Abstract
Indirect impacts of climate change, mediated by new species interactions (including pathogens or parasites) will likely be key drivers of biodiversity reorganization. In addition, direct effects of extreme weather events remain understudied. Simultaneous investigation of the significance of ectoparasites on host populations and extreme weather events is lacking, especially in the Arctic. Here we document the consequences of recent black fly outbreaks and extreme precipitation events on the reproductive output of an arctic top predator, the peregrine falcon (Falco peregrinus tundrius) nesting at the northern range limit of ornithophilic black flies in Nunavut, Canada. Overall, black fly outbreaks and heavy rain reduced annual nestling survival by up to 30% and 50% respectively. High mortality caused by ectoparasites followed record-breaking spring snow precipitation, which likely increased stream discharge and nutrient runoff, two key parameters involved in growth and survival of black fly larvae. Using the RCP4.5 intermediate climate scenario run under the Canadian Global Climate Model, we anticipate a northward expansion of black fly distribution in Arctic regions. Our case study demonstrates that, in the context of climate change, extreme weather events can have substantial direct and indirect effects on reproductive output of an arctic top-predator population.
Introduction
Recent climate change has been associated with higher temperatures, and long-term projections indicate that an increase in the frequency of extreme weather events is likely1. These abiotic changes can have direct and/or indirect ecological consequences including trophic mismatch2, demographic effects3, and shifts in species distribution4 that generate new species assemblages5. Species’ range expansion can alter trophic relationships and contribute to novel host-parasite systems that can facilitate emergence of infectious diseases6–8 and increase host mortality.
Climate change has occurred at a faster rate in the Arctic compared to any other ecoregion globally9, resulting in increased temperature, melting permafrost and sea ice reduction1,10. Increased frequency of extreme weather events and wetter winters are also predicted for some arctic regions11. Warmer temperatures facilitate range expansion and establishment of pathogens and parasites in the Arctic7,12,13. For example, warmer conditions likely facilitated range expansion and establishment of a parasitic nematode of muskoxen Ovibos moschatus allowing the completion of the parasite life cycle in a single summer12. Emergence of diseases and parasites has been reported in arctic birds13–15 with potential interplay with changes in winter temperature affecting the host-parasite system in arctic-nesting birds16. Despite exposure and sensitivity of arctic host-parasite systems to climate change, empirical and model-based studies describing climate impacts on life history traits of parasites17,18 and fitness consequences on hosts19 in arctic regions are lacking7. This is surprising given that tundra ecosystems comprise low diversity of pathogens and parasites, relatively few species interactions20, and minimal anthropogenic disturbance, thus greatly facilitating understanding of the climate-driven mechanisms potentially affecting host-parasite interactions.
Here we examine the significance of ectoparasite outbreaks and heavy precipitation events on nestling survival in an arctic population of peregrine falcons monitored between 1982–1995 and 2008–2015 near Rankin Inlet, Nunavut, Canada (62°49′N, 92°05′W). Hematophagous ornithophilic black flies (Diptera: Simuliidae) are ectoparasites that can reduce breeding success in avian species by affecting adult nesting behaviour and increasing juvenile mortality21. Black fly outbreaks have rarely been implicated as a population-level source of mortality, however, the negative direct effects of increased frequency of heavy rain events on nestling survival is well established3 and partly explained the long-term decline in reproductive success in our study population22.
Our objectives were to: (i) illustrate the potential significance of ornithophilic black fly outbreaks at their northern range limit and heavy rain on nestling mortality in peregrine falcons; (ii) investigate the potential mechanisms (such as changes in temperature, precipitation and snow regimes) involved in promoting local black fly outbreaks and (iii) estimate the northern limit of black fly distribution using climate data (1990–2010) and project shifts in the northern limit using Canadian Global Climate Models with the RCP4.5 scenario23.
