Summary
Background/Objective
T1ρ and T2 relaxation mapping in knee cartilage have been used extensively at 3 Tesla (T) as markers for proteoglycan and collagen, respectively. The objective of this study was to evaluate the feasibility of T1ρ and T2 imaging of knee cartilage at 7T in comparison to 3T and to evaluate the ability of T1ρ and T2 to determine differences between normal and osteoarthritis (OA) patients.
Materials and methods
Twenty patients, seven healthy patients (Kellgren–Lawrence = 0), and 13 patients with signs of radiographic OA (Kellgren–Lawrence > 0) were scanned at 3T and 7T. The knee cartilage was segmented into six compartments and the T1ρ and T2 values were fit using a two-parameter model. Additionally, patients were stratified by the presence of cartilage lesions using the modified Whole Organ Magnetic Resonance Imaging Score classification of the knee. One-way analysis of variance was used to compare the healthy and OA groups at 3T and 7T. The specific absorption ratio was kept under Food and Drug Administration limits during all scans.
Results
T1ρ and T2 values at 3T and 7T were significantly higher in the lateral femoral condyle and patella in patients with OA. However, more regions were significant or approached significance at 7T compared with 3T, with the differences between healthy and OA patients also larger at 7T. The signal to noise ratio across all cartilage and meniscus compartments was 60% higher on average at 7T compared to 3T.
Conclusion
T1ρ imaging at 7T has been established as a viable imaging method for the differentiation of degenerated cartilage despite previous concerns over specific absorption rate and imaging time. The potential increased sensitivity of T1ρ and T2 imaging at 7T may be useful for future studies in the development of OA.
Keywords: cartilage, T1ρ, T2, ultra high field imaging
Introduction
Magnetic resonance imaging (MRI) is widely used for cartilage imaging due to its soft tissue contrast and wide array of imaging markers for cartilage integrity. T2 relaxation values have been used extensively to investigate early cartilage degeneration [1], [2], [3], [4], [5], with T2 being correlated with changes in water content, collagen anisotropy, and concentration [6], [7], and to a lesser extent proteoglycan content [8], [9]. T1ρ relaxation values tend to increase in degenerated cartilage [10], [11], [12], [13] and have been associated with the proteoglycan content of cartilage [14], [15], [16] and to a lesser extent collagen [17], [18].
While T1ρ and T2 imaging have primarily been performed on 3 Tesla (3T) MR scanners, there has been increasing interest in 7 Tesla (7T) imaging of the knee, typically due to increased signal-to-noise ratio (SNR) at 7T. Many 7T studies have focused on sodium imaging [19], [20], chemical exchange saturation transfer [21], [22], or high resolution morphological imaging [23], [24]. Mlynarik et al [25] performed an in vitro comparison of T1ρ and T2 at 3T and 7T. In vivo T2 mapping of the knee at 7T has been performed by Welsch et al [26] and Chang et al [27], but only a limited number of studies have been performed for T1ρ at 7T. These include a study by Kogan et al [28] that investigated T1ρ dispersion at 7T, as well as a study by Singh et al [29] where in vivo imaging of T1ρ was performed at 3T and 7T. Unfortunately, all the quantitative studies performed at 7T have focused on healthy volunteers, with no studies focused on patients with osteoarthritis (OA) or cartilage damage.
In this study, healthy controls and patients with knee OA were scanned at 3T and 7T and the T1ρ and T2 relaxation values between the two groups were compared to determine possible differences between field strengths. The objective of this study was therefore to evaluate the feasibility of T1ρ and T2 imaging of knee cartilage at 7T in comparison with 3T and to determine the ability of T1p and T2 obtained at 3T and 7T to determine differences between normal and OA patients.
Materials and methods
Participant recruitment
Twenty volunteers (11 women, nine men), ranging in age from 37 years to 72 years, were recruited under an Institutional Review Board approved protocol. All participants underwent weight-bearing postero-anterior fixed flexion radiograph using the SynaFlexer device (Synarc, Newark, CA, USA). A musculoskeletal radiologist with more than 20 years' experience performed Kellgren–Lawrence (KL) grading [30] of the tibio-femoral compartment using these radiographs. Seven of the volunteers were healthy controls (KL = 0) and the remaining 13 volunteers had OA (KL = 2, 3). The inclusion criteria for OA patients were age >35 years, knee pain, aching, or stiffness on most days per month during the past year, or use of medication for knee pain on most days per month during the past year, and definite radiographic evidence of knee OA (KL > 1). The inclusion criteria for controls were age >35 years, no knee pain or stiffness in either knee or use of medications for knee pain in the last year, and no radiographic evidence of OA (KL ≤ 1) on either knee. The exclusion criteria for all participants were: (1) concurrent use of an investigational drug; (2) history of fracture or surgical intervention in the study knee; and (3) contraindications to MRI. All participants signed a written informed consent approved by the University of California, San Francisco Committee on Human Research.
