Abstract
Water transport across lipid membranes is fundamental to all forms of life and plays a major role in health and disease. However, not only typical water facilitators like aquaporins facilitate water flux, but also transporters, ion channels or receptors represent potent water pathways. The efforts directed towards a mechanistic understanding of water conductivity determinants in transmembrane proteins, the development of water flow inhibitors, and the creation of biomimetic membranes with incorporated membrane proteins or artificial water channels depend on reliable and accurate ways of quantifying water permeabilities Pf. A conventional method is to subject vesicles to an osmotic gradient in a stopped-flow device: Fast recordings of scattered light intensity are converted into the time course of vesicle volume change. Even though an analytical solution accurately acquiring Pf from scattered light intensities exists, approximations potentially misjudging Pf by orders of magnitude are used. By means of computational and experimental data we point out that erroneous results such as that the single channel water permeability pf depends on the osmotic gradient are direct results of such approximations. Finally, we propose an empirical solution of which calculated permeability values closely match those calculated with the analytical solution in the relevant range of parameters.
Subject terms: Data processing, Imaging techniques, Biophysical methods, Permeation and transport, Biomimetics
Introduction
Alterations in the single channel water permeability (pf) of aquaporin’s (AQPs) are directly related to human diseases1,2. Alongside with AQPs, different classes of proteins like transporters3, ion channels4 and receptors5 represent highly efficient water pathways. To get an idea about the impact of each of these transmembrane proteins on the water permeability of the respective tissue, accurate ways of quantifying unitary water permeability values pf of wild-type and mutant proteins are of particular interest and importance. Increased computer performance currently enables micro-second long MD simulations of transmembrane proteins. Despite their indisputable value to uncover molecular details of the substrate transport process, in silico data lack credibility without experimental validation. Furthermore, accurate pf values promote further development of more precise in silico algorithms and models. Fresh water scarcity boosts the field of biomimetic membranes6 which seeks to generate highly selective and efficient lipid or polymer based filter membranes. Selectivity and permeability can be tuned by the incorporation of aquaporins (AQPs)7–10 and supra-11 or single-molecular12 artificial water channels. Carbon nanotubes are potential candidates as simulations already predicted high rates of waterflux13–15, which is only awaiting experimental verification16. However the performance of these biological or synthetic water channels as well as the membrane matrix itself, which is critical for channel stability and functionality6,17, can only be tested by accurately determining membrane water permeabilities Pf.
Different methods18 exist, which are capable of retrieving information on water flux through lipid or polymer based membranes and incorporated transmembrane proteins or artificial water channels. On the one hand, in vivo techniques are available that can be divided into single cell swelling experiments with e.g. oocytes19–21 and transcellular flux through polarized cells grown on porous support22. While oocyte swelling experiments maybe limited by the unfolding of the oolemma3, measurements of transcellular flux through polarized cells offer the unique advantage of online inhibition and channel manipulation22,23. On the other hand, in vitro systems like planar lipid bilayers24 or lipid vesicles25 exist. Dilution or up-concentration of reporter ions in stagnant water layers close to planar lipid bilayers due to an osmotic water flux can be measured by ion sensitive microelectrodes26–28. This so called scanning electrochemical microscopy technique is time consuming and experimentally challenging, but it allows simultaneous counting of embedded electrically active channels29–31. However, measurements on transmembrane proteins have notably different success rates, since some proteins hardly incorporate into these planar lipid bilayers for unknown reasons. Therefore, experiments are most commonly performed with giant unilamellar vesicles (GUV)32 that are tens of μm in diameter or large unilamellar vesicles (LUV) with a typical size of about 100–120 nm. In contrast to GUV’s dimensions, LUV’s size cannot be determined with conventional light microscopy. A suitable signal to assess time driven size changes of liposomes is fluorescence self-quenching33, absorption/turbidity34 and scattering intensity35. Due to superior sensitivity and ease of use, the latter method became the standard method implemented in today’s stopped-flow experiments. The scattering intensity depends on the vesicle’s size and the refractive index. After a hyperosmotic shock, the concentration of entrapped osmolytes increases due to a decrease in vesicle volume. This increase in inner osmolarity increases the vesicle’s refractive index which elevates their light scattering ability. At the same time, the loss of vesicle size, reducing its scattering ability, counteracts this effect. To relate vesicle volume to scattering intensity, empirical approximations ranged from double logarithmic25, over quadratic36 to simple linear37 relations. We found that the computed solution of the scattering intensity in dependence of vesicle volume is well described by a second degree polynomial function of the vesicular volume38.
In a stopped-flow device, vesicles in osmotic equilibrium are subjected to a hyperosmotic buffer with impermeable solutes of concentration cout at time zero. The vesicle shrinkage depends on the water permeability of the vesicular membrane Pf:
| 1 |
| 2 |
where Vw, V0, A, cin,0 and cout are the molar volume of water, vesicle volume at time zero, constant surface area of the vesicle, the initial osmolyte concentration inside the vesicles, and osmolyte concentration in the external solution, respectively. We assume that the osmolarities change indirectly proportional to the vesicle volume. This is justified by an almost linear relation between concentrations and osmolarities for various substances in the relevant concentration range39.