Results
Nestling survival and heavy rain
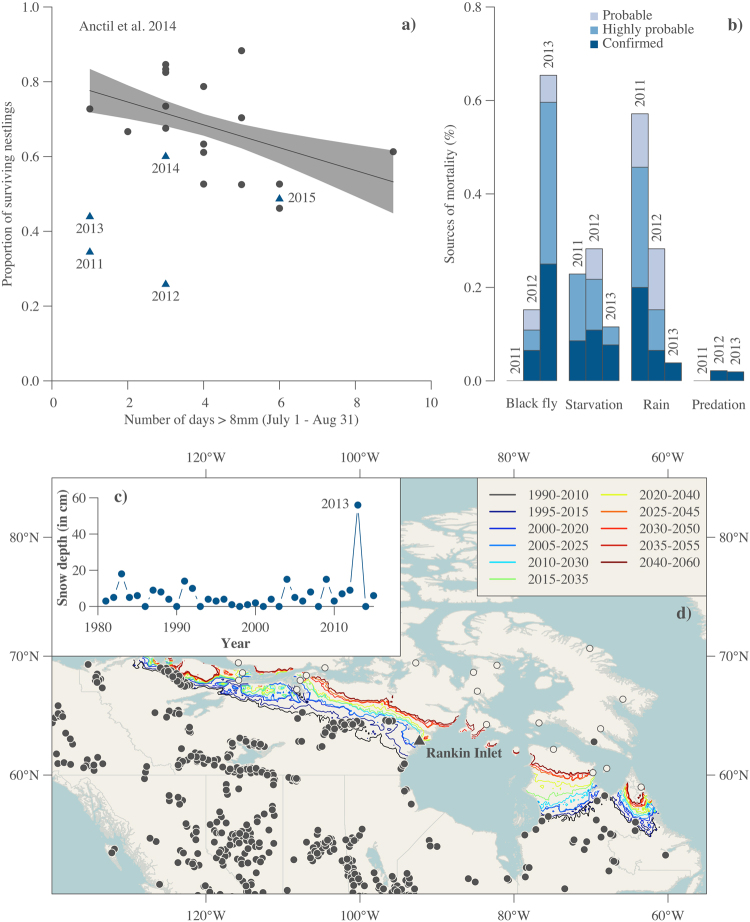
We first extended previously published data that describe the proportion of peregrine falcon nestlings surviving to 25 days by incorporating five additional years (2011–2015) from long-term population monitoring3. Nestling survival from 1982–1995 and 2008–2010 was negatively related to the number of days with heavy rain (≥8 mm.day−1) recorded in July and August (Fig. 1a). Four years were identified as outliers (1985: Studentized residuals = 2.40, P = 0.03; 2011, 2012 and 2013: all Studentized residuals ≤−2.46, all P = 0.02). A peak in microtine abundance likely explained the high productivity observed in 198524. Despite relatively few days of heavy rain between 2011 and 2013, nestlings experienced lower survival (Fig. 1a). Difference between predicted survival based on long-term monitoring and observed survival indicate a reduction by up to 30–50% in nestling survival during these specific years (Fig. 1a).
Figure 1.
Identification of the climate-mediated mechanisms involved in peregrine falcon nestling mortality in the Canadian Low Arctic. (a) Relationship between the number of days with heavy rain (≥8 mm.day−1) recorded in July and August, and the proportion of peregrine falcon nestlings surviving up to 25 days old in the Rankin Inlet population (1982–1995 and 2008–2015). The solid black line and the shaded gray area correspond to the linear regression of Anctil et al.3 for the period 1982–1995 and 2008–2010 (black dots). We expanded the work of Anctil et al.3 by adding five extra years (2011–2015; blue triangles). (b) Contribution of black flies, starvation, rain and predation as causes of mortality in years with exceptionally low nestling survival (2011–2013). Additional (mostly unknown) causes of mortality are described in the Methods. The inner graph (c) indicates snow depth (cm) on the last day of May for the period 1981–2015 at Rankin Inlet. (d) Distribution map of ornithophilic black fly species in Canada. Dots refer to the presence or absence (filled and open dots respectively) of black flies. Black line represents the summer isotherm for the reference period (1990–2010) that best matches the northern limit of black flies (9.5 °C isotherm, see Methods). Color lines illustrate future potential predicted isotherms for 20-year periods from 1995 to 2060 based on the Canadian Global Climate Model (RCP4.5).
Causes of nestling mortality in extreme years (2011–2013)
Nestling mortality in 2011 was mostly due to a single heavy rain event with >19 mm precipitation in one day (Fig. 1a,b). Three episodes of heavy rain (10, 23 and 24 mm.day−1) caused high mortality in 2012 (Fig. 1a,b). Starvation mortality was observed in all years (Fig. 1b). Black flies represented a major source of nestling mortality over a very short period (<24 h) during the chick-rearing period only in 2013 (Fig. 1b). Evidence of nestling mortality from the biting effects of black flies was also found in 2012 (Fig. 1b), however the severity of this outbreak was much lower compared to 2013.
