Abstract
Proteostasis alterations are proposed as a transversal hallmark of several pathological conditions, including metabolic disorders, mechanical injury, cardiac malfunction, neurodegeneration, and cancer. Strategies to improve proteostasis aim to reduce the accumulation of specific disease-related misfolded proteins or bolster the endogenous mechanisms to fold and degrade abnormal proteins. Endoplasmic reticulum (ER) stress is a common pathological signature of a variety of diseases, which engages the unfolded protein response (UPR) as a cellular reaction to mitigate ER stress. Pharmacological modulation of the UPR is challenging considering the physiological importance of the pathway in various organs. However, local targeting of ER stress responses in the affected tissue using gene therapy is emerging as a possible solution to overcome side effects. The delivery of ER chaperones or active UPR components using adeno-associated virus (AAV) has demonstrated outstanding beneficial effects in several disease models (e.g., neurodegenerative conditions, eye disorders, and metabolic diseases). Here, we discuss current efforts to design and optimize gene therapy strategies to improve ER proteostasis in different disease contexts.
Keywords: ER stress, UPR, protein misfolding, AAV, gene therapy, neurodegeneration, diabetes
Proteostasis impairment is a common factor in several diseases. Gene therapy approaches may target specific cells in each disease. Here, we summarize successful efforts to modulate proteostasis alterations by gene therapy approaches in several preclinical disease models and discuss the possibility to extend these results to clinical trials.
Main Text
Proteostasis is defined as the dynamic interrelation of all cellular processes that ensures the generation of functional proteins, including their synthesis, quality control, localization, and degradation.1 Maintenance of the health of the proteome is central to sustain cell function, reflected in the fact that proteostasis imbalance contributes to a wide spectrum of human diseases.1 Approximately 30% of proteins are synthesized and processed in the endoplasmic reticulum (ER), representing a key compartment of the proteostasis machinery.2 A variety of noxious inputs to the cell may alter the function of this fundamental organelle, triggering a condition known as ER stress. Chronic accumulation of misfolded proteins at the ER and subsequent events, such as abnormal protein aggregation and the collapse of protein degradation systems, are deleterious to the cell and can result in cell dysfunction and death.3
A central node of the proteostasis network is a signaling pathway known as the unfolded protein response (UPR), which is activated in response to ER stress.4 Activation of the UPR have been extensively described as an early event in several pathological conditions, suggesting a causative role in the progression of disease.5
Although ER stress is emerging as an attractive target for disease intervention, the use of small molecules has many disadvantages,6 including side effects due to the fundamental physiological roles of the UPR, especially in specialized secretory cells.7 To overcome this limitation, several gene therapy strategies have been developed to target the UPR and ER stress, demonstrating outstanding results in preclinical models of disease. In this review, we discuss current evidence supporting the potential of gene therapy to improve proteostasis in various disease contexts.
ER Stress and the Unfolded Protein Response
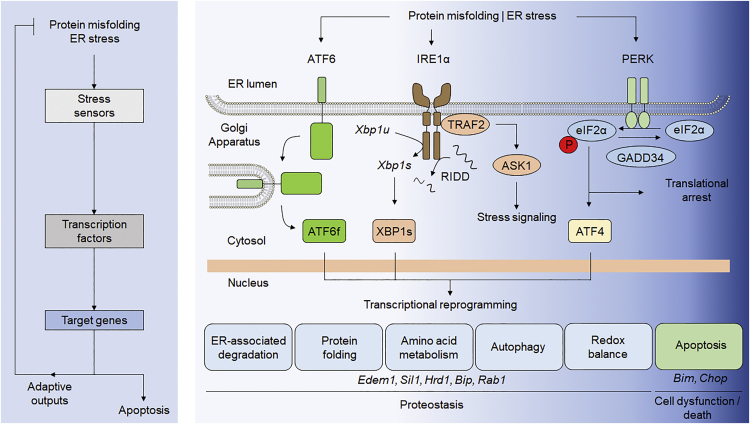
The ER is the main organelle where secretory-pathway-associated proteins are synthesized and folded. Multiple post-translational modifications and protein quality control systems ensure the correct folding and localization of proteins.8 The folding capacity of the cell is constantly adjusted according to demand, and the UPR is essential to sustain the activity of specialized secretory cells, including B lymphocytes, salivary glands, and pancreatic beta cells.9 Additionally, environmental changes at the ER lumen also influence the efficiency of protein synthesis and folding rates. These changes can include the redox state of the cell, lumenal calcium levels, and alterations in lipid or energy metabolism. Activation of the UPR enhances the basal capacity of the cell by enforcing pro-adaptive responses, such as the adjustment of protein synthesis and the upregulation of genes involved in folding, quality control mechanisms, and protein degradation of pathways (Figure 1).10 Conversely, under chronic or irreversible ER stress, the UPR activates pro-apoptotic signals. Thus, the UPR represents a central pathway controlling cell fate under stress.11, 12
Figure 1.
ER Stress and the UPR
Left: flowchart showing the hallmarks of the UPR activation under ER stress. Right: principal components and potential targets of the UPR to modulate in disease by AAV-mediated gene therapy approaches. ASK1, apoptosis signal-regulating kinase 1; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; ATF6f, fragmented activating transcription factor 6; BiP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; EDEM1, ER degradation-enhancing alpha-mannosidase-like 1; eIF2α, eukaryotic translation initiation factor-2 alpha; GADD34, growth arrest and DNA damage-inducible 34; Hrd1, hypoxia responsive domain-1; IRE1, inositol-requiring enzyme 1; JNK, c-Jun N-terminal kinase; PERK, protein kinase RNA-like endoplasmic reticulum kinase; PP1, protein phosphatase 1; Rab1, Ras-associated binding protein 1; Sil1, SIL1 nucleotide exchange factor 1; TRAF2, TNF receptor-associated factor 2; Xbp1s, spliced X-box binding protein 1; Xbp1u, unspliced X-box binding protein 1.
