Abstract
Increased urbanization and increase in population has led to an increased demand for fuels. The result is the prices of fuels are reaching new heights every day. Using low-cost feedstocks such as rendered animal fats in biodiesel production will reduce biodiesel expenditures. One of the low-cost feedstocks for biodiesel production from poultry feathers. This paper describes a new and environmentally friendly process for developing biodiesel production technology from feather waste produced in poultry industry. Transesterification is one of the well-known processes by which fats and oils are converted into biodiesel. The reaction often makes use of acid/base catalyst. If the material possesses high free fatty acid then acid catalyst gives better results. The data resulted from gas chromatography (GC) revealed these percentages for fatty acid compositions: myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid and arachidonic acid. The biodiesel function group was analyzed by using FTIR. This study concluded that the rooster feathers have superior potential to process them into biodiesel than broiler chicken feathers fat because of fatty acid composition values and it has important properties of biodiesel.
Keywords: Feather, Transesterification, Biodiesel, GC, FTIR
Highlights
-
•
Production of biodiesel from low cost feedstock poultry feathers.
-
•
Conversion of fat and oil in to biodiesel by transesterification process.
-
•
Composition of fatty acid in two different feathers were compared.
1. Introduction
Energy is the single most important resource capable of sustaining life on earth. Energy not only is the engine of economic growth but also the cause of important life threatening outcomes [4]. The strong interest in liquid biofuel is due to the fact that it can be used as a supplement, or alternative, to gasoline or diesel fuel derived from petroleum fossil fuel [1], [7]. Over time with entrance of oil as new and cheap fuel, tendency to this fuel increased. Biodiesel, a replacement of petroleum diesel, derived from biological sources received increasing attention globally as it lessens the dependence on petroleum products, the energy crisis and environmental pollution [6]. Biodiesel is a non petroleum-based alternative diesel fuel that consists of alkyl esters derived from renewable feedstocks such as plant oils or animal fats. A successful biofuel industry will not be based on digestible starch from staple crops such as corn. The main problem the biodiesel industry frequently faces is the availability of cheap and abundant, high-quality feedstock. Thus, finding alternative, nonfood, feedstocks such as waste vegetable oil, grease, and animal fats (beef tallow) is considered a necessity for the industry. Through continued research to produce biofuels from nonfood sources, it has been discovered that poultry feather offers another promising feedstock source for biodiesel production [4], [5], [11]. Feather fat is a low-cost feedstock for biodiesel production compared to high-grade vegetable oils [3], [9]. Feathers are byproducts of poultry processing plant and produced in large amount. Worldwide 24 billion chickens are killed annually and around 8.5 billion tones of poultry feather are produced. Currently the poultry feathers are treated in some ways such as dumping, landfilling, composting and incinerating, which involve problems in storage, handling, emissions control and ash disposal [2], [10]. Moreover feather meal is used as an animal feed, given its high protein content, and also as a fertilizer because of its high nitrogen content [11]. The utilization of feather fats for biodiesel production is a good alternative to recycle these wastes [8]. The transesterification method is commonly used for biodiesel production because of its higher yield and lower energy consumption. Transesterification is a chemical process of reacting triglycerides with alcohol in the presence of a catalyst. If the reaction is not completed, then there will be mono-, di- and tri-glycerides left in the reaction mixture. Alcohols such as methanol, ethanol or butanol can be used in the transesterification [12], [13] It generally involves a three step reversible reaction in which initial triglycerides yields a mixture of fatty acid methyl ester (FAME) and glycerol in the presence of three catalysts: acid, alkali and enzyme. The present study is designed to emphasize on processing and characterization of biodiesel from poultry waste (i.e. chicken's feather meal) by the process of transesterification and then characterized its fuel properties according to standard test methods.
2. Materials and methods
2.1. Sample collection
The chicken and rooster waste feather samples were collected from SD poultry farm (Gollamadugu, Chittoor District, Latitude- 13.213386°N, Longitude 79.094836°E), Sample was collected in a sterile plastic bags and it was brought to the laboratory for further processing. The collected sample was washed with sterile distilled water and air dried under the room temperature. The dried feathers were stored in refrigerator for further use.