Variation in monitoring effort among nesting sites (i.e., presence of a camera; see Methods) prevented confirmation of the cause of nestling mortality at nesting sites that were not monitored using cameras (Fig. 1b). To address this, we compared mortality at wind-exposed and wind-sheltered nesting sites during the short period that encompassed the black fly outbreak. Black fly activity and abundance are negatively affected by wind velocity25 and we predicted that wind-exposed nesting sites would experience lower mortality compared to wind-sheltered nesting sites during the outbreak. We found that mortality was lower for nesting sites exposed to the prevailing wind (north oriented nesting sites) compared to sheltered ones during the outbreak in 2013 (χ2 = 3.84; P < 0.05, N = 27 nesting sites). The same analysis conducted prior to, and after, the outbreak showed no effect of exposure to the prevailing wind direction on mortality (χ2 < 1.3; P > 0.25; N = 30 and 23 nesting sites, respectively). Decreased mortality at wind-exposed nesting sites strongly suggests that blood-feeding black flies were probably responsible for deaths of nestlings at sites that were not monitored with cameras but that experienced a complete brood reduction during the 1-day black fly outbreak in 2013 (Fig. 1b).
Weather, climate and black fly distribution
We examined weather variables that potentially explained high mortality rates associated with the 2013 outbreak. Analysis of weather variables available from 1981 to 2015 (temperature, precipitation and snow pack) indicated that snow depth recorded in late May 2013 was exceptionally high (56.0 cm in 2013 compared to 16.3 cm on average for the period 1981–2015; Fig. 1c)26 and was detected as an outlier (Studentized residuals = 9.89, P < 0.0001).
We constructed a distribution map of ornithophilic black fly species across North America from presence-absence data27, and estimated their northern distribution limit. The 9.5 °C summer isotherm of mean temperature for the months of June, July and August best matched the northern limit of the range of ornithophilic black fly species for the reference period (1990–2010). Forward projection of this summer reference isotherm by 20-year periods with five-year lags (e.g. 1995–2015, 2000–2020, 2005–2025, etc.) up to the period 2040–2060 predicted that ornithophilic black flies could become firmly established at latitudes consistent with our study area between 2010–2030 and 2015–2035 (Fig. 1d).
Discussion
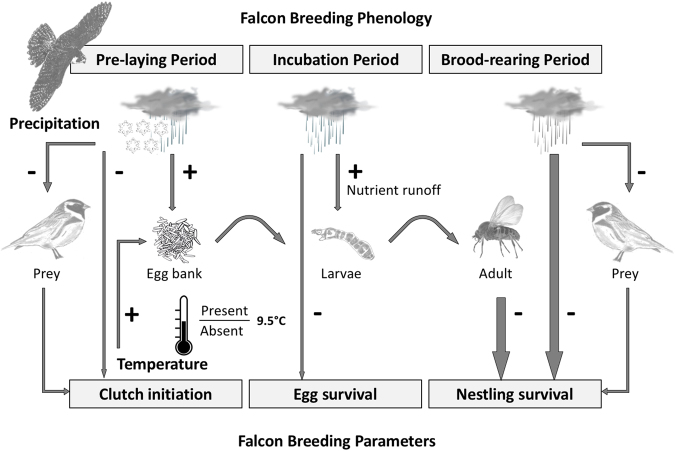
Arctic species, including parasites and hosts have evolved under limiting environmental conditions and therefore, their life history traits are sensitive to even small changes in climatic conditions28. High exposure and sensitivity without any particular high adaptive capacity29 make arctic vertebrates particularly vulnerable to parasites12,19 and extreme weather events3,30. Our results confirm that increased frequency of heavy precipitation can reduce reproductive output of arctic-nesting peregrine falcons directly (through exposure to cold, wet weather), and indirectly through the outbreak of black flies described here and elsewhere31. We qualitatively summarized the frequency and severity of these effects on the reproduction of arctic-nesting peregrine falcon during major phases of the breeding cycle (Fig. 2). Heavy precipitation can have negative direct effects at any stage of the reproductive cycle and result in reduced reproductive output (Fig. 2). The frequency of heavy precipitation has increased over the past three decades and partly explained the long-term decline in falcon reproductive success3. The frequency of severe black fly effects is currently extremely rare but may increase in the Arctic as a result of climate warming (Figs 1 and 2).
Figure 2.
A qualitative assessment of direct and indirect effects of precipitation and temperature on reproduction of peregrine falcons nesting in the Canadian Low Arctic. Effects can be severe (wide arrows) or slight (narrow arrows) and occur during different phases of the breeding cycle. Curved arrows represent metamorphosis among life history stages of black flies. Mean summer temperature ≥9.5 °C likely reflects the isotherm at which populations of ornithophilic black fly species can become firmly established (Fig. 1). The magnitude and signs of the presented effects are justified in the discussion section.
