Abstract
Over the last 60 years an eclectic collection of microbes has been tested in a variety of pre-clinical models as anti-cancer agents. At the forefront of this research are a number of virus-based platforms that have shown exciting activity in a variety of pre-clinical models and are collectively referred to as oncolytic viruses. Our true understanding of the potential and limitations of this therapeutic modality has been substantially advanced through clinical studies carried out over the last 25 years. Perhaps not surprising, as with all other cancer therapeutics, it has become clear that current oncolytic virus therapeutics on their own are unlikely to be effective in the majority of patients. The greatest therapeutic gains will therefore be made through thoughtful combination strategies built upon an understanding of cancer biology.
Keywords: oncolytic virus, immunotherapy, cancer, combination therapy
Oncolytic viruses are anti-cancer agents that can exert multi-pronged attacks on tumors through direct lysis of cancer cells, destroying the tumors vasculature and by inducing anti-tumor immunity. Martin and Bell propose that their potential can be drastically expanded by combination with other cancer therapeutics, and the authors highlight current approaches.
Main Text
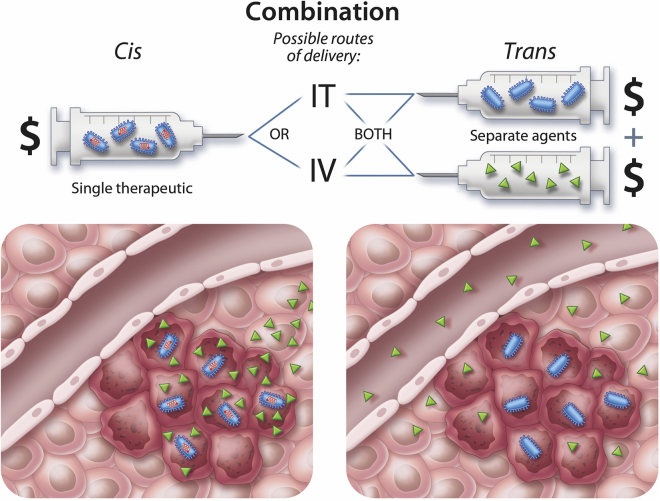
Oncolytic or “cancer-lysing” viruses (OVs) have been designed or selected to kill cancer cells but have their replication blunted in normal tissues (extensively reviewed elsewhere1, 2, 3). Several interesting OV platforms are being evaluated pre-clinically and clinically including, but not limited to, adenovirus (AdV),4 coxsackievirus,5 herpes simplex virus (HSV),6, 7, 8 Maraba virus,9, 10 measles virus,11, 12 Newcastle disease virus,13 parvovirus,14 reovirus,15 vaccinia virus (VACV),16 and vesicular stomatitis virus (VSV).17, 18 The mechanism of tumor selectivity of these viruses is variable, ranging from control of expression of viral genes by transcriptional elements that are uniquely/primarily used in cancer cells,19, 20, 21 to cancer-specific virus receptor expression (e.g. Martin et al.,22 Petrovic et al.,23 and Bhatia et al.24), metabolic over-activity of tumor cells,25 tumor-specific defects in antiviral responses,26 and combinations thereof. In theory, OVs are the ultimate cancer-targeted therapeutic because they are able to selectively amplify themselves within the tumor milieu increasing therapeutic dose over time. However, when considered as stand-alone, purely oncolytic agents, there is a clash between theory and clinical reality. For an OV to be curative, as a purely cytolytic agent, it would have to infect and kill the vast majority, if not all, cancer cells within the tumor. Human tumors are well known to be genetically27 and phenotypically28 heterogeneous and built of mixtures of normal and malignant cell types, making it unlikely that an OV could find, infect, and completely kill widespread disseminated disease in the majority of patients. However, the therapeutic activity of OVs is not limited to their tumor oncolytic activity but rather is multi-faceted, including interactions within the stromal cells of the tumor microenvironment (TME),29 as well as the vascular30 and immune system31 within the patients. Each of these points of interaction provides an opportunity for combining orthogonal therapeutics to complement OVs and improve outcomes for cancer patients. Furthermore, many of the OV platforms under development can be engineered to express transgenic payloads vastly expanding their therapeutic potential.32 For the purposes of this review, we borrow from the genetics world and use cis combinations to describe approaches that involve encoding of transgenes within the virus backbone and trans combinations to describe the coupling of an OV with another stand-alone therapeutic (e.g., drugs, antibodies, cells). Advantages and limitations of cis and trans combinations are depicted in Figure 1.
Figure 1.
cis versus trans Combination: Advantages and Limitations
OV therapy in cis (left) involves encoding a gene product or therapeutic payload (green) directly in the virus backbone (blue). When a productive infection takes place within cancer cells, the payload is locally expressed at high levels with minimal systemic exposure. This has the advantage of avoiding systemic toxicities and reducing costs because only a single therapeutic (i.e., OV) is required. The disadvantage of this approach is that the expression of the therapeutic payload is linked to the longevity of the virus replication within the tumor. If a therapeutic requires sustained expression over several months, then this approach may be suboptimal. Additionally, if a virus can only be administered by direct intratumoral injection (IT), then the payload will not be optimally expressed in disseminated tumors. Providing a therapeutic in trans with OV administration is required if the complementary treatment is a chemical, small molecule, or form of radiation. It may also be desirable if the therapeutic needs to be dosed for a shorter or longer period than the virus that is found within the tumor. In the setting of trans administration, the costs of using therapeutics together is additive. In order to achieve therapeutically effective doses within the tumor, systemic administration of large amounts of therapeutic with associated toxicities will be required.
OVs Mount a Multi-pronged Attack on the Tumor
Although the primary target of all OV products is the tumor cell, it has become clear that much like many natural virus infections of normal tissues, OVs cause collateral damage as they multiply within the TME. For instance, certain OVs can attack and destroy tumor neo-vasculature, creating areas of tumor hypoxia that culminate in extensive bystander killing of uninfected cells.30, 33 In addition, because of the cytokines expressed within the TME, stromal cells that support cancer cell growth can be infected and destroyed.29 However, it has become evident that perhaps the most impactful therapeutic byproduct of OV infection is stimulation of the host’s immune system. OVs have been shown to initiate immunogenic cell death (ICD), and thus releasing damage-associated molecular patterns (DAMPs) such as HMGB1, heat shock proteins, and ATP, as well as virus-derived pathogen-associated molecular patterns (PAMPs). ICD culminates in the recruitment/activation of both the innate and adaptive immune systems within the TME, usually a site of immune tolerance, and in combination with proteasome blockade can sensitize tumors to adjuvant NK cell therapy by enhancing necroptosis and autophagy.34 Additionally, by inducing an antiviral immune response both through Fc-fragment-dependent NK cell activation35 as well as pre-existing immunity against the OV, a systemic antitumor immune response can be observed.36 This “adjuvant effect” of virus infection is recognized to be a key component of the long-term efficacy of OV therapy (reviewed in Kaufman et al.,3 Aurelian,37 Aitken et al.,38 Guo et al.,39 De Munck et al.,40 and van Vloten et al.41). Despite the multiple OV platforms that are in development, to date only one has been approved as a monotherapy by the US Food and Drug Administration (FDA) and the European Commission. The HSV-based talimogene laherparepvec (T-Vec) was approved in 2015 for the treatment of inoperable malignant melanoma42 and represented a tremendous breakthrough for the field overall. However, as discussed below, the ultimate clinical application of T-Vec is likely to be in the setting of combination therapy.