2.2. Extraction of fat from feather
About Twenty grams of feather sample was taken and stirred it continuously with 200 mL of 5% NaOH at 90 °C for 45 min on hot plate. The feather completely dissolved and absorbed fat melted and floated on the surface of layer. The dissolved feathers were transfer to plate and cooled at room temperature. The plates were transfer to hot air oven for 1 h and evaporate the excess of Water. The dried feather meal was collected and stored at 4 °C for further use [25].
2.3. Extraction of Lipid
The total lipid content was extracted by Bligh & Dyer extraction method [23]. In a 100 mL conical flask containing 5 g or 5 mL of sample, 20 mL methanol and 10 mL chloroform were added and the mixture was vortexed for 2 min 10 mL of chloroform was added and the mixture was shaked vigorously for 2 min 18 mL of distilled water was added and the mixture was vortexed again for 2 min. The chloroform layer was separated by centrifugation at 2000 rpm for 10 min. The lower layer was transferred to a pear-shaped flask with a Pasteur pipette. Second extraction was made with 20 mL 10% (v/v) methanol in chloroform by vortexing for 2 min. After centrifugation, the chloroform phase was added to the first extract. Evaporation was done with rotary vapor and the residue was further dried at 104 °C for 1 h [26].
2.4. Biofuel extraction
The feather oil content was extracted from feather meal samples using a modified Folch and Lee's method. Dried feather meal (10 g) were taken and added with 20 mL of chloroform/ methanol (2:1 v/v) at 25 °C after cell disruption in a 25 W sonicator (Branson model 2510) and incubated. Tubes were incubated for 10 min at room temperature to allow the organic and aqueous layers to separate. After removing and saving the bottom (organic) layer, the aqueous layer was re-extracted by adding chloroform (6–8 mL), to separate the layer. The resulting extracts (6–10 mL each) were stored at 4 °C prior to removing aliquots for oil analysis. Fatty acid composition analysis was performed using gas chromatography (GC) method.
2.5. Biodiesel separation: (transesterfication method)
Base catalyzed transesterification method was carried out in a 250 mL glass beaker equipped with a magnetic stirrer. Feather meal lipid (100 mL) was taken in flask and potassium hydroxide (1.8 g) dissolved in 33.5 mL methanol was added to flask. Stirring was continued for 2 h at 55 °C, the mixture was transferred to a separatory funnel and glycerol was allowed to separate for a minimum of 3 h. After draining off the glycerol, methyl ester was washed twice with 1:1 vol of water for 1 h to remove excess methanol [21], [22].
2.6. FTIR (Fourier Transform Infra Red Spectroscopy) analysis of lipid
The FTIR were recorded in the mid-IR region 4000–400 cm−1 at room resolution 4 cm−1 with scans using Thermo Nicolet FTIR Nexus spectrometer coupled with TGS (Tri-glycine sulphate) detector. The interferometer and the detector chamber were purged with dry nitrogen to remove spectral interference due to atmospheric carbon dioxide and water vapor. Air background spectrum was recorded before each sample and all experiments were performed in six triplicates (six pellets KBr with three scan each).
2.7. GC-MS analysis of lipid and FAME
Analysis of biodiesel was carried out on GC [24]. FAME produced from various oil was performed on mass spectrometer (Shimadzu GCMS-TQ8030) with two narrow-bore capillary columns, coupled to a gas chromatograph (Shimadzu GCMS-TQ8030) equipped with an auto sampler. The GC column used was fused with silica capillary column (TQ8030, 30 m × 250 μmi.d., film thickness 0.25 µm). The pressure of the carrier gas (helium) was 6.0799 Psi at the initial oven temperature with flow rate 67 mL min −1. All standards and samples were injected in the split mode (split/column flow ratio 60:1). The injector temperature was 240 °C; the oven temperature was 55 °C, rose to 210 °C at rate of 15 °C min −1 (total run time 34 min). The mass spectrometer was operated in the electron impact (EI) mode at 70 eV in the scan range of 50–650 m/z. The temperature of the transfer line and of the ion source was set to a value of 320 and 280 °C,respectively. The injection sample volume was 1.0 μL. Peak identification of a oil was performed by comparison with retention times of standards and the mass spectra obtained compared with those available in the Wiley and NIST libraries (Wiley Registry TM, 8th Edition Mass Spectral Library and the NIST 08 Mass Spectral Library (NIST/EPA/NIH) 2008 version) with an acceptance criterion of a match above a critical factor of 80%.