The species of black fly (Metacnephia saskatchewana) likely responsible for mortality of nestlings in 2013 had not been reported in Rankin Inlet prior to 201631. However, other ornithophilic species have been reported in Rankin Inlet (Simulium silvestre) and other nearby communities; (Simulium anatinum and Simulium annulus at Arviat and Baker Lake, Nunavut, Canada)27. Black fly outbreaks may be a rarely occurring natural phenomenon, or may represent an emerging source of mortality in our peregrine falcon population that could be related to climate change (i.e., changes in precipitation regimes and temperature). However, predicting shifts in geographic distribution of parasite species within the food web is challenging, and requires notably climate-driven projection18. We found that the measured and anticipated increase in summer temperature represents a projection of climatic conditions required for black fly species to expand their current northern range limit. In our opinion, the record-breaking spring snow depth recorded in 2013 may have contributed to increased stream discharge and nutrient runoff, two key parameters involved in growth and survival of black fly larvae32,33. The link between water flow and black fly larvae growth and survival combined with the expected increase in temperature and the frequency of extreme weather events with climate change could lead to an increase in the frequency of arctic black fly outbreaks in the near future. Although establishment of ornithophilic black flies in the Arctic under warmer temperatures is a prerequisite for an outbreak to occur, the amount of running water in spring is likely the proximate driver that influenced the severity of an outbreak.
Our results also demonstrate that single extreme weather events can reduce nestling survival in arctic-nesting birds. This adds to the growing body of literature showing that extreme weather conditions can reduce reproductive success during different stages of the breeding cycle34–36. Repeated heavy rain events across the brood rearing period can have direct consequences on peregrine falcon nestling survival3. Peregrine falcon nestlings younger than three weeks of age are sensitive to hypothermia during heavy rain events because they are unable to thermoregulate without being brooded or without the presence of sibling37. We also found that a single ectoparasite outbreak (2013) or extreme event of heavy rain (2011) can also have substantial negative effect on annual reproductive success by reducing offspring survival. In 2013, ectoparasitism (likely an indirect effect of precipitation; Fig. 2) is more likely driving the observed low nestling survival that year while in 2012, a combination of heavy rain and black fly outbreak are responsible for the low reproductive success. During the outbreaks, the biting effect of black flies resulted in widespread mortality due to the effects hemorrhagic coalescent lesions on the head and body of affected nestlings31.
Spring precipitation during the pre-laying period directly reduces clutch initiation. Precipitation can also indirectly reduce reproductive output, likely through lower body condition38 due to low prey availability. Precipitation during the pre-laying period likely triggers the hydrological conditions33 and nutrient runoff levels32 required for black fly reproduction. This ultimately results in indirect nestling mortality once adult black flies emerged (this study) and begin blood feeding (i.e., the proximate cause of mortality) on nestlings31. During incubation, precipitation directly reduces egg survival (Franke, unpublished data). During the brood rearing period, nestling survival is negatively affected by the direct effects of precipitation3 and by the indirect effects mediated through availability of prey39. Those direct and indirect linkages are summarized in Fig. 2.
One limitation of our study is that high mortality caused by ectoparasite was detected only during one year of our long-term population monitoring study and occurred over a very short period (<24 h), thus preventing an overall analysis of the mechanisms that may drive black fly outbreaks. Note that little is known about the biology of northern black flies and their interactions with wild birds; however, evidence suggests that the influence is likely to be as severe as that observed in the poultry and exotic bird industries21. Deaths from massive attacks are known to occur from toxemic shock or withdrawal of excessive blood27. For example, the deaths of more than 240 chickens and 20 turkeys in southwestern Manitoba in a single day were attributed to black fly attacks40. Although the duration of the outbreak was very short at Rankin Inlet in 2013, it was recorded in real time. Moreover, the severity of this single outbreak was remarkable and was supported by analysis of nesting site orientation, which is known to affect reproductive performance of cliff nesting birds41. In our study, we found that north-facing nesting sites (i.e. wind exposed nesting sites) experienced lower mortality only during black fly outbreak. This result is further supported by findings that show reproductive output is usually lower on north-facing nesting sites due mainly to lower solar radiation and exposure to cold northerly wind and storms41,42.
This study is one of the first to quantify the impact of an ectoparasite at its northern limit on the survival of a host population and illustrates how extreme weather events and increased temperature can have direct and indirect impacts on arctic wildlife. Despite increased efforts to model direct and indirect impacts of climate change on host-parasite systems17,18, long-term ecosystem-based monitoring will be required to detect climate-driven effects on host-parasites systems in the Arctic7,18.