cis and trans Combinations to Increase Oncolytic Activity
An initial critical step in OV therapy is the ability of virus to infect and kill cancer cells. It has been well documented that there is heterogeneity in the ability of a range of OVs to infect a broad spectrum of cancer cell lines and patient samples. Jean-Simon Diallo has championed the identification and mechanism of action of small molecules and chemotherapeutics that selectively improve virus replication within cancer cells. The trans combination of OV therapy with organic or inorganic molecules (so-called viral sensitizers) to potentiate virus infection and spreading within the tumor as well as antitumor immune response has been successfully demonstrated in several syngeneic and xenograft tumor models.43, 44, 45 For example, Selman et al.46 demonstrated that dimethyl fumarate inhibits type I interferon (IFN) production and response by blocking nuclear translocation of NF-κB, thus potentiating oncolytic virotherapy. Similarly, Arulanandam et al.33 showed an improved bystander killing effect caused by reduction of IFN expression and secretion when combining VSV with microtubule-destabilizing agents. VSV as well as VACV have also been synergistically combined with histone deacetylase inhibitors47, 48 as well as second mitochondrial activator of caspase (Smac)-mimetics.49 The chemotherapeutic cyclophosphamide has been shown to enhance oncolytic HSV efficacy by inhibiting the innate immune response when combined in trans;50 therefore, Currier et al.51 have successfully constructed an oncolytic HSV encoding a prodrug converting enzyme for cyclophosphamide and demonstrated the safety and efficacy of this cis combination in xenograft models. Another important aspect of the innate immunity is the activation of macrophages and microglia, causing the expression and secretion of tumor necrosis factor alpha (TNF-α), which in turn induces both intrinsic and extrinsic apoptosis and necroptosis as demonstrated for Newcastle disease virus (NDV)-infected (tumor) cells.52 The effect of TNF-α on OV therapy is being investigated because although it enhances the bystander effect, especially when encoded under a late promoter in HSV,53 it can also inhibit virus replication by inducing apoptosis of infected cells. This effect, however, can be counteracted by transient TNF-α blockade.54 The innate antiviral defense is not the only factor diminishing OV efficacy: as already discussed, a tumor is usually highly heterogenic, comprising fibrotic barriers, extracellular matrix, and other physical barriers inhibiting OV spread and infection of tumor cells.55 Several groups have pursued a variety of cis combinations to enhance virus replication, e.g., encoding fusogenic glycoproteins to enhance virus spread via syncytia formation as demonstrated for HSV,56, 57 as well as VSV and measles virus,58 or by encoding enzymes actively remodeling the extracellular matrix as demonstrated by AdV encoding relaxin59 or hyaluronidase,60 the latter demonstrating improved efficacy over the parental virus in orthotopic murine models for osteosarcoma,61 as well as glioma.62 Several groups have also successfully combined antiangiogenic therapies with OVs, both in trans as well as in cis. Tan et al.,63 for example, showed synergistic effects, i.e., improved viral distribution throughout the tumor, as well as enhanced tumor hypoxia of HSV trans-combined with the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab in a human breast cancer xenograft mouse model. Similarly, Buckel et al.64 successfully cis-combined VACV with a single-chain variant of bevacizumab together with radiotherapy for the treatment of a subcutaneous patient-derived glioma xenograft model. Using a comparable model of glioblastoma, Zhang et al.65 demonstrated that treatment with bevacizumab is augmented by trans combination with an HSV armed with angiostatin, an antiangiogenic polypeptide. The observed synergistic effects of this combination are only partly caused by the antiangiogenic effect, but also by modulation of the innate immune response as demonstrated for an HSV encoding different anti-VEGF antibodies.66 Not pursuing an antibody-based approach, Bolyard et al.67 showed significant antitumor efficacy of a cis-combination of HSV and vasculostatin, a secreted angiogenesis inhibitor, in an ovarian cancer xenograft mouse model, additionally combined in trans with the approved chemotherapeutic doxorubicin.
The combination of OVs with approved chemotherapeutics holds several benefits, foremost the known safety profile and indications of an approved drug. Also, when it comes from bench to bedside, patients may have already been treated with chemotherapeutics, and it is therefore important to determine whether OVs can work in combination or not. Several pre-clinical and clinical studies have been conducted investigating the combination of OVs with approved chemotherapeutics, and also a few reviews have been written especially on this topic (e.g. Simpson et al.68). Recently, Binz et al.69 demonstrated the successful combination of the two chemotherapeutics nab-paclitaxel and gemcitabine with oncolytic VACV GLV-1h68 for the treatment of pancreatic cancer in vitro, Tanaka et al.70 investigated a combination therapy with oncolytic HSV and dacarbazine in a mouse melanoma model, and Bourgeois-Daigneault et al.71 were able to show synergistic cytopathic activity, and therefore reduced tumor growth and prolonged survival, in a murine breast cancer model treated with a combination of Maraba MG1 and paclitaxel.
Priming and Boosting T Cell Responses with OV Vaccine Combinations
cis Approaches
Our immune systems have evolved to recognize and vigorously respond to virus infection, and thus in many ways a virus is the perfect immunological adjuvant. With this in mind, investigators have created OVs that encode and express tumor-associated antigens (TAAs) with the goal of coupling oncolytic activity with targeted T cell stimulation. In early iterations of this approach, OVs encoding TAAs (e.g., Her2) were directly injected into tumor beds to locally stimulate immune responses against the TAA in a milieu where cancer cells are being lysed and releasing inflammatory cytokines.72 As a further advancement on this strategy, OVs expressing a TAA can be used to synergize with OVs armed with cytokines to enhance systemic antitumor immunity either with cis encoding of cytokines and TAAs or by trans combinations (e.g., VACV-granulocyte-macrophage colony-stimulating factor [GM-CSF] with VACV armed with HER2/neu16, 73). Although this approach shows promise, it may be limited because multiple treatments with the same virus encoding a tumor antigen are likely to skew the immune response toward the vector and away from the TAA. More recently, Bridle et al.74 introduced the concept of heterologous prime-boost regimens to focus immune responses on the TAA and away from the OV backbone. In their initial studies they used a non-replicating AdV vector expressing a TAA “self-antigen,” namely dopachrome tautomerase or DCT to prime animals and generate a central memory (TCM) response. This was followed 2 weeks later by systemic administration of an oncolytic rhabdovirus also encoding DCT.74 This approach provided outstanding systemic T cell responses against DCT while simultaneously recruiting anti-DCT immune cells into the TME. The unprecedented T cell responses appear to be related to the natural tropism of rhabdoviruses for follicular B cells that leads ultimately to boosting of central memory T cells harbored within the splenic follicle.75 Since the first description of this concept, several studies investigating possible antigens and combinations have been conducted and recently summarized in two comprehensive reviews by Aitken et al.38 and Meyers et al.76 The promising results of prime-boost regimens in pre-clinical experiments resulted in two clinical phase I/II studies (NCT02285816 and NCT02879760), both employing a prime with a non-replicative AdV encoding the antigen MAGE-A3 followed by a boost with MG1 Maraba/MAGE-A3. Notably, NCT02879760 also includes the combination with the immune checkpoint inhibitor (ICI) pembrolizumab.
An alternative approach to OV vaccination has been described by Lemay et al.,77 who generated “infected cell” vaccines (ICV) through ex vivo infection of γ-irradiated tumor cells with oncolytic VSV. This approach can generate potent antitumor immune response in immunocompetent mouse models and provide long-term protection against future tumor challenges. Recently, Alkayyal et al.78 described potent NK cell activation induced by an Maraba MG1-interleukin-12 (IL-12) ICV for the treatment of peritoneal carcinomatosis.
trans Approaches
So far, there is only one approach described that combines TAAs with OV therapy in trans. Capasso et al.79 coated an oncolytic AdV with tumor-specific major histocompatibility (MHC) class I peptides and observed an increase in antitumor cytotoxic T cells, as well as epitope-specific dendritic cells (DCs), and consequently an improved therapeutic efficacy of this peptide-coated conditionally replicating AdV (PeptiCRAd) in a humanized mouse melanoma model.