2.8. Measurement of viscosity and density
Viscosity is defined as the resistance to flow of a fluid. The viscosity of biodiesel was determined at 40 °C by ASTM standard D445 test method. Cannon-Fenske viscometer tube and Selecta (VB 1423) viscosity bath were used for the viscosity measurement.
Density can be defined as the mass of an object divided by its volume. The density of the biodiesel was measured by ASTM standard D941 test method. The measurement were done at 15 °C used Anton Paar (DMA 35N) density meter.
3. Results
3.1. Collection of feather meal
The feather meal was collected from the 200 mL of 0.2 gm NaOH degradation and was measured in the terms of dry weight. The results showed better feather meal biomass yield of 10.51 g/200 mL as dry weight.
3.2. Estimation of fat
The fat contents were measured the dry feather meal. In the fat content 218.98 mg/mL was extracted in feather meal.
3.3. Separation of biodiesel by using transesterification process
The transesterification process under the room temperature, there are two layer form in the separating funnel. In the bottom layer as a glycerol and upper layer is biodiesel. The bottom layer was removed and upper layer was collected in the conical flask. Biodiesel was transfer into plate and evaporated in under room temperature remove the excess amount of solvent. 4.5 mL of biodiesel was extracted from 10.51 g of dried rooster feather meal. The Fig. 1a. and b.depicts biodiesel production at different level of experimental components.
Fig. 1.

Transesterification process (a. Chicken and b. Rooster).
3.4. FTIR analysis of fat
Fourier transform infrared spectroscopy (FTIR) spectroscopy provides a tool by which one can access to many of the key functional groups which are related to biodiesel content, the loss and the formation of ester linkages, production and loss of OH, etc. With the product changing these functional groups undergo well marked changes and can be used to qualify the biodiesel, the lipid FTIR spectra can be distinguished from each other. The fat of feather meal oil has several absorption features which can be used to differentiate it. In mid-IR region, lipid has several characteristic peaks at around 4443.18, 4333.23, 4314.9, 4215.57, 4120.09, 4187.6,3450.77, 3429.55, 3402.54, 3010.98, 2956.01, 2923.22, 2852.81, 2358.93, 2375.42, 2342.62, 1383.01, 1299.68, 1284.63, 982.76, 723.33 and 614.35 cm−1. When the amount of lipid increases, these peaks increase correspondently. The peak at around 1008.17 cm−1 to 1540.02 is assigned to O-CH3 initial methyl group stretch. The peak at around 1638.86 cm−1 is considered to be the -CH3 asymmetric vibrations. The peak at around 4000–4450 cm−1 is considered as aAmide N-H Stretch groups. The dominant feature is the much larger absorbance band between 3500 and 3000 cm−1 which is typically associated with hydroxyl groups. The two intense bands at 2928 cm−1 and 2852 cm−1 are due to CH2 asymmetric and symmetric stretching vibrations. At around 1377 cm−1, the absorbance of lipid decreases with the increase of lipid amount. Results show that the obtained liquid has a good quality as a biodiesel fuel (Table 1 and Fig. 2(a) and (b)).
Table 1.