Methods
Population monitoring
The population of peregrine falcons breeding near Rankin Inlet, Nunavut, Canada (62°49′N, 92°05′W) has been monitored continuously since 1980. However, we limited our analysis to 22 years (1982–1995 and 2008–2015) as the number of nestlings to hatch was not recorded in other years3. This population represents the highest known density of breeding peregrine falcons in the Arctic and the second highest density recorded worldwide43. Within the Rankin Inlet study area that encompasses approximately 455 km2, most rocky outcrops are oriented along a northwest-southwest axis and, consequently, most nesting cliffs used by peregrine falcons face southwest24. Nest monitoring procedures are extensively described in Franke et al.22. Since 2008, we deployed motion sensitive cameras at nests from egg-laying until fledging to document breeding phenology (lay and hatch dates), reproductive behavior (e.g. incubation duration, food delivery) and causes of mortality. We programmed cameras to capture one to three images when triggered by motion, after which they remained insensitive to movement for 5–15 seconds. In addition, camera recorded one image every 15 minutes and we replaced memory cards and batteries every 5–10 days during the nestling-rearing period. At each nest visit during this period, we systematically recorded growth of nestlings by assessment of body mass. Continuous monitoring by motion sensitive cameras during the reproductive season allowed the detection of two black fly outbreaks in the population. The first outbreak (25 July 2012) was less severe than the second outbreak (20 July 2013)31. We found no evidence of black fly outbreak in any other year in which cameras were deployed at nest sites (2008–2015) and we did not report nestling mortality associated with black flies for the 1982–1995 period31.
Nestling survival and heavy rain
We followed Anctil et al.3 and defined “heavy rain” as any precipitation event ≥8 mm.day−1 (Fig. 1a). We compared the proportion of peregrine falcon nestlings surviving up to 25 days of age in the Rankin Inlet population as a function of the number of days with heavy rain recorded in July and August in recent years (2011–2015) to the regression model based on long-term monitoring (1982–1995 and 2008–2010)3. Weather data were available from the Environment Canada weather station located centrally within the study area26. We identified years with extremely high or low survival using Studentized residuals to test for the presence of outliers44. In addition, we examined the specific causes of nestling mortality observed in years with low nestling survival.
Causes of nestling mortality between 2011–2013
Mortality events were recorded and classified into one of the following causes: (Fig. 1b).
Black fly
During the 2013 black fly outbreak, mortality events from black fly at camera monitored nests (N = 10) were confirmed through inspection of images. Descriptions and pictures of nestling mortality related to black fly are available in Franke et al.31. Remaining active nests (n = 20) were not monitored by camera but were visited weekly (every 5–7 days) during the chick-rearing period. At these nests, mortality from black flies was classified as highly probable when the full brood disappeared (dead nestlings are presumably carried away and cached by the adults) or probable when the brood partially disappeared during the period encompassing the date of the black fly outbreak. Decreased mortality recorded at wind-exposed nesting sites during the 1-day outbreak also indicate that blood-feeding black flies were very likely responsible for deaths of nestlings at nesting sites that were not monitored with cameras but that experienced a complete brood reduction during the outbreak (see results). The same methodology was used to assign mortalities during black fly outbreak in 2012. No evidence of nestling mortality related to black fly occurred in other years.
Starvation
Nestlings with growth curves below average growth curves for peregrine falcon nestlings in our population24 that did not die during or soon (≤2 days) after a rainstorm or black fly outbreak were confirmed to have died from starvation3. Mortality from starvation was classified as highly probable for nestlings that did not die during or soon after a rainstorm or black fly outbreak, that were fed less than three occasions per day prior to mortality and for which information on growth was not available. Mortality events from starvation that would have normally been classified as highly probable were classified probable when the event was not recorded due to improper camera placement or recording. Hence, the relative importance of starvation may have been slightly overestimated.
Rain
At camera monitored nests, mortality events from exposure to rain were confirmed for nestlings dying during rain event that were fed on three or more occasions per day3. At camera monitored nests where information on food delivery rate was not available, mortality from rain was classified as highly probable if nestlings died during a rain event. Mortality due to rain at nests that were visited weekly but were not monitored by camera was classified as probable if an episode of heavy rain occurred between two nest visits.
Predation
Nestling predation in the Rankin Inlet population is marginal and accounted for <1% of annual nestling mortality between 2009 and 2015. Predation events reported in Fig. 1b were all confirmed by camera.
Other
Nestlings that died of known causes that were not related to black fly, rain, starvation or predation were classified as Other (N = 5). Mortality events that could not be assigned to any known causes and that were not recorded on camera were classified as Unknown (2011: N = 7, 2012: N = 16, 2013: N = 9).