Combinatorial Application of Microbes
In contrast with the described prime-boost regimen where two viruses encode the same antigen, it is also possible to combine viruses with different immune profiles to overcome antiviral immune responses and exert synergistic effects, even when applied sequentially. For example, Le Boeuf et al.80 demonstrated improved antitumor response of VSV combined with VACV in immunodeficient and immunocompetent mouse tumor models, as well as ex vivo studies in primary human cancer samples compared with each virus alone; Tysome et al.81 successfully applied a sequential treatment regimen of oncolytic AdV followed by VACV for the treatment of pancreatic cancer in a Syrian hamster model; and recently Nistal-Villan et al.82 demonstrated the feasibility of sequential intratumor administrations of AdV and NDV, the latter in cis combination with the immunostimulatory cytokine, oncostatin M. Recently, Ilett et al.83 demonstrated the feasibility of the combination of a heterologous prime-boost model using reovirus and VSV together with anti-programmed cell death protein 1 (anti-PD1) checkpoint blockade. To date there have been no clinical studies testing the sequential administration of two distinct OVs; however, there is plenty of potential for using rationally engineered OV combinations.
Similar to the previously discussed trans combination of OVs with other viruses, it has been demonstrated that bacteria can synergize with OVs. For example, Cronin et al.84 showed that intravenous (i.v.) application of nonpathogenic E. coli expressing the vaccinia type 1 IFN antagonist B18R augments subsequent therapy with oncolytic VSV in an athymic nude mouse model by overcoming innate immunity against the OV. Recently, Aitken et al.85 demonstrated the feasibility of a heterologous prime-boost regimen in immunocompetent mice. In this proof-of-concept study the intracellular bacteria Listeria monocytogenes was used as a priming agent in an exemplary prime-boost regimen together with Maraba virus. This study demonstrated that L. monocytogenes not only directly lyses tumor cells, but also primes an immune response comparable with the commonly used adenoviral vectors. Because bacteria are well-described (cancer) vaccination agents,86 the use of other bacteria, e.g., Salmonella typhimurium,87, 88 warrants further investigation.
Teaching OVs to Drive a CAR
Probably one of the most substantial advancements in cancer immunotherapy was the description of chimeric antigen receptor (CAR) T cells. In this approach, cytotoxic T cells are genetically modified to express an engineered recombinant antigen receptor, usually comprising a single-chain variable fragment of an antibody, directed against a surface antigen on malignant cells, thus inducing cell death.89 Given the promising results of this technique so far, it makes sense to combine OVs with CAR T cells (for a review, see Ajina and Maher90). Nishio et al.91 demonstrated that an AdV armed with the chemokine RANTES and the cytokine IL-15 not only exerts oncolytic function on its own, but also enhances migration and proliferation of CAR T cells specific for the TAA GD2 in a xenograft human neuroblastoma mouse model. As a result, tumor burden was reduced and overall survival was improved compared with each treatment alone, encouraging further combination approaches of OVs and CAR T cells. Tanoue et al.92 demonstrated the feasibility of trans-combining an AdV armed with an anti-programmed death ligand 1 (PD-L1) minibody with HER2/neu CAR-T cells in a xenograft model in nude mice. A similar concept of complementing HER2/neu-specific CAR T cells with oncolytic Ad expressing PD-L1 blockade and combining cytokine (IL-12) expression in cis for the treatment of head and neck squamous cell carcinoma in a human xenograft mouse model was recently described.93 These few studies so far demonstrate the feasibility and the promising potential of combining CAR T cell therapy with OVs, and they encourage further studies.
Strategic Combinations of Immune Modulators with OVs
Since the description of costimulatory receptors and their ligands acting as immune checkpoints, envisioned as “brakes” during an immune response, several of these “brakes” have been identified and antibodies blocking them have been described (for a review, see Pardoll94). These ICIs have proven themselves very promising tools in the therapeutic toolbox, even when used as monotherapies (for a review, see Wilson et al.95). So far, the monoclonal antibodies blocking the Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD1, as well as the corresponding ligand, PD-L1, were the first ICIs to be approved by the US FDA. The blockade of CTLA-4 enhances T cell responses, whereas the blockage of PD1/PD-L1 interaction dismantles the tumor defense; therefore, it is obvious that these ICIs can be synergistically combined in trans with OVs96, 97 or even combined in cis as a transgene for localized expression within the TME, as demonstrated for CTLA-4,98 CTLA-4 and PD-L1,99 the ICOS ligand (combined in trans with anti-CTLA-4),100 and soluble PD1.101, 102 It has also been demonstrated that influenza A virus can be engineered to express an ICI (single-chain anti-CTLA-4 antibody) within the infected tumor tissue in a mouse melanoma model.103 Recently, Saha et al.104 demonstrated the treatment of glioblastoma with a triple combination of CTLA-4 and PD-L1 blockade together with an IL-12 cis-armed HSV. In their study they also confirmed that the observed enhanced therapeutic efficacy is caused by CD8+ and CD4+ T cells, as well as macrophage polarization within the tumor. In a neo-adjuvant setting, two groups reported that OVs (reovirus and Maraba MG1, respectively) can precondition tumors and metastases to ICI therapy and synergize with surgery in breast cancer and glioblastoma models in mouse.105, 106 In the clinical setting, Ribas et al.107 trans-combined the oncolytic HSV T-Vec with pembrolizumab and demonstrated increased infiltration of the tumor by CD8+ cytotoxic T cells and elevated levels of PD-L1 and IFNγ, leading to a synergistic improvement in therapeutic efficacy with a complete response in a third of the patients according to immune-related response criteria. Similarly, the oncolytic Coxsackievirus A21 is being trans-combined with ipilimumab (anti-CTLA-4),108 as well as pembrolizumab,109 in phase 1b clinical trials for the treatment of advanced melanoma. Both trials demonstrate high overall response rates. Taken together, it is clear that OVs and ICIs can work synergistically together when combined both in cis and in trans.
Taking a BiTE out of Cancer with OVs
In 2014, the US FDA approved the use of a novel type of immune modulator for the treatment of acute lymphoblastic leukemia, the CD3/CD19 bispecific T cell engager (BiTE) blinatumomab. This new family of very promising immune modulators, which exert direct effect on T cells, are artificial fusion proteins composed of two single-chain variable fragments designed to bind CD3 on T cells and a known antigen on the target cell, effectively overruling TCR specificity, MHC expression, and costimulatory signals, forming a so-called pseudoimmunological synapse and triggering CTL activation. Upon activation, CTLs release perforins and granzymes, effectively inducing apoptosis of the target cell. Several BiTEs targeting different TAAs such as the epithelial cell adhesion molecule EpCAM (solitomab or AMG 110, MT110), the carcinoembryonic antigen CEA (AMG 211, MT111), and prostate-specific membrane antigen PSMA (AMG 212) are currently being evaluated in clinical trials (for a review, see Krishnamurthy and Jimeno110). A major shortcoming of BiTEs, however, is the need for continuous i.v. perfusion due to their rather short half-life in circulation (approximately 1.25 hr for blinatumomab111). A way to overcome this is to encode the BiTE as a transgene within an OV to sustain production, as described in several pre-clinical studies (see Scott et al.112). In a pioneering approach, Yu et al.113 constructed a thymidine kinase (TK)-deleted VACV encoding a BiTE specific for ephrin type-A receptor 2 (EphA2) and observed not only oncolytic activity and BiTE secretion from infected cells, but also potent CTL activation and a bystander killing effect on uninfected cells both in in vitro co-culture assays and in vivo in an A549 lung carcinoma xenograft mouse model. In a xenograft model, Fajardo et al.114 made supporting observations with an oncolytic AdV encoding an epidermal growth factor receptor (EGFR)-specific BiTE. Freedman et al.115 observed potent oncolysis and CTL mediated anti-tumor effect both in vitro and in primary human tumor samples using the oncolytic group B AdV EnAdenotucirev (EnAd) encoding a BiTE targeted to EpCAM. These findings together suggest that the combination of the novel immune modulator BiTE with an OV, especially in a transgenic approach, can work in a synergistic fashion. Although both CAR T cells and BiTEs are yielding very promising results in vitro and in vivo, especially in combination with OVs, and undoubtedly are the most powerful tools in the immune modulator toolbox so far, they share the common shortcoming of being dependent on known TAAs.