Imaging protocol
Each volunteer underwent a single knee scan on 3T (GE MR750w; GE Healthcare, Waukesha, WI, USA) and 7T (GE MR950, GE Healthcare) MR scanners. Scans were performed within 3 months of each other, so little progression of disease should have occurred between scans. An eight-channel phased array knee coil (In Vivo, Gainesville, FL, USA) was used for the 3T scans while a 28-channel phased array knee coil (Quality Electrodynamics, Mayfield Village, OH, USA) was used for the 7T scans. Two participants were scanned twice at 7T to assess reproducibility of the T1ρ and T2 imaging methods.
For each participant, three-dimensional (3D) fast-spin-echo (FSE)-CUBE, T1ρ, and T2 images were acquired at both 3T and 7T. At 7T, B1 maps were acquired using a modified version of the Bloch–Siegert method [31]. The images for the B1 maps were acquired with TR = 350 ms, TE = 10 ms, flip angle = 10°, BW = 31.25 kHz, slice thickness = 3 mm, and 64 × 64 matrix. The T1ρ and T2 images were acquired with the 3D magnetisation prepared angle modulated partitioned k-space (MAPSS) acquisition [32]. The MAPSS sequence was acquired with the following parameters: field of view = 14 cm, 256 × 128 matrix, slice thickness = 4 mm, 28–32 slices, spin-lock pulse times (TSL) = 0 ms, 2 ms, 4 ms, 8 ms, 12 ms, 20 ms, 40 ms, 80 ms, spin lock frequency = 500 Hz, echo times (TE) = 0 ms, 1.6 ms, 3.2 ms, 6.5 ms, 12.9 ms, 25.9 ms, 38.8 ms, 51.7 ms, and repetition time (TR)/TE = 5.2/2.9 ms. However, 7T scans were acquired with TSL = 0 ms, 2 ms, 4 ms, 8 ms, 12 ms, 20 ms, 40 ms, 60 ms and TE = 0 ms, 3.4 ms, 6.8 ms,10.3 ms, 20.5 ms, 34.2 ms, 47.8 ms, 61.5 ms to reduce the duty cycle of the radio frequency (RF) amplifier at 7T. Additionally, the slice thickness at 7T was 3 mm to match a previous study. Composite tip-down and tip-up RF pulses were used to compensate for B0 and B1 inhomogeneities [33], [34]. The composite pulses consisted of a 90° hard pulse along the x-axis and a 135° hard pulse along the y-axis.
Image analysis
The 3T 3D FSE images were graded using a modified Whole Organ Magnetic Resonance Imaging Score (WORMS) classification of the knee [35], [36]. The participants were then separated by the WORMS cartilage score in each compartment. Participants with WORMS > 1 were considered as cartilage lesion positive (CL+) for that compartment and those with WORMS ≤ 1 were cartilage lesion negative (CL−).
The 3D FSE images were rigidly registered to the T1ρ images using the VTK CISG Registration Toolkit (Kitware Inc., Clifton Park, NY, USA). Additionally, the individual T1ρ and T2 echoes were rigidly registered to the first T1ρ echo (TSL = 0) to remove any movement between echoes. The cartilage was then semiautomatically segmented into six compartments on the high resolution 3D FSE images using in-house software and adjusted as necessary on the TSL = 0 image of the T1ρ sequence. Six compartments were segmented, including the lateral femoral condyle (LFC), the lateral tibial condyle, the medial femoral condyle, the medial tibial condyle, the patella, and the trochlea (TRO).
T1ρ and T2 relaxation maps were generated using a two-parameter nonlinear monoexponential fit of the signal in each pixel over all TSL or TE times.
SNR ratio was calculated for each participant at 3T and 7T using the difference method described by Dietrich et al [37]. The TSL = 0 ms T1ρ image and the TE = 0 T2 image for each individual were subtracted from each other (since they have the same preparation) and the standard deviation of one slice in each of the cartilage compartments was calculated. The SNR was then calculated using the following equation:
The difference method was used to compensate for the noise variation present due to the use of parallel acceleration.
Statistical analysis
A multivariate one-way analysis of variance was performed between the T1ρ or T2 values of the healthy controls and patients with OA for each compartment. Age and body mass index (BMI) were adjusted for when significantly different between populations. Similar statistical analysis was performed using the CL− and CL+ groups in each knee cartilage compartment. However, because age and BMI were not significantly different between the groups differentiated by CL, they were not adjusted for in the analysis of variance. A p value < 0.05 was considered significant for each comparison. In addition to calculating the p values, the effect size of each comparison was calculated to determine sensitivity of each field strength to changes in cartilage degeneration.
Results
Participant characteristics
The age, BMI, sex, and KL score of the healthy volunteers and the knee OA populations are shown in Table 1. One participant was not considered in the results due to excessive motion in the 7T scans. There were significant differences in BMI between the control and OA groups. However, there were no significant differences in age or BMI between any of the CL− and CL+ groups.
Table 1.