The UPR can be viewed as a three-component system mediated by (1) stress transducers or sensors, (2) transcription factors, and (3) downstream targets (Figure 1). UPR signal transducers are located at the ER membrane and directly or indirectly detect the accumulation of misfolded proteins inside the ER, including inositol-requiring transmembrane kinase/endonuclease 1 (IRE1) alpha and beta, PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) alpha and beta.10 Each transducer controls specific downstream transcription factors. IRE1α is a ubiquitously expressed protein, whereas IRE1β is specifically expressed in the gut and lung. IRE1α is a kinase and endoribonuclease that, upon activation, catalyzes the processing of the pre-mRNA encoding the transcription factor X-box-binding protein 1 (XBP1), deleting a 26-nt intron that changes the open reading frame to express a potent transcriptional activator termed XBP1s (for the spliced form).13 XBP1s controls the expression of genes involved in protein folding, secretion, quality control, and ER-associated degradation (ERAD), among other processes.14, 15 Additionally, IRE1α directly cleaves a subset of mRNAs and microRNAs, having important regulatory function on inflammation, apoptosis, and other pathways.16 IRE1α mediates the signaling crosstalk with other pathways through the binding of adaptor proteins, like TRAF2, to engage alarm genes, including ASK1 and JNK that regulate autophagy and apoptosis.17
Activation of PERK leads to the transient inhibition of protein translation in the ER by the phosphorylation of eukaryotic translation initiator factor 2α (eIF2α), which attenuates the overload of misfolded proteins.18 eIF2α phosphorylation allows the selective translation of the transcription factor ATF4, which mediates the upregulation of foldases, proteins involved in autophagy, chaperones, and proteins involved in the regulation of the redox and metabolic status of the cell.19 Under prolonged ER stress, ATF4 has an important pro-apoptotic role through the upregulation of the transcription factor CHOP and several members of the BCL-2 family of proteins (e.g., BIM).12, 20 eIF2α phosphorylation is negatively regulated by two phosphatase complexes, including the ER-stress-inducible regulatory subunit GADD34, which is controlled by the ATF4/CHOP pathway.21 In addition, this UPR signaling branch regulates apoptosis by enhancing protein synthesis and the production of reactive oxygen species on the stressed cell.22 Importantly, in addition to PERK, eIF2α phosphorylation is a convergent point of several stress responses mediated by general control nonderepressible 2 (GCN2), protein kinase R (PKR), and heme-regulated inhibitor (HRI), collectively known as the integrated stress response (ISR).23
Under ER stress, ATF6 is transported to the Golgi apparatus, where it is cleaved to release a cytosolic fragment (ATF6f), which operates as a transcription factor that regulates a cluster of genes, including ERAD components and XBP1.24 Importantly, the universe of target genes governed by each of these three transcription factors depends on the cell type affected and the context (i.e., stress stimuli), which may be determined by the interaction with other transcription factors, the formation of heterodimers, genetic backgrounds, post-translational modifications, and chromatin remodeling.11 In addition, the threshold to activate UPR stress transducers and the kinetic pattern of their signaling is regulated by the selective binding of different cofactors.10 Thus, activation of the UPR has distinct consequences to gene expression and cell physiology. Selective modulation of the stress mitigation (adaptation) responses may offer new therapeutic targets for disease intervention.
Contribution of ER Stress to Disease
ER stress is emerging as a pathological hallmark of a variety of human diseases, ranging from cancer, diabetes, obesity, and inflammatory diseases to neurodegenerative conditions.5 Furthermore, the UPR has been linked to the aging process, a major risk factor to develop neurodegenerative diseases.25, 26 Importantly, functional studies using genetic or pharmacological manipulation of the UPR in preclinical models of disease have demonstrated a relevant contribution of ER stress to disease pathogenesis, placing the UPR as an attractive target for future interventions.27
A large proportion of studies have focused on developing strategies to reduce the pathological consequences of ER stress in models of neurodegenerative diseases. Although the clinical manifestations of most neurodegenerative conditions are dissimilar, most of them share the accumulation of abnormal protein aggregates as a common disease feature (e.g., Alzheimer’s disease [AD], amyotrophic lateral sclerosis [ALS], Parkinson’s disease [PD], Huntington’s disease [HD], Creutzfeldt-Jakob disease [a prion-related disorder (PrD)], retinitis pigmentosa, etc.) and are now classified as protein misfolding disorders.28, 29 Importantly, the presence of chronic ER stress markers is an emerging signature of most of these diseases,30, 31, 32 as well as other conditions, such as spinal cord injury, brain ischemia,33 and multiple sclerosis.34 Thus, strategies to reduce the load of protein aggregates have been tested in various preclinical models of disease, including increasing UPR-dependent transcriptional responses, improving protein folding, or enhancing the clearance of misfolded proteins. In the next sections, we discuss the potential use of gene therapy as a strategy to target the UPR in a tissue- or cell-type-specific manner. We will focus on the use of adeno-associated viruses (AAVs) and highlight the available evidence supporting the applicability of these vectors for human use.
AAV-Mediated Gene Therapy
AAVs are small, non-enveloped viruses of the Parvoviridae family that package a single-stranded linear DNA genome. These vectors are attractive for use in gene therapy, as they are non-pathogenic; AAVs have not been associated with any human disease. They have a very low rate of integration, and the recombinant virus even lacks the site-specific integration machinery of the native virus.35 They do not trigger a significant immune response,36 and transgene expression can persist for years; expression has been shown for up to 4 years37, 38 in the human CNS and 15 years in non-human primates.39
Advances in the engineering of recombinant AAVs has generated methods for fast production with standards for human use, in a reliable, highly pure, and affordable manner.36, 40 Recently, the use of AAVs for gene therapy has been significantly increased with the US Food and Drug Administration (FDA) approval of two gene therapies for blood cancer and an AAV-based gene therapy for a genetic form of blindness (Box 1).41, 42
Box 1. A Promising Outlook for AAV-Based Gene Therapies.