FTIR analysis of biodiesel for rooster and chicken.
| Characteristic absorptions (cm−1) |
Functional group | Type of vibration | |
|---|---|---|---|
| Rooster | Chicken | ||
| 3700–4500 | – | Amide | N-H Stretch |
| 3550–3200 | – | Phenols & Alcohols | Hydrogen-bonded O-H Stretch |
| 3100–3010 | – | Alkynes | =C-H Stretch |
| 3000–2500 | 3000–2500 | Carboxylic Acids | Hydrogen-bonded O-H Stretch |
| 2950–2850 | – | Alkenes | C˭C-H Asymmetric Stretch |
| 2600–2800 | – | Alkynyl | C˭C Stretch |
| 2300–2500 | – | Carbonyl | C˭O Stretch |
| 2000–2200 | – | Ketones | C-C˭C Symmetric Stretch |
| 1300–1500 | 1300–1500 | Aromatic | C˭C Bending Stretch |
| 900–1200 | 900–1200 | Aromatic | C-O Stretch |
| 860–680 | 860–680 | Aromatic | C-H Bending Stretch |
| 850–550 | 850–550 | Stretch | C–Cl alkyl halides |
Fig. 2.
FTIR analysis of biodiesel (a) Rooster and (b) Chicken.
3.5. GC-MS analysis of fat
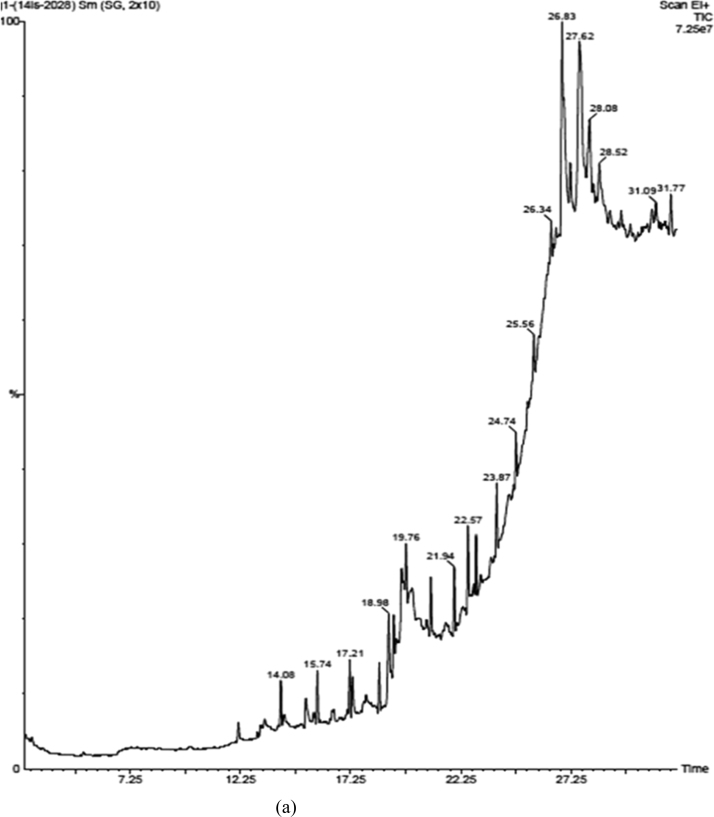
In a feather meal lipid composition, 4-Hydroxy-4-methyl-2-Pentanone, 1-Octanol,Decane, Naphthalene, Dichloroacetic acid, Dodecane, beta- Caryophyllene, 2,6-Di-tert-butylphenol, Hexadecane, Eicosane, 4-Methyl-2-tert-octylphenol, Tridecane, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol), 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione, Methyl propionate, Hexadecanoic acid, Phthalic acid, cis-11 14-eicosadienoic acid methyl ester (Eicosadienoic acid), 2,3-dihydroxypropyl ester (Glycerol monostearate), 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol), 9-Octadecenoic acid (Oleic acid), octadecanoic acid (Stearic acid), Hexadecane, Tetracosane were recognized as the most common lipid contained in GC-MS analysis. As indicated, Decane, Dodecane, Hexadecane, Eicosane, Hexadecanoic acid, cis-11 14-eicosadienoic acid methyl ester (Eicosadienoic acid), 2,3-dihydroxypropyl ester (Glycerol monostearate), 9-Octadecenoic acid (Oleic acid), octadecanoic acid (Stearic acid), Hexadecane, Tetracosane were commonly dominant. Decane-1.96%, Dodecane-1.85%, Hexadecane-2.19%, Eicosane-1.69%, Hexadecanoic acid-2.35%, cis-11 14-eicosadienoic acid methyl ester (Eicosadienoic acid) − 2.37%, 2, 3-dihydroxypropyl ester (Glycerol monostearate) − 3.30%, 9-Octadecenoic acid (Oleic acid) − 17.55%, octadecanoic acid (Stearic acid) − 8.79%, Hexadecane-5.43%, Tetracosane-2.41% (based on dry weight of lipid). Among the feather meal showed the highest oleic acid (17.55%) and Stearic acid (8.79%) content, made it the most suitable for the production of good quality biodiesel. Reported that oil with a high oleic acid content have a reasonable balance of fuel properties. As such, higher oleic acid content increases the oxidative stability for longer storage and decreases the cold filter plugging point for use in cold regions (Table 2 and Fig. 3).
