Abstract
Plants control organ growth rate by adjusting the rate and duration of cell division and expansion. Surprisingly, there have been few studies where both parameters have been measured in the same material, and thus we have little understanding of how division and expansion are regulated interdependently. We have investigated this regulation in the root meristem of the stunted plant 1 (stp1) mutation of Arabidopsis, the roots of which elongate more slowly than those of the wild type and fail to accelerate. We used a kinematic method to quantify the spatial distribution of the rate and extent of cell division and expansion, and we compared stp1 with wild type and with wild type treated with exogenous cytokinin (1 μm zeatin) or auxin (30 nm 2,4-dichlorophenoxyacetic acid). All treatments reduced average cell division rates, which reduced cell production by the meristem. Auxin lowered root elongation by narrowing the elongation zone and reducing the time spent by a cell in this zone, but did not decrease maximal strain rate. In addition, auxin increased the length of the meristem. In contrast, cytokinin reduced root elongation by lowering maximal strain rate, but did not change the time spent by a cell within the elongation zone; also, cytokinin blocked the increase in length and cell number of the meristem and elongation zone. The cytokinin-treated wild type phenocopied stp1 in nearly every detail, supporting the hypothesis that cytokinin affects root growth via STP1. The opposite effects of auxin and cytokinin suggest that the balance of these hormones may control the size of the meristem.
How does a plant regulate the rate at which its organs grow? This question is important because as a plant develops and responds to the environment, organ growth rate is regulated carefully. The rate at which organs grow depends on the rates of cell division and expansion. However, this straightforward answer belies considerable complexity. Division and expansion are not alternative or sequential processes, but instead are interdependent processes whose coordinated regulation we scarcely understand.
The regulation of organ growth rate involves the rate at which new cells are produced and how fast these cells expand. Strictly, cell division does not enlarge an organ, but builds partitions within component cells (Green, 1976). Nevertheless, meristematic cells typically expand and divide at comparable rates, maintaining an approximately constant average cell size. Through this expansion, dividing cells do contribute to organ growth, albeit to a much lesser extent than non-meristematic cells (Volenec and Nelson, 1981; Volenec and Nelson, 1983). Beyond the small direct contribution to growth, cell division supplies the cells whose subsequent expansion causes the majority of organ expansion. If the rate of cell supply changes, then it is reasonable to expect the rate of organ expansion will change in parallel (Beemster and Baskin, 1998). Therefore, there are three processes that can modify organ growth rate: cell division, expansion of meristematic cells, and expansion of non-meristematic cells. One must distinguish the contribution of each of these processes to understand how an organ grows at a defined rate.
It is also not always appreciated that a change in how fast cells are supplied does not require a change in the rate of cell division; instead, cells may remain mitotically active for a different period, thus changing the number of cells in the meristem and hence the number of daughter cells produced. We will refer to the rate at which cells are supplied by a meristem as the rate of cell production. To understand how a plant adjusts the growth rate of an organ requires understanding the linked regulation of division and expansion: understanding changes in division includes distinguishing between changes in division rate and the duration of mitotic activity.
Regulating the duration of mitotic activity and hence meristem size seems to be particularly important for the root. In a review of cell division in the root meristem, it was argued that cell division rate changes rarely, except when the root is exposed to extreme conditions, and hence that cell production rate depends principally on meristem size (Baskin, 2000). For example, using a kinematic method developed specifically for the root of Arabidopsis, we analyzed how root elongation accelerates in this species following germination (Beemster and Baskin, 1998). There was little change in cell division rate or in cellular elongation; instead, nearly all of the acceleration could be explained by the number of meristematic cells increasing. Similarly, when maize roots responded to changing levels of carbohydrate supplied by the shoot, division rate stayed constant, whereas the number of meristem cells changed (Muller et al., 1998).
To understand how division and expansion contribute to determining how fast the root elongates, and in particular to assess the role of meristem size, we took advantage of a semi-dwarf mutant in Arabidopsis, stunted plant 1 (stp1). Root cells in this mutant are produced more slowly and reach lower maximal relative elongation rates than in the wild type, and notably, stp1 roots do not accelerate following germination (Baskin et al., 1995). Along with constant root elongation rate, estimated meristem size remained constant, suggesting that meristem size can regulate root elongation rate. To confirm this suggestion, it is necessary to do more than estimate meristem behavior, and an objective of this work was to quantify the growth parameters of the stp1 meristem. In addition, because root growth in stp1 responds normally to auxin, abscisic acid, ethylene, and gibberellin, but weakly to cytokinin, it was hypothesized that cytokinin levels inhibit root elongation by down-regulating STP1 (Baskin et al., 1995). If so, treating the wild type with exogenous cytokinin can be predicted to phenocopy the growth parameters of the mutant and block the increase in meristem size; in contrast, exogenous auxin should induce a different pattern of response. A further objective of this work was to test this prediction.