The promise of gene therapy to revolutionize the treatment of disease has finally come to fruition. Gene therapies using AAV have recently gained approval by regulatory agencies (e.g., Glybera by the European Medicine Agency and Luxturna by the US Food and Drug Administration [FDA]). A strong commitment to gene therapy is underscored by the number of companies as well as academic and government institutions that are pursuing clinical trials. Over 2,200 gene therapy clinical trials have been initiated, of which at least 110 are active trials using AAVs117 (see https://clinicaltrials.gov/). Moreover, the FDA has given breakthrough or fast track designations to several gene therapy clinical trials. These designations allow for a faster review process for life-threatening diseases, suggesting confidence in gene therapy by regulatory agencies.
AAV-mediated gene delivery has emerged as the most effective and safe tool for both preclinical and clinical studies.36, 43 No side effects have yet been reported in humans treated with AAVs, including the entrance of Glybera as the first commercial AAV-based gene therapy in Europe44 and seven gene therapies with disclosed FDA breakthrough designation, including three accepted in 2017.42 Moreover, data obtained in several clinical trials in PD patients revealed outstanding safety profiles of AAVs.45, 46, 47
In parallel to the development of pharmacological approaches to attenuate ER stress in preclinical models of disease (reviewed in Maly and Papa27 and Hetz et al.48), AAV-mediated gene therapy to improve ER proteostasis has also demonstrated success in multiple disease models. Table 1 summarizes most of the advances in the development of AAV-mediated gene therapies to attenuate ER stress levels in vivo. Basically, four main concepts have been tested to target proteostasis alterations: (1) improving the folding process by overexpressing ER chaperones or the calcium pump SERCA (ER calcium is a cofactor of most chaperones); (2) enforcing adaptive gene programs by overexpressing XBP1s or ATF6f; (3) enhancing ERAD activity; or (4) the delivery of the eIF2α phosphatase GADD34 to reduce translational repression. In the following section, we discuss recent efforts to develop AAV-mediated gene therapy strategies to target ER stress in various pathologies.
Table 1.
A Summary of Successful Studies Using AAV-Based Gene Therapy to Target Proteostasis Alterations in Preclinical Models of Disease
| Organ | Disease/Condition | Vehicle | Administration/Target | Phenotype | References |
|---|---|---|---|---|---|
| Eye | glaucoma | AAV2-XBP1s | intravitreal | increases neuronal survival | Hu et al.84 |
| MS/optic neuritis | AAV2-XBP1s in chop KO context | intravitreal | preserved visual function | Huang et al.85 | |
| optic nerve crush | AAV2-Hsp70 | intravitreal | increased retinal ganglion cells | Kwong et al.118 | |
| retinal degeneration | AAV2-XBP1s | intravitreal | reduces axonal degeneration | Hu et al.84 | |
| retinitis pigmentosa | AAV5-BiP | sub-retinal | restores visual function in mutant rhodopsin transgenic rats | Gorbatyuk et al.83 | |
| Heart | myocardial ischemia/reperfusion | AAV9-ATF6f | i.v. | decreased injury and improved function | Jin et al.91 |
| CNS | amyotrophic lateral sclerosis | AAV6-SIL1 | i.c.v. | delayed muscle denervation and prolonged survival | Filézac de L’Etang et al.70 |
| Huntington’s disease | AAV2-XBP1s | striatum | reduces aggregation of mutant huntingtin | Zuleta et al.72 | |
| memory | AAV6-XBP1s | hippocampus | increased learning and memory | Martinez et al.61 | |
| Parkinson’s disease | AAV5-BiP | SNpc | reduces toxicity and aggregation of α-synuclein | Gorbatyuk et al.54 | |
| AAV2-XBP1s | SNpc | protected dopaminergic neurons and reduces striatal denervation against 6-OHDA | Valdes et al.52 | ||
| AAV6-Rab1A | SNpc | motor improvement | Coune et al.56 | ||
| spinal cord injury | AAV2-XBP1s | T12-13 level | improves locomotor recovery | Valenzuela et al.76 | |
| PNS | sciatic nerve injury | AAV2-XBP1s | DRG | enhanced axonal regeneration | Onate et al.78 |
6-OHDA, 6-hydroxydopamine; AAV, adeno-associated virus; BiP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; DRG, dorsal root ganglia; HSP70, heat shock protein 70; i.c.v., intracerebroventricular; i.v., intravenous; MS, multiple sclerosis; PNS, peripheral nervous system; Rab1A, Rab GTPase 1A; SIL1, SIL1 nucleotide exchange factor 1; SNpc, substantia nigra pars compacta; XBP1s, X-box binding protein 1 (spliced form).
CNS and Peripheral Nervous System Disorders
Neurodegenerative diseases involve the accumulation of protein aggregates, in addition to synaptic loss, neuronal death, axonal degeneration, and inflammation. The experimental manipulation of the UPR has demonstrated that ER stress signaling impacts many different aspects of the disease process. In this section, we summarize key findings supporting the potential of gene therapy strategies to improve ER proteostasis in the context of different brain disease conditions affecting the nervous system.
PD
PD is the second most common age-related neurodegenerative disease, affecting 1% of the population over 60 years of age. Although more than 90% of PD cases are sporadic, mutations in several genes have been linked to familial parkinsonian syndromes and account for 5%–10% of the total PD cases.49 Currently, PD is one of the best candidate diseases to apply gene therapy because the neuronal populations affected are spatially restricted, the volume of the brain that they cover is small, and the circuits involved are also well-characterized. PD involves the progressive degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNpc).49 At the histological level, the presence of intracellular inclusions known as Lewy bodies is a hallmark of PD. Lewy bodies are formed of fibrillar and ubiquitinated aggregates of alpha-synuclein. Consequently, the involvement of ER stress in PD has been extensively studied in multiple cellular and animal models, including successful pharmacological treatments.50
A few studies have demonstrated positive outcomes of UPR-based gene therapy in the progression of experimental PD. Adeno-viral-mediated expression of XBP1s in the SNpc significantly reduced the degeneration of dopaminergic neurons in a pharmacological model of PD.51 We confirmed these results by injecting AAVs to express active XBP1s into the SNpc of adult mice in a PD model, which also attenuated the denervation of the striatum.52 In contrast, the overexpression of ATF4 in the SNpc induced spontaneous cell death.53
AAV-based gene therapy strategies have been also developed to enhance the folding capacity of the ER by overexpressing the chaperone BiP. Remarkably, BiP gene transfer into the SNpc reduced dopaminergic neuron loss triggered by alpha-synuclein overexpression.54 Additionally, one of the major targets of alpha-synuclein is Rab1, impairing ER to Golgi trafficking, with resultant ER stress.55 An AAV gene therapy to overexpress Rab1 was developed and tested in PD models. Injection of AAV6-Rab1 into the SNpc significantly attenuated motor deficits triggered by alpha-synuclein overexpression.56 However, this strategy did not rescue dopaminergic neuron survival.