Table 2.
GC-MS analysis of biodiesel rooster and chicken.
| S. No | Peak value (RT) |
Compound name | |
|---|---|---|---|
| Rooster | Chicken | ||
| 1 | – | 4.26 | 4-Hydroxy-4-methyl-2-Pentanone |
| 2 | – | 10.58 | 1-octanol |
| 3 | – | 12.06 | 3-methyl-4-isopropylphenol |
| 4 | 13.02 | 2-Benzothiazolamine, 6-Methoxy | |
| 5 | 13.96 | Tetracosane | |
| 6 | 14.08 | – | Dodecane |
| 7 | – | 15.15 | Pyrimidinone |
| 8 | – | 15.67 | Ethyl Ester |
| 9 | 15.74 | – | beta- Caryophyllene |
| 10 | 17.21 | – | 2,6-Di-tert-butylphenol |
| 11 | 17.59 | Tetrahydroimidazo[1,2-A]Pyrimidine | |
| 12 | 17.72 | Dihydrogmelinol Isomer | |
| 13 | 18.98 | – | Hexadecane |
| 14 | 19.58 | – | Eicosane |
| 15 | 19.63 | Pyrimidinone | |
| 16 | 19.77 | Diethyl Methyl | |
| 17 | 19.67 | – | 4-Methyl-2-tert-octylphenol |
| 18 | 21.29 | Hexadecyl Ester | |
| 19 | 21.64 | Pentadecyl Ester | |
| 20 | 21.94 | – | Tridecane |
| 21 | 22.57 | – | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) |
| 22 | 23.55 | Bromocriptine | |
| 23 | 23.87 | – | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione |
| 24 | 24.74 | Methyl propionate | |
| 25 | 25.12 | Hexadecyl Ester | |
| 26 | 25.25 | Glycyl-L-Proline | |
| 27 | 25.56 | – | Hexadecanoic acid |
| 28 | 26.34 | – | Phthalic acid |
| 29 | 26.83 | 26.84 | cis-11 14-eicosadienoic acid methyl ester (Eicosadienoic acid) |
| 30 | 27.62 | – | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester (Glycerol monostearate) |
| 31 | 28.08 | – | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) |
| 32 | 28.52 | – | 9-Octadecenoic acid (Oleic acid) |
| 33 | 31.09 | – | Octadecanoic acid (Stearic acid) |
| 34 | 31.77 | – | Hexadecane |
Note: RT – Retention Time.
Fig. 3.
(a) GC-MS analysis of biodiesel (Rooster). (b) GC-MS analysis of biodiesel (Chicken).
3.6. FAME value
In a feather mealfatty acid composition, palmitic, stearic, oleic, and linolenic acid were recognized as the most common fatty acids contained in biodiesel. As indicated, for the four tested biofuel, palmitic acid (C16:0) and oleic acid (C18:1) were commonly dominant. Linoleic acid (C18:2) was highest rooster feather mealin at 23.6% (based on dry weight), while other contained a low amount of poly unsaturated fatty acid methyl esters linoleic acid (C18:2), α-linolenic acid (C18:3), eicosenoic acid (C20:5) and docosenoic acid (C22:6). Which is a significant difference compared to edible oils such as soybean and sunflower. Among the feather meal showed the highest oleic acid (45.4%) content, making it the most suitable for the production of good quality biodiesel. As such, higher oleic acid content increases the oxidative stability for longer storage and decreases the cold filter plugging point for use in cold regions. A suite of FAME standards were used for chromatographic peak identification. The following were chosen as a representation of FAMEs that are expected in feather samples: methyl myristate (C14:0), methyl palmitate (C16:0), methyl stearate (C18:0), methyl arachidate (C20:0), methyl oleate (C18:1), methyl linoleate (C18:2), methyl linolenate (C18:3), methyl eicosapentaenoate (C20:5), and methyl docosahexaenoate (C22:6). An internal standard, methyl laurate (C12:0), were used to monitor concentration changes over time in samples and mixed standards (Table 3, Table 4).