Nesting site exposure and mortality
We performed a chi-square test to compare nesting site mortality between wind-exposed and wind-sheltered nesting site during the 1-day black fly outbreak on 20 July 2013. During the outbreak, wind speed was approximately 30 km.hour−1 blowing from the north26. Nesting sites oriented between 270° and 90° (0° = north) and nesting sites with nest located on the very top of a cliff were classified as wind-exposed while nesting sites oriented between 90° and 270° were classified as wind-sheltered. We first compared mortality at sheltered and wind-exposed nesting sites before (N = 30 nesting sites) during (N = 27) and after (N = 23) black fly outbreak in 2013.
Weather, climate and black fly distribution
Data on snow depth on the last day of May for the period 1981–2015 (Fig. 1c) were available from the Government of Canada26 (Fig. 1c,d). We also used the Studentized residuals measure to test the presence of outlier(s) following a Student’s t distribution for a linear regression between snow depth on the last day of May and year44. To relate the distribution of ornithophilic black flies to a projected temperature increase across the North American Arctic (Fig. 1d), we mapped the presence and absence of 17 species with a boreal, subarctic and arctic distribution across North America for the period 1823–200327. We then identified the summer isotherm of mean temperature for June, July and August that best matched the northern distribution of black flies (i.e. the isotherm with minimal distance from the observed northern distribution limit of black flies) for the reference period (1990–2010). Global climate models are complex mathematical representations of the Earth’s climate system that incorporate physical processes such as atmospheric flux, ocean circulation, land surface and sea ice dynamics, snow cover, and permafrost. We used the CGCM model because it is centered in Canada, and is more likely to represent future climate scenarios in that country relative to GCMs centered in other geographic regions. To estimate the northern shift of the isotherm that best matched the northern distribution of black flies, the CGCM model was coupled with the Representative Concentration Pathway (RCP) 4.523, an IPCC scenario available through CMIP5 multi-model ensemble45 that represents an intermediate greenhouse gas concentration. These data were interpolated at a 10 km × 10 km horizontal resolution and we then projected the potential summer isotherms by 20-year periods with a lag window of five years (e.g. 1995–2015, 2000–2020, 2005–2015, etc.) up to the period 2040–2060 to predict the expansion of the northern limit of black flies. The map (Fig. 1d) was produced using the open-source statistical software R (version 3.4.0 available at https://www.R-project.org/) with the use of the following R packages: sp46,47, raster48, rgdal49 and rgeos50.
Acknowledgements
This work was funded by the ArcticNet Network of Centers of Excellence of Canada, the Government of Nunavut (Department of Environment), C/Bar and Agnico Eagle Mines Limited (AEM). We are very grateful to Erik Hedlin and Andy Aliyak who contributed to field work, and to the Department of Environment (Government of Nunavut), the Kangiqliniq Hunters and Trappers Organization, the Nunavut Arctic College and the residents of Rankin Inlet for ongoing project support. We also thank AEM for logistic support. We acknowledge the World Climate Research Programme’s Working Group on Coupled Modelling, which is responsible for CMIP, and we thank the climate modeling groups for producing and making available their model output. All drawings from Fig. 2 were kindly provided by Aurélie Mélot and David Pinaud. V.L. was supported by the Natural Sciences and Engineering Research Council of Canada, the Fond de Recherche du Québec, Nature et Technologies, the Garfield Weston Foundation and Mitacs Accelerate with AEM as the industry partner.
Author Contributions
V.L., P.L., A.F. and J.B. designed the project. V.L. and A.F. contributed to data collection. V.L. and P.L. analyzed the data and interpreted the results with contribution from A.F., J.B., D.C. and D.B. N.C. modeled the northward expansion of black fly distribution with climate data. V.L. and P.L. drafted the manuscript and all authors provided input and revisions.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collins, M. et al. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. Stocker, T. F. et al.) Ch. 12, 1029–1136 (Cambridge University Press, 2013).
- 2.Post E, Pedersen C, Wilmers CC, Forchhammer MC. Warming, plant phenology and the spatial dimension of trophic mismatch for large herbivores. Proc. R. Soc. B. 2008;275:2005–2013. doi: 10.1098/rspb.2008.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anctil A, Franke A, Bêty J. Heavy rainfall increases nestling mortality of an Arctic top predator: experimental evidence and long-term trend in peregrine falcons. Oecologia. 2014;174:1033–1043. doi: 10.1007/s00442-013-2800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 5.Post E, et al. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- 6.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 7.Van Hemert C, Pearce JM, Handel CM. Wildlife health in a rapidly changing North: focus on avian disease. Front. Ecol. Environ. 2014;12:548–556. doi: 10.1890/130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 9.Arctic Climate Impact Assessment. Impacts of a Warming Arctic Vol. 1 (Cambridge University Press, 2004).