AD and Synaptic Function
AD is the most common neurodegenerative condition, leading to memory loss and dementia involving a progressive alteration to synaptic function. Although AD is a major health problem in the world, strategies to alleviate ER stress in mouse models have been poorly explored.57 Of note, a polymorphism on the XBP1 promoter has been linked to AD,58 in addition to bipolar disorders59 and schizophrenia,60 suggesting ER stress may be of particular importance to these diseases. In an attempt to study the possible physiological contribution of the UPR to the nervous system, we recently reported a new function of XBP1 in the brain in learning and memory processes.61 Using local administration of AAV6-XBP1s into the hippocampus of wild-type animals (rats and mice), we demonstrated an improvement in the basal performance in learning and memory tasks.61 A recent report demonstrated that the ectopic expression of XBP1 using lentivirus in the hippocampus restored synaptic and cognitive function in transgenic models of AD.62 However, the possible consequences of XBP1s overexpression on the accumulation of amyloid plaques were not described.
ALS
ALS is a fatal adult-onset neurodegenerative disease that affects motoneurons and thus the motor capabilities of patients.63 5%–10% of total cases are defined as familial ALS, and the remainder are sporadic.64 Many studies have shown signatures of ER stress and activation of the UPR in preclinical models of ALS as well as familial and sporadic induced pluripotent stem cell (iPSC)-derived motoneurons from ALS patients.65, 66 Multiple reports have shown the consequences of pharmacological modulation of the UPR in ALS mouse models, including the use of eIF2α phosphatase inhibitors.30, 67 Studies to identify factors that explain the differential neuronal vulnerability of certain motoneuron populations in ALS uncovered ER stress as a major pathological response,68, 69 detecting the expression of the BiP cofactor SIL1 in resistant motoneurons.70 Interestingly, AAV-mediated delivery of SIL1 restored ER homeostasis, delayed muscle denervation, and prolonged survival in mutant SOD1 transgenic mice.70 Of note, SIL1 was described as a direct target of XBP1s in myotubes and secretory cells.14
HD
HD is an inheritable neurodegenerative disease generated by the expansion of a trinucleotide repeat sequence in the gene encoding huntingtin, resulting in an abnormal polyglutamine expansion that triggers aggregation and neurotoxicity.71 AAV-mediated XBP1s gene transfer into the striatum of a HD mouse model significantly reduced the accumulation of mutant huntingtin inclusions.72 However, the possible consequences of XBP1s overexpression to the clinical progression of HD remain to be determined.
Spinal Cord Injury and Peripheral Neuropathy
The UPR has been extensively characterized in several models of tissue damage, including spinal cord injury.73, 74, 75 Several pharmacological approaches that reduce ER stress have been evaluated at the preclinical level.33 The efficacy of UPR-based gene transfer approaches to reduce ER stress levels after mechanical damage to the nervous system has been also explored. Local administration of AAV2-XBP1s directly into the spine in a model of spinal cord injury after damage enhanced locomotor recovery.76 This therapeutic effect was associated with an increased number of oligodendrocytes in the injured region.76
Peripheral neuropathies are the major cause of chronic pain associated with systemic conditions, such as infections (e.g., HIV and hepatitis C), metabolic problems (e.g., diabetes), inherited causes (e.g., Charcot-Marie-Tooth diseases), and exposure to toxins (e.g., chemotherapy).77 We recently delivered AAV2-XBP1s to the dorsal root ganglia and tested the impact of artificially enforcing the UPR in models of peripheral nerve degeneration. Overexpression of XBP1s accelerated axonal regeneration in the sciatic nerve, demonstrating a novel activity of the UPR in the nervous system.78
Prion-Related Disorders
A few reports have provided promising evidence, indicating that targeting ER proteostasis with gene therapy strategies can delay neurodegeneration. In prion disease models (scrapie), the sustained phosphorylation of eIF2α leads to decreased expression of synaptic proteins, resulting in neuronal dysfunction.79 Overexpression of the eIF2α phosphatase subunit GADD34 into the brain using lentiviral vectors reduced neurodegeneration of prion-infected mice.80 In summary, strategies to attenuate ER stress levels using gene therapy may have beneficial consequences on a variety of diseases and conditions that are associated with altered ER proteostasis in common despite disparate etiology.