RESULTS
Root Elongation Rate over Time
The growth of wild-type Arabidopsis primary roots accelerated over time after germination; in contrast, roots of the semi-dwarf mutant, stp1, grew at a constant rate (Fig. 1; Baskin et al., 1995). Because the roots of stp1 responded normally to auxin and selected other hormones, but only weakly to cytokinin, Baskin et al. (1995) hypothesized that cytokinin inhibits root elongation by down-regulating the STP1 gene product, assumed to promote expansion within the elongation zone specifically. When wild-type seedlings were exposed to the synthetic auxin, 2,4-dichlorophenoxyacetic acid (2,4-D), at 30 nm, root elongation was decreased to a similar extent throughout the first 10 d after sowing, hence these roots accelerated normally (Fig. 1). At higher concentrations of 2,4-D, some roots stopped elongating almost completely within a few days, whereas growth of the remaining roots on the plates still accelerated. At no concentration of 2,4-D did roots elongate at a steady rate. By contrast, when seedlings were exposed to the cytokinin, zeatin, at 1 μm, roots grew slowly and did not accelerate. At higher concentrations of zeatin, elongation decelerated or stopped, and at lower concentrations, root elongation did accelerate. Thus treating the wild type with cytokinin mimicked the root elongation behavior of stp1.
Figure 1.
Time course of primary root elongation for Arabidopsis wild type, stp1, and wild type treated with 1 μm zeatin or 30 nm 2,4-D. Data are means ± se of 15 replicate plates, with five to eight seedlings per plate. In this and subsequent figures, data for the wild type are replotted from Beemster and Baskin (1998).
Spatial Analysis of Elongation Rates
To determine the effect of the stp1 mutation and of 1 μm zeatin and 30 nm 2,4-D on cell expansion and division, we used a kinematic approach. The analysis was one-dimensional and simplified the root into a single file of a specific cell type, in this case, cortical cells. We determined the spatial profile of velocity throughout the whole growth zone by measuring the displacement of graphite particles, and of cell length by using Nomarski optics on living roots. Measurements were made at 6, 8, and 10 d after sowing. Data for wild-type roots are replotted from Beemster and Baskin (1998). For wild-type roots treated with zeatin and the roots of the stp1 mutant, the spatial distributions of velocity and of cell length were the same on each of these days (data not shown), indicating steady-state growth. To increase the number of replicates for those treatments, data from d 6 and 8 were pooled. For wild-type roots treated with 2,4-D, velocity and cell length profiles changed through time. Therefore the data were analyzed separately for each observation day, and the local rate of change in cell density was included in all calculations of cell division parameters, presented here for d 8.
In each treatment the velocity profiles were reproducible between roots, as seen from the smallness of the standard deviations (Fig. 2A). The overall root elongation rates obtained from the marked roots used for kinematics did not differ from the rates obtained for undisturbed roots (Fig. 2A, ticks on the right y axis). Once measured, the velocity profile was differentiated to give the profile of relative elongation rate, termed “strain rate” because strain is defined as a relative deformation. The profile of strain rate in stp1 was truncated apically and had a much lower maximum than the wild type (Fig. 2B). These direct measurements confirm the previous indirect ones (Baskin et al., 1995). Consistent with the prediction that treating the wild type with cytokinin will phenocopy stp1, the strain rate profile of stp1 roots closely resembled the wild type treated with zeatin, although the stp1 mutation affected the maximal strain rate slightly, but consistently, more severely than did the zeatin treatment. In contrast, the strain rate profile for 2,4-D was narrowed, but the maximal strain rate was not significantly reduced. Over time on 2,4-D, the strain rate profile widened, but did not reach higher values, thus accelerating root elongation in the same manner as reported previously for wild type (Beemster and Baskin, 1998).
Figure 2.
Spatial profiles of velocity (A) and strain rate (B) versus distance from the quiescent center on d 8 for Arabidopsis wild type, stp1, and wild type treated with 1.0 μm zeatin or 30 nm 2,4-D. In A, ticks at right give the final velocity measured for unmarked roots on d 8. C enlarges the apical 250 μm of B. Symbols are means ± se of five (2,4-D) or 10 roots, from d 8 (wild type and 2,4-D) or pooled from d 6 and 8 (stp1 and zeatin).
In the meristem, strain rates in the wild type over the first 200 μm had a slight minimum at 100 μm (Fig. 2C). Similarly, in the apical 200 μm of 2,4-D-treated roots, the strain rate profile retained the gentle minimum, but was lower throughout the region, showing that auxin affected strain rates differently in zones of division and elongation. In contrast, strain rates in the apical 200 μm of stp1 rose steadily and by 50 μm from the tip were greater than those of wild-type roots. Thus the diminished expansion conditioned by the mutation was specific for the elongation zone. Again, the strain rate profile of cytokinin-treated roots in the apical 200 μm resembled the mutant in steadily increasing with distance from the quiescent center. Although strain rates in the first 100 μm of zeatin-treated roots were significantly lower than those of the mutant, they increased more steeply with distance.
Cell Length
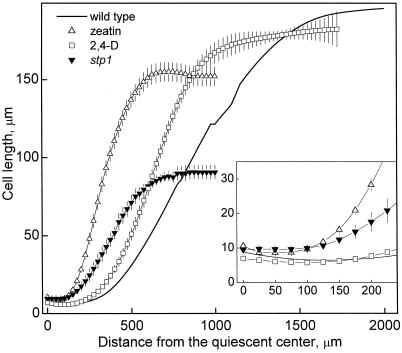
On the same roots used for the determination of the velocity profiles we also measured the spatial distribution of cortical cell length. Final cell length in the mutant was less than one-half that of the wild type, whereas in zeatin-treated roots it was reduced by only 20% and the effect of 2,4-D was negligible (Fig. 3). The shorter mature cells in stp1 compared with zeatin-treated wild type resulted from the mutant's having a lower maximal strain rate and a greater cell production rate (see below) and point to the importance of lowered strain rate for the decreased root elongation rate in the mutant. In contrast, the weak or absent effect of 2,4-D on final cell length and maximal strain rate suggests that the reduced root elongation of the auxin-treated roots resulted primarily from reduced cell production. In the apical part of the meristem, cell length was nearly constant with position for a considerable distance (Fig. 3, inset). In this region, average cell length in zeatin-treated and in stp1 roots was significantly higher than in wild-type and 2,4-D-treated roots. Over time, the region with roughly constant cell length extended basally in wild type and 2,4-D treatment (not shown), suggesting that in these treatments the size of the meristem increased over time.