Table 3.
FAME analysis of biodiesel (Rooster).
| S.No | FAME | Transesterfication method |
|---|---|---|
| 1 | C14:0 | 6.2 ± 0.42 |
| 2 | C16:0 | 13 ± 2.3 |
| 3 | C18:0 | 1 ± 0.26 |
| 4 | C18:1 | 0.32 ± 0.10 |
| 5 | C18:2 | 1.9 ± 0.18 |
| 6 | C18:3 | 0.84 ± 0.01 |
| 7 | C20:5 | 3.0 ± 0.45 |
| 8 | C22:6 | 3.0 ± 0.72 |
Table 4.
FAME analysis of biodiesel (Chicken).
| S.No | FAME | Transesterfication method |
|---|---|---|
| 1 | C16:0 | 10 ± 1.3 |
| 2 | C18:2 | 2.1 ± 0.83 |
| 3 | C22:6 | 1.6 ± 0.52 |
3.7. Properties of feather biodiesel
The biodiesel samples were made from rooster and chicken feather. Only there were minor deviations in both the samples. The properties of biodiesel samples are within the ASTM 6751 standards shown in Table 5.
Table 5.
Specification of feather biodiesel samples.
| S. No | Properties | UNIT | Testing Procedure ASTM | ASTM Standard 6751 | Feather biodiesel |
|
|---|---|---|---|---|---|---|
| Chicken | Rooster | |||||
| 1 | Viscosity at 40 °C | mm2/s | D445 | 3.5–5.0 | 3.9 | 4.3 |
| 2 | Density at 15 °C | g/m3 | D941 | 0.86–0.90 | 0.81 | 0.87 |
| 3 | Flash Point | °C | D93 | 130 | 120 | 126 |
| 4 | Cetane number | – | D613 | 47 min | 50 | 55 |
| 5 | Specific gravity | – | D4052 | 0.87–0.90 | 0.86 | 0.88 |
| 6 | Acid value | mg/gm | D664 | 0.5 max | 0.3 | 0.2 |
| 7 | Water Content | mg/kg | D1796 | 500 max | 90 | 95 |
| 8 | Cloud Point | °C | D2500 | − 3–12 | 2 | 3 |
| 9 | Total sulfur | ppmw | D5453 | 15 max | 17 | 15 |
The viscosity of chicken and rooster feathers found to be 3.9 and 4.1 mm2/s at 40 °C by using ASTM D445 method. The density of chicken and rooster feathers is 0.81 and 0.87 g/m3 at 15 °C by ASTM D941 method. These two physical properties are major important for biodiesel specification.
4. Discussions
The biodiesel production from the chicken feather meal by the action of transesterification method by using methanol and NaOH as a catalyst. The total fat content of the feather meal sample were determined, and has been reported in 40 mg/mL, prior to synthesize them into biodiesel in order to assess whether they have enough potential to into biodiesel and amount of extracted fat from feather meal was evaluated whose values ranged between 200 and 220 mg/mL. The high amount of extracted fat is achieved from chicken feather meal. Recovered fat was obtained from extracted fat by the process of transesterification as to esterification extracted fat and reduced its FFA level into desired level. The amount of recovered fat lied between 180 to 185 mg/mL. Subsequently, that recovered fat was used further for the synthesis of biodiesel.
The production of biodiesel using chicken feather meal by transesterification method and using such as methanol, KOH and NaOH a catalyst the biodiesel productivity at 88.5%, 71.3% respectively [3].