Eye Disease
ER stress has been linked to several eye disorders.81 Retinitis pigmentosa is a genetic disorder of the eyes that causes vision loss. Mutations in the rhodopsin gene that cause retinitis pigmentosa are associated with rhodopsin misfolding and ER stress, leading to photoreceptor degeneration.82 Subretinal delivery of AAV5 to overexpress BiP in rhodopsin transgenic rats led to a reduction in CHOP levels, attenuating apoptosis. The administration of AAV-BiP had outstanding effects, resulting in a sustained increase in electro-retinogram amplitudes, suggesting the recovery of vision in the mutant rhodopsin transgenic rats.83
Other diseases of the eye, including glaucoma and optic neuritis, have been also linked with the occurrence of pathological ER stress levels. AAV-mediated XBP1s overexpression dramatically protected retinal ganglion cells from axon-injury-induced death from glaucoma.84 Additionally, AAV-mediated XBP1s overexpression led to increased retinal ganglion cell survival and axonal protection in optic neuritis from experimental autoimmune encephalomyelitis (a model of multiple sclerosis) in mice.85
Heart Pathology
Most ER chaperones have a high capacity to bind calcium with low affinity, operating as a major cofactor for optimal folding activity.86 The sarco-ER calcium ATPase (SERCA) family of proteins pumps calcium from the cytosol to the sarcoplasmic reticulum (SR). SERCA is essential to maintain steady-state levels of SR and ER calcium and hence muscle function. In heart failure, several efforts have been made to develop a SERCA2a AAV-gene therapy and are currently under clinical testing, including CUPID (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease).87 The results from these clinical trials suggested a significant improvement of heart function;88, 89 however, these observations could not be replicated later. The authors suggested that reduced transduction efficiency may explain the failure of the second study.90
Other strategies have been investigated to target other heart conditions. AAV-mediated ATF6f overexpression restored heart function under an ischemia/reperfusion paradigm.91 In heart disease, delivery of the ER foldase PDIA1 into the heart using adenoviruses yielded protection against acute myocardial infarction.92 Similarly, overexpression of Hdr1, a critical component of protein quality control and ERAD, by AAV9 injections showed beneficial effects in a heart hypertrophy model.93
Metabolic Disorders
ER stress has been proposed as a central pathological event linked to insulin resistance and obesity.94, 95 Aberrant lipid metabolism disrupts calcium homeostasis, causing liver ER stress in obesity.96 Diabetes is a group of disorders characterized by chronic high blood glucose levels (hyperglycemia) due to a failure to produce insulin or regulate its action. Treatment of diabetic mice with adenoviruses to express XBP1s in the liver improves glucose homeostasis.97 In this line, XBP1s is proposed as a master modulator of insulin action and also as an anti-lipogenic protein.98, 99, 100 In addition, adenovirus-mediated delivery of BiP to the liver of obese mice or wild-type mice exposed to a high-fat diet led to a decrease of steatosis correlating with a reduction on ER stress levels.101
Current Challenges
ER stress and the activation of the UPR is a feature of normal physiology (i.e., specialized secretory cells) and many pathological conditions. UPR modulation is emerging as an attractive alternative to target multiple diseases, and gene therapy approaches to modulate ER stress levels have greatly advanced in the last five years. However, there are still many critical challenges in the field, ranging from the optimization of AAV technologies for large-scale production, improvement of transduction efficiency and spreading of the virus, and the targeted expression of the transgene. The complexity of UPR signaling offers multiple levels of intervention from stress sensors, modulators, transcription factors, and hundreds of downstream targets. A complete understanding of the specific outputs of the UPR and how it relates to disease is key to designing successful future therapeutic strategies.
Developing an effective gene therapy by modulating the UPR represents a complex challenge, considering its roles in stress mitigation, cell physiology, and apoptosis. Most functional studies using small molecules or genetic manipulation of the UPR in disease conditions indicate that, depending on disease context, its stage of evolution (pre-symptomatic and symptomatic versus late stage), the cell type affected, and the specific signaling branch analyzed, the pathway may have contrasting and even opposite effects.30 In addition to cell-type-specific modulation, temporal regulation of transgene expression might produce specific effects in the proteostasis network. Because the UPR is also emerging as a central driver of cancer,102 including brain cancer,103 it remains to be determined whether the long-term expression of other active UPR components in the brain, such as XBP1s, increases the probability of developing this disease. Until now, no reports have shown the possible efficacy of UPR-based gene therapy for cancer treatment in preclinical models.
A major challenge in the AAV field is to increase the spread of the AAVs to multiple regions within the CNS. This is particularly relevant for neurodegenerative diseases that affect larger portions of the brain. For example, although PD is often thought of as a nigrostriatal disease, it is now well accepted that it affects many regions of the brain. A single conventional injection of AAV to the striatum or cortex is unlikely to transduce the entire brain area. A variety of techniques have been described to attempt to overcome this problem. One method is to use multiple injection sites to achieve a broader area of viral transduction; however, this may increase the risk of infection, hemorrhage, and mechanical damage to the brain. Another approach for increased viral spread is convection-enhanced delivery. This technique uses a pressure gradient combined with physiological interstitial flow to increase AAV distribution.104 Broader routes of administration, including intra-cerebroventricular or intravenous administration, have also been tested to target the entire CNS.105 Methods to increase blood-brain barrier permeability, including mannitol or focused ultrasound, also require much higher vector doses and increase the potential for peripheral organ transduction and thus side effects.
New engineered capsid proteins are under development to improve efficacy, target larger neuronal populations,106, 107, 108 and display enhanced blood-brain barrier (BBB) penetrance.45, 109, 110, 111, 112 A variety of natural AAV serotypes and modified recombinant versions are available to allow specific tropism for selected cell types (i.e., astrocytes, oligodendrocytes, neurons, liver cells, muscle, etc.).35, 36, 45 Moreover, the site of AAV injection can determine the specific population of cells transduced, as demonstrated, for example, after injection in gastrocnemius muscle to deliver transgenes into lumbar motoneurons.113
The combination of selected serotypes with cell-type or condition-dependent promoters to drive gene expression can be used to further ensure a restricted expression pattern in the desired cell type or even subpopulations of cells.114, 115 The minimal human synapsin I promoter has been frequently used to ensure neuronal expression, and a variety of other cell-type-specific promoters are under preclinical development.116 One of the continuing challenges for cell-type-specific promoters within the context of AAVs is the large size of most promoters and the small packaging capacity of AAV. Minimal or partial promoters need to be generated. Additionally, considering the early activation of the UPR in some diseases, the use of ER-stress-responsive cis elements (ERSE) as an expression regulator may be used as a therapeutic alternative to activate the transgene only in cells suffering stress.