Figure 3.
The spatial profile of cortical cell length versus distance from the quiescent center on d 8 for Arabidopsis wild type, stp1, and wild type treated with 1.0 μm zeatin or 30 nm 2,4-D. Cell length was measured in the same roots as used to obtain velocity data, and symbols are means ± se, as given for Figure 2. The wild-type cell length at positions greater than 800 μm from the quiescent center was not measured but was estimated by dividing the local velocity by the flux rate determined at the base of the meristem.
Cell Production and Meristem Size
To characterize the production of cells by the meristem, we used the measured profiles of velocity and cell length to calculate the spatial profile of cell flux, which gives the rate cells move past each position. Cell flux increased in the apical part of the growth zone as a consequence of cell division there, and reached a roughly constant value, indicating the cessation of division (Fig. 4). All treatments lowered maximal cell flux and affected the position where cell flux became roughly constant, with that position being more apical compared with wild type for stp1 and zeatin-treated roots and more basal for 2,4-D-treated roots. Over time, cell flux profiles were constant for stp1 and the zeatin treatment, but for wild type and the auxin treatment, maximal flux increased and the position where roughly steady flux was reached moved basally (not shown). These results indicate that the extent of the meristem was truncated in stp1 and by cytokinin treatment and in contrast was extended by auxin.
Figure 4.
Spatial profile of cortical cell flux versus distance from the quiescent center on d 8 for Arabidopsis wild type, stp1, and wild type treated with 1.0 μm zeatin or 30 nm 2,4-D. A cell flux profile was obtained for each root and averaged; symbols are means ± se, as given for Figure 2.
To more fully characterize cell production in the treatments we calculated local rates of cell production by evaluating the derivative of the spatial flux profile. This derivative has dimensions of cells produced per unit time and length, and is therefore distinct from the typical cell division rate, which has dimensions of cells produced per unit time and cell. We previously called this derivative a cell production rate (Beemster and Baskin, 1998); however, that term is easily confused with the cell production rate of the entire meristem. Therefore, we now call this derivative a cell deposition rate, which is consistent with notation used for other rates calculated with the continuity equation (Silk, 1992). The derivative of the spatial cell flux profile gives the rate of cell deposition at each position exactly when division and expansion are at steady state; however, when growth is not steady, the derivative must be corrected by adding the local time-dependent rate of change of cell density (Eq. 1), and this was done for both wild-type and auxin-treated roots. Note that for these treatments the time-dependent correction was small: When integrated over the whole meristem, it amounted to 5% (auxin) and 9% (wild type) of total cell production.
The spatial profile of cell deposition in the wild type was bell-shaped, and fell to a low level by 400 μm (Fig. 5). Because of small measurement errors in either velocity or cell length, calculated rates of cell deposition rarely fell exactly to zero. In stp1, the profile of cell deposition had a similar shape to that of the wild type, but peaked at roughly one-half the maximal rate and reached essentially zero at approximately one-half the distance from the quiescent center. The profile in zeatin-treated roots closely resembled that of stp1. In contrast, rates of cell deposition in the 2,4-D-treated roots were rather constant over the apical 300 μm of the root, and the meristematic activity extended somewhat farther basally than in the wild type. Thus lowered cell production by the meristem of 2,4-D-treated roots can be attributed to the lower maximal rates of cell deposition, whereas in stp1and zeatin-treated roots it was due to lower cell deposition rates and a reduction in the size of the meristem (Table I). For stp1 and zeatin treatment, these results, together with the constant cell length profile, suggest that the inhibition of acceleration of root elongation was caused by the cell number remaining constant in the meristem.
Figure 5.
Spatial profile of cortical cell deposition rate versus distance from the quiescent center on d 8 for Arabidopsis wild type, stp1, and wild type treated with 1.0 μm zeatin or 30 nm 2,4-D. Cell deposition rates were calculated for each root from Equation 1 and averaged. The local time-dependent change in cell density was zero for stp1 and zeatin-treated roots. Symbols are means ± se, as given for Figure 2.
Table I.
Spatial and temporal dimensions of the meristem and elongation zone in response to external application of 30 nm 2,4-D, 1.0 μm zeatin and in the stp1 mutant
| Treatment | Length
|
Cell
No.
|
Residence Time
|
|||
|---|---|---|---|---|---|---|
| Meristem | Elongation | Meristem | Elongation | Meristem | Elongation | |
| μm | h | |||||
| Wild type | 488 ± 42 | 1,362 ± 92 | 52 ± 3 | 16 ± 2 | 107 ± 9 | 8 ± 1 |
| 2,4-D | 530 ± 42 | 710 ± 52 | 50 ± 1 | 6 ± 1 | 158 ± 7 | 5 ± 1 |
| Zeatin | 273 ± 35 | 418 ± 37 | 20 ± 1 | 4 ± 1 | 113 ± 5 | 7 ± 1 |
| stp1 | 306 ± 37 | 327 ± 54 | 23 ± 2 | 7 ± 2 | 84 ± 7 | 7 ± 1 |
Data report the means ± se of 10 roots on d 8 for the wild type (data from Beemster and Baskin, 1998), 10 roots combined from d 6 and 8 for the zeatin and stp1 treatments, and 5 roots on d 8 for the 2,4-D treatment.