Several decades ago these differential-algebraic equations were solved numerically36,40 to calculate V(t) for various Pf values. Time constants τ of mono-exponential fits to the permeability dependent V(t) were matched to experimentally fitted τ values to obtain Pf. However, in the meantime different approximations25,41,42 were proposed to calculate Pf directly from τ. These models only differ in the way the osmotic conditions in the vesicles interior and exterior are considered (Fig. 1b–d). An analytical solution exists for Eq. (1) at hyperosmotic conditions38 (Fig. 1a), but still approximations are applied. Even though an exponential function (Fig. 2, black line) is not an exact solution to Eq. (1), it resembles the analytical solution. However, these approximations based on exponential fits to the scattering data lead to systematic errors and false positive effects as described below. Therefore we question all published approximations of estimating Pf from light scattering traces of vesicle shrinkage and search for a new relation based on the time constant of exponential fits to the scattering data. To do so, we compare the discrepancies between the different methods by combined experimental and computational approaches. Permeability values obtained from different approximations are analyzed in respect to the absolute concentration of osmolyte, the osmotic gradient, the actual permeability and the vesicle radius. Finally we propose a new relation based on τ values which closely resembles Pf,analyt calculated with our analytical solution.
Figure 1.
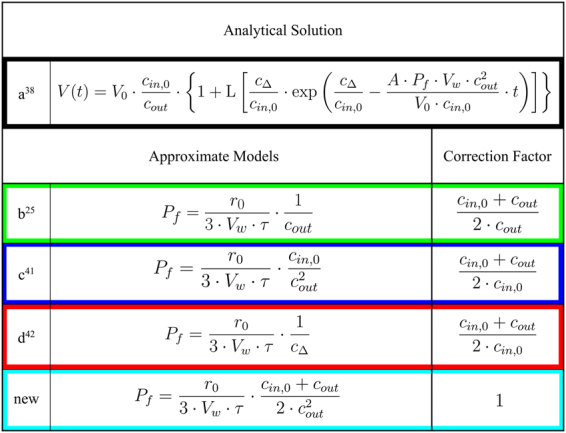
Models used to estimate Pf values from light-scattering data. Water permeability’s Pf of exponential approximations depend on the outer and initial inner osmolarities. Correction factors can be used to recalculate erroneous Pf values. The different models are color coded throughout the entire manuscript.
Figure 2.
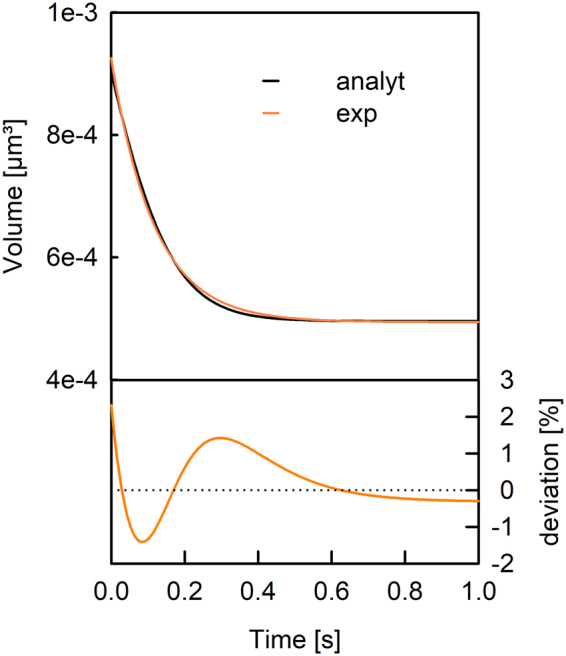
Comparison of an exponential fit to the analytically calculated decrease in vesicle volume. The fit of an exponential function with initial and final volume as fit parameter (orange line) to the analytical solution (black) of a vesicle with a radius r0 of 60 nm exposed to a hyperosmotic buffer at time zero. The deviation of the exponential fit to the analytical solution is depicted below. An initial inner osmolarity of 200 mOsm, an outer osmolarity of 300 mOsm and a permeability of 20 µm/s are used.
Results
The rate of vesicle shrinkage depends on Pf (Eq. 1). This differential equation was analytically solved by Horner et al.38 (Eq. 7) and is depicted in Fig. (2) (black line). It may be fitted by an exponential function (Fig. 2, orange line) even though the latter is not an exact solution to Eq. (1). Three different approximations (Fig. 1b–d) connect Pf with the exponential time constant τ (Eq. 6) of vesicle shrinkage. They only differ in the way cin,0 and cout are considered. To test these models for their deviation from the analytical solution depending on Pf,analyt, we first overexpressed, purified and reconstituted the aqua-glyceroporin of E.Coli, GlpF, into large unilamellar vesicles (LUVs). We subjected these proteoliposomes to a hyperosmotic solution and followed the decrease in vesicle volume by light scattering (Fig. 3). The raw data are either fitted by the analytical solution (Eqs 7 and 8) considering two vesicle populations38 or by a double exponential function where the slow time constant corresponded to bare lipid vesicles and the fast time constant to GlpF containing vesicles. By adjusting the protein to lipid ratio during reconstitution, we experimentally varied the water permeability calculated with the analytical solution, Pf,analyt, between 5 and 175 µm/s. The deviation of Pf,exp from Pf,analyt depends crucially on the approximation used (Fig. 4). However, the relative deviation is independent of Pf,analyt. Comparing these approximations (Fig. 1b–d) for the relation between τ and Pf,exp revealed that the arithmetic mean of the two approximations (b, c) closely resembles Pf,analyt in the investigated range of parameters with a maximal error of 5% (Fig. 1, new):
| 3 |
Figure 3.