Conclusions
Overall, gene therapy-mediated delivery of therapeutic agents represents a unique strategy to provide cellular specificity, temporal activation/blockage, and dosage. Targeting ER stress and the UPR is emerging as a promising avenue for disease intervention with possible applications in neurodegeneration, cancer, metabolic diseases, stroke, and heart disease. For monogenic diseases, co-treatments involving mutant gene correction or replacement plus proteostatic modulators may enhance therapeutic outcomes. For non-familial cases or multifactorial diseases, ER stress targeting represents a potential disease-modifying agent by altering pathological mechanisms of disease. The concept of intervining homeostatic networks (biological processes) rather than single genes may offer the possibility of treating a larger population of patients independent of the etiology of the disease (genetic or sporadic forms). However, additional systematic studies are needed to assess the long-term efficacy of such approaches and define possible side effects of manipulating the proteostasis network.
Author Contributions
V.V., K.L.J., S.P.S., and C.H. developed and corrected the manuscript. C.H. devised the manuscript.
Acknowledgments
This work is funded by the Millennium Institute (P09-015-F), FONDAP (15150012), the ALS Therapy Alliance (2014-F-059), the Muscular Dystrophy Association (382453, CONICYT-USA 2013-0003), the Michael J. Fox Foundation for Parkinson’s Research – Target Validation (grants. 9277 and 12473, Ecos-Conicyt (C13S02), FONDECYT (11180186), the Office of Naval Research-Global (ONR-G) (N62909-16-1-2003), an ALSRP Therapeutic Idea Award (AL150111), the Leading House for the Latin American Region (seed money grant) to C.H.; Fondecyt 3170622, and ALSA 17-PDF-32 to V.V.
References
- 1.Balch W.E., Morimoto R.I., Dillin A., Kelly J.W. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Braakman I., Bulleid N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 3.Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 4.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 5.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers E.T., Morimoto R.I., Dillin A., Kelly J.W., Balch W.E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 7.Dufey E., Sepúlveda D., Rojas-Rivera D., Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am. J. Physiol. Cell Physiol. 2014;307:C582–C594. doi: 10.1152/ajpcell.00258.2014. [DOI] [PubMed] [Google Scholar]
- 8.Xu C., Ng D.T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 2015;16:742–752. doi: 10.1038/nrm4073. [DOI] [PubMed] [Google Scholar]
- 9.Cornejo V.H., Pihán P., Vidal R.L., Hetz C. Role of the unfolded protein response in organ physiology: lessons from mouse models. IUBMB Life. 2013;65:962–975. doi: 10.1002/iub.1224. [DOI] [PubMed] [Google Scholar]
- 10.Hetz C., Papa F.R. The unfolded protein response and cell fate control. Mol. Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Hetz C., Chevet E., Oakes S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C., Lennon C.J., Kluger Y., Dynlacht B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurel M., Chevet E., Tavernier J., Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Hetz C., Glimcher L.H. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 19.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 20.Urra H., Dufey E., Lisbona F., Rojas-Rivera D., Hetz C. When ER stress reaches a dead end. Biochim. Biophys. Acta. 2013;1833:3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Tsaytler P., Bertolotti A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS J. 2013;280:766–770. doi: 10.1111/j.1742-4658.2012.08535.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto K., Sato T., Matsui T., Sato M., Okada T., Yoshida H., Harada A., Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Taylor R.C. Aging and the UPR(ER) Brain Res. 2016;1648(Pt B):588–593. doi: 10.1016/j.brainres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Martínez G., Duran-Aniotz C., Cabral-Miranda F., Vivar J.P., Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16:615–623. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maly D.J., Papa F.R. Druggable sensors of the unfolded protein response. Nat. Chem. Biol. 2014;10:892–901. doi: 10.1038/nchembio.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertram L., Tanzi R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soto C. Transmissible proteins: expanding the prion heresy. Cell. 2012;149:968–977. doi: 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetz C., Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017;13:477–491. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 31.Smith H.L., Mallucci G.R. The unfolded protein response: mechanisms and therapy of neurodegeneration. Brain. 2016;139:2113–2121. doi: 10.1093/brain/aww101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheper W., Hoozemans J.J. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 2015;130:315–331. doi: 10.1007/s00401-015-1462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela V., Oñate M., Hetz C., Court F.A. Injury to the nervous system: a look into the ER. Brain Res. 2016;1648(Pt B):617–625. doi: 10.1016/j.brainres.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 34.Clayton B.L.L., Popko B. Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 2016;1648(Pt B):594–602. doi: 10.1016/j.brainres.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samulski R.J., Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 36.Wang D., Gao G. State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov. Med. 2014;18:67–77. [PMC free article] [PubMed] [Google Scholar]
- 37.Mittermeyer G., Christine C.W., Rosenbluth K.H., Baker S.L., Starr P., Larson P., Kaplan P.L., Forsayeth J., Aminoff M.J., Bankiewicz K.S. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum. Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartus R.T. Gene therapy for Parkinson’s disease: a decade of progress supported by posthumous contributions from volunteer subjects. Neural Regen. Res. 2015;10:1586–1588. doi: 10.4103/1673-5374.167783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehara Y., Fujimoto K.I., Ikeguchi K., Katakai Y., Ono F., Takino N., Ito M., Ozawa K., Muramatsu S.I. Persistent expression of dopamine-synthesizing enzymes 15 years after gene transfer in a primate model of Parkinson’s disease. Hum. Gene Ther. Clin. Dev. 2017;28:74–79. doi: 10.1089/humc.2017.010. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Gao G. State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov. Med. 2014;18:151–161. [PMC free article] [PubMed] [Google Scholar]