Numbers of Dividing Cells and Cell Division Rates
To explain the cellular basis of lowered cell production rates and altered meristem size we determined for each root the number of dividing cortical cells per file in the meristem and their average rate of division. To do this we defined the basal boundary of the meristem as the location where cell deposition rate first became zero (or reached its minimal value; Beemster and Baskin, 1998). Using the change in cell number for the region between this position and the quiescent center over time, and the cell flux out of the meristem, we could calculate an average cell division rate (Eq. 4).
In zeatin-treated wild-type and stp1 roots, the shortened meristem comprised cells that on average were longer than those of the wild type (Fig. 3, inset) and as a result contained fewer than one-half the number of dividing cells of the wild type (Table I). In wild-type and 2,4-D-treated roots, the number of dividing cells increased concurrently with the length of the meristem (data not shown), and on any day, the number of meristematic cells was essentially the same in the two treatments, as illustrated for d 8 (Table I).
Average cell division rate in stp1 and 2,4-D-treated roots was 15% to 20% lower than in the wild type (Table II). Although cell division rates were similarly affected in these two treatments, the increase in the extent of the meristem was inhibited in stp1, but not in 2,4-D-treated roots. Thus the control of meristem size appears to be independent of the control of cell division rate. Zeatin decreased cell division rate by over 30%. Because zeatin application reduced cell division rate more than the other treatments did and also reduced the number of dividing cells, overall cell production was lowered to a greater extent than it was in the mutant or in 2,4-D-treated roots.
Table II.
Cell division rate and cell cycle duration in response to external application of 30 nm 2,4-D, 1.0 μm zeatin and in the stp1 mutant
| Treatment | Division Rate | Cell Cycle Duration |
|---|---|---|
| cell cell−1 h−1 | h | |
| Wild type | 0.047 ± 0.009 | 16.9 ± 1.2 |
| 2,4-D | 0.034 ± 0.003 | 20.1 ± 0.9 |
| Zeatin | 0.020 ± 0.005 | 24.8 ± 1.3 |
| stp1 | 0.034 ± 0.005 | 19.1 ± 1.1 |
Cell division rate was averaged over the whole meristem and cell cycle duration calculated as described in “Material and Methods.” Data report means ± se from 10 roots on d 8 for the wild type (data from Beemster and Baskin, 1998), 10 roots combined from d 6 and 8 for the zeatin and stp1 treatments, and five roots on d 8 for the 2,4-D treatment.
Temporal Analysis
The spatial pattern of cell division and expansion could be a reflection of a temporal regulation of the underlying processes in individual cells as they migrate through the growth zone. To determine the time taken for each cell to move through the meristem or the elongation zone, we calculated the residence times in these regions. The time spent in the elongation zone was remarkably similar in all but the 2,4-D-treated roots (Table I), for which the shortened period of elongation after leaving the meristem contributed to the reduced root elongation rate. Residence times in the meristem were also most divergent for the 2,4-D treatment. Interestingly, the prolonged residence time for 2,4-D roots and the reduced cell division rate combined to give about the same number of dividing cells per file as in the wild type. Likewise, in stp1 and zeatin-treated roots, residence time in the meristem and cell division rate balanced to produce about the same number of dividing cortical cells. Consequently, a decreased meristem residence time failed to account for decreased cell production in any treatment.
DISCUSSION
As predicted, root growth in stp1 was phenocopied by treating the wild type with cytokinin. Comparable behavior of stp1 and zeatin-treated wild type included the absence of a developmental increase in root elongation rate, strain rate increasing with position across the meristem, reduced maximal strain rate, cell size in the apical one-half of the meristem that was increased and strictly constant with position, decreased cell division rate, and failure to increase the number of meristematic cells (Table III). Externally applied 2,4-D also reduced root elongation rate, but the meristem behaved unlike stp1: The curve of cell deposition rate versus position was flat, the curves of strain rate and cell length versus position had the same shape as the wild type, and the meristem expanded developmentally. Therefore, our results suggest that STP1 controls organ growth by acting in the meristem, as well as in the elongation zone, and that cytokinin acts on growth by reducing the activity of this protein.
Table III.
Comparison of the effects of the stp1 mutation, 1 μm zeatin, and 30 nm 2,4-D on elongation and division parameters of the primary root
| Elongation
Parameters
|
Cell Division Parameters
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Root growth rate | Meristem strain rate | Maximal strain rate | Time in elongation zone | Cell production | Cell division rate | Time in meristem | Meristem cell number | |
| Development | ++ | 0 | 0 | 0 | ++ | 0 | ++ | ++ |
| 2,4-D | − | − | 0 | −− | − | − | ++ | 0 |
| Zeatin | −−− | −− | −− | 0 | −−− | −− | 0 | −− |
| stp1 | −−− | − | −− | 0 | −− | − | −− | −− |
The first row describes the changes in wild-type root growth that occur during development as reported by Beemster and Baskin (1998). The symbols in the table are as follows: 0, no effect; −, slight reduction in the value of the parameter; −−, intermediate reduction; −−−, severe reduction; and ++, intermediate increase in the parameter.