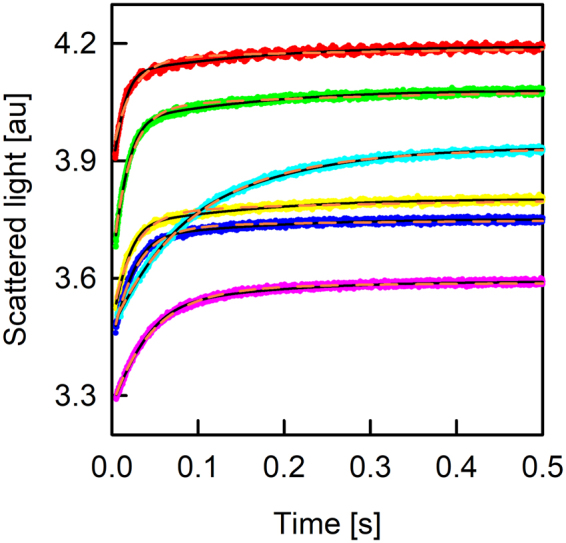
The osmotic shrinkage of GlpF containing proteoliposomes. Stopped-flow raw data (colored spline lines), fits (black lines) according to Eqs (7) and (8) and exponential fits (orange short dashed) for varying amounts of GlpF (on average number of monomers/PL; 4.5 - red line; 4.1 - green line; 3.5 - yellow line; 2.7 - blue line; 2.0 - pink line; 1.5 - cyan line). Equal volumes of vesicle suspension (100 mM NaCl, 20 mM MOPS, pH 7.5) and hyperosmotic solution (300 mM sucrose, 100 mM NaCl, 20 mM MOPS, pH 7.5) were mixed at 4 °C.
Figure 4.
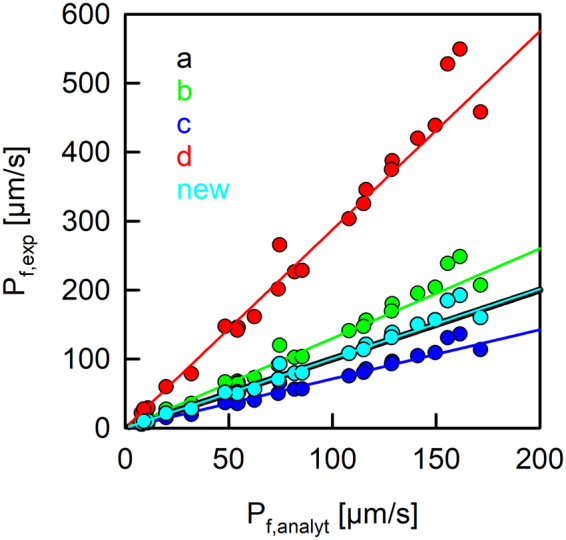
Correlation of permeabilities obtained from exponential fits, Pf,exp, and the analytical solution, Pf,analyt. Permeability values obtained by the fit of an exponential function to the analytical solution as in Fig. (2) (solid lines) deviate depending on the model used (b: Π = cout−1, green; c: Π = (cout − cin,0)−1, red; d: Π = cin,0·cout−2, blue). Our new relation (new: Π = (cout + cin,0)·(2·cout2)−1, cyan) closely tracks the black line for the analytical solution. Dots represent permeability values obtained by fitting scattering raw data obtained from GlpF containing proteoliposomes (as in Supplementary Fig. S1) either with an exponential fit together with the different approximations (y-data) or with the analytical solution (x-data). The buffer conditions were the same as in Fig. (3).
To computationally verify this result we computed the analytical solution V(t) for a series of Pf values between 2 and 200 µm/s. The time constants of the exponential fits to the data and the three published approximations (Fig. 1b–d) as well as our newly found approximation (Fig. 1, new) were used to calculate the corresponding membrane permeabilities Pf. These permeability values are consistent with the experimentally found discrepancy between the approximations based on exponential time constants and the analytical solution (Fig. 4).