Arming OVs with Cytokines or Chemokines
One of the first cis-combination therapies with any OV involved encoding cytokines, most notably GM-CSF, which has been introduced as a transgene in several different backbones. GM-CSF promotes the maturation of monocytes into DCs and increases antigen presentation on DCs, and therefore enhances the activation of NK cells and CD8-mediated T cell response downstream.116, 117, 118 So far, multiple OVs have been armed with different cytokines, all following the rationale to “heat up” the TME, either by attracting immune cells to the tumor bed or by activating cells already present within the tumor. The arming of OVs with cytokines is a well-established method119 to improve therapeutic outcome. Notable examples apart from GM-CSF are VSV-IL-15,120 VSV-IFNγ,121 VACV expressing CCL5,122 and MG1-IL-12.31 Viruses armed with two cytokines are possible, for example, AdV in cis combination with TNF-α and IL-2 and in trans combination with adoptive cell therapy.123 Notably, OVs can also be successfully armed with cytokines not specifically targeted toward cells of the innate or adaptive immune system: by cis-combining oncolytic Maraba MG1 with fibroblast growth factor 2 (FGF-2), Ilkow et al.29 showed improved therapeutic efficacy in tumor-bearing mice by exploiting the crosstalk between cancer-associated fibroblasts (CAFs) and malignant cells, thus rendering them more susceptible to OV infection. These studies demonstrate that OV platforms can work synergistically in combination with other immune effectors.
cis/trans Combination Therapies
Currently, most clinical trials already use a combination of OVs with other therapeutic agents in cis and in trans. For example, T-Vec already is cis-combined with GM-CSF and can be synergistically trans-combined with ICIs.107 However, the idea of cis/trans-combination therapies becomes clearer when the OV is armed with a prodrug converting enzyme and then trans-combined with the prodrug, allowing for localized action of the drug and an improved bystander effect. Notable examples for this cis/trans strategy apart from the already discussed HSV encoding a prodrug converting enzyme for cyclophosphamide51 are OVs cis-armed with the HSV TK and trans-combined with ganciclovir.124 Also feasible is the cis combination of two prodrug converting enzymes in a single OV as demonstrated by Gibson et al.125 for an AdV armed with GM-CSF, HSV TK, and cytosine deaminase (CD) converting 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU) (notably, this virus did not show significant improvement over AdV-GM-CSF in a mouse mammary tumor model) and also demonstrated by Tyminski et al.126 for oncolytic HSV armed with two enzymes, YP2B1 and secreted human intestinal carboxylesterase, trans-combined with cyclophosphamide and irinotecan in a xenograft glioma mouse model. On the clinical side, a phase 1b trial is investigating the therapeutic efficacy and safety of a nonlytic replicating retrovirus encoding yeast CD, converting the prodrug Toca-FC (extended-release 5-FC) into 5-FU for the treatment of recurrent or progressive high-grade glioma.127 OVs can also be cis/trans-combined with radiotherapy. Radiotherapy can cause an anti-tumor immune response by inducing ICD similar to OVs (reviewed in Levy et al.128). It is therefore logical to investigate synergistic effects129, 130 of this established therapy with novel approaches like ICIs. The combination of radiotherapy with OVs, so-called radiovirotherapy, has been well described. For example, oncolytic AdV can be used as radiosensitizers,131 and other OVs can be armed with the gene for the sodium iodide symporter (NIS). This method has been demonstrated to augment localized radiotherapy and imaging by actively transporting radioactive iodine into infected (cancer) cells.132 Dingli et al.133 employed an oncolytic measles virus encoding NIS for the treatment of multiple myeloma in a mouse xenograft model, and Gholami et al.134 demonstrated therapeutic efficacy in triple-negative breast cancer using VACV encoding NIS in combination with radioactive iodide. Pursuing a combination approach, Markert et al.135 demonstrated safety and efficacy of oncolytic HSV in combination with radiotherapy of glioblastoma in a phase 1 clinical trial, and an ongoing phase 2 clinical trial (NCT02819843) is investigating the efficacy of trans-combining T-Vec with radiotherapy for the treatment of melanoma, Merkel cell carcinoma, and other solid tumors.
Conclusions
In summary, OV therapy, and even more so the combination of OVs and immunotherapy, is a rapidly expanding field. There is considerable reason for optimism because combinations of OVs with innovative immune modulators like ICIs, CAR T cells, and BiTEs are accelerating the field toward our long-term goal of achieving lasting cures for cancer patients. To take advantage of these exciting opportunities, there are some clear hurdles and knowledge gaps we need to overcome. For instance, we need a more comprehensive understanding of the multi-mechanistic actions of OVs and how they interact with the finely tuned regulatory pathways of the immune system. At the molecular level, unraveling of the details of the interactions between OVs, the TME, and the immune system are crucial to optimization of combination strategies. One important example is understanding the molecular aspects of “epitope spreading” in the context of OV therapy and how to maximize this phenomena in order to potentiate long-term immunotherapeutic attack on the tumor. Coupling this with a thorough analysis of the many ways cancer cells evade immune surveillance will help in the design of multiplexed immunotherapeutic approaches to target the heterogeneity of cancer. Finally translating this knowledge into the clinic remains a challenge. Pre-clinical animal models are of limited value when using therapeutics that have species-specific interactions. The field needs more rationally designed, high-content clinical trials exploring thoughtful therapeutic combinations. Substantial benefit is likely to be gleaned with a better understanding of the optimal doses and timing of therapeutic combinations.
As multiple studies demonstrate, for the majority of patients with systemic disease, the only way to fight cancer is through combinations with multiple therapeutics. It seems that it is not only important to hit the enemy hard and early,136 but also necessary to throw more than two stones to finally kill the bird.
Conflicts of Interest
J.C.B. is a founder and on the Board of Directors of Turnstone Biologics.
Acknowledgments
J.C.B. is supported by Canadian Cancer Society Research Institute, Ontario Institute of Cancer Research, Prostate Cancer Canada, and the Canadian Institute for Cancer Research.