- 41.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 42.Morrison C. Landmark gene therapy poised for US approval. Nat. Rev. Drug Discov. 2017;16:739–741. doi: 10.1038/nrd.2017.212. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg M.S., Samulski R.J., McCown T.J. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology. 2013;69:82–88. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senior M. After Glybera’s withdrawal, what’s next for gene therapy? Nat. Biotechnol. 2017;35:491–492. doi: 10.1038/nbt0617-491. [DOI] [PubMed] [Google Scholar]
- 45.Bourdenx M., Dutheil N., Bezard E., Dehay B. Systemic gene delivery to the central nervous system using Adeno-associated virus. Front. Mol. Neurosci. 2014;7:50. doi: 10.3389/fnmol.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coune P.G., Schneider B.L., Aebischer P. Parkinson’s disease: gene therapies. Cold Spring Harb. Perspect. Med. 2012;2:a009431. doi: 10.1101/cshperspect.a009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo V., Mercado G., Hetz C. Gene therapy in Parkinson’s disease: targeting the endplasmic reticulum proteostasis network. Neural Regen. Res. 2015;10:1053–1054. doi: 10.4103/1673-5374.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hetz C., Chevet E., Harding H.P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 49.Martin I., Dawson V.L., Dawson T.M. Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genomics Hum. Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercado G., Valdés P., Hetz C. An ERcentric view of Parkinson’s disease. Trends Mol. Med. 2013;19:165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Sado M., Yamasaki Y., Iwanaga T., Onaka Y., Ibuki T., Nishihara S., Mizuguchi H., Momota H., Kishibuchi R., Hashimoto T. Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 52.Valdés P., Mercado G., Vidal R.L., Molina C., Parsons G., Court F.A., Martinez A., Galleguillos D., Armentano D., Schneider B.L., Hetz C. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl. Acad. Sci. USA. 2014;111:6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gully J.C., Sergeyev V.G., Bhootada Y., Mendez-Gomez H., Meyers C.A., Zolotukhin S., Gorbatyuk M.S., Gorbatyuk O.S. Up-regulation of activating transcription factor 4 induces severe loss of dopamine nigral neurons in a rat model of Parkinson’s disease. Neurosci. Lett. 2016;627:36–41. doi: 10.1016/j.neulet.2016.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorbatyuk M.S., Shabashvili A., Chen W., Meyers C., Sullivan L.F., Salganik M., Lin J.H., Lewin A.S., Muzyczka N., Gorbatyuk O.S. Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol. Ther. 2012;20:1327–1337. doi: 10.1038/mt.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coune P.G., Bensadoun J.C., Aebischer P., Schneider B.L. Rab1A over-expression prevents Golgi apparatus fragmentation and partially corrects motor deficits in an alpha-synuclein based rat model of Parkinson’s disease. J. Parkinsons Dis. 2011;1:373–387. doi: 10.3233/JPD-2011-11058. [DOI] [PubMed] [Google Scholar]
- 57.Gerakis Y., Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018;285:995–1011. doi: 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- 58.Liu S.Y., Wang W., Cai Z.Y., Yao L.F., Chen Z.W., Wang C.Y., Zhao B., Li K.S. Polymorphism -116C/G of human X-box-binding protein 1 promoter is associated with risk of Alzheimer’s disease. CNS Neurosci. Ther. 2013;19:229–234. doi: 10.1111/cns.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kakiuchi C., Iwamoto K., Ishiwata M., Bundo M., Kasahara T., Kusumi I., Tsujita T., Okazaki Y., Nanko S., Kunugi H. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat. Genet. 2003;35:171–175. doi: 10.1038/ng1235. [DOI] [PubMed] [Google Scholar]
- 60.Kakiuchi C., Ishiwata M., Umekage T., Tochigi M., Kohda K., Sasaki T., Kato T. Association of the XBP1-116C/G polymorphism with schizophrenia in the Japanese population. Psychiatry Clin. Neurosci. 2004;58:438–440. doi: 10.1111/j.1440-1819.2004.01280.x. [DOI] [PubMed] [Google Scholar]
- 61.Martínez G., Vidal R.L., Mardones P., Serrano F.G., Ardiles A.O., Wirth C., Valdés P., Thielen P., Schneider B.L., Kerr B. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 2016;14:1382–1394. doi: 10.1016/j.celrep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Cissé M., Duplan E., Lorivel T., Dunys J., Bauer C., Meckler X., Gerakis Y., Lauritzen I., Checler F. The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model. Mol. Psychiatry. 2017;22:1562–1575. doi: 10.1038/mp.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner M.R., Hardiman O., Benatar M., Brooks B.R., Chio A., de Carvalho M., Ince P.G., Lin C., Miller R.G., Mitsumoto H. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruegsegger C., Saxena S. Proteostasis impairment in ALS. Brain Res. 2016;1648(Pt B):571–579. doi: 10.1016/j.brainres.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 66.Medinas D.B., Valenzuela V., Hetz C. Proteostasis disturbance in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2017;26(R2):R91–R104. doi: 10.1093/hmg/ddx274. [DOI] [PubMed] [Google Scholar]
- 67.Kim H.J., Raphael A.R., LaDow E.S., McGurk L., Weber R.A., Trojanowski J.Q., Lee V.M., Finkbeiner S., Gitler A.D., Bonini N.M. Therapeutic modulation of eIF2α phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat. Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saxena S., Cabuy E., Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 69.Rozas P., Bargsted L., Martínez F., Hetz C., Medinas D.B. The ER proteostasis network in ALS: Determining the differential motoneuron vulnerability. Neurosci. Lett. 2017;636:9–15. doi: 10.1016/j.neulet.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 70.Filézac de L’Etang A., Maharjan N., Cordeiro Braña M., Ruegsegger C., Rehmann R., Goswami A., Roos A., Troost D., Schneider B.L., Weis J., Saxena S. Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat. Neurosci. 2015;18:227–238. doi: 10.1038/nn.3903. [DOI] [PubMed] [Google Scholar]
- 71.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 72.Zuleta A., Vidal R.L., Armentano D., Parsons G., Hetz C. AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2012;420:558–563. doi: 10.1016/j.bbrc.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 73.Paschen W., Yatsiv I., Shoham S., Shohami E. Brain trauma induces X-box protein 1 processing indicative of activation of the endoplasmic reticulum unfolded protein response. J. Neurochem. 2004;88:983–992. doi: 10.1046/j.1471-4159.2003.02218.x. [DOI] [PubMed] [Google Scholar]