- 10.Larsen, J. N. et al. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. Barros, V. R. et al.) Ch. 28, 1567–1612 (Cambridge University Press, 2014).
- 11.Hewitson, B. et al. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. Barros, V. R. et al.) Ch. 21, 1133–1197 (Cambridge University Press, 2014).
- 12.Kutz SJ, et al. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Glob. Change Biol. 2013;19:3254–3262. doi: 10.1111/gcb.12315. [DOI] [PubMed] [Google Scholar]
- 13.Loiseau C, et al. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PLoS One. 2012;7:e44729. doi: 10.1371/journal.pone.0044729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harriman VB, Alisauskas RT. Of fleas and geese: the impact of an increasing nest ectoparasite on reproductive success. J. Avian Biol. 2010;41:573–579. doi: 10.1111/j.1600-048X.2010.05013.x. [DOI] [Google Scholar]
- 15.Solheim R, Jacobsen K-O, Øien IJ, Aarvak T, Polojärvi P. Snowy Owl nest failures caused by blackfly attacks on incubating females. Ornis Norv. 2013;36:1–5. doi: 10.15845/on.v36i0.394. [DOI] [Google Scholar]
- 16.Descamps S. Winter temperature affects the prevalence of ticks in an Arctic seabird. PLoS One. 2013;8:e65374. doi: 10.1371/journal.pone.0065374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnár PK, Kutz SJ, Hoar BM, Dobson AP. Metabolic approaches to understanding climate change impacts on seasonal host‐macroparasite dynamics. Ecol. Lett. 2013;16:9–21. doi: 10.1111/ele.12022. [DOI] [PubMed] [Google Scholar]
- 18.Dobson A, Molnár PK, Kutz S. Climate change and Arctic parasites. Trends Parasitol. 2015;31:181–188. doi: 10.1016/j.pt.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Laaksonen S, et al. Climate change promotes the emergence of serious disease outbreaks of filarioid nematodes. EcoHealth. 2010;7:7–13. doi: 10.1007/s10393-010-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legagneux P, et al. Arctic ecosystem structure and functioning shaped by climate and herbivore body size. Nat. Clim. Change. 2014;4:379–383. doi: 10.1038/nclimate2168. [DOI] [Google Scholar]
- 21.Currie, D. C. & Hunter, D. B. In Parasitic Diseases of Wild Birds (eds Atkinson, C. T., Thomas, N. J. & Hunter, D. B.) 537–545 (Wiley-Blackwell, 2008).
- 22.Franke A, Setterington M, Court G, Birkholz D. Long-term trends of persistent organochlorine pollutants, occupancy and reproductive success in peregrine falcons (Falco peregrinus tundrius) breeding near Rankin Inlet, Nunavut, Canada. Arctic. 2010;63:442–450. doi: 10.14430/arctic3333. [DOI] [Google Scholar]
- 23.IPCC. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2013).
- 24.Court GS, Gates CC, Boag DA. Natural history of the peregrine falcon in the Keewatin District of the northwest territories. Arctic. 1988;41:17–30. doi: 10.14430/arctic1688. [DOI] [Google Scholar]
- 25.Martínez‐de la Puente J, et al. Does weather affect biting fly abundance in avian nests? J. Avian Biol. 2009;40:653–657. doi: 10.1111/j.1600-048X.2009.04726.x. [DOI] [Google Scholar]
- 26.Government of Canada. Historical Data, http://climate.weather.gc.ca/historical_data/search_historic_data_e.html (2016).
- 27.Adler, P. H., Currie, D. C. & Wood, D. M. The black flies (Simuliidae) of North America (Cornell University Press, 2004).