Comparison of Cytokinin and Auxin Effects on Root Growth
An additional outcome of our results is the ability to compare the effects of auxin and cytokinin on the parameters of root growth. Despite the centrality of these hormones to plant physiology, we know of no other study that measured the spatial distribution of division and expansion concurrently, even for one of the hormones. The major contrasts are that cytokinin reduced maximal strain rate by nearly 50%, whereas auxin did not affect this parameter, and that cytokinin blocked the increase in meristem size, whereas auxin appeared to enhance it (Table III). Treatment with auxin or cytokinin continues to be used in influential experiments (e.g. Sabatini et al., 1999); consequently, the comparison here helps to fill an outstanding gap in our knowledge of how these hormones act to direct plant development.
Auxin typically inhibits root elongation, although under appropriate conditions it may stimulate it (Scott, 1972). Because root elongation slows within minutes after treatment (Evans, 1976; Tanimoto and Watanabe, 1986) and cell production is little affected, if at all (Street et al., 1954; Davidson and MacLeod, 1966; Van't Hof, 1968; Burström, 1969), the slow elongation is attributed mainly to the capacity for cellular expansion diminishing. Yet when cellular expansion is quantified by measuring the spatial distribution of strain rate, which has been done for several grass species (Hejnowicz, 1961; Goodwin, 1972; Ishikawa and Evans, 1993), moderate concentrations of auxin, following a transient phase of extreme inhibition, narrowed the elongation zone, but did not lower the maximal strain rate, similar to the pattern reported here for Arabidopsis. Thus when auxin reduces root elongation rate it does not necessarily reduce the capacity of cells for elongation, but instead can limit the extent of the elongation zone.
In Arabidopsis this narrowing appeared to be caused by decreased cellular elongation, as well as cell production. Auxin reduced cellular elongation because cells spent less time traversing the elongation zone, which has been previously reported for several other species on the basis of less precise measurements (Scott, 1972). However, in Arabidopsis auxin also cut the rate of supply of cells to the elongation zone, which reduced the number of cells elongating at any one time and thereby narrowed the elongation zone.
Throughout the apical one-half of the Arabidopsis meristem, auxin decreased strain rate, but did not change the concave downward shape of the curve for strain rate versus position. To our knowledge, ours are the first published strain rate data within an auxin-treated root meristem, so their generality remains to be determined. Nevertheless, the results show that cells in the meristem and in the elongation zone can respond differently to auxin, which supports the hypothesis that elongation of dividing cells differs mechanistically from that of cells elongating without dividing (Baskin et al., 1995).
Like auxin, cytokinin generally inhibits root elongation (Stenlid, 1982; Nissen, 1988), but the hormone has been inferred to stimulate (Guttman, 1956; MacLeod, 1968), as well as to inhibit cell production (Butcher and Street, 1960; Van't Hof, 1968). We found that cytokinin reduced maximal strain rate significantly and so diminished the magnitude of cellular elongation. In addition, cytokinin blocked the meristem from increasing in size developmentally. This stabilization of meristem size by cytokinin was opposite to the effect of auxin, which increased the length of the meristem, although the number of meristem cells was similar to that of the wild type. This suggests that auxin and cytokinin influence meristem size antagonistically. Understanding how hormones control the size of the meristem will require understanding how the cell cycle is regulated within intact organs.
Regulation of Cell Division Rate
Average cell division rate in the root meristem was reduced moderately in stp1 and by auxin-treatment, and more severely by cytokinin. In addition, for stp1 and cytokinin, cell division rate can be inferred to have increased with position in the apical one-half of the meristem because the curve of cell deposition rate versus distance increased, whereas cell size stayed constant. Although finding changed cell division rates might sound unremarkable, in a recent review on the root meristem, Baskin (2000) concluded that (except when the root was exposed to extremes) well-supported examples of changes in cell division rate are rare. This is because on the one hand, cell division rate often does remain constant (e.g. Beemster and Baskin, 1998; Muller et al., 1998), and on the other hand, the contradictions arising with the conventional “labeled mitoses” methods prevent drawing firm conclusions (Green and Bauer, 1977; Webster and MacLeod 1980; Ivanov and Dubrovsky, 1997). It should be noted that the decreased cell division rates reported here are consistent with the accompanying measurements of strain rate and cell length, and hence not strictly dependent on the validity of the equation (Eq. 4) used to calculate them. Given that cell division rate varies, the next step is to identify the signals that guide the cell cycle machinery in the root meristem past each checkpoint, and hence that set cell division rate.
One possible signal might come from cell size. In fission yeast, cell size is well known to help control when a cell enters mitosis (Nurse, 1975). In plant roots the length at which a cell divides at any given position had a strikingly narrow range (Ivanov, 1971); moreover, the distribution of mature cell length was also narrow (Beemster and Baskin, 1998). Both observations suggest that cell size in root meristems may help regulate the cell cycle; however, even in yeast, it is still not understood how size is sensed by the cell cycle machinery.