References
- 1.Russell S.J., Peng K.-W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell S.J., Peng K.-W. Oncolytic virotherapy: a contest between apples and oranges. Mol. Ther. 2017;25:1107–1116. doi: 10.1016/j.ymthe.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niemann J., Kühnel F. Oncolytic viruses: adenoviruses. Virus Genes. 2017;53:700–706. doi: 10.1007/s11262-017-1488-1. [DOI] [PubMed] [Google Scholar]
- 5.Bradley S., Jakes A.D., Harrington K., Pandha H., Melcher A., Errington-Mais F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 2014;3:47–55. doi: 10.2147/OV.S56322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchala D.S., Bhatt L.K., Prabhavalkar K.S. Oncolytic herpes simplex viral therapy: a stride toward selective targeting of cancer cells. Front. Pharmacol. 2017;8:270. doi: 10.3389/fphar.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockle J.V., Brüning-Richardson A., Scott K.J., Thompson J., Kottke T., Morrison E., Ismail A., Carcaboso A.M., Rose A., Selby P. Oncolytic herpes simplex virus inhibits pediatric brain tumor migration and invasion. Mol. Ther. Oncolytics. 2017;5:75–86. doi: 10.1016/j.omto.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schvartsman G., Perez K., Flynn J.E., Myers J.N., Tawbi H. Safe and effective administration of T-VEC in a patient with heart transplantation and recurrent locally advanced melanoma. J. Immunother. Cancer. 2017;5:45. doi: 10.1186/s40425-017-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Boeuf F., Selman M., Son H.H., Bergeron A., Chen A., Tsang J., Butterwick D., Arulanandam R., Forbes N.E., Tzelepis F. Oncolytic Maraba virus MG1 as a treatment for sarcoma. Int. J. Cancer. 2017;141:1257–1264. doi: 10.1002/ijc.30813. [DOI] [PubMed] [Google Scholar]
- 10.Brun J., McManus D., Lefebvre C., Hu K., Falls T., Atkins H., Bell J.C., McCart J.A., Mahoney D., Stojdl D.F. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol. Ther. 2010;18:1440–1449. doi: 10.1038/mt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aref S., Bailey K., Fielding A. Measles to the rescue: a review of oncolytic measles virus. Viruses. 2016;8:1–16. doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson S., Galanis E. Potential and clinical translation of oncolytic measles viruses. Expert Opin. Biol. Ther. 2017;17:353–363. doi: 10.1080/14712598.2017.1288713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tayeb S., Zakay-Rones Z., Panet A. Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncolytic Virother. 2015;4:49–62. doi: 10.2147/OV.S78600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelova A.L., Geletneky K., Nüesch J.P.F., Rommelaere J. Tumor selectivity of oncolytic parvoviruses: from in vitro and animal models to cancer patients. Front. Bioeng. Biotechnol. 2015;3:55. doi: 10.3389/fbioe.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson A., Bentham M.J., Scott K., Nuovo G., Bloy A., Appleton E., Adair R.A., Dave R., Peckham-Cooper A., Toogood G. Oncolytic reovirus as a combined antiviral and anti-tumour agent for the treatment of liver cancer. Gut. 2018;67:562–573. doi: 10.1136/gutjnl-2016-312009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp D.W., Lattime E.C. Recombinant poxvirus and the tumor microenvironment: oncolysis, immune regulation and immunization. Biomedicines. 2016;4:19. doi: 10.3390/biomedicines4030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melzer M.K., Lopez-Martinez A., Altomonte J. Oncolytic vesicular stomatitis virus as a viro-immunotherapy: defeating cancer with a “hammer” and “anvil”. Biomedicines. 2017;5:8. doi: 10.3390/biomedicines5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felt S.A., Grdzelishvili V.Z. Recent advances in vesicular stomatitis virus-based oncolytic virotherapy: a 5-year update. J. Gen. Virol. 2017;98:2895–2911. doi: 10.1099/jgv.0.000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E., Kim J.-H., Shin H.-Y., Lee H., Yang J.M., Kim J., Sohn J.H., Kim H., Yun C.O. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum. Gene Ther. 2003;14:1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- 20.Wirth T., Zender L., Schulte B., Mundt B., Plentz R., Rudolph K.L., Manns M., Kubicka S., Kühnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- 21.Parato K.A., Breitbach C.J., Le Boeuf F., Wang J., Storbeck C., Ilkow C., Diallo J.S., Falls T., Burns J., Garcia V. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012;20:749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin N.T., Wrede C., Niemann J., Brooks J., Schwarzer D., Kühnel F., Gerardy-Schahn R. Targeting polysialic acid-abundant cancers using oncolytic adenoviruses with fibers fused to active bacteriophage borne endosialidase. Biomaterials. 2018;158:86–94. doi: 10.1016/j.biomaterials.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Petrovic B., Leoni V., Gatta V., Zaghini A., Vannini A., Campadelli-Fiume G. Dual ligand insertion in gB and gD of oncolytic herpes simplex viruses for retargeting to a producer Vero cell line and to cancer cells. J. Virol. 2018;92 doi: 10.1128/JVI.02122-17. e02122-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia S., O’Bryan S.M., Rivera A.A., Curiel D.T., Mathis J.M. CXCL12 retargeting of an adenovirus vector to cancer cells using a bispecific adapter. Oncolytic Virother. 2016;5:99–113. doi: 10.2147/OV.S112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puhlmann M., Gnant M., Brown C.K., Alexander H.R., Bartlett D.L. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum. Gene Ther. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 26.Stojdl D.F., Lichty B.D., tenOever B.R., Paterson J.M., Power A.T., Knowles S., Marius R., Reynard J., Poliquin L., Atkins H. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Ilkow C.S., Marguerie M., Batenchuk C., Mayer J., Ben Neriah D., Cousineau S., Falls T., Jennings V.A., Boileau M., Bellamy D. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 30.Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Tai L.-H., Ilkow C.S., Alkayyal A.A., Ananth A.A., de Souza C.T., Wang J., Sahi S., Ly L., Lefebvre C. Maraba MG1 virus enhances natural killer cell function via conventional dendritic cells to reduce postoperative metastatic disease. Mol. Ther. 2014;22:1320–1332. doi: 10.1038/mt.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maroun J., Muñoz-Alía M., Ammayappan A., Schulze A., Peng K.W., Russell S. Designing and building oncolytic viruses. Future Virol. 2017;12:193–213. doi: 10.2217/fvl-2016-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arulanandam R., Batenchuk C., Varette O., Zakaria C., Garcia V., Forbes N.E., Davis C., Krishnan R., Karmacharya R., Cox J. Microtubule disruption synergizes with oncolytic virotherapy by inhibiting interferon translation and potentiating bystander killing. Nat. Commun. 2015;6:6410. doi: 10.1038/ncomms7410. [DOI] [PubMed] [Google Scholar]
- 34.Yoo J.Y., Jaime-Ramirez A.C., Bolyard C., Dai H., Nallanagulagari T., Wojton J., Hurwitz B.S., Relation T., Lee T.J., Lotze M.T. Bortezomib treatment sensitizes oncolytic HSV-1-treated tumors to NK cell immunotherapy. Clin. Cancer Res. 2016;22:5265–5276. doi: 10.1158/1078-0432.CCR-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai H.S., Griffin N., Bolyard C., Mao H.C., Zhang J., Cripe T.P., Suenaga T., Arase H., Nakano I., Chiocca E.A. The Fc domain of immunoglobulin is sufficient to bridge NK cells with virally infected cells. Immunity. 2017;47:159–170.e10. doi: 10.1016/j.immuni.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricca J.M., Oseledchyk A., Walther T., Liu C., Mangarin L., Merghoub T., Wolchok J.D., Zamarin D. Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol. Ther. 2018;26:1–12. doi: 10.1016/j.ymthe.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aurelian L. Oncolytic viruses as immunotherapy: progress and remaining challenges. OncoTargets Ther. 2016;9:2627–2637. doi: 10.2147/OTT.S63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aitken A.S., Roy D.G., Bourgeois-Daigneault M.-C. Taking a stab at cancer; oncolytic virus-mediated anti-cancer vaccination strategies. Biomedicines. 2017;5:3. doi: 10.3390/biomedicines5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Z.S., Liu Z., Kowalsky S., Feist M., Kalinski P., Lu B., Storkus W.J., Bartlett D.L. Oncolytic immunotherapy: conceptual evolution, current strategies, and future perspectives. Front. Immunol. 2017;8:555. doi: 10.3389/fimmu.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Munck J., Binks A., McNeish I.A., Aerts J.L. Oncolytic virus-induced cell death and immunity: a match made in heaven? J. Leukoc. Biol. 2017;102:631–643. doi: 10.1189/jlb.5RU0117-040R. [DOI] [PubMed] [Google Scholar]