Age, body mass index, Kellgren–Lawrence, sex, and knee side for all patient groups in the study. + and − indicate the presence and absence of cartilage lesions in that compartment.
| CNT | OA | LFC− | LFC+ | MFC− | MFC+ | LT− | LT+ | MT− | MT+ | PAT− | PAT+ | TRO− | TRO+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 50.1 ± 9.5 | 58.5 ± 8.8 | 55.6 ± 10.6 | 54.3 ± 3.5 | 57.0 ± 10.7 | 51.0 ± 4.6 | 54.0 ± 10.3 | 57.9 ± 8.8 | 55.9 ± 10.4 | 52.7 ± 5.0 | 56.6 ± 10.6 | 55.0 ± 9.8 | 56.6 ± 8.9 | 52.0 ± 12.2 |
| p | 0.070 | 0.840 | 0.248 | 0.421 | 0.608 | 0.762 | 0.375 | |||||||
| BMI | 21.7 ± 2.1 | 25.1 ± 3.0 | 24.1 ± 3.4 | 22.6 ± 1.5 | 23.5 ± 3.6 | 24.8 ± 1.4 | 24.6 ± 3.2 | 22.6 ± 2.9 | 23.6 ± 3.4 | 25.2 ± 1.2 | 25.2 ± 3.1 | 23.4 ± 3.1 | 23.6 ± 3.3 | 24.6 ± 2.8 |
| p | 0.017 | 0.455 | 0.437 | 0.180 | 0.448 | 0.266 | 0.633 | |||||||
| Sex | ||||||||||||||
| Male | 2 | 7 | 8 | 1 | 5 | 4 | 7 | 2 | 7 | 2 | 3 | 6 | 6 | 3 |
| Female | 5 | 5 | 8 | 2 | 9 | 1 | 5 | 5 | 9 | 1 | 2 | 8 | 8 | 2 |
| KL | ||||||||||||||
| 0 | 7 | 0 | 6 | 1 | 5 | 2 | 6 | 1 | 7 | 0 | 1 | 6 | 5 | 2 |
| 1 | 0 | 6 | 6 | 0 | 6 | 0 | 4 | 2 | 6 | 0 | 2 | 4 | 4 | 2 |
| 2 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 0 |
| 3 | 0 | 4 | 3 | 1 | 2 | 2 | 1 | 3 | 2 | 2 | 2 | 2 | 3 | 1 |
| Knee side | ||||||||||||||
| Right | 4 | 4 | 10 | 1 | 5 | 3 | 5 | 3 | 7 | 1 | 3 | 5 | 6 | 2 |
| Left | 3 | 8 | 6 | 2 | 9 | 2 | 7 | 4 | 9 | 2 | 2 | 9 | 8 | 3 |
BMI = body mass index; CNT = control; KL = Kellgren–Lawrence; LFC = lateral femoral condyle; LT = lateral tibial; MFC = medial femoral condyle; MT = medial tibial; OA = osteoarthritis; PAT = patella; TRO = trochlea.
Comparison between healthy and radiographic OA groups
The mean cartilage T1ρ and T2 values for the groups separated by KL score are shown in Table 2 for the 3T scans. T1ρ and T2 values were significantly higher in OA patients in the patella, while no other regions were significantly different. The T1ρ and T2 values at 7T are shown in Table 3. While no significant differences were found between control and radiographic OA patients at 7T, the T1ρ in the LFC and patella were higher and were approaching significance. Additionally, the higher T2 of the TRO in OA patients was also approaching significance.
Table 2.
3 Tesla T1ρ and T2 relaxation values for the healthy control, osteoarthritis, cartilage lesion positive (+), and cartilage lesion negative (−) groups for the six segmented cartilage compartments used in the study.
| LFC | MFC | LT | MT | PAT | TRO | Average | ||
|---|---|---|---|---|---|---|---|---|
| T1p | CNT | 38.8 ± 3.0 | 41.0 ± 3.7 | 37.3 ± 5.0 | 36.6 ± 4.8 | 44.0 ± 3.0 | 44.7 ± 4.5 | |
| OA | 39.7 ± 3.4 | 42.0 ± 3.4 | 38.2 ± 2.6 | 37.0 ± 2.5 | 46.6 ± 3.7 | 42.9 ± 2.1 | ||
| p | 0.365 | 0.198 | 0.738 | 0.969 | 0.001 | 0.321 | ||
| Effect size | 0.118 | 0.183 | 0.037 | 0.004 | 0.590 | 0.132 | 0.178 | |
| CL− | 38.9 ± 2.7 | 41.4 ± 4.0 | 37.8 ± 3.8 | 37.1 ± 3.5 | 45.6 ± 2.8 | 43.7 ± 3.3 | ||
| CL+ | 43.5 ± 5.1 | 42.4 ± 1.2 | 38.5 ± 2.2 | 34.8 ± 0.6 | 45.8 ± 4.8 | 42.0 ± 3.3 | ||
| p | 0.049 | 0.6 | 0.76 | 0.377 | 0.89 | 0.477 | ||
| Effect size | 0.209 | 0.017 | 0.006 | 0.046 | 0.001 | 0.030 | 0.051 | |
| T2 | CNT | 28.9 ± 2.2 | 30.3 ± 3.1 | 27.0 ± 3.1 | 27.9 ± 4.6 | 30.8 ± 2.1 | 33.4 ± 2.7 | |
| OA | 29.7 ± 3.3 | 30.8 ± 2.4 | 27.5 ± 1.8 | 28.4 ± 2.6 | 32.1 ± 2.5 | 32.2 ± 2.3 | ||
| p | 0.532 | 0.256 | 0.8 | 0.945 | 0.04 | 0.195 | ||
| Effect size | 0.081 | 0.157 | 0.029 | 0.007 | 0.332 | 0.185 | 0.132 | |
| CL− | 28.9 ± 2.4 | 30.8 ± 2.9 | 27.1 ± 2.3 | 28.2 ± 3.5 | 31.8 ± 2.2 | 32.8 ± 2.6 | ||
| CL+ | 33.5 ± 4.2 | 30.1 ± 1.8 | 28.7 ± 1.4 | 28.2 ± 2.5 | 31.3 ± 2.8 | 31.9 ± 1.3 | ||
| p | 0.029 | 0.618 | 0.262 | 0.984 | 0.685 | 0.645 | ||
| Effect size | 0.264 | 0.015 | 0.078 | 0.000 | 0.010 | 0.013 | 0.063 |
Data are presented as the mean ± standard deviation.