Another possible signal for regulating cell division rate is strain rate. In the apical one-half of the meristem, rates of cell division and strain were similar to each other in all treatments, although this similarity might reflect both parameters responding in parallel. Despite their overall correlation, division and strain rates in the meristem were not correlated perfectly. This can be seen by considering the profiles of strain rate and cell length over the first 250 μm from the quiescent center in wild-type and auxin-treated roots. The profiles of strain rate and cell length had slight, but reproducible minima between 100 and 150 μm from the quiescent center. As cells moved away from the quiescent center and elongated more slowly, were division rates to have fallen proportionally, then cell length would have remained constant. In fact, our previous calculations for the wild type showed that cell division rates were roughly constant over this region (Beemster and Baskin, 1998). In agreement, within the meristem of the maize root, spatial profiles of strain rate and cell length had comparable minima, whereas cell division rate was constant (Muller et al., 1998). Evidently, a cell's strain rate can change, at least within certain limits, without there being a corresponding change in the cell's division rate. Therefore, strain rate may not be sensed by the cell cycle machinery, or perhaps may be sensed only when it changes beyond certain limits.
How Is the Size of the Growth Zone Determined?
In general, differences in organ elongation rates are mediated at least partly through the sizes of meristem and elongation zone. How the sizes of these zones are specified is not clear. Size might be determined spatially, through some as yet undiscovered carrier of positional information (Barlow, 1976). However, as pointed out by Silk (1984) and exemplified by others (Bradford and Trewavas, 1994; Beemster et al., 1996), such zones can be determined temporally, that is, by regulating the time a given cell is allowed to spend in each zone.
Temporally, although it is easy to visualize a cell passing through the elongation zone, it is difficult to visualize one passing through the meristem because a cell only exists from one cytokinesis to the next. We avoided this problem by calculating the time to move through the meristem of the first-formed cell wall. However, it is not clear how the residence time of a cell wall applies to an actual meristem cell. What's more, the products of any given division are unequal, one being apical and the other basal, and each cell will have different fates because of this positional difference. Therefore, it is ambiguous what a temporal approach means for cells traversing the meristem.
Despite this ambiguity, the results suggest that meristem size might be regulated in part by the number of times a cell and its progeny divide. The meristems of wild-type and 2,4-D-treated roots had identical numbers of cells, even though they differed in average cell division rate and residence time. Similarly, the meristems of stp1 and cytokinin-treated roots had the same number of cells despite having different average cell division rates and residence times, whereas the number of meristem cells was lower than it was in the wild type. These results are explained most simply by saying that the number of cell divisions in the meristem was reduced by high cytokinin levels (or low STP1 activity). To otherwise explain the equal cell numbers requires opposite and compensatory changes in average cell division rate and meristem residence time. That cells carry information on how many times to divide was suggested long ago for the onion root tip (González-Fernández et al., 1968) and more recently supported by experiments on mammalian tissue culture cells (Temple and Raff, 1986). However, the mechanism by which cells count the number of their divisions remains unknown.
In contrast to the meristem, a temporal perspective can be applied readily to the elongation zone. Despite the size of this zone varying among the studied treatments, a cell tended to spend about the same time traversing the zone. This was also true for wild-type roots whose elongation zone expanded during development (Beemster and Baskin, 1998) or narrowed in response to either salt or ethylene (G.T.S. Beemster, unpublished data). This supports the hypothesis that a cell enters the elongation zone with a programmed duration for expansion activity, and consequently, that the size of this zone depends to an important degree on the number of cells supplied by the meristem per unit time. The alternative hypothesis of spatial control over the size of the elongation zone requires that its size is adjusted to maintain a steady duration for cells to elongate, and that for the stp1 and cytokinin treatments, the mechanisms regulating how far the meristem and elongation zone extend in space are affected in parallel.
If, as we hypothesize, a cell's passage through the growth zone is regulated by controlling how much time it spends, first in division and second in elongation without division, then it becomes paramount to discover the hands, gears, and escarpment used for telling cellular time.
MATERIALS AND METHODS
Wild-type seedlings of Arabidopsis ecotype Columbia and seedlings from the same background carrying the stp1 mutation (Baskin et al., 1995) were grown on agar-solidified medium (1.2% [w/v] Bacto agar, Difco Laboratories, Detroit) containing a modified Hoagland solution and 3% (w/v) Suc in vertically oriented 90- × 90-mm square tissue culture plates under constant temperature (20°C) and light (80 μmol m−2 s−1, filtered to remove UV and blue wavelengths) as described previously (Baskin and Wilson, 1997). For hormone treatments, filter-sterilized stock solutions were added to media after autoclaving to give final concentrations of 1 to 100 nm 2,4-D or 0.3 to 5 μm zeatin. The zeatin stock was made in dimethyl sulfoxide and the 2,4-D stock was aqueous.
Root elongation rates were determined by scoring the bottom of the plate with a razor blade at the position of the root tip at known times (once per day). The average growth rate for each day was determined from enlarged photocopies of the plates as the distance between successive marks along the root, measured with a digitizing tablet, divided by the corresponding time interval. Kinematic analysis of cell division and elongation was performed on selected treatments at d 6, 8, and 10, using the methods described earlier (Beemster and Baskin, 1998). Briefly, on each observation day, selected roots on several plates were marked with graphite particles, and a series of overlapping images of the root surface was captured hourly through a horizontal compound microscope by a charge-coupled device camera connected to a computer fitted with a frame grabber board. After five to six observations, overlapping images along the root of cortical cells were obtained using Nomarski optics. In addition, the distance between the quiescent center and the end of the root cap was measured for all roots within a treatment and used to register the cell length and velocity data sets, taking the quiescent center as zero on the x axis. The analysis used five plants per treatment selected from separate plates on each observation day. The exception was zeatin-treated roots on d 6, of which two grew at less than one-half the speed of the other roots and for this reason were excluded from further analysis.