- 41.van Vloten J.P., Workenhe S.T., Wootton S.K., Mossman K.L., Bridle B.W. Critical interactions between immunogenic cancer cell death, oncolytic viruses, and the immune system define the rational design of combination immunotherapies. J. Immunol. 2018;200:450–458. doi: 10.4049/jimmunol.1701021. [DOI] [PubMed] [Google Scholar]
- 42.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 43.Dornan M.H., Krishnan R., Macklin A.M., Selman M., El Sayes N., Son H.H., Davis C., Chen A., Keillor K., Le P.J. First-in-class small molecule potentiators of cancer virotherapy. Sci. Rep. 2016;6:26786. doi: 10.1038/srep26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selman M., Rousso C., Bergeron A., Son H.H., Krishnan R., El-Sayes N.A., Varette O., Chen A., Le Boeuf F., Tzelepis F. Multi-modal potentiation of oncolytic virotherapy by vanadium compounds. Mol. Ther. 2018;26:56–69. doi: 10.1016/j.ymthe.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diallo J.S., Le Boeuf F., Lai F., Cox J., Vaha-Koskela M., Abdelbary H., MacTavish H., Waite K., Falls T., Wang J. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther. 2010;18:1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selman M., Ou P., Rousso C., Bergeron A., Krishnan R., Pikor L., Chen A., Keller B.A., Ilkow C., Bell J.C., Diallo J.S. Dimethyl fumarate potentiates oncolytic virotherapy through NF-κB inhibition. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao1613. eaao1613. [DOI] [PubMed] [Google Scholar]
- 47.MacTavish H., Diallo J.-S., Huang B., Stanford M., Le Boeuf F., De Silva N., Cox J., Simmons J.G., Guimond T., Falls T. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS ONE. 2010;5:e14462. doi: 10.1371/journal.pone.0014462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyên T.L.-A., Abdelbary H., Arguello M., Breitbach C., Leveille S., Diallo J.-S., Yasmeen A., Bismar T.A., Kirn D., Falls T. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D.S., Dastidar H., Zhang C., Zemp F.J., Lau K., Ernst M., Rakic A., Sikdar S., Rajwani J., Naumenko V. Smac mimetics and oncolytic viruses synergize in driving anticancer T-cell responses through complementary mechanisms. Nat. Commun. 2017;8:344. doi: 10.1038/s41467-017-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulci G., Breymann L., Gianni D., Kurozomi K., Rhee S.S., Yu J., Kaur B., Louis D.N., Weissleder R., Caligiuri M.A., Chiocca E.A. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Currier M.A., Gillespie R.A., Sawtell N.M., Mahller Y.Y., Stroup G., Collins M.H., Kambara H., Chiocca E.A., Cripe T.P. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol. Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y., Wang H.-X., Mao X., Fang H., Wang H., Li Y., Sun Y., Meng C., Tan L., Song C. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget. 2017;8:43201–43217. doi: 10.18632/oncotarget.17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Z.Q., Assenberg M., Liu B.L., Wang Y.B., Simpson G., Thomas S., Coffin R.S. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J. Gene Med. 2007;9:99–106. doi: 10.1002/jgm.999. [DOI] [PubMed] [Google Scholar]
- 54.Meisen W.H., Wohleb E.S., Jaime-Ramirez A.C., Bolyard C., Yoo J.Y., Russell L., Hardcastle J., Dubin S., Muili K., Yu J. The impact of macrophage- and microglia-secreted TNFα on oncolytic HSV-1 therapy in the glioblastoma tumor microenvironment. Clin. Cancer Res. 2015;21:3274–3285. doi: 10.1158/1078-0432.CCR-14-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vähä-Koskela M., Hinkkanen A. Tumor restrictions to oncolytic virus. Biomedicines. 2014;2:163–194. doi: 10.3390/biomedicines2020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson G.R., Han Z., Liu B., Wang Y., Campbell G., Coffin R.S. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 57.Fu X., Tao L., Jin A., Vile R., Brenner M.K., Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol. Ther. 2003;7:748–754. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 58.Ayala-Breton C., Russell L.O.J., Russell S.J., Peng K.-W. Faster replication and higher expression levels of viral glycoproteins give the vesicular stomatitis virus/measles virus hybrid VSV-FH a growth advantage over measles virus. J. Virol. 2014;88:8332–8339. doi: 10.1128/JVI.03823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J.H., Lee Y.S., Kim H., Huang J.H., Yoon A.R., Yun C.O. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J. Natl. Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 60.Rodríguez-García A., Giménez-Alejandre M., Rojas J.J., Moreno R., Bazan-Peregrino M., Cascalló M., Alemany R. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015;21:1406–1418. doi: 10.1158/1078-0432.CCR-14-2213. [DOI] [PubMed] [Google Scholar]
- 61.Martínez-Vélez N., Xipell E., Vera B., Acanda de la Rocha A., Zalacain M., Marrodán L., Gonzalez-Huarriz M., Toledo G., Cascallo M., Alemany R. The oncolytic adenovirus VCN-01 as therapeutic approach against pediatric osteosarcoma. Clin. Cancer Res. 2016;22:2217–2225. doi: 10.1158/1078-0432.CCR-15-1899. [DOI] [PubMed] [Google Scholar]
- 62.Vera B., Martínez-Vélez N., Xipell E., Acanda de la Rocha A., Patiño-García A., Saez-Castresana J., Gonzalez-Huarriz M., Cascallo M., Alemany R., Alonso M.M. Characterization of the antiglioma effect of the oncolytic adenovirus VCN-01. PLoS ONE. 2016;11:e0147211. doi: 10.1371/journal.pone.0147211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan G., Kasuya H., Sahin T.T., Yamamura K., Wu Z., Koide Y., Hotta Y., Shikano T., Yamada S., Kanzaki A. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int. J. Cancer. 2015;136:1718–1730. doi: 10.1002/ijc.29163. [DOI] [PubMed] [Google Scholar]
- 64.Buckel L., Advani S.J., Frentzen A., Zhang Q., Yu Y.A., Chen N.G., Ehrig K., Stritzker J., Mundt A.J., Szalay A.A. Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium. Int. J. Cancer. 2013;133:2989–2999. doi: 10.1002/ijc.28296. [DOI] [PubMed] [Google Scholar]
- 65.Zhang W., Fulci G., Buhrman J.S., Stemmer-Rachamimov A.O., Chen J.W., Wojtkiewicz G.R., Weissleder R., Rabkin S.D., Martuza R.L. Bevacizumab with angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol. Ther. 2012;20:37–45. doi: 10.1038/mt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Currier M.A., Eshun F.K., Sholl A., Chernoguz A., Crawford K., Divanovic S., Boon L., Goins W.F., Frischer J.S., Collins M.H. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Mol. Ther. 2013;21:1014–1023. doi: 10.1038/mt.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolyard C., Yoo J.Y., Wang P.-Y., Saini U., Rath K.S., Cripe T.P., Zhang J., Selvendiran K., Kaur B. Doxorubicin synergizes with 34.5ENVE to enhance antitumor efficacy against metastatic ovarian cancer. Clin. Cancer Res. 2014;20:6479–6494. doi: 10.1158/1078-0432.CCR-14-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simpson G.R., Relph K., Harrington K., Melcher A., Pandha H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother. 2016;5:1–13. doi: 10.2147/OV.S66083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binz E., Berchtold S., Beil J., Schell M., Geisler C., Smirnow I., Lauer U.M. Chemovirotherapy of pancreatic adenocarcinoma by combining oncolytic vaccinia virus GLV-1h68 with nab-paclitaxel plus gemcitabine. Mol. Ther. Oncolytics. 2017;6:10–21. doi: 10.1016/j.omto.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka R., Goshima F., Esaki S., Sato Y., Murata T., Nishiyama Y., Watanabe D., Kimura H. The efficacy of combination therapy with oncolytic herpes simplex virus HF10 and dacarbazine in a mouse melanoma model. Am. J. Cancer Res. 2017;7:1693–1703. [PMC free article] [PubMed] [Google Scholar]