Next, we studied the deviation of Pf,exp for different inner and outer osmolarities. Therefore, we varied cin,0 (97 to 919 mOsm) and cout (133 to 1778mOsm) of control vesicles (bare lipid vesicles) resulting in a gradient factor G = cout/cin,0 between 1.2 and 8.3. These experiments (Supplementary Fig. S1) revealed that the deviation of the approximate models from the analytical solution do not depend on the absolute inner osmolarity cin,0, but only on the gradient factor G (Fig. 5). Computational analysis confirmed these results (Fig. 5 and Supplementary Fig. S2). The deviation of permeabilies Pf,exp obtained from exponential fits to the analytical solution Pf,analyt for the osmotic shrinkage of vesicles with radius r0 = 60 nm and a water permeability of 20 µm/s depends on the relative inner and outer osmolarities but not their absolute values (Fig. 5 and Supplementary Fig. S2). To ensure comparability with experimental data we normalized all permeabilities Pf,exp with Pf,analyt. By calculating G for the proteoliposomes shown in Figs (3) and (4) and plotting them into Fig. (5), we strengthen the argument that the relative deviations between Pf,exp and Pf,analyt are independent of Pf,analyt. The normalized permeability values perfectly match our control measurements. Hence Fig. (5) illustrates that the accuracy of the different models (Fig. 1b–d) only depends on G and in the range of investigated ratios, Pf,exp may be decreased or increased compared to Pf,analyt by a factor of six or several orders of magnitude, respectively. Our new approximation (Eq. 3, Fig. 1, new) gave similar values to Pf,analyt, independent of the experimental conditions. The simple dependence of the error on the osmotic conditions allows recalculating an erroneous Pf obtained by one of the old models (Fig. 1b–d) with the corresponding correction factor depicted in Fig. (1).
Figure 5.
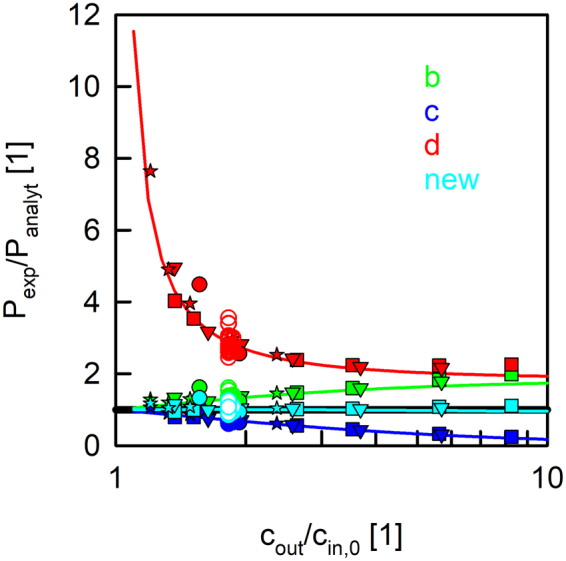
Permeabilities calculated from the time constant of exponential fits, Pf,exp, depend on the gradient factor G = cout/cin,0. Permeability values are normalized to Pf,analyt. The analytical solution (black line) is independent of G. Computed permeability values obtained by a fit of an exponential function to the analytical solution as in Fig. (2) (solid lines) deviate from Pf,analyt depending on the model (color code as in Figs 1 and 3) used. Control vesicles with four different cin,0 (filled square, 97 mOsm; filled triangle, 171 mOsm; filled star, 538 mOsm, filled circles, 919 mOsm) are subjected to different cout. Proteoliposomes containing GlpF from Figs (3) and (4) (empty circles) comprising a cin,0 of 200 mOsm are subjected to a hyperosmotic solution containing 150 mM sucrose (365 mOsm). Computational data were generated with control vesicles by keeping cin,0 constant at 200 mOsm and varying cout between 220 and 2000 mOsm.
Discussion
Direct pf measurements are subject to large inherent technical difficulties. They include effects of stagnant water layers in membrane vicinity and uncertainties in the actual channel density43. Additional errors are introduced by using approximations (Fig. 1b–d) to calculate Pf from the time constant τ of a single exponential fit to the scattering data. For example model d depicted in Fig. (1) produces an error that depends on the osmotic gradient44,45 (see Fig. 5d), which can neither be explained by a structural resistance of LUVs to volume changes33, nor by an intra-vesicular unstirred layer35,38. Alternative methods, like scanning electrochemical microscopy29 and fluorescence self-quenching experiments combined with a numerical solution33, confirm that Pf is independent of the osmotic gradient. Still this model has widely been used to report for example water permeabilities for KcsA4, several AQPs8,17,42,46–52 and artificial water channels11,12. Models b and c shown in Fig. (1) exhibit a smaller relative error when changing the ratio cout to cin,0. Figure (5) depicts that approximation b and c represent a systematic over- or underestimation of Pf,analyt with an increasing relative osmotic gradient. To conclude, varying G from 1 to 10, models b, c and d produce a systematic relative error up to a factor of 2, 6 and several orders of magnitude, respectively. We further demonstrate by means of computational and experimental data that the deviations of Pf,exp from Pf,analyt do not depend on Pf,analyt or cin,0 but solely on G, the ratio cout/cin,0. This is evident as models b to d only differ in the way they consider cout and cin,0.