For quantifying expansion and division parameters kinematically, the spatial distributions of velocity and cell length must be measured. These data were first smoothed and interpolated using a specially developed algorithm (Beemster and Baskin, 1998) and then used to calculate strain rate, cell flux, and cell deposition rate.
Strain rates were calculated as the derivative of velocity versus position. To calculate cell division, the kinematic method compares the rate at which cells flow into and out of a region and attributes any increased outflow to production of cells within that region (Silk and Erickson 1979; Gandar 1980; Baskin 2000). The rate at which cells move past a position, x, is called cell flux, F(x), with dimensions of cells per unit time, and was calculated as the product of velocity and cell density (ρ, the reciprocal of cell length) at x. The spatial derivative of cell flux at x, added to the local rate of change of cell density over time measures the rate at which cells are produced at that position:
 |
1 |
The quantity, P(x), which has dimensions of cells per unit time and length, is called cell deposition rate.
The basal endpoint of the elongation zone was defined as the position where strain rate first equaled zero. The basal endpoint of the meristem (Xdiv) was defined for each root as the position where cell deposition rate first became zero. In some roots this rate became small, but not zero, presumably because of measurement error. For these roots the end of the meristem was defined where the deposition rate reached its minimal value. From these endpoints it was then possible to determine the number of cells in the meristem and elongation zone from the cell length profile, as well as a residence time for cells traversing these zones, as described in Beemster and Baskin (1998).
Because a cell in the meristem is extant only until it divides again, the calculated residence time for the meristem applies to the first cell wall created by a cell located at the most apical position in the file. The calculations assume that cell division rate is constant over time, which was true for wild-type (Beemster and Baskin, 1998) and 2,4-D-treated roots and nearly true for the other treatments (data not shown). For the calculation of residence time in the elongation zone, root elongation rate was divided by the cell flux out of meristem, assuming both to be constant. In fact, both parameters increased over time under the non-steady conditions studied here (2,4-D and wild type); however, they increased in parallel. This fact and the relatively short period that cells resided in the elongation zone suggest that the calculated residence time is a reasonable approximation under the non-steady conditions encountered here.
An average cell division rate for the whole meristem at a given time was estimated as follows. First, Equation 1 is recast to a finite form to refer to the rate of cell production out of the entire meristem:
 |
2 |
where Xtdiv equals the length of the meristem at time t, Ndiv equals the number of cells in the meristem (i.e. from x = 0 to x = Xtdiv) and Fm equals the flux of cells out of the meristem (i.e. cell flux at x = Xtdiv). The temporal derivative accounts for the change in cell density over a constant length interval defined by the size of the meristem at time t, and because the length interval (Xtdiv) is a constant, it may be taken out of the derivative. Second, to convert this cell deposition rate (Eq. 2) into an average cell division rate, recall that:
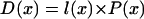 |
3 |
where D(x) is the rate of cell division (cells per cell and time) and l(x) is cell length at x (Beemster and Baskin, 1998). Therefore, Equation 2 is multiplied through by the average cell length within the meristem, L̄. Then substituting 1/Ndiv for L̄/Xtdiv, we obtain:
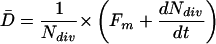 |
4 |
Note that when the number of cells within the meristem is constant over time, this equation reduces to D̄ = Fm/Ndiv, which has been used before to calculate average cell division rate (Ivanov and Dubrovsky, 1997). Average cell cycle duration was then obtained as ln(2)/D̄.
To evaluate the temporal derivative of cell number in the meristem (i.e. dNdiv/dt), we applied the three-point differentiation formula (Erickson, 1976) to the average cell number between x = 0 and x = Xdiv for days 6, 8, and 10.
ACKNOWLEDGMENTS
We gratefully acknowledge technical assistance by Jan Wilson and the preliminary work defining the dose-response relationship of root elongation to zeatin by Bethany Stone. Corine van der Weele commented insightfully on the manuscript.
Footnotes
This project was supported in part by the U.S. Department of Energy (grant no. 94ER20146 to T.I.B.), which does not constitute endorsement by that Department of views expressed herein, and by the U.S. National Science Foundation (grant no. IBN 9817132 to T.I.B.). G.T.S.B. was supported by a postdoctoral fellowship from the Molecular Biology Program of the University of Missouri, Columbia.