The reports of Abdoli [1] explains maximum fatty acid content such as myristic 3%, palmitic 30%, stearic 22%, oleic 8.1%, linoleic 3%, linolenic 25%, arashidic acid 7% respectively and obtained by using gas chromotatograph mass specteram analysis.
Viscosity is the most important factor, which affects the fluidity of biodiesel [17]. The viscosities of biodiesel vary in the range of 3.5 and 5.0 mm2/s. The viscosity of rooster feather biodiesel is higher than the chicken feather biodiesel as seen in Table 5. In ASTM standard the density of biodiesel ranges between 0.86 and 0.90 gm/cm3. In many studies, it was observed that biodiesel's density has not changed a lot, because densities of methanol and oil are close to the density of the produced biodiesel [14], [15]. Biodiesel from chicken feather has the low density than the rooster feather, as presented in Table 5. Densities of biodiesel will vary with the fatty acid composition and their purity [16].
The Flash Point of chicken feather biodiesel is 120 °C and rooster feather biodiesel is 126 °C. The higher flash points makes storage and transport issues less important [18], [19]. Specific gravity of chicken feather biodiesel is 0.86 and 0.88 of specific gravity in rooster feather biodiesel. This value indicates the effective removal of glycerin [20] Cetane number of chicken feather biodiesel is 50 min lower than rooster feather biodiesel which is 55 min.
In our study, we achieved high feather meal fat content and purified biodiesel at 10 g and 2.5 mL respectively using transesterification method. To the best of our knowledge, it is the first report which used Methanol and NaOH catalyst for biodiesel production and analysis in gas chromotatograph mass specteram from chicken feather meal. In this study has given a basis for further study with large scale production of biodiesel from this waste chicken feather.
5. Conclusion
Biodiesel has become alternative fuel due to its environmental benefits and easily available renewable resources. The present study concluded that the rooster feather fat have enough potential to process them into biodiesel than broiler chicken feather fat because of high energy, fatty acid composition values and important properties of biodiesel. Rooster feather biodiesel is very competitive to the chicken feather biodiesel by the presence of important fatty acids in rooster feather which is suitable to produce more and potential biodiesel. The cost of transesterification processing is low when compared to other process and also the recovery of quality of glycerol. Fat extracted from the feather meal sample was processed into biodiesel by the process of transesterification after the significant reduction of free fatty acid (FFA) by saponification used separator. As well as the quality of obtained biodiesel samples were evaluated according to GC-MS, FTIR and also determined the physical properties of biodiesel which was presented that all the measured parameters results were in acceptable range. Slaughter houses can use these projects to solve their ecological problems by disposal of feather wastes.
Acknowledgement
We are thankful to Department of Biotechnology, Thiruvalluvar University and Fermentation lab, Department of Microbiology, Periyar University for providing lab, equipments for this study.
Acknowledgments
Conflict of interest
The author doesn’t have any conflict of interest.
References
- 1.Abdoli M.A., Mohamadi F., Ghobadian B., Fayyazi E. Effective parameters on biodiesel production from feather fat oil as a cost-effective feedstock. Int. J. Environ. Res. 2014;8(1):139–148. [Google Scholar]
- 2.Abduli M.A., Azimi E. Municipal waste reduction potential and related strategies in Tehran. Int. J. Environ. Res. 2010;4(4):901–912. [Google Scholar]
- 3.Alptekin E., Canakci M., Sanli H. Methyl ester production from chicken fat with high FFA. Fuel. 2011;90:2630–2638. [Google Scholar]
- 4.Bhatti H.N., Hanif M.A., Qasim M., Rehman A. Biodiesel production from waste tallow. Fuel. 2008;87(13–14):2961–2966. [Google Scholar]
- 5.Canakci M., Sanli H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008;35(31):431–434. doi: 10.1007/s10295-008-0337-6. [DOI] [PubMed] [Google Scholar]
- 6.Chicken Fat Supercritical Transesterification, Energy & Fuels, 24(1), 2009, pp. 253–260.
- 7.Dias J.M., Alvim-Ferraz M.C., Almeida M.F. Production of biodiesel from acid waste lard. Bioresour. Technol. 2009;100(24):6355–6361. doi: 10.1016/j.biortech.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Encinar J.M., Snchez N., Martnez G., Garcia L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011;102:10907–10914. doi: 10.1016/j.biortech.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 9.Alptekin Ertan, Canakci Mustafa. Optimization of transesterification for methyl ester production from chicken fat. Fuel. 2011;90:2630–2638. [Google Scholar]
- 10.Fukuda H., Kondo A., Noda H.J. Biodiesel fuel production by transesterification of oils. Biosci. Bioeng. 2001;91:405–416. doi: 10.1263/jbb.92.405. [DOI] [PubMed] [Google Scholar]
- 11.Kondamudi N., Strull J., Misra M., Mohapatra S.K. A green process for producing biodiesel from feather meal. J. Agric. food Chem. 2009;57(14):6163–6166. doi: 10.1021/jf900140e. [DOI] [PubMed] [Google Scholar]
- 12.Lang X., Dalai A.K., Bakshi N.N., Reaney M.J., Hertz P.B. Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001;80:53–62. doi: 10.1016/s0960-8524(01)00051-7. [DOI] [PubMed] [Google Scholar]
- 13.Najafi G., Ghobadian B., Tavakoli T., Buttsworth D.R., Yusaf T., Faizollahnejad M. Performance and exhaust emissions of a gasoline engine with ethanol blended gasoline fuels using artificial neural network. Appl. Energy. 2009;86:630–639. [Google Scholar]
- 14.Graboski M.S., McCormik R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998;24:125–164. [Google Scholar]
- 15.Encinar J.M., Gonzalez J.F., Rodriquez-Reinares A. Biodiesel from used frying variables affecting the yields and Characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005;44:5491–5499. [Google Scholar]
- 16.Tat M.E., Gerpen J.V. The specific gravity of biodiesel and its blends with diesel fuels. JAOCS. 2000;77(2):115–119. [Google Scholar]
- 17.Islam M.N., Islam M.N., Beg M.R.A. The fuel properties of pyrolysis liquid derived from urban solid waste in Bangladesh. Bioresour. Technol. 2004;92(2):181–186. doi: 10.1016/j.biortech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Krawczyk T. Biodiesel alternative fuel makes inroads but hurdles remain. Inform. 1996;7(8):800–815. [Google Scholar]
- 19.Wardle D.A. Global sale of green air travel supported using biodiesel. Renew. Sustain. Energy Rev. 2008;7(1):1–64. [Google Scholar]
- 20.Sharma Y.C., Singh B., Upadhya S.A. Advancements in development and characterization of biodiesl, a review. Fuel. 2008;87(12) (2355-53) [Google Scholar]
- 21.B. Rice, A. Frohlich, R. Leonard, Teagasc, W. Korbitz, Bio-Diesel Production Based on Waste Cooking oil: Promotion of the Establishment of An Industry in Ireland, Altener Contract No. XVII/4.1030/AL/77/95/IRL. Final Report, 1997.
- 22.Bhatti Haq Nawaz, Hanif Muhammad Asif, Faruq Umar, Sheikh Munir Ahmad. Acid and base Catalyzed transesterification of animal fats to biodiesel. Iran. J. Chem. Eng. 2008;27(4):41–48. [Google Scholar]
- 23.Bligh E.G., Dyer W.J. Can. J. Biochem. Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho Ana p., Xavier malcata F. J. Agric. Food Chem. 2005;53:5049. doi: 10.1021/jf048788i. [DOI] [PubMed] [Google Scholar]
- 25.Abdoli M.A., Mohamadi F., Ghobadian B., Fayyazi E. Effective parameters on biodiesel production from feather fat oil as a cost-effective feedstock. Int. J. Environ. Res. 2014;8(1):139–148. [Google Scholar]
- 26.Amber Aleem, Fakhra Aslam, Ammara Shahid. Quantification of fat in chicken's feather meal for its conversion into biodiesel. Int. Res. J. Environ. Sci. 2014;3(6):67–74. [Google Scholar]