The above discussed approximations tend to systematically deviate from Pf,analyt, due to oversimplifications in the respective derivations. For example, model b linearly approximates V(t) based on the initial change in volume25. The elaborate calculation yields a similar result as a heuristic approach that assumes an exponential volume change with the final volume depending on the ratio cin,0/cout:
| 4 |
Comparing the derivative of Eq. (4) at time zero
| 5 |
with the initial change in volume (Eq. 1 at t = 0), one obtains approximation b. Yet the best-fit global time constant does not necessarily give the best fit to the initial rate of volume change which ill-poses the assumption of a strict exponential shrinkage. But, the flatter the kinetics, the better this approximation is. An example is provided by experiments with small relative osmotic gradients (Fig. 5). Approximation d can also be obtained heuristically, but with the assumption that the volume drops exponentially to zero instead to the ratio cin,0/cout. Especially at very low osmotic gradients or gradient factors close to 1 model d leads to a divergent solution since only for large gradients cin,0/cout approaches zero. Approximation c results in rather reliable values for small relative osmotic gradients as its derivation relies on small volume changes41 (Fig. 5).
Generally Pf is independent of the initial vesicle radius r038. Any vesicle population with a unimodal radius distribution can be fitted by considering only one mean vesicle diameter. Still under- or overestimation of r0 leads to an error in Pf, which linearly depends on the ratio of assumed to real vesicle radius (Supplementary Fig. S3). Hence, r0 must be known with even higher accuracy to resolve small changes in Pf. It is important to note that for a scattering technique it is mandatory to estimate the mean radius of the intensity distribution and not the mean radius of the volume or particle distribution.
Finally, we established a new relation to calculate accurate Pf values from the time constant of an exponential fit to scattered light of vesicle shrinkage. Our new model (Fig. 1, new) yields an osmotic gradient independent permeability value consistent with the analytical solution of Eq. (1), which was already published in 2015. However, the analytical solution was only applied in our work group so far to calculate pf values of AQP1, GlpF, AqpZ, KcsA38, AQP453 and hSGLT13. We attribute this to the fact that the Lambert function as part of the analytical solution is not implemented in common tools for stopped-flow data analysis. This survey enables the community to obtain correct permeability values from exponential fits or recalculate previous erroneous results.
Methods
Protein Overexpression, Purification and Reconstitution
The sequence of GlpF (Escherichia coli) is inserted in a pTrc plasmid, which is transformed into C43 (DE3) cells. The cells are grown in LB overnight, diluted 40-fold and grown until OD 0.6. Expression is induced by 1 mM IPTG for 3 hours. Cells are harvested and pellets frozen at −80 °C. Purification and Atto488 labeling is performed as we described elsewhere38,54,55. In brief, cell pellets are lysed and the pelleted cell fraction is solubilized in a detergent buffer. After the removal of the insoluble material by ultracentrifugation, the supernatant is further purified using affinity (Ni2+-column). GlpF is reconstituted into proteoliposomes (PLs) with small modifications as previously described38,54,55. E.coli polar lipids (PLE, Avanti Polar Lipids) doped with 0.004 m% Atto633PPE are dried on a rotary evaporator. The thin lipid film is rehydrated in Reko buffer (100 mM NaCl, 20 mM MOPS, 1.4% OG, pH 7.4) to attain a final lipid concentration of 20 mg/ml. Subsequent to bath sonication, the clear suspension is incubated with equal amounts of protein diluted in Reko buffer at room temperature for an hour. With stepwise addition of Biobeads SM-2 (Bio-Rad), we remove the detergent within 36 hours. PLs are harvested by ultracentrifugation. The resuspended vesicles are centrifuged to remove aggregates and put through 21 extrusion cycles stacked with two polycarbonate filters with 100-nm pore sizes using a mini-extruder from Avanti Polar Lipids. This results in a unimodal radius distribution as seen from dynamic light scattering measurements. In addition protein reconstitution results in a fraction of bare lipid vesicles and a fraction of proteoliposomes with a heterogeneous but very sharp distribution of proteins between the lipid vesicles38. Control vesicles are treated similarly. All samples are assayed without delay.
Bare Lipid Vesicle Preparation
Large unilamellar vesicles (LUVs) were prepared from an E.coli polar lipid extract (PLE) mixture in chloroform as described elsewhere56. In brief, PLE was dried on a rotary evaporator, hydrated in a solution containing 100 mM NaCl and 20 mM MOPS buffered at pH 7.4, and extruded through 100 nm polycarbonate filters as described above. The final stock solution subsequently contained 10 mg/ml lipids. For experiments with different cin,0 the NaCl concentration was varied accordingly.
