (289-1,491) | 717 (424-1,213) | ‡ |
| CCL3 (pg/mL) | 85.5 (7.7-948.3) | 90.7 (53.3-154.3) | 27.1 (12.9-56.9) | 67.3 (34.1-132.6) | 32.7 (11.4-93.3) | 80.2 (43.5-147.6) | |
| CCL4 (pg/mL) | 410 (56-2,995) | 1,401 (898-2,183) | 955 (410-2,223) | 1,688 (1,066-2,673) | 662 (242-1814) | 1,053 (576-1,925) | |
| CCL5 (pg/mL) | 54.9 (25.6-117.5) | 10.0 (6.9-14.3)∗ | 8.5 (3.6-20.1) | 3.2 (1.9-5.3)∗ | 22.2 (8.4-58.4) | 7.4 (4.2-13.0)∗ | †‡ |
| CCL13 (pg/mL) | 9.1 (6.6-12.7) | 11.3 (9.0-14.2) | 22.3 (12.1-40.8) | 36.4 (25.3-52.3) | 15.5 (9.2-26.2) | 18.7 (12.4-28.1) | ‡ |
| CCL17 (pg/mL) | 12.1 (6.3-23.5) | 3.7 (2.2-6.4) | 69.7 (27.4-177.7) | 71.8 (45.6-113.1) | 11.2 (6.4-19.6) | 10.4 (7.0-15.4) | †‡ |
| CCL26 (pg/mL) | 3.1 (1.1-9.0) | 2.1 (1.7-2.7) | 39.8 (16.6-95.2) | 20.4 (15.4-26.9) | 5.2 (2.4-11.3) | 3.5 (2.4-5.2) | †‡ |
| TNF-α (pg/mL) | 290.9 (90.2-937.5) | 109.0 (65.8-180.5) | 2.4 (1.0-5.7) | 2.6 (1.2-5.7) | 10.3 (2.7-37.9) | 14.2 (5.7-35.5) | †‡ |
| TNF-R1 (pg/mL) | 5,430 (3,932-7,499) | 5,643 (4,303-7,400) | 640 (311-1,314) | 860 (627-1,179) | 552 (267-1,144) | 898 (661-1,220) | †‡ |
| TNF-R2 (pg/mL) | 2,746 (1,715-4,398) | 1,785 (1,236-2,578) | 373 (173-801) | 330 (227-481) | 513 (190-1,385) | 377 (214-663) | †‡ |
| VEGF (pg/mL) | 2,880 (1,428-5,807) | 2,323 (1,829-2,950) | 1,372 (927-2,031) | 1,076 (812-1,426) | 1,099 (751-1,607) | 1,057 (889-1,257) | †‡ |
| IFN-γ (pg/mL) | 6.9 (1.6-30.9) | 0.6 (0.4-1.0)∗ | 0.3 (0.3-0.3) | 0.4 (0.3-0.4) | 5.4 (0.9-31.1) | 2.6 (1.0-6.9) | †‡ |
Data are presented as geometric means with 95% CIs. Values in boldface indicate statistical significance.
Significant difference between asthmatic patients and patients with COPD within clusters.
Overall significant difference between clusters in asthmatic patients or patients with COPD, respectively.
Table E6.
Serum mediators for asthmatic patients and patients with COPD across the 3 identified biologic exacerbation clusters
| Cluster 1 |
Cluster 2 |
Cluster 3 |
|||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients (n = 7) | Patients with COPD (n = 27) | Asthmatic patients (n = 10) | Patients with COPD (n = 17) | Asthmatic patients (n = 15) | Patients with COPD (n = 29) | ||
| IL-1β (pg/mL) | 2.21 (1.19-4.13) | 6.98 (5.90-8.26)∗ | 3.57 (1.30-9.82) | 7.55 (5.99-9.51) | 3.76 (2.01-7.03) | 7.48 (5.91-9.46)∗ | |
| IL-5 (pg/mL) | 2.76 (0.91-8.31) | 2.20 (1.94-2.49) | 4.42 (2.74-7.16) | 3.00 (2.46-3.65)∗ | 5.31 (3.71-7.58) | 2.44 (2.13-2.79)∗ | ‡ |
| IL-6 (pg/mL) | 2.6 (1.4-4.8) | 28.5 (17.3-46.7)∗ | 1.8 (1.4-2.3) | 16.6 (10.7-25.8)∗ | 3.8 (1.9-7.4) | 19.6 (13.5-28.3)∗ | |
| IL-8 (pg/mL) | 13.8 (6.5-29.3) | 6.5 (4.9-8.7)∗ | 7.8 (4.0-15.3) | 6.1 (4.7-7.9) | 12.7 (9.1-17.6) | 6.2 (4.6-8.3)∗ | |
| CXCL10 (pg/mL) | 148 (102-213) | 49 (41-58)∗ | 130 (80-212) | 51 (39-68)∗ | 223 (154-322) | 76 (55-106)∗ | ‡ |
| CXCL11 (pg/mL) | 164 (92-294) | 55 (45-67)∗ | 227 (90-569) | 66 (51-85)∗ | 225 (152-332) | 74 (58-96)∗ | |
| CCL2 (pg/mL) | 566 (400-801) | 551 (483-628) | 516 (357-745) | 572 (426-769) | 607 (468-786) | 665 (549-805) | |
| CCL4 (pg/mL) | 116 (75-180) | 365 (310-430)∗ | 156 (87-280) | 396 (340-462)∗ | 240 (175-328) | 408 (333-501)∗ | † |
| CCL13 (pg/mL) | 539 (368-789) | 528 (385-726) | 759 (511-1,127) | 708 (561-893) | 512 (412-635) | 552 (454-670) | |
| CCL17 (pg/mL) | 516 (233-1,142) | 370 (270-508) | 507 (279-921) | 427 (290-628) | 832 (462-1,498) | 336 (251-449)∗ | |
| CCL26 (pg/mL) | 3.2 (1.7-6.0) | 9.2 (7.8-10.8)∗ | 33.1 (11.0-99.3) | 12.5 (9.8-16.1) | 39.2 (15.2-100.8) | 9.1 (7.8-10.7)∗ | †‡ |
| TNF-α (pg/mL) | 3.6 (2.0-6.4) | 3.4 (3.0-4.0) | 5.0 (2.2-10.9) | 3.6 (3.0-4.3) | 9.3 (4.7-18.8) | 3.5 (2.9-4.1)∗ | |
| TNF-R1 (pg/mL) | 4,216 (3,161-5,623) | 8,981 (7,819-10,316)∗ | 3,936 (3,029-5,115) | 7,169 (5,756-8,927)∗ | 4,209 (3,509-5,049) | 9,822 (8,360-11,539)∗ | ‡ |
| TNF-R2 (pg/mL) | 6,022 (4,154-8,731) | 5,164 (4,633-5,756) | 5,055 (4,225-6,048) | 4,622 (4,017-5,317) | 5,535 (4,224-7,253) | 6,004 (5,123-7,036) | ‡ |
| VEGF (pg/mL) | 1,324 (877-1,998) | 832 (707-978)∗ | 542 (222-1,324) | 910 (712-1,163) | 962 (659-1,403) | 720 (619-838) | |
Data are presented as geometric means with 95% CIs. Values in boldface indicate statistical significance.
Significant difference between asthmatic patients and patients with COPD within clusters.
Overall significant difference by ANOVA between clusters in asthmatic patients or patients with COPD, respectively.
Table E7.
Change in levels of serum mediators between stable and exacerbation states within each identified biologic exacerbation cluster
| Cluster 1 (asthmatic patients, n = 7; patients with COPD, n = 27) |
Cluster 2 (asthmatic patients, n = 10; patients with COPD, n = 17) |
Cluster 3 (asthmatic patients, n = 15; patients with COPD, n = 29) |
||||
|---|---|---|---|---|---|---|
| P value | P value | P value | ||||
| IL-1β (pg/mL) | 0.88 (0.68-1.16) | .356 | 0.73 (0.49-1.09) | .119 | 0.87 (0.66-1.15) | .328 |
| IL-5 (pg/mL) | 0.86 (0.64-1.17) | .326 | 1.14 (0.89-1.45) | .284 | 1.10 (0.90-1.35) | .343 |
| IL-6 (pg/mL) | 1.84 (1.14-2.97) | .014↑ | 1.08 (0.76-1.53) | .671 | 1.24 (0.91-1.70) | .170 |
| IL-8 (pg/mL) | 1.03 (0.74-1.42) | .867 | 0.70 (0.50-0.98) | .040↓ | 1.01 (0.79-1.30) | .931 |
| CXCL10 (pg/mL) | 1.06 (0.88-1.27) | .537 | 0.84 (0.68-1.03) | .090 | 1.34 (1.06-1.70) | .017↑ |
| CXCL11 (pg/mL) | 1.02 (0.85-1.22) | .826 | 0.98 (0.75-1.29) | .891 | 1.23 (0.96-1.58) | .093 |
| CCL2 (pg/mL) | 0.86 (0.76-0.96) | .010↓ | 0.87 (0.78-0.96) | .010↓ | 0.94 (0.85-1.04) | .241 |
| CCL4 (pg/mL) | 0.92 (0.81-1.03) | .141 | 0.86 (0.72-1.02) | .080 | 0.97 (0.85-1.11) | .630 |
| CCL13 (pg/mL) | 0.80 (0.70-0.90) | .001↓ | 1.08 (0.94-1.24) | .256 | 0.87 (0.78-0.97) | .017↓ |
| CCL17 (pg/mL) | 0.75 (0.60-0.94) | .013↓ | 0.72 (0.49-1.07) | .103 | 0.75 (0.57-0.97) | .030↓ |
| CCL26 (pg/mL) | 0.69 (0.52-0.91) | .009↓ | 1.14 (0.81-1.62) | .433 | 1.06 (0.79-1.42) | .711 |
| TNF-α (pg/mL) | 0.93 (0.78-1.12) | .426 | 0.77 (0.55-1.07) | .109 | 1.08 (0.82-1.44) | .563 |
| TNF-R1 (pg/mL) | 1.17 (1.06-1.29) | .002↑ | 0.92 (0.83-1.01) | .082 | 1.00 (0.92-1.09) | .924 |
| TNF-R2 (pg/mL) | 1.12 (1.05-1.20) | .002↑ | 0.88 (0.81-0.96) | .007↓ | 1.00 (0.92-1.10) | .930 |
| VEGF (pg/mL) | 1.19 (0.98-1.44) | .079 | 0.86 (0.62-1.18) | .340 | 0.99 (0.85-1.16) | .940 |
Data are presented as geometric means with 95% CIs. Values in boldface indicate statistical significance.
Table E8.
Change in clinical parameters and levels of sputum mediators between the stable and exacerbation states for asthmatic patients and patients with COPD within each identified biologic exacerbation cluster
| Cluster 1 |
Cluster 2 |
Cluster 3 |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients (n = 7) | Patients with COPD (n = 27) | Asthmatic patients (n = 10) | Patients with COPD (n = 17) | Asthmatic patients (n = 15) | Patients with COPD (n = 29) | ||
| Pre-FEV1 (L)∗ | −0.12 (0.07) | −0.14 (0.05) | −0.15 (0.18) | −0.20 (0.06) | −0.16 (0.08) | −0.10 (0.04) | |
| Post-FEV1 (L)∗ | −0.17 (0.17) | −0.13 (0.04) | −0.06 (0.13) | −0.11 (0.04) | −0.33 (0.11) | −0.21 (0.04) | |
| Pre-FEV1 (% predicted)∗ | −5.80 (2.96) | −6.32 (2.14) | −5.94 (5.41) | −8.38 (2.76) | −6.28 (3.14) | −3.69 (1.53) | |
| Post-FEV1 (% predicted)∗ | −7.89 (7.25) | −5.75 (1.65) | −1.11 (3.35) | −4.17 (1.63) | −11.32 (3.52) | −7.36 (1.43) | |
| FEV1/FVC ratio (%)∗ | 0.24 (1.94) | 1.43 (2.24) | 2.14 (1.60) | 4.05 (1.87) | 3.15 (2.56) | 1.36 (2.29) | |
| VAS, cough (mm)∗ | 23.80 (7.01) | 16.19 (5.80) | 45.20 (8.88) | 29.65 (7.69) | 45.29 (7.20) | 18.45 (4.26)¶ | |
| VAS, dyspnea (mm)∗ | 16.40 (13.04) | 14.15 (5.97) | 41.20 (9.83) | 36.94 (7.86) | 26.36 (8.25) | 22.34 (3.72) | ‡ |
| Blood neutrophils (× 109/L)∗ | 0.92 (0.92) | 2.28 (0.60) | 0.68 (0.74) | 0.83 (0.47) | 0.31 (0.54) | 0.74 (0.49) | |
| Blood eosinophils (× 109/L) | 0.68 (0.11-4.08) | 0.70 (0.48-1.01) | 1.10 (0.40-3.01) | 1.10 (0.87-1.41) | 0.60 (0.22-0.62) | 0.61 (0.44-0.83) | |
| TCC (× 106 cells/g sputum) | 11.68 (2.39-57.20) | 2.64 (1.59-4.37)¶ | 0.78 (0.32-1.90) | 1.64 (0.82-3.31) | 2.26 (0.61-8.36) | 1.46 (0.75-2.85) | † |
| Sputum neutrophil count (%)∗ | 21.35 (14.05) | 14.00 (5.80) | −11.94 (6.77) | 2.09 (5.89) | −5.20 (9.32) | −2.12 (3.64) | |
| Sputum eosinophil count (%) | 0.66 (0.20-2.21) | 0.49 (0.30-0.81) | 4.41 (0.73-26.67) | 1.46 (0.55-3.82) | 0.39 (0.18-0.84) | 0.91 (0.45-1.86) | † |
| Bacterial load (CFU/mL) | 3.4 (0.03-442) | 1.5 (0.3-6.8) | 0.5 (0.14-1.9) | 0.8 (0.1-4.7) | 0.4 (0.01-12) | 1.7 (0.4-7.4) | |
| IL-1β (pg/mL) | 17.32 (4.93-60.87) | 9.13 (3.94-21.14) | 1.22 (0.30-5.02) | 1.55 (0.46-5.25) | 1.22 (0.56-2.66) | 1.20 (0.60-2.40) | †‡ |
| IL-5 (pg/mL) | 0.09 (0.02-0.42) | 0.59 (0.35-1.00)¶ | 1.91 (0.45-8.20) | 2.76 (1.53-4.96) | 0.69 (0.25-1.90) | 1.07 (0.59-1.95) | †‡ |
| IL-6 (pg/mL) | 2.06 (0.68-6.29) | 1.35 (0.75-2.43) | 0.97 (0.33-2.92) | 1.12 (0.52-2.39) | 3.47 (0.95-12.74) | 1.69 (0.76-3.76) | |
| IL-6R (pg/mL) | 1.87 (0.94-3.71) | 3.36 (1.94-5.82) | 1.75 (0.88-3.51) | 1.52 (0.91-2.52) | 1.42 (0.72-2.78) | 0.92 (0.56-1.50) | ‡ |
| IL-8 (pg/mL) | 1.67 (0.76-3.68) | 1.67 (1.12-2.47) | 1.33 (0.45-3.92) | 1.35 (0.72-2.55) | 1.43 (0.76-2.67) | 0.62 (0.37-1.05) | ‡ |
| IL-10 (pg/mL) | 11.85 (2.64-53.14) | 2.38 (1.40-4.03)¶ | 0.73 (0.40-1.33) | 0.96 (0.56-1.63) | 9.44 (2.32-38.39) | 2.65 (1.49-4.72) | †‡ |
| IL-13 (pg/mL) | 0.61 (0.18-2.04) | 0.89 (0.73-1.09) | 0.72 (0.33-1.55) | 1.32 (1.02-1.69) | 1.36 (1.00-1.84) | 1.16 (0.98-1.38) | ‡ |
| CXCL10 (pg/mL) | 0.27 (0.03-2.21) | 0.52 (0.26-1.04) | 0.70 (0.25-1.93) | 1.24 (0.51-3.02) | 6.52 (2.40-17.77) | 2.88 (1.26-6.57) | †‡ |
| CXCL11 (pg/mL) | 0.08 (0.01-1.22) | 0.30 (0.13-0.69) | 0.37 (0.12-1.19) | 1.50 (0.48-4.74) | 7.29 (0.98-54.34) | 7.77 (2.13-28.38) | †‡ |
| CCL2 (pg/mL) | 0.49 (0.09-2.71) | 0.46 (0.26-0.79) | 0.75 (0.41-1.37) | 0.95 (0.52-1.73) | 3.08 (1.17-8.11) | 1.26 (0.76-2.12) | †‡ |
| CCL3 (pg/mL) | 1.44 (0.11-18.32) | 1.02 (0.60-1.76) | 0.51 (0.13-1.92) | 1.23 (0.45-3.37) | 1.37 (0.41-4.62) | 1.58 (0.83-3.03) | |
| CCL4 (pg/mL) | 0.63 (0.12-3.27) | 1.22 (0.80-1.86) | 2.06 (0.61-6.93) | 1.72 (0.88-3.40) | 2.36 (0.83-6.68) | 1.26 (0.63-2.55) | |
| CCL5 (pg/mL) | 1.90 (0.55-6.48) | 1.97 (1.25-3.11) | 0.97 (0.30-3.17) | 1.28 (0.68-2.40) | 3.88 (1.61-9.32) | 2.64 (1.46-4.75) | |
| CCL13 (pg/mL) | 0.30 (0.15-0.62) | 0.38 (0.25-0.57) | 0.92 (0.40-2.13) | 0.81 (0.51-1.29) | 0.58 (0.32-1.06) | 0.69 (0.48-1.00) | ‡ |
| CCL17 (pg/mL) | 0.22 (0.03-1.44) | 0.24 (0.13-0.44) | 2.10 (0.86-5.11) | 1.65 (0.82-3.31) | 0.55 (0.27-1.15) | 0.60 (0.37-0.96) | †‡ |
| CCL26 (pg/mL) | 0.20 (0.04-1.14) | 0.69 (0.45-1.05) | 2.81 (0.90-8.75) | 2.30 (1.47-3.58) | 0.59 (0.25-1.39) | 1.34 (0.88-2.06)¶ | †‡ |
| TNF-α (pg/mL) | 21.90 (5.35-89.70) | 6.08 (2.78-13.32) | 0.65 (0.17-2.49) | 1.77 (0.64-4.91) | 5.45 (1.43-20.74) | 3.31 (1.22-9.00) | † |
| TNF-R1 (pg/mL) | 2.22 (0.92-5.36) | 3.18 (2.04-4.94) | 1.34 (0.59-3.05) | 1.22 (0.75-1.97) | 1.48 (0.74-2.98) | 0.80 (0.55-1.18) | ‡ |
| TNF-R2 (pg/mL) | 2.12 (1.06-4.23) | 3.18 (1.94-5.23) | 1.58 (0.58-4.29) | 1.63 (0.92-2.89) | 2.81 (1.05-7.56) | 1.14 (0.58-2.25) | ‡ |
| VEGF (pg/mL) | 1.03 (0.59-1.79) | 1.57 (1.14-2.15) | 1.07 (0.59-1.94) | 1.01 (0.68-1.49) | 0.84 (0.50-1.42) | 0.83 (0.67-1.04) | ‡ |
| IFN-γ (pg/mL) | 5.12 (0.91-28.81) | 1.32 (0.77-2.26)¶ | 0.56 (0.15-2.01) | 0.81 (0.44-1.49) | 17.5 (2.6-117) | 6.73 (2.48-18.24) | †‡ |
Data are presented as geometric means with 95% CIs, unless otherwise stated. Values in boldface indicate statistical significance.
CFU, Colony-forming unit; FVC, forced vital capacity; TCC, total sputum cell count.
Mean (SEM).
Overall significant difference between clusters in asthmatic patients or patients with COPD, respectively.
Significant difference between asthmatic patients and patients with COPD within clusters.
Table E9.
Change in levels of serum mediators between stable and exacerbation states for asthmatic patients and patients with COPD within each identified biologic exacerbation cluster
| Cluster 1 |
Cluster 2 |
Cluster 3 |
|||||
|---|---|---|---|---|---|---|---|
| Asthmatic patients (n = 7) | Patients with COPD (n = 27) | Asthmatic patients (n = 10) | Patients with COPD (n = 17) | Asthmatic patients (n = 15) | Patients with COPD (n = 29) | ||
| IL-1β (pg/mL) | 0.32 (0.17-0.59) | 1.17 (0.95-1.43)∗ | 0.41 (0.13-1.27) | 0.96 (0.71-1.30) | 0.64 (0.34-1.18) | 1.03 (0.78-1.36) | |
| IL-5 (pg/mL) | 0.32 (0.10-1.03) | 1.09 (0.86-1.37)∗ | 0.59 (0.24-1.48) | 1.32 (1.07-1.63)∗ | 1.11 (0.49-2.53) | 1.10 (0.90-1.34) | |
| IL-6 (pg/mL) | 1.41 (0.75-2.65) | 1.98 (1.09-3.60) | 0.72 (0.48-1.08) | 1.30 (0.81-2.10) | 1.07 (0.57-2.00) | 1.35 (0.92-1.96) | |
| IL-8 (pg/mL) | 0.90 (0.42-1.92) | 1.07 (0.73-1.56) | 0.54 (0.24-1.18) | 0.79 (0.54-1.17) | 1.25 (0.92-1.72) | 0.90 (0.63-1.28) | † |
| CXCL10 (pg/mL) | 1.20 (0.71-2.02) | 1.02 (0.83-1.26) | 0.63 (0.44-0.89) | 0.96 (0.75-1.23)∗ | 1.74 (1.17-2.58) | 1.16 (0.86-1.57) | † |
| CXCL11 (pg/mL) | 1.23 (0.79-1.89) | 0.98 (0.80-1.20) | 1.21 (0.26-5.54) | 0.93 (0.71-1.22) | 1.22 (0.50-2.96) | 1.24 (0.96-1.60) | |
| CCL2 (pg/mL) | 1.06 (0.78-1.43) | 0.81 (0.72-0.92) | 0.68 (0.56-0.83) | 0.97 (0.89-1.06)∗ | 0.86 (0.73-1.01) | 0.99 (0.86-1.13) | †‡ |
| CCL4 (pg/mL) | 0.78 (0.49-1.25) | 0.96 (0.86-1.07) | 0.77 (0.42-1.40) | 0.91 (0.84-0.98) | 1.04 (0.82-1.33) | 0.93 (0.79-1.10) | |
| CCL13 (pg/mL) | 0.73 (0.52-1.02) | 0.82 (0.71-0.94) | 1.33 (0.90-1.98) | 0.98 (0.89-1.07) | 0.88 (0.70-1.10) | 0.87 (0.75-0.99) | † |
| CCL17 (pg/mL) | 0.77 (0.33-1.78) | 0.75 (0.60-0.93) | 0.53 (0.20-1.41) | 0.84 (0.54-1.29) | 0.82 (0.44-1.54) | 0.71 (0.54-0.92) | |
| CCL26 (pg/mL) | 0.30 (0.10-0.86) | 0.86 (0.71-1.04) | 1.19 (0.34-4.12) | 1.12 (0.95-1.33) | 1.20 (0.52-2.79) | 0.99 (0.83-1.18) | |
| TNF-α (pg/mL) | 0.79 (0.38-1.64) | 0.97 (0.82-1.15) | 0.44 (0.16-1.16) | 1.00 (0.82-1.21) | 1.14 (0.50-2.57) | 1.06 (0.90-1.24) | |
| TNF-R1 (pg/mL) | 0.97 (0.73-1.27) | 1.23 (1.12-1.36) | 0.88 (0.71-1.09) | 0.93 (0.82-1.06) | 0.95 (0.84-1.08) | 1.03 (0.92-1.16) | ‡ |
| TNF-R2 (pg/mL) | 1.09 (0.88-1.36) | 1.13 (1.04-1.22) | 0.81 (0.68-0.98) | 0.92 (0.82-1.02) | 0.93 (0.78-1.10) | 1.05 (0.94-1.16) | ‡ |
| VEGF (pg/mL) | 1.42 (0.55-3.63) | 1.13 (0.98-1.30) | 0.50 (0.22-1.14) | 1.11 (0.86-1.44)∗ | 1.01 (0.65-1.57) | 0.98 (0.88-1.10) | |
Data are presented as geometric means with 95% CIs.
Significant difference between asthmatic patients and patients with COPD within clusters.
Overall significant difference by ANOVA between clusters in asthmatic patients or patients with COPD.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldar P., Pavord I.D., Shaw D.E., Berry M.A., Thomas M., Brightling C.E. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bafadhel M., McKenna S., Terry S., Mistry V., Reid C., Haldar P. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Siroux V., Basagana X., Boudier A., Pin I., Garcia-Aymerich J., Vesin A. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J. 2011;38:310–317. doi: 10.1183/09031936.00120810. [DOI] [PubMed] [Google Scholar]
- 6.Burgel P.R., Paillasseur J.L., Caillaud D., Tillie-Leblond I., Chanez P., Escamilla R. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36:531–539. doi: 10.1183/09031936.00175109. [DOI] [PubMed] [Google Scholar]
- 7.Ghebre M.A., Bafadhel M., Desai D., Cohen S.E., Newbold P., Rapley L. Biological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;135:63–72. doi: 10.1016/j.jaci.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bafadhel M., McCormick M., Saha S., McKenna S., Shelley M., Hargadon B. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma Global strategy for asthma management and prevention, 2017. http://www.ginasthma.org/ Available at:
- 10.From the global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. http://www.goldcopd.org/ Available at:
- 11.Desai D., Newby C., Symon F.A., Haldar P., Shah S., Gupta S. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188:657–663. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bafadhel M., McKenna S., Terry S., Mistry V., Pancholi M., Venge P. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal T.A., Wilkinson T.M., Hurst J.R., Perera W.R., Sapsford R.J., Wedzicha J.A. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 15.Albert R.K., Connett J., Bailey W.C., Casaburi R., Cooper J.A., Jr., Criner G.J. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nouira S., Marghli S., Belghith M., Besbes L., Elatrous S., Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001;358:2020–2025. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]
- 17.Vollenweider D.J., Jarrett H., Steurer-Stey C.A., Garcia-Aymerich J., Puhan M.A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(12):CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 18.Brusselle G.G., Vanderstichele C., Jordens P., Deman R., Slabbynck H., Ringoet V. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 19.Gibson P.G., Yang I.A., Upham J.W., Reynolds P.N., Hodge S., James A.L. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 20.Johnston S.L., Szigeti M., Cross M., Brightling C., Chaudhuri R., Harrison T. Azithromycin for acute exacerbations of asthma: the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630–1637. doi: 10.1001/jamainternmed.2016.5664. [DOI] [PubMed] [Google Scholar]
- 21.Johnston S.L., Blasi F., Black P.N., Martin R.J., Farrell D.J., Nieman R.B. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Bafadhel M., Haldar K., Spivak A., Mayhew D., Miller B.E. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47:1082–1092. doi: 10.1183/13993003.01406-2015. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Singh R., Miller B.E., Tal-Singer R., Van Horn S., Tomsho L. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: an analysis of the COPDMAP study. Thorax. 2018;73:331–338. doi: 10.1136/thoraxjnl-2017-210741. [DOI] [PubMed] [Google Scholar]
- 24.Haldar K., Bafadhel M., Lau K., Berg A., Kwambana B., Kebadze T. Microbiome balance in sputum determined by PCR stratifies COPD exacerbations and shows potential for selective use of antibiotics. PLoS One. 2017;12:e0182833. doi: 10.1371/journal.pone.0182833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brightling C.E., Monteiro W., Ward R., Parker D., Morgan M.D., Wardlaw A.J. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 26.Siva R., Green R.H., Brightling C.E., Shelley M., Hargadon B., McKenna S. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 27.Haldar P., Brightling C.E., Hargadon B., Gupta S., Monteiro W., Sousa A. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 29.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 30.Castro M., Zangrilli J., Wechsler M.E., Bateman E.D., Brusselle G.G., Bardin P. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 31.Bjermer L., Lemiere C., Maspero J., Weiss S., Zangrilli J., Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Bleecker E.R., FitzGerald J.M., Chanez P., Papi A., Weinstein S.F., Barker P. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 34.FitzGerald J.M., Bleecker E.R., Nair P., Korn S., Ohta K., Lommatzsch M. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 35.Brightling C.E., Bleecker E.R., Panettieri R.A., Jr., Bafadhel M., She D., Ward C.K. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med. 2014;2:891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta A., Kjarsgaard M., Capaldi D., Radford K., Aleman F., Boylan C. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur Respir J. 2017;49 doi: 10.1183/13993003.02486-2016. [DOI] [PubMed] [Google Scholar]
- 37.Pavord I.D., Chanez P., Criner G.J., Kerstjens H.A.M., Korn S., Lugogo N. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377:1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 38.Wark P.A., Bucchieri F., Johnston S.L., Gibson P.G., Hamilton L., Mimica J. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quint J.K., Donaldson G.C., Goldring J.J., Baghai-Ravary R., Hurst J.R., Wedzicha J.A. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest. 2010;137:812–822. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallia P., Message S.D., Gielen V., Contoli M., Gray K., Kebadze T. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]