- 71.Bourgeois-Daigneault M.-C., St-Germain L.E., Roy D.G., Pelin A., Aitken A.S., Arulanandam R., Falls T., Garcia V., Diallo J.S., Bell J.C. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016;18:83. doi: 10.1186/s13058-016-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masuelli L., Fantini M., Benvenuto M., Sacchetti P., Giganti M.G., Tresoldi I., Lido P., Lista F., Cavallo F., Nanni P. Intratumoral delivery of recombinant vaccinia virus encoding for ErbB2/Neu inhibits the growth of salivary gland carcinoma cells. J. Transl. Med. 2014;12:122. doi: 10.1186/1479-5876-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Vries C.R., Monken C.E., Lattime E.C. The addition of recombinant vaccinia HER2/neu to oncolytic vaccinia-GMCSF given into the tumor microenvironment overcomes MDSC-mediated immune escape and systemic anergy. Cancer Gene Ther. 2015;22:154–162. doi: 10.1038/cgt.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bridle B.W., Boudreau J.E., Lichty B.D., Brunellière J., Stephenson K., Koshy S., Bramson J.L., Wan Y. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bridle B.W., Nguyen A., Salem O., Zhang L., Koshy S., Clouthier D., Chen L., Pol J., Swift S.L., Bowdish D.M. Privileged antigen presentation in splenic B cell follicles maximizes T cell responses in prime-boost vaccination. J. Immunol. 2016;196:4587–4595. doi: 10.4049/jimmunol.1600106. [DOI] [PubMed] [Google Scholar]
- 76.Meyers D.E., Wang A.A., Thirukkumaran C.M., Morris D.G. Current immunotherapeutic strategies to enhance oncolytic virotherapy. Front. Oncol. 2017;7:114. doi: 10.3389/fonc.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemay C.G., Rintoul J.L., Kus A., Paterson J.M., Garcia V., Falls T.J., Ferreira L., Bridle B.W., Conrad D.P., Tang V.A. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol. Ther. 2012;20:1791–1799. doi: 10.1038/mt.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alkayyal A.A., Tai L.-H., Kennedy M.A., de Souza C.T., Zhang J., Lefebvre C., Sahi S., Ananth A.A., Mahmoud A.B., Makrigiannis A.P. NK-cell recruitment is necessary for eradication of peritoneal carcinomatosis with an IL12-expressing Maraba virus cellular vaccine. Cancer Immunol. Res. 2017;5:211–221. doi: 10.1158/2326-6066.CIR-16-0162. [DOI] [PubMed] [Google Scholar]
- 79.Capasso C., Hirvinen M., Garofalo M., Romaniuk D., Kuryk L., Sarvela T., Vitale A., Antopolsky M., Magarkar A., Viitala T. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. OncoImmunology. 2015;5:e1105429. doi: 10.1080/2162402X.2015.1105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Boeuf F., Diallo J.-S., McCart J.A., Thorne S., Falls T., Stanford M., Kanji F., Auer R., Brown C.W., Lichty B.D. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tysome J.R., Li X., Wang S., Wang P., Gao D., Du P., Chen D., Gangeswaran R., Chard L.S., Yuan M. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- 82.Nistal-Villan E., Bunuales M., Poutou J., Gonzalez-Aparicio M., Bravo-Perez C., Quetglas J.I., Carte B., Gonzalez-Aseguinolaza G., Prieto J., Larrea E., Hernandez-Alcoceba R. Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol. Cancer. 2015;14:210. doi: 10.1186/s12943-015-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ilett E., Kottke T., Thompson J., Rajani K., Zaidi S., Evgin L., Coffey M., Ralph C., Diaz R., Pandha H. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther. 2017;24:21–30. doi: 10.1038/gt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cronin M., Le Boeuf F., Murphy C., Roy D.G., Falls T., Bell J.C., Tangney M. Bacterial-mediated knockdown of tumor resistance to an oncolytic virus enhances therapy. Mol. Ther. 2014;22:1188–1197. doi: 10.1038/mt.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aitken A.S., Roy D.G., Martin N.T., Sad S., Bell J.C., Bourgeois-Daigneault M.-C. Brief communication; a heterologous oncolytic bacteria-virus prime-boost approach for anticancer vaccination in mice. J. Immunother. 2018;41:125–129. doi: 10.1097/CJI.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toussaint B., Chauchet X., Wang Y., Polack B., Le Gouëllec A. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev. Vaccines. 2013;12:1139–1154. doi: 10.1586/14760584.2013.836914. [DOI] [PubMed] [Google Scholar]
- 87.Wall D.M., Srikanth C.V., McCormick B.A. Targeting tumors with salmonella Typhimurium—potential for therapy. Oncotarget. 2010;1:721–728. doi: 10.18632/oncotarget.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark-Curtiss J.E., Curtiss R. Salmonella vaccines: conduits for protective antigens. J. Immunol. 2018;200:39–48. doi: 10.4049/jimmunol.1600608. [DOI] [PubMed] [Google Scholar]
- 89.Maher J. Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells. ISRN Oncol. 2012;2012:278093. doi: 10.5402/2012/278093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ajina A., Maher J. Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer. 2017;5:90. doi: 10.1186/s40425-017-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V., Bouchier-Hayes L., Savoldo B., Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanoue K., Rosewell Shaw A., Watanabe N., Porter C., Rana B., Gottschalk S., Brenner M., Suzuki M. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. 2017;77:2040–2051. doi: 10.1158/0008-5472.CAN-16-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson R.A.M., Evans T.R.J., Fraser A.R., Nibbs R.J.B. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin. Exp. Immunol. 2018;191:133–148. doi: 10.1111/cei.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008095. 226ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Z., Ravindranathan R., Kalinski P., Guo Z.S., Bartlett D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du T., Shi G., Li Y.M., Zhang J.F., Tian H.W., Wei Y.Q., Deng H., Yu D.C. Tumor-specific oncolytic adenoviruses expressing granulocyte macrophage colony-stimulating factor or anti-CTLA4 antibody for the treatment of cancers. Cancer Gene Ther. 2014;21:340–348. doi: 10.1038/cgt.2014.34. [DOI] [PubMed] [Google Scholar]
- 99.Engeland C.E., Grossardt C., Veinalde R., Bossow S., Lutz D., Kaufmann J.K., Shevchenko I., Umansky V., Nettelbeck D.M., Weichert W. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zamarin D., Holmgaard R.B., Ricca J., Plitt T., Palese P., Sharma P., Merghoub T., Wolchok J.D., Allison J.P. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 2017;8:14340. doi: 10.1038/ncomms14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartee M.Y., Dunlap K.M., Bartee E. Tumor-localized secretion of soluble PD1 enhances oncolytic virotherapy. Cancer Res. 2017;77:2952–2963. doi: 10.1158/0008-5472.CAN-16-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kleinpeter P., Fend L., Thioudellet C., Geist M., Sfrontato N., Koerper V., Fahrner C., Schmitt D., Gantzer M., Remy-Ziller C. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology. 2016;5:e1220467. doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamilton J.R., Vijayakumar G., Palese P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model. Cell Rep. 2018;22:1–7. doi: 10.1016/j.celrep.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 104.Saha D., Martuza R.L., Rabkin S.D. Curing glioblastoma: oncolytic HSV-IL12 and checkpoint blockade. Oncoscience. 2017;4:67–69. doi: 10.18632/oncoscience.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourgeois-Daigneault M.-C., Roy D.G., Aitken A.S., El Sayes N., Martin N.T., Varette O., Falls T., St-Germain L.E., Pelin A., Lichty B.D. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao1641. eaao1641. [DOI] [PubMed] [Google Scholar]
- 106.Samson A., Scott K.J., Taggart D., West E.J., Wilson E., Nuovo G.J., Thomson S., Corns R., Mathew R.K., Fuller M.J. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam7577. eaam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Curti B.D., Richards J.M., Hallmeyer S., Faries M.B., Andtbacka R.H.I., Daniels G.A., Grose M., Shafren D. Activity of a novel immunotherapy combination of intralesional Coxsackievirus A21 and systemic ipilimumab in advanced melanoma patients previously treated with anti-PD1 blockade therapy. J. Clin. Oncol. 2017;35:3014. [Google Scholar]
- 109.Silk, A.W., Kaufman, H.L., Faries, M., O’Day, S., Gabrail, N., Mehnert, J., Bryan, J., Norrell, J., Haider, A., Bommareddy, P.K., et al. (2017). CAPRA: A Phase Ib study of intratumoral oncolytic Coxsackievirus A21 (CVA21) and systemic pembrolizumab in advanced melanoma patients. https://viralytics.com/wp-content/uploads/2017/03/Final-SITC-CAPRA-10-11-2017.pdf.