Stopped-Flow & Data Analysis
PLs and LUVs are subjected to a hyperosmotic solution in a stopped-flow apparatus (SFM-300, Bio-Logic, Claix, France) at 4 °C. As previously described3,4,38,53, we monitor the intensity of scattered light at 90° at a wavelength of 546 nm. To calculate water permeability values from light scattering we use our recently found analytical solution38 and three common approximations25,41,42 as well as our new approximation all based on single-exponential functions with a time constant τ
| 6 |
where depending on different models Π is equal to cout−1, (cout – cin,0)−1, cin,0·cout−2 or (cin,0 + cout)/(2· cout2). The analytical solution for Eq. (1) at hyperosmotic conditions can be written as38:
| 7 |
where cΔ, and L are the incremental osmolyte concentration in the external solution due to osmolyte addition (cΔ = cout − cin,0) and the Lambert function defined by L(x)·eL(x) = x, respectively. This solution only holds for hyperosmotic conditions since the Lambert function is not defined for hypoosmotic solutions where cout < 0.841 · cin,0. Anyway, the surface area of a vesicle can only expand by a few percent57 before the vesicle bursts and the condition of a constant amount of encapsulated osmolytes is violated. The vesicle volume is experimentally accessible by measuring the scattering intensity. Careful treatment of scattering intensity I(t) of vesicle shrinkage by employing the Rayleigh-Gans-Debye relation and acknowledging the change in size and refractive index revealed that a second order Taylor series with coefficients a, b, d is of high accuracy (see SI of Horner et al.)38:
| 8 |
However, in the relevant range of r0 (~30 to ~100 nm) Eq. (6) can be approximated with a linear dependence of V(t) on I(t) with acceptable accuracy (Fig. S138). In combination with our new approximation (Eq. 3, Fig. 1, new) this results in Pf values deviating only a few percent from Pf,analyt (Fig. 4, cyan dots). In addition we take into account the fraction of bare lipid vesicles in a proteoliposome preparation as described elsewhere38,53. Osmolarities of cin,0 and cout were routinely checked using a vapor pressure osmometer 5500 from (Wescor, EliTechGroup, Utah).
Computational Analysis
All mathematical computation is done with Mathematica 11.2 (Wolfram Research 2017). A function was implemented which takes cin,o, cout, Pf and r0 as an argument, tabulates the numerical V(t) values of the analytical solution (Eq. 7) to these arguments and returns the time constant τ of an exponential fit to the numerical values. An exponential fit to the analytical solution with initial and final volume as fit parameters violates the initial volume change (Eq. 1) as the fit overestimates V(t) at time zero as seen from the initial slopes in Fig. (2) However, we are only interested in the kinetic parameter τ, which is subsequently used in conjunction with Eq. (6) to derive approximate Pf,exp values. These purely computationally derived Pf,exp values are subsequently compared to Pf,exp values obtained by the time constants of exponential fits to the scattering raw data, which comprises a linear dependence of V(t) on I(t). If not varied for systematic comparison, Pf,analyt and r0 are set to 20 µm/s and 60 nm. If not stated differently cin,0 is set to 200 mOsm and cout to 300 mOsm.
Availability of materials and data
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by grant P31074 of the Austrian Science Fund to A.H.
Author Contributions
T.B. and A.H. performed the in vitro experiments. C.H. performed the computational work. C.S. performed protein overexpression and purification. T.B. and A.H. analyzed the experimental data. C.H. and A.H. designed the project. C.H. and A.H. wrote the paper. All authors discussed and have given approval to the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/15/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26946-9.
References
- 1.Agre P, et al. Aquaporin water channels–from atomic structure to clinical medicine. J. Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S, et al. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 3.Erokhova, L., Horner, A., Ollinger, N., Siligan, C. & Pohl, P. The Sodium Glucose Cotransporter SGLT1 is an Extremely Efficient Facilitator of Passive Water Transport. J. Biol. Chem. 291, jbc.M115.706986 (2016). [DOI] [PMC free article] [PubMed]
- 4.Hoomann T, Jahnke N, Horner A, Keller S, Pohl P. Filter gate closure inhibits ion but not water transport through potassium channels. Proc. Natl. Acad. Sci. USA. 2013;110:10842–7. doi: 10.1073/pnas.1304714110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan S, Hu Z, Filipek S, Vogel H. W246 Opens a Gate for a Continuous Intrinsic Water Pathway during Activation of the Adenosine A2AReceptor. Angew. Chemie - Int. Ed. 2014;53:1–5. doi: 10.1002/anie.201409679. [DOI] [PubMed] [Google Scholar]
- 6.Shen, Y., Saboe, P. O., Sines, I. T., Erbakan, M. & Kumar, M. Biomimetic membranes: A review. 454, 359–381 (2014).