LITERATURE CITED
- Barlow PW. Towards an understanding of the behavior of root meristems. J Theor Biol. 1976;57:433–451. doi: 10.1016/0022-5193(76)90014-x. [DOI] [PubMed] [Google Scholar]
- Baskin TI. On the constancy of cell division rate in the root meristem. Plant Mol Biol. 2000;43:545–554. doi: 10.1023/a:1006383921517. [DOI] [PubMed] [Google Scholar]
- Baskin TI, Cork A, Williamson RE, Gorst JR. STUNTED PLANT 1, A gene required for expansion in rapidly elongating but not in dividing cells and mediating root growth responses to applied cytokinin. Plant Physiol. 1995;107:233–243. doi: 10.1104/pp.107.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI, Wilson JE. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997;113:493–502. doi: 10.1104/pp.113.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:515–526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Masle J, Williamson RE, Farquhar GD. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot. 1996;47:1663–1678. [Google Scholar]
- Bradford KJ, Trewavas AJ. Sensitivity thresholds and variable time scales in plant hormone action. Plant Physiol. 1994;105:1029–1036. doi: 10.1104/pp.105.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burström HG. Influence of the tonic effect of gravitation and auxin on cell elongation and polarity in roots. Am J Bot. 1969;56:679–684. [Google Scholar]
- Butcher DN, Street HE. Effects of kinetin on the growth of excised tomato roots. Physiol Plant. 1960;13:46–55. [Google Scholar]
- Davidson D, MacLeod RD. Changes in mitotic indices in roots of Vicia faba: I. Antagonistic effects of colchicine and IAA. Chromosoma. 1966;18:421–437. doi: 10.1007/BF00332546. [DOI] [PubMed] [Google Scholar]
- Erickson RO. Modeling of plant growth. Annu Rev Plant Physiol. 1976;27:407–434. [Google Scholar]
- Evans ML. A new sensitive root auxanometer: preliminary studies on the interaction of auxin and acid pH in the regulation of intact root elongation. Plant Physiol. 1976;58:599–601. doi: 10.1104/pp.58.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandar PW. The analysis of growth and cell production in root apices. Bot Gaz. 1980;141:131–138. [Google Scholar]
- González-Fernández A, López-Sáez JF, Moreno P, Giménez-Martín G. A model for dynamics of cell division cycle in onion roots. Protoplasma. 1968;65:263–276. doi: 10.1007/BF01682532. [DOI] [PubMed] [Google Scholar]
- Goodwin RH. Studies on roots: V. Effects of indoleacetic acid on the standard root growth pattern of Phleum pratense. Bot Gaz. 1972;133:224–229. [Google Scholar]
- Green PB. Growth and cell pattern formation on an axis: critique of concepts, terminology and modes of study. Bot Gaz. 1976;137:187–202. [Google Scholar]
- Green PB, Bauer K. Analyzing the changing cell cycle. J Theor Biol. 1977;68:299–315. doi: 10.1016/0022-5193(77)90167-9. [DOI] [PubMed] [Google Scholar]
- Guttman R. Effects of kinetin on cell division, with special reference to initiation and duration of mitosis. Chromosoma. 1956;8:341–350. doi: 10.1007/BF01259506. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z. The response of the different parts of the cell elongation zone in root to external B-indolylacetic acid. Acta Soc Bot Pol. 1961;30:25–42. [Google Scholar]
- Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VB. Critical size of the cell and its transition to division. Sov J Dev Biol. 1971;2:421–428. [Google Scholar]
- Ivanov VB, Dubrovsky JG. Estimation of the cell-cycle duration in the root apical meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. Int J Plant Sci. 1997;158:757–763. [Google Scholar]
- MacLeod R. Changes in the mitotic cycle in lateral root meristems of Vicia faba following kinetin treatment. Chromosoma. 1968;24:177–187. [Google Scholar]
- Muller B, Stosser M, Tardieu F. Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant Cell Environ. 1998;21:149–158. [Google Scholar]
- Nissen P. Dose responses of cytokinins. Physiol Plant. 1988;74:450–456. [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in the fission yeast Schizosaccharomyces pombe. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Scott TK. Auxins and roots. Annu Rev Plant Physiol. 1972;23:235–258. [Google Scholar]
- Silk WK. Quantitative descriptions of development. Annu Rev Plant Physiol. 1984;35:479–518. [Google Scholar]
- Silk WK. Steady form from changing cells. Int J Plant Sci. 1992;153:S49–S58. [Google Scholar]
- Silk WK, Erickson RO. Kinematics of plant growth. J Theor Biol. 1979;76:481–501. doi: 10.1016/0022-5193(79)90014-6. [DOI] [PubMed] [Google Scholar]
- Stenlid G. Cytokinins as inhibitors of root growth. Physiol Plant. 1982;56:500–506. [Google Scholar]
- Street HE, McGregor SM, Sussex IM. Effects of 3-indolylacetic acid and 3-indolylacetonitrile on the growth of excised tomato roots. J Exp Bot. 1954;5:204–214. [Google Scholar]
- Tanimoto E, Watanabe J. Automated recording of lettuce root elongation as affected by auxin and acid pH on a new rhizometer with minimum mechanical contact to roots. Plant Cell Physiol. 1986;27:1475–1487. [Google Scholar]
- Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Van't Hof J. The action of IAA and kinetin on the mitotic cycle of proliferative and stationary phase excised root meristems. Exp Cell Res. 1968;51:167–176. doi: 10.1016/0014-4827(68)90167-5. [DOI] [PubMed] [Google Scholar]
- Volenec JJ, Nelson CJ. Cell dynamics in leaf meristems of contrasting tall fescue genotypes. Crop Sci. 1981;21:381–385. [Google Scholar]
- Volenec JJ, Nelson CJ. Responses of tall fescue leaf meristems to N fertilization and harvest frequency. Crop Sci. 1983;23:720–724. [Google Scholar]
- Webster PL, MacLeod RD. Characteristics of root apical meristem cell population kinetics: a review of analyses and concepts. Environ Exp Bot. 1980;20:335–358. [Google Scholar]