- 74.Aufenberg C., Wenkel S., Mautes A., Paschen W. Spinal cord trauma activates processing of xbp1 mRNA indicative of endoplasmic reticulum dysfunction. J. Neurotrauma. 2005;22:1018–1024. doi: 10.1089/neu.2005.22.1018. [DOI] [PubMed] [Google Scholar]
- 75.Penas C., Guzman M.S., Verdu E., Fores J., Navarro X., Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 76.Valenzuela V., Collyer E., Armentano D., Parsons G.B., Court F.A., Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cashman C.R., Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oñate M., Catenaccio A., Martínez G., Armentano D., Parsons G., Kerr B., Hetz C., Court F.A. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep. 2016;6:21709. doi: 10.1038/srep21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duran-Aniotz C., Martínez G., Hetz C. Memory loss in Alzheimer’s disease: are the alterations in the UPR network involved in the cognitive impairment? Front. Aging Neurosci. 2014;6:8. doi: 10.3389/fnagi.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno J.A., Radford H., Peretti D., Steinert J.R., Verity N., Martin M.G., Halliday M., Morgan J., Dinsdale D., Ortori C.A. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature. 2012;485:507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan P., Stolz J., Kohl S., Chiang W.C., Lin J.H. Endoplasmic reticulum stress in human photoreceptor diseases. Brain Res. 2016;1648(Pt B):538–541. doi: 10.1016/j.brainres.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griciuc A., Aron L., Ueffing M. ER stress in retinal degeneration: a target for rational therapy? Trends Mol. Med. 2011;17:442–451. doi: 10.1016/j.molmed.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Gorbatyuk M.S., Knox T., LaVail M.M., Gorbatyuk O.S., Noorwez S.M., Hauswirth W.W., Lin J.H., Muzyczka N., Lewin A.S. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. USA. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Y., Park K.K., Yang L., Wei X., Yang Q., Cho K.S., Thielen P., Lee A.H., Cartoni R., Glimcher L.H. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang H., Miao L., Liang F., Liu X., Xu L., Teng X., Wang Q., Ridder W.H., 3rd, Shindler K.S., Sun Y., Hu Y. Neuroprotection by eIF2α-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017;8:e2936. doi: 10.1038/cddis.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halperin L., Jung J., Michalak M. The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life. 2014;66:318–326. doi: 10.1002/iub.1272. [DOI] [PubMed] [Google Scholar]
- 87.Hayward C., Banner N.R., Morley-Smith A., Lyon A.R., Harding S.E. The current and future landscape of SERCA gene therapy for heart failure: a clinical perspective. Hum. Gene Ther. 2015;26:293–304. doi: 10.1089/hum.2015.018. [DOI] [PubMed] [Google Scholar]
- 88.Jaski B.E., Jessup M.L., Mancini D.M., Cappola T.P., Pauly D.F., Greenberg B., Borow K., Dittrich H., Zsebo K.M., Hajjar R.J., Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jessup M., Greenberg B., Mancini D., Cappola T., Pauly D.F., Jaski B., Yaroshinsky A., Zsebo K.M., Dittrich H., Hajjar R.J., Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Desai A.S., Barnard D., Bouchard A., Jaski B., Lyon A.R. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 91.Jin J.K., Blackwood E.A., Azizi K., Thuerauf D.J., Fahem A.G., Hofmann C., Kaufman R.J., Doroudgar S., Glembotski C.C. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ. Res. 2017;120:862–875. doi: 10.1161/CIRCRESAHA.116.310266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toldo S., Severino A., Abbate A., Baldi A. The role of PDI as a survival factor in cardiomyocyte ischemia. Methods Enzymol. 2011;489:47–65. doi: 10.1016/B978-0-12-385116-1.00003-0. [DOI] [PubMed] [Google Scholar]
- 93.Doroudgar S., Völkers M., Thuerauf D.J., Khan M., Mohsin S., Respress J.L., Wang W., Gude N., Müller O.J., Wehrens X.H. Hrd1 and ER-associated protein degradation, ERAD, are critical elements of the adaptive ER stress response in cardiac myocytes. Circ. Res. 2015;117:536–546. doi: 10.1161/CIRCRESAHA.115.306993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 96.Fu S., Yang L., Li P., Hofmann O., Dicker L., Hide W., Lin X., Watkins S.M., Ivanov A.R., Hotamisligil G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y., Lee J., Reno C.M., Sun C., Park S.W., Chung J., Lee J., Fisher S.J., White M.F., Biddinger S.B., Ozcan U. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herrema H., Zhou Y., Zhang D., Lee J., Salazar Hernandez M.A., Shulman G.I., Ozcan U. XBP1s is an anti-lipogenic protein. J. Biol. Chem. 2016;291:17394–17404. doi: 10.1074/jbc.M116.728949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park S.W., Zhou Y., Lee J., Lu A., Sun C., Chung J., Ueki K., Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J., Ibi D., Taniguchi K., Lee J., Herrema H., Akosman B., Mucka P., Salazar Hernandez M.A., Uyar M.F., Park S.W. Inflammation improves glucose homeostasis through IKKβ-XBP1s interaction. Cell. 2016;167:1052–1066.e18. doi: 10.1016/j.cell.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kammoun H.L., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T., Ferré P., Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang M., Kaufman R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 103.Obacz J., Avril T., Le Reste P.J., Urra H., Quillien V., Hetz C., Chevet E. Endoplasmic reticulum proteostasis in glioblastoma-From molecular mechanisms to therapeutic perspectives. Sci. Signal. 2017;10:eaal2323. doi: 10.1126/scisignal.aal2323. [DOI] [PubMed] [Google Scholar]
- 104.Bankiewicz K.S., Eberling J.L., Kohutnicka M., Jagust W., Pivirotto P., Bringas J., Cunningham J., Budinger T.F., Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 105.Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.L., Sánchez-Guardado L., Lois C., Mazmanian S.K., Deverman B.E., Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choudhury S.R., Harris A.F., Cabral D.J., Keeler A.M., Sapp E., Ferreira J.S., Gray-Edwards H.L., Johnson J.A., Johnson A.K., Su Q. Widespread central nervous system gene transfer and silencing after systemic delivery of novel AAV-AS vector. Mol. Ther. 2016;24:726–735. doi: 10.1038/mt.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murlidharan G., Sakamoto K., Rao L., Corriher T., Wang D., Gao G., Sullivan P