- 7.Kumar, M., Grzelakowski, M., Zilles, J., Clark, M. & Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. 104, 20719–20724 (2007). [DOI] [PMC free article] [PubMed]
- 8.Kumar M, Habel JEO, Shen Y, Meier WP, Walz T. High-Density Reconstitution of Functional Water Channels into Vesicular and Planar Block Copolymer Membranes. JACS. 2012;134:18631–18637. doi: 10.1021/ja304721r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groot BL, Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- 10.Wang, Y. & Tajkhorshid, E. Molecular mechanisms of conduction and selectivity in aquaporin water channels. J. Nutr. 137, 1509S–1515S, discussion 1516S–1517S (2007). [DOI] [PubMed]
- 11.Licsandru E, et al. Salt-Excluding Artificial Water Channels Exhibiting Enhanced Dipolar Water and Proton Translocation. J. Am. Chem. Soc. 2016;138:5403–5409. doi: 10.1021/jacs.6b01811. [DOI] [PubMed] [Google Scholar]
- 12.Shen Yue-xiao, Si Wen, Erbakan Mustafa, Decker Karl, De Zorzi Rita, Saboe Patrick O., Kang You Jung, Majd Sheereen, Butler Peter J., Walz Thomas, Aksimentiev Aleksei, Hou Jun-li, Kumar Manish. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proceedings of the National Academy of Sciences. 2015;112(32):9810–9815. doi: 10.1073/pnas.1508575112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–190. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 14.Berezhkovskii A, Hummer G. Single-file transport of water molecules through a carbon nanotube. Phys. Rev. Lett. 2002;89:64503. doi: 10.1103/PhysRevLett.89.064503. [DOI] [PubMed] [Google Scholar]
- 15.Zuo G, Shen R, Ma S, Guo W. Transport Properties of single-flile water molecules inside a carbon nanotube biomimicking water channel. ACS Nano. 2010;4:205–210. doi: 10.1021/nn901334w. [DOI] [PubMed] [Google Scholar]
- 16.Horner, A. & Pohl, P. Comment on ‘Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins’. Science (80-.). 359, (2018). [DOI] [PubMed]
- 17.Ren T, et al. Membrane Protein Insertion into and Compatibility with Biomimetic. Membranes. 2017;1700053:1–10. doi: 10.1002/adbi.201700053. [DOI] [PubMed] [Google Scholar]
- 18.To J, Torres J. Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes? Membranes (Basel). 2015;5:352–368. doi: 10.3390/membranes5030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Logree KA, Verkman AS. Expression of mRNA Coding for Kidney and Red Cell Water Channels in Xenopus Oocytes. J. Biol. Chem. 1990;265:15375–15378. [PubMed] [Google Scholar]
- 20.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28. Protein. Science (80-.). 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 21.Németh-Cahalan KL, Clemens DM, Hall JE. Regulation of AQP0 water permeability is enhanced by cooperativity. J. Gen. Physiol. 2013;141:287–95. doi: 10.1085/jgp.201210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erokhova L, Horner A, Kügler P, Pohl P. Monitoring single-channel water permeability in polarized cells. J. Biol. Chem. 2011;286:39926–39932. doi: 10.1074/jbc.M111.291864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollajew R, et al. Routes of epithelial water flow: Aquaporins versus cotransporters. Biophys. J. 2010;99:3647–3656. doi: 10.1016/j.bpj.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindler, H. In Biomembranes Part R (ed. Enzymology, B. T.-M. in) 171, 225–253 (Academic Press, 1989).
- 25.van Heeswijk MP, van Os CH. Osmotic water permeabilities of brush border and basolateral membrane vesicles from rat renal cortex and small intestine. J. Membr. Biol. 1986;92:183–193. doi: 10.1007/BF01870707. [DOI] [PubMed] [Google Scholar]
- 26.Pohl P, Antonenko YN, Rosenfeld E. Effect of ultrasound on the pH profiles in the unstirred layers near planar bilayer lipid membranes measured by microelectrodes. Biochim. Biophys. Acta - Biomembr. 1993;1152:155–160. doi: 10.1016/0005-2736(93)90242-r. [DOI] [PubMed] [Google Scholar]
- 27.Pohl P, Saparov SM. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophys. J. 2000;78:2426–2434. doi: 10.1016/S0006-3495(00)76786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saparov SM, et al. Mobility of a one-dimensional confined file of water molecules as a function of file length. Phys. Rev. Lett. 2006;96:1–4. doi: 10.1103/PhysRevLett.96.148101. [DOI] [PubMed] [Google Scholar]
- 29.Saparov SM, Pohl P. Beyond the diffusion limit: Water flow through the empty bacterial potassium channel. Proc. Natl. Acad. Sci. USA. 2004;101:4805–4809. doi: 10.1073/pnas.0308309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saparov SM, et al. Determining the Conductance of the SecY Protein Translocation Channel for Small Molecules. Mol. Cell. 2007;26:501–509. doi: 10.1016/j.molcel.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Saparov SM, Antonenko YN, Koeppe RE, Pohl P. Desformylgramicidin: a model channel with an extremely high water permeability. Biophys. J. 2000;79:2526–2534. doi: 10.1016/S0006-3495(00)76493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boytsov D, Hannesschlaeger C, Horner A, Pohl P. Determining Unitary Water Permeability of Membrane Proteins Reconstituted into Giant Unilamellar Vesicles. Biophys. J. 2016;110:215a. [Google Scholar]
- 33.Chen PY, Pearce D. & Verkman, a S. Membrane water and solute permeability determined quantitatively by self-quenching of an entrapped fluorophore. Biochemistry. 1988;27:5713–5718. doi: 10.1021/bi00415a048. [DOI] [PubMed] [Google Scholar]
- 34.Bangham AD, Gier JDE, Greville GD. Osmotic Properties and Water Permeability of Phospholipid Liquid Crystals. Chem. Phys. Lipids. 1967;1:225–246. [Google Scholar]
- 35.ShaAfi RI, Rich GT, Sidel VW, Bossert W, Solomon AK. The Effect of the Unstirred Layer on Human Red Cell Water Permeability. J. Gen. Physiol. 1967;50:1377–1399. doi: 10.1085/jgp.50.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Illsley NP. & Verkman, a S. Serial permeability barriers to water transport in human placental vesicles. J. Membr. Biol. 1986;94:267–78. doi: 10.1007/BF01869722. [DOI] [PubMed] [Google Scholar]
- 37.Van Hoek AN, Verkman AS. Functional Reconstitution of the Isolated Erythrocyte Water Chanel CHIP28. J. Biol. Chem. 1992;267:18267–18269. [PubMed] [Google Scholar]
- 38.Horner A, et al. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015;1:1–5. doi: 10.1126/sciadv.1400083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weast, R. C. & Astle, M. J. Handbook of Chemistry and Physics. (CRC Press, 1982).