- 28.Kutz S, Hoberg EP, Polley L, Jenkins E. Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. B. 2005;272:2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berteaux D, Réale D, McAdam AG, Boutin S. Keeping pace with fast climate change: can arctic life count on evolution? Integr. Comp. Biol. 2004;44:140–151. doi: 10.1093/icb/44.2.140. [DOI] [PubMed] [Google Scholar]
- 30.Gaston AJ, Hipfner JM, Campbell D. Heat and mosquitoes cause breeding failures and adult mortality in an Arctic‐nesting seabird. Ibis. 2002;144:185–191. doi: 10.1046/j.1474-919X.2002.00038.x. [DOI] [Google Scholar]
- 31.Franke A, Lamarre V, Hedlin E. Rapid nestling mortality in arctic peregrine falcons due to the biting effects of black flies. Arctic. 2016;69:281–285. doi: 10.14430/arctic4580. [DOI] [Google Scholar]
- 32.Hiltner AL, Hershey AE. Black fly (Diptera: Simuliidae) response to phosphorus enrichment of an arctic tundra stream. Hydrobiologia. 1992;240:259–265. doi: 10.1007/BF00013467. [DOI] [Google Scholar]
- 33.Hershey, A. E. et al. In Freshwaters of Alaska. Ecological Studies (Analysis and Synthesis) Vol. 119, 107–129 (Springer, 1997).
- 34.Robinson BG, Franke A, Derocher AE. Weather‐mediated decline in prey delivery rates causes food‐limitation in a top avian predator. J. Avian Biol. 2017;48:748–758. doi: 10.1111/jav.01130. [DOI] [Google Scholar]
- 35.Blomberg EJ, Sedinger JS, Gibson D, Coates PS, Casazza ML. Carryover effects and climatic conditions influence the postfledging survival of greater sage‐grouse. Ecol. Evol. 2014;4:4488–4499. doi: 10.1002/ece3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanner EP, et al. Extreme climatic events constrain space use and survival of a ground‐nesting bird. Glob. Change Biol. 2017;23:1832–1846. doi: 10.1111/gcb.13505. [DOI] [PubMed] [Google Scholar]
- 37.Hovis J, Snowman T, Cox V, Fay R, Bildstein K. Nesting behavior of Peregrine Falcons in west Greenland during the nestling period. Raptor Res. 1985;19:15–19. [Google Scholar]
- 38.Lamarre V, Franke A, Love OP, Legagneux P, Bêty J. Linking pre-laying energy allocation and timing of breeding in a migratory arctic raptor. Oecologia. 2017;183:653–666. doi: 10.1007/s00442-016-3797-9. [DOI] [PubMed] [Google Scholar]
- 39.Robinson BG, Franke A, Derocher AE. The influence of weather and lemmings on spatiotemporal variation in the abundance of multiple avian guilds in the Arctic. PLoS One. 2014;9:e101495. doi: 10.1371/journal.pone.0101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riegert, P. W. A history of outbreaks of black flies and their control in western Canada 1886–1980. Entomology Series Vol. 14 (Rampeck Publishers, 1999).
- 41.Beardsell A, Gauthier G, Therrien J-F, Bêty J. Nest site characteristics, patterns of nest reuse, and reproductive output in an Arctic-nesting raptor, the Rough-legged Hawk. Auk. 2016;133:718–732. doi: 10.1642/AUK-16-54.1. [DOI] [Google Scholar]
- 42.Ontiveros D, Pleguezuelos JM. Physical, environmental and human factors influencing productivity in Bonelli’s eagle Hieraaetus fasciatus in Granada (SE Spain) Biodivers. Conserv. 2003;12:1193–1203. doi: 10.1023/A:1023056102216. [DOI] [Google Scholar]
- 43.Court G, Gates C, Boag D. Turnover and recruitment in a tundra population of Peregrine Falcons Falco peregrinus. Ibis. 1989;131:487–496. doi: 10.1111/j.1474-919X.1989.tb04785.x. [DOI] [Google Scholar]
- 44.Cook RD, Weisberg S. Residuals and influence in regression. New York: Chapman and Hall; 1982. [Google Scholar]
- 45.Meehl GA, et al. The WCRP CMIP3 multimodel dataset: A new era in climate change research. B. Am. Meteorol. Soc. 2007;88:1383–1394. doi: 10.1175/BAMS-88-9-1383. [DOI] [Google Scholar]
- 46.Bivand, R. S., Pebesma, E. & Gomez-Rubio, V. Applied Spatial Data Analysis with R, 2nd ed., (Springer, 2013).
- 47.Pebesma EJ, Bivand RS. Classes and methods for spatial data in R. R News. 2005;5:9–13. [Google Scholar]
- 48.Hijmans, R. J. raster: Geographic Data Analysis and Modeling. R package version 2.5-8 (2016).
- 49.Bivand, R., Keitt, T. & Rowlingson, B. rgdal: Bindings for the Geospatial Data Abstraction Library. R package version 1.2-7 (2017).
- 50.Bivand, R. & Rundel, C. rgeos: Interface to Geometry Engine - Open Source (GEOS). R package version 0.3-23 (2017).