CL = cartilage lesion; CNT = control; LFC = lateral femoral condyle; MFC = medial femoral condyle; OA = osteoarthritis; PAT = patella; TRO = trochlea.
Table 3.
7T T1ρ and T2 relaxation values for the healthy control, osteoarthritis, cartilage lesion positive (+), and cartilage lesion negative (−) groups for the six segmented cartilage compartments used in the study.
| LFC | MFC | LT | MT | PAT | TRO | Average | ||
|---|---|---|---|---|---|---|---|---|
| T1p | CNT | 40.2 ± 2.9 | 41.6 ± 3.9 | 38.9 ± 5.5 | 37.9 ± 4.6 | 42.3 ± 3.5 | 42.0 ± 5.7 | |
| OA | 44.4 ± 3.7 | 43.5 ± 3.8 | 42.0 ± 5.4 | 37.3 ± 4.3 | 50.5 ± 6.7 | 47.5 ± 5.7 | ||
| p | 0.0707 | 0.286 | 0.434 | 0.864 | 0.0551 | 0.148 | ||
| Effect size | 0.298 | 0.145 | 0.105 | 0.018 | 0.339 | 0.225 | 0.188 | |
| CL− | 42.1 ± 3.5 | 42.3 ± 3.9 | 40.4 ± 5.8 | 37.4 ± 4.5 | 47.2 ± 7.0 | 45.7 ± 6.1 | ||
| CL+ | 48.2 ± 2.1 | 44.2 ± 3.8 | 42.9 ± 4.1 | 38.7 ± 1.7 | 48.0 ± 7.3 | 42.5 ± 1.7 | ||
| p | 0.0305 | 0.374 | 0.48 | 0.691 | 0.819 | 0.506 | ||
| Effect size | 0.260 | 0.047 | 0.032 | 0.010 | 0.004 | 0.028 | 0.063 | |
| T2 | CNT | 28.0 ± 2.0 | 28.1 ± 2.5 | 27.3 ± 3.8 | 27.4 ± 2.8 | 28.0 ± 2.6 | 28.1 ± 2.3 | |
| OA | 31.0 ± 2.7 | 31.0 ± 3.8 | 29.4 ± 3.9 | 29.1 ± 4.0 | 31.0 ± 3.9 | 30.8 ± 2.7 | ||
| p | 0.0616 | 0.149 | 0.52 | 0.341 | 0.209 | 0.0638 | ||
| Effect size | 0.294 | 0.212 | 0.079 | 0.126 | 0.178 | 0.291 | 0.196 | |
| CL− | 29.4 ± 2.4 | 29.1 ± 2.9 | 28.3 ± 4.1 | 28.3 ± 3.8 | 29.4 ± 3.7 | 30.0 ± 2.9 | ||
| CL+ | 34.8 ± 1.9 | 32.2 ± 4.7 | 30.4 ± 2.8 | 29.7 ± 2.4 | 30.5 ± 3.8 | 28.3 ± 1.0 | ||
| p | 0.00682 | 0.107 | 0.419 | 0.636 | 0.549 | 0.434 | ||
| Effect size | 0.358 | 0.146 | 0.039 | 0.013 | 0.021 | 0.036 | 0.102 |
Data are presented as mean ± standard deviation.
CL = cartilage lesion; CNT = control; LFC = lateral femoral condyle; LT = lateral tibial; MFC = medial femoral condyle; MT = medial tibial; OA = osteoarthritis; PAT = patella; TRO = trochlea.
Comparison between healthy and CL groups
The T1ρ and T2 values for participants differentiated by WORMS score in each compartment are shown in Table 2 for 3T. T1ρ and T2 values were significantly higher in patients with CL in the LFC, while no other regions were significantly different. When examining the 7T values, shown in Table 3, the T1ρ and T2 values were also significantly higher in patients with cartilage lesions in the LFC. However, the average effect size for the 7T T1ρ and T2 values were higher than the average effect size at 3T. The same effect size increase at 7T was seen with radiographic OA comparisons.
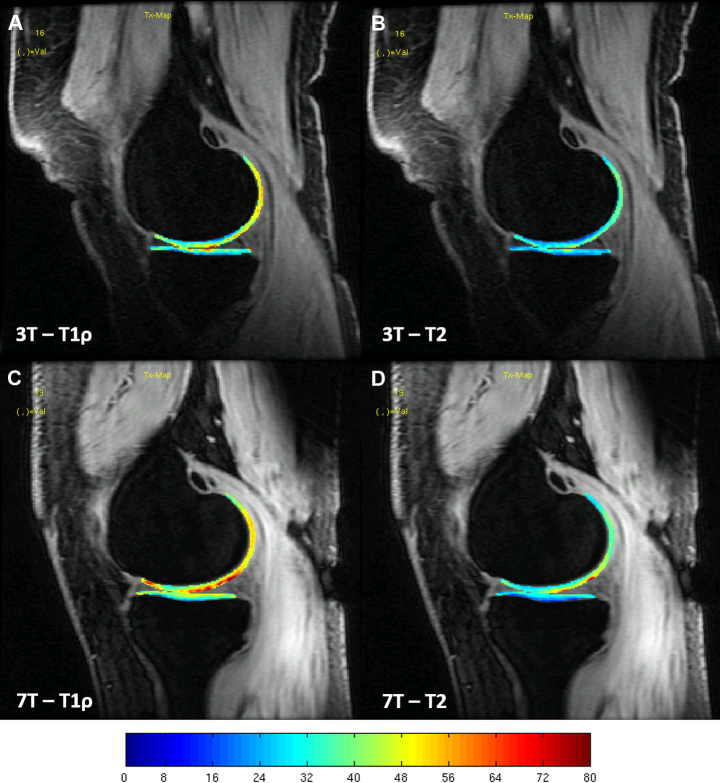
Example images of the T1ρ and T2 maps overlaid on the TSL = 0 image for the knee of a patient with WORMS = 3 in the medial femoral condyle are shown in Figure 1. These maps show extensive spatial similarities present between 3T and 7T T1ρ and T2 values for the same volunteers.
Figure 1.
T1ρ relaxation colour maps for the cartilage of the right knee of a healthy volunteer: (A) 3 Tesla (T) lateral; (B) 7T lateral; (C) 3T medial; and (D) 7T medial. The colour maps have the same scale, which is displayed in milliseconds. 3T = 3 Tesla; 7T = 7 Tesla.
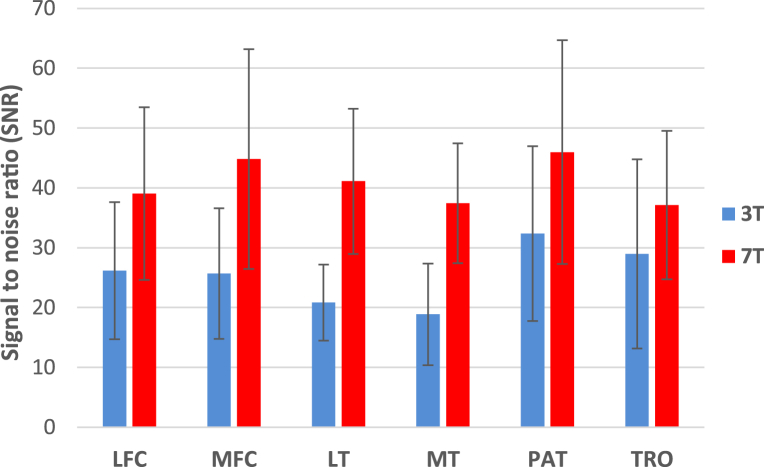
The SNR in the six cartilage compartments at 3T and 7T is shown in Figure 2. The SNR at 7T was higher than that at 3T for all cartilage compartments, without consideration for the differences in slice thickness, but the differences were not significant. On average, the SNR at 7T was 60% higher than the SNR at 3T.
Figure 2.
Signal to noise ratio at the six cartilage compartments at 3 Tesla and 7 Tesla. LFC = lateral femoral condyle; LT = lateral tibial; MFC = medial femoral condyle; MT = medial tibial; PAT = patella; TRO = trochlea; 3T = 3 Tesla; 7T = 7 Tesla.
The correlation coefficients between the 3T and 7T T1ρ and T2 values in each cartilage compartment are shown in Table 4. Significant correlations were found for T1ρ in every compartment except the medial tibia. However, significant correlations were only found in the medial tibia for the T2 values.
Table 4.
Correlation between 3 Tesla and 7 Tesla relaxation values and their p values.
| LFC | MFC | LT | MT | PAT | TRO | ||
|---|---|---|---|---|---|---|---|
| T1p | Correlation | 0.620 | 0.682 | 0.579 | 0.304 | 0.569 | 0.494 |
| p | 0.006 | 0.001 | 0.012 | 0.207 | 0.017 | 0.037 | |
| T2 | Correlation | 0.439 | 0.400 | 0.360 | 0.496 | 0.279 | 0.265 |
| p | 0.069 | 0.090 | 0.142 | 0.031 | 0.247 | 0.273 |
LT = lateral tibial; LFC = lateral femoral condyle; MFC = medial femoral condyle; MT = medial tibial; PAT = patella; TRO = trochlea.
The coefficients of variation of T1ρ and T2 at 7T averaged across all six cartilage compartments were approximately 6.5% and 6.7%, respectively.
Discussion
In this study, we have demonstrated the feasibility of T1ρ and T2 mapping of knee cartilage at 7T and compared the values to 3T in patients with knee OA. T1ρ and T2 mapping at higher field strengths benefits from increased SNR as well as increased chemical shifts. These chemical shifts can result in increased chemical exchange, which should result in increased sensitivity to changes in the concentration of macromolecules. There has been a limited number of in vivo cartilage studies at 7T, with all of them focused on healthy volunteers with minimal cartilage degeneration. To our knowledge, this is the first in vivo study of patients with OA.
While the implementation of T1ρ mapping at 7T has typically met resistance due to the large amounts of RF energy required by the spin lock pulse, T1ρ, and T2 imaging at 7T has potential advantages over 3T imaging. First, 7T imaging typically provides increased SNR due to the increase in magnetisation. Secondly, the sensitivity of T1ρ and T2 relaxation times to changes in macromolecular protein and water concentration should be increased at 7T, due to an increase in the chemical exchange component of the relaxation mechanisms. The chemical exchange component of T1ρ and T2 are defined from the following equations [38], [39]:
In which pa and pb are the concentrations of the two interactions groups (EX water and proteoglycan), k is the chemical exchange rate constant, δ is the chemical shift between groups a and b, τCPMG is the time between 180° pulses in the Carr–Purcell–Meiboom–Gill train, and ω1 is the spin lock frequency. For both relaxation times, the chemical exchange component increases with increases in the field strength, which is related to the chemical shift (δ). The increase in this component should create larger changes for similar changes in the macromolecule concentrations (pa and pb). This increased sensitivity could improve the ability of T1ρ and T2 imaging to identify early degenerative changes in cartilage during the development of OA at higher field strengths. However, more studies are needed with patients with OA and cartilage damage to determine the effects.
As shown in Table 2, T1ρ and T2 relaxation values at 3T were higher for patients with radiographic OA as well as patients with cartilage lesions, which have been shown in previous studies, especially at 3T. However, the differences were only significant in the patella for patients with radiographic OA and the LFC for patients with cartilage lesions. Similar trends were seen with 7T relaxation times, showing higher values with radiographic OA and WORMS scores. The same significant differences were seen in the LFC, but the differences were not significant in the patella. However, several regions (LFC, patella, and TRO) approached significance and in general the p values were lower at 7T. Also, the effect size was slightly higher at the 7T for all comparisons, which is partially due to increased differences between healthy and OA populations at 7T compared to 3T. First of all, these results suggest that T1ρ and T2 relaxation times at 7T can detect differences in cartilage degeneration similar to those at 3T. While slightly less significant differences were found at 7T, it can be argued that the larger magnitude differences and lowered p values at 7T are the result of increased sensitivity, especially when comparing effect size. The increased differences at 7T are most likely due to an increase of the chemical exchange, which increases sensitivity to changes in the concentration of proteoglycan or collagen.
When analysing the correlations in Table 4, moderate correlations were seen when the T1ρ and T2 values were compared from 3T to 7T. However, only the majority of the T1ρ correlation values were significant, with little significance for the T2 correlations. A previous study has shown correlations between T1ρ and T2 at 3T10, which is expected due to the similar relaxation mechanisms between T1ρ and T2. The correlations were weaker at 7T, but this is most likely due to B1 and B0 inhomogeneities, which created signal inhomogeneities in some of the images. However, the disparity in correlation could also be due to the different changes in chemical exchange that occurs for T1ρ and T2 at 7T compared to 3T.
Magnetic field (B0) and RF magnetic field (B1) inhomogeneities can increase substantially at 7T and can affect the quantification of T1p and T2 relaxation times. Composite pulses were used to alleviate these issues for T1ρ and T2 imaging, reducing the occurrence of banding in the relaxation maps. However, despite possible inhomogeneities, the T1ρ and T2 maps at 7T were similar to those at 3T and similar laminar behaviour was also observed, as shown in Figure 1.
As shown in Figure 2, the SNR of the relaxation weighted images at 7T were significantly higher than the 3T images. The increase is due to the inherent increased signal at higher field strengths, which has also been shown in previous studies. While some studies have shown much larger increases in SNR at 7T compared to 3T [23], work is still being performed to optimize the SNR performance of the coil used in the study on the 7T system. With more testing we expect larger increases in the SNR that will be more in line with the increases expected from increasing field strength and a larger number of coil elements.
The reproducibility variations of the 7T T1ρ and T2 imaging protocol were reasonable, but are slightly increased when compared to values found at 3T with previous cartilage studies [32], [40].
Increases in the specific absorption rate (SAR) have been an on-going concern for sequence development at 7T. It is one of the main reasons for the lack of 7T T1ρ development, since the spin lock pulses are high energy RF pulses and SAR is already a restraint on 3T systems. However, the SAR limit was never reached during any of the scans performed for this study at 3T or 7T. This can be attributed to the implementation of the MAPSS sequence, with its long T1 recovery time between segments (1200 ms for all scans), which allows for less spin lock pulses and a large span of time between them. Additionally, a TSL time of 60 ms and an effective TE time of approximately 60 ms were achieved in vivo at 7T, which should allow for enough range to accurately measure elevated T1ρ and T2 values in patients with cartilage damage.
There are some limitations to this study, including the small number of volunteers. While more participants could have provided more power to the statistical tests, the focus of this study was to evaluate the feasibility of T1ρ and T2 imaging at 7T in individuals with OA, where even with the small cohort significant differences between T1ρ and T2 values at 3T and 7T were found. In addition, part of the study was intended to examine whether 7T imaging could provide improved detection of changes in smaller cohorts, to facilitate the use of 7T to reduce overall study size. In terms of the imaging, the TSL and TE parameters were slightly different between 3T and 7T, which was only to alleviate hardware constraints on the 7T scanner. However, the changes mostly affected the last echo and the last echo time was still longer than the measured T1ρ and T2 values, so the changes most likely had little effect on the relaxation measurements. Additionally, only the correlations were performed between 3T and 7T values, so any variations from scan parameters should be similar between groups in each cohort at each field strength. Lastly, different coils were used on the two scanners, with the 7T coil having many more channels than the 3T coil. This can result in differences in B1 homogeneity and SNR, which can influence results. While having near identical coils would be ideal, smaller arrays at 7T are not feasible at this time.
In conclusion, T1ρ and T2 imaging at 7T have been established as viable imaging for the detection of degeneration of cartilage in knee OA despite previous concerns over SAR and imaging time. Additionally, 7T relaxation values had slightly more significant differences when compared to 3T values when evaluating the differences between healthy and degenerated cartilage in vivo.
Conflicts of interest
The authors have nothing to disclose.
Funding/support
This work was supported by NIH/NIAMS grants P50AR060752, P0046859, R01AR046905, 1F32AR062964-01A1, a UCSF Department of Radiology and Biomedical Imaging seed grant, and a UCSF REAC Pilot for Junior Investigators in Basic and Clinical/Translational Sciences.
Acknowledgements
We thank Melissa Guan and Mary McPolin for assistance in recruiting and scanning the volunteers.
References
- 1.Bachmann G.F., Basad E., Rauber K., Damian M.S., Rau W.S. Degenerative joint disease on MRI and physical activity: a clinical study of the knee joint in 320 patients. Eur Radiol. 1999;9:145–152. doi: 10.1007/s003300050646. [DOI] [PubMed] [Google Scholar]
- 2.Blumenkrantz G., Lindsey C.T., Dunn T.C., Jin H., Ries M.D., Link T.M. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:997–1005. doi: 10.1016/j.joca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Regatte R.R., Akella S.V., Lonner J.H., Kneeland J.B., Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 4.David-Vaudey E., Ghosh S., Ries M., Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 5.Dunn T.C., Lu Y., Jin H., Ries M.D., Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin D.W., Wadghiri Y.Z., Zhu H., Vinton C.J., Smith E.D., Dunn J.F. Macroscopic structure of articular cartilage of the tibial plateau: Influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y., Moody J.B., Burton-Wurster N., Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 8.Wayne J.S., Kraft K.A., Shields K.J., Yin C., Owen J.R., Disler D.G. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology. 2003;228:493–499. doi: 10.1148/radiol.2282012012. [DOI] [PubMed] [Google Scholar]
- 9.Menezes N.M., Gray M.L., Hartke J.R., Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Benjamin Ma C., Link T.M. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Han E.T., Ma C.B., Link T.M., Newitt D.C., Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 12.Regatte R.R., Akella S.V., Wheaton A.J., Lech G., Borthakur A., Kneeland J.B. 3D-T1rho-relaxation mapping of articular cartilage: In vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Stahl R., Luke A., Li X., Carballido-Gamio J., Ma C.B., Majumdar S. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients – a 3.0-tesla MRI study. Eur Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 14.Akella S.V., Regatte R.R., Gougoutas A.J., Borthakur A., Shapiro E.M., Kneeland J.B. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 15.Duvvuri U., Reddy R., Patel S.D., Kaufman J.H., Kneeland J.B., Leigh J.S. T1rho–relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 16.Regatte R.R., Akella S.V., Borthakur A., Kneeland J.B., Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 17.Keenan K.E., Besier T.F., Pauly J.M., Han E., Rosenberg J., Lane Smith R. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C.S., Yan C.H., Gong N.J., Li T., Chan Q., Chu Y.C. Imaging biomarker with T1rho and T2 mappings in osteoarthritis—in vivo human articular cartilage study. Eur J Radiol. 2013;82:647–650. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Staroswiecki E., Bangerter N.K., Gurney P.T., Grafendorfer T., Gold G.E., Hargreaves B.A. In vivo sodium imaging of human patellar cartilage with a 3D cones sequence at 3 T and 7 T. J Magn Reson Imaging. 2010;32:446–451. doi: 10.1002/jmri.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., Wu Y., Chang G., Oesingmann N., Schweitzer M.E., Jerschow A. Rapid isotropic 3D-sodium MRI of the knee joint in vivo at 7T. J Magn Reson Imaging. 2009;30:606–614. doi: 10.1002/jmri.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A., Haris M., Cai K., Kassey V.B., Kogan F., Reddy D. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012;68:588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt B., Zbyn S., Stelzeneder D., Jellus V., Paul D., Lauer L. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)na MR imaging at 7 T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 23.Regatte R.R., Schweitzer M.E. Ultra-high-field MRI of the musculoskeletal system at 7.0T. J Magn Reson Imaging. 2007;25:262–269. doi: 10.1002/jmri.20814. [DOI] [PubMed] [Google Scholar]
- 24.Krug R., Carballido-Gamio J., Banerjee S., Stahl R., Carvajal L., Xu D. In vivo bone and cartilage MRI using fully-balanced steady-state free-precession at 7 tesla. Magn Reson Med. 2007;58:1294–1298. doi: 10.1002/mrm.21429. [DOI] [PubMed] [Google Scholar]
- 25.Mlynarik V., Szomolanyi P., Toffanin R., Vittur F., Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169:300–307. doi: 10.1016/j.jmr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Welsch G.H., Apprich S., Zbyn S., Mamisch T.C., Mlynarik V., Scheffler K. Biochemical (T2, T2* and magnetisation transfer ratio) MRI of knee cartilage: feasibility at ultra–high field (7T) compared with high field (3T) strength. Eur Radiol. 2011;21:1136–1143. doi: 10.1007/s00330-010-2029-7. [DOI] [PubMed] [Google Scholar]
- 27.Chang G., Wiggins G.C., Xia D., Lattanzi R., Madelin G., Raya J.G. Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7T. J Magn Reson Imaging. 2012;35:441–448. doi: 10.1002/jmri.23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogan F., Singh A., Cai K., Haris M., Hariharan H., Reddy R. Investigation of chemical exchange at intermediate exchange rates using a combination of chemical exchange saturation transfer (CEST) and spin-locking methods (CESTrho) Magn Reson Med. 2012;68:107–119. doi: 10.1002/mrm.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A., Haris M., Cai K., Kogan F., Hariharan H., Reddy R. High resolution T1rho mapping of in vivo human knee cartilage at 7T. PLoS One. 2014;9:e97486. doi: 10.1371/journal.pone.0097486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo–arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalighi M.M., Rutt B.K., Kerr A.B. RF pulse optimization for Bloch–Siegert B (1)(+) mapping. Magn Reson Med. 2012;68:857–862. doi: 10.1002/mrm.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Han E.T., Busse R.F., Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon W.T., Oshinski J.N., Trudeau J.D., Arnold B.C., Pettigrew R.I. Myocardial suppression in vivo by spin locking with composite pulses. Magn Reson Med. 1996;36:90–94. doi: 10.1002/mrm.1910360116. [DOI] [PubMed] [Google Scholar]
- 34.Chen W., Takahashi A., Han E. Quantitative T(1)(rho) imaging using phase cycling for B0 and B1 field inhomogeneity compensation. Magn Reson Imaging. 2011;29:608–619. doi: 10.1016/j.mri.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Peterfy C.G., Guermazi A., Zaim S., Tirman P.F., Miaux Y., White D. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Stehling C., Lane N.E., Nevitt M.C., Lynch J., McCulloch C.E., Link T.M. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non–symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietrich O., Raya J.G., Reeder S.B., Reiser M.F., Schoenberg S.O. Measurement of signal-to-noise ratios in MR images: Influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J.H., Gore J.C., Armitage I.M. Relative contributions of chemical exchange and other relaxation mechanisms in protein solutions and tissues. Magn Reson Med. 1989;11:295–308. doi: 10.1002/mrm.1910110304. [DOI] [PubMed] [Google Scholar]
- 39.Trott O., Palmer A.G., 3rd R1rho relaxation outside of the fast-exchange limit. J Magn Reson. 2002;154:157–160. doi: 10.1006/jmre.2001.2466. [DOI] [PubMed] [Google Scholar]
- 40.Pakin S.K., Xu J., Schweitzer M.E., Regatte R.R. Rapid 3D-T1rho mapping of the knee joint at 3.0T with parallel imaging. Magn Reson Med. 2006;56:563–571. doi: 10.1002/mrm.20982. [DOI] [PubMed] [Google Scholar]