- 40.Zeidel ML, Albalak a, Grossman E, Carruthers a. Role of glucose carrier in human erythrocyte water permeability. Biochemistry. 1992;31:589–596. doi: 10.1021/bi00117a041. [DOI] [PubMed] [Google Scholar]
- 41.Farmer REL, Macey RI. Pertubation of Red Cell Volume: Rectification of Osmotic Flow. Biochim. Biophys. Acta. 1970;196:53–65. doi: 10.1016/0005-2736(70)90165-3. [DOI] [PubMed] [Google Scholar]
- 42.Borgnia Mario J, Kozono David, Calamita Giuseppe, Maloney Peter C, Agre Peter. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. Journal of Molecular Biology. 1999;291(5):1169–1179. doi: 10.1006/jmbi.1999.3032. [DOI] [PubMed] [Google Scholar]
- 43.Bellissent-Funel Marie-Claire, Hassanali Ali, Havenith Martina, Henchman Richard, Pohl Peter, Sterpone Fabio, van der Spoel David, Xu Yao, Garcia Angel E. Water Determines the Structure and Dynamics of Proteins. Chemical Reviews. 2016;116(13):7673–7697. doi: 10.1021/acs.chemrev.5b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tunuguntla RH, et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science (80-.). 2017;796:792–796. doi: 10.1126/science.aan2438. [DOI] [PubMed] [Google Scholar]
- 45.Grzelakowski M, Cherenet MF, Shen Y, Kumar M. A framework for accurate evaluation of the promise of aquaporin based biomimetic membranes. J. Memb. Sci. 2015;479:223–231. [Google Scholar]
- 46.Kai L, et al. Preparative Scale Production of Functional Mouse Aquaporin 4 Using Different Cell-Free Expression Modes. PLoS One. 2010;5:2–9. doi: 10.1371/journal.pone.0012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kai L, Kaldenhoff R. A refined model of water and CO2 contribution of sterols and proteins. Sci. Rep. 2014;4:1–6. doi: 10.1038/srep06665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gan, H. X., Zhou, H., Lin, Q. & Tong, Y. W. Quantification of Aquaporin-Z reconstituted into vesicles for biomimetic membrane fabrication. Sci. Rep. 1–13 10.1038/s41598-017-11723-x (2017). [DOI] [PMC free article] [PubMed]
- 49.Wree D, Wu B, Zeuthen T, Beitz E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011;278:740–748. doi: 10.1111/j.1742-4658.2010.07993.x. [DOI] [PubMed] [Google Scholar]
- 50.Xie W, et al. Y. Regulation of Aquaporin Z osmotic permeability in ABA tri-block copolymer. AIMS Biophys. 2015;2:381–397. [Google Scholar]
- 51.Calamita G, et al. The Inner Mitochondrial Membrane Has Aquaporin-8 Water Channels and Is Highly Permeable to Water. J. Biol. Chem. 2005;280:17149–17153. doi: 10.1074/jbc.C400595200. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt V, Sturgis JN, Macromoleculaires S, Universite A, Aiguier CJ. Making Monomeric Aquaporin Z by Disrupting the Hydrophobic Tetramer Interface. ACS Omega. 2017;2:3017–3027. doi: 10.1021/acsomega.7b00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horner Andreas, Siligan Christine, Cornean Alex, Pohl Peter. Positively charged residues at the channel mouth boost single-file water flow. Faraday Discussions. 2018;209:55–65. doi: 10.1039/c8fd00050f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preiner J, et al. High-Speed AFM Images of Thermal Motion Provide Stiffness Map of Interfacial Membrane Protein Moieties. Nano Lett. 2015;15:759–763. doi: 10.1021/nl504478f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karner A, et al. Tuning membrane protein mobility by confinement into nanodomains. Nat. Nanotechnol. 2016;12:1–7. doi: 10.1038/nnano.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horner A, Goetz F, Tampé R, Klussmann E, Pohl P. Mechanism for targeting the A-kinase anchoring protein AKAP18δ to the membrane. J. Biol. Chem. 2012;287:42495–42501. doi: 10.1074/jbc.M112.414946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwok R, Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys. J. 1981;35:637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