- 110.Krishnamurthy A., Jimeno A. Bispecific antibodies for cancer therapy: a review. Pharmacol. Ther. 2017 doi: 10.1016/j.pharmthera.2017.12.002. Published online December 18, 2017. [DOI] [PubMed] [Google Scholar]
- 111.Klinger M., Brandl C., Zugmaier G., Hijazi Y., Bargou R.C., Topp M.S., Gökbuget N., Neumann S., Goebeler M., Viardot A. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 112.Scott E.M., Duffy M.R., Freedman J.D., Fisher K.D., Seymour L.W. Solid tumor immunotherapy with T cell engager-armed oncolytic viruses. Macromol. Biosci. 2018;18:1700187. doi: 10.1002/mabi.201700187. [DOI] [PubMed] [Google Scholar]
- 113.Yu F., Wang X., Guo Z.S., Bartlett D.L., Gottschalk S.M., Song X.-T. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol. Ther. 2014;22:102–111. doi: 10.1038/mt.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fajardo C.A., Guedan S., Rojas L.A., Moreno R., Arias-Badia M., de Sostoa J., June C.H., Alemany R. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77:2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 115.Freedman J.D., Hagel J., Scott E.M., Psallidas I., Gupta A., Spiers L., Miller P., Kanellakis N., Ashfield R., Fisher K.D. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol. Med. 2017;9:1067–1087. doi: 10.15252/emmm.201707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mastrangelo M.J., Maguire H.C., Jr., Eisenlohr L.C., Laughlin C.E., Monken C.E., McCue P.A., Kovatich A.J., Lattime E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 117.Liu B.L., Robinson M., Han Z.-Q., Branston R.H., English C., Reay P., McGrath Y., Thomas S.K., Thornton M., Bullock P. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 118.Ren J., Gwin W.R., Zhou X., Wang X., Huang H., Jiang N., Zhou L., Agarwal P., Hobeika A., Crosby E. Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy. OncoImmunology. 2016;6:e1264563. doi: 10.1080/2162402X.2016.1264563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Gruijl T.D., Janssen A.B., van Beusechem V.W. Arming oncolytic viruses to leverage antitumor immunity. Expert Opin. Biol. Ther. 2015;15:959–971. doi: 10.1517/14712598.2015.1044433. [DOI] [PubMed] [Google Scholar]
- 120.Stephenson K.B., Barra N.G., Davies E., Ashkar A.A., Lichty B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 121.Bourgeois-Daigneault M.-C., Roy D.G., Falls T., Twumasi-Boateng K., St-Germain L.E., Marguerie M., Garcia V., Selman M., Jennings V.A., Pettigrew J. Oncolytic vesicular stomatitis virus expressing interferon-γ has enhanced therapeutic activity. Mol. Ther. Oncolytics. 2016;3:16001. doi: 10.1038/mto.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J., O’Malley M., Urban J., Sampath P., Guo Z.S., Kalinski P., Thorne S.H., Bartlett D.L. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Havunen R., Siurala M., Sorsa S., Grönberg-Vähä-Koskela S., Behr M., Tähtinen S., Santos J.M., Karell P., Rusanen J., Nettelbeck D.M. Oncolytic adenoviruses armed with tumor necrosis factor alpha and interleukin-2 enable successful adoptive cell therapy. Mol. Ther. Oncolytics. 2016;4:77–86. doi: 10.1016/j.omto.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wildner O., Blaese R.M., Morris J.C. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 125.Gibson H., Munns S., Freytag S., Barton K., Veenstra J., Bettahi I., Bissonette J., Wei W.Z. Immunotherapeutic intervention with oncolytic adenovirus in mouse mammary tumors. OncoImmunology. 2015;4:e984523. doi: 10.4161/2162402X.2014.984523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tyminski E., Leroy S., Terada K., Finkelstein D.M., Hyatt J.L., Danks M.K., Potter P.M., Saeki Y., Chiocca E.A. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005;65:6850–6857. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 127.Cloughesy T.F., Landolfi J., Hogan D.J., Bloomfield S., Carter B., Chen C.C., Elder J.B., Kalkanis S.N., Kesari S., Lai A. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad9784. 341ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levy A., Chargari C., Marabelle A., Perfettini J.-L., Magné N., Deutsch E. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur. J. Cancer. 2016;62:36–45. doi: 10.1016/j.ejca.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 129.Mansfield A.S., Park S.S., Dong H. Synergy of cancer immunotherapy and radiotherapy. Aging (Albany N.Y.) 2015;7:144–145. doi: 10.18632/aging.100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai M.H., Liu S.L., Chen N.G., Zhang T.P., You L., Q Zhang F., Chou T.C., Szalay A.A., Fong Y., Zhao Y.P. Oncolytic vaccinia virus in combination with radiation shows synergistic antitumor efficacy in pancreatic cancer. Cancer Lett. 2014;344:282–290. doi: 10.1016/j.canlet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 131.O’Cathail S.M., Pokrovska T.D., Maughan T.S., Fisher K.D., Seymour L.W., Hawkins M.A. Combining oncolytic adenovirus with radiation—a paradigm for the future of radiosensitization. Front. Oncol. 2017;7:153. doi: 10.3389/fonc.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J., Arulanandam R., Wassenaar R., Falls T., Petryk J., Paget J., Garson K., Cemeus C., Vanderhyden B.C., Wells R.G. Enhancing expression of functional human sodium iodide symporter and somatostatin receptor in recombinant oncolytic vaccinia virus for in vivo imaging of tumors. J. Nucl. Med. 2017;58:221–227. doi: 10.2967/jnumed.116.180463. [DOI] [PubMed] [Google Scholar]
- 133.Dingli D., Peng K.W., Harvey M.E., Greipp P.R., O’Connor M.K., Cattaneo R., Morris J.C., Russell S.J. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 134.Gholami S., Chen C.-H., Lou E., Belin L.J., Fujisawa S., Longo V.A., Chen N.G., Gönen M., Zanzonico P.B., Szalay A.A., Fong Y. Vaccinia virus GLV-1h153 in combination with 131I shows increased efficiency in treating triple-negative breast cancer. FASEB J. 2014;28:676–682. doi: 10.1096/fj.13-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Markert J.M., Razdan S.N., Kuo H.-C., Cantor A., Knoll A., Karrasch M., Nabors L.B., Markiewicz M., Agee B.S., Coleman J.M. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014;22:1048–1055. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruf B., Lauer U.M. Assessment of current virotherapeutic application schemes: “hit hard and early” versus “killing softly”? Mol. Ther. Oncolytics. 2015;2:15018. doi: 10.1038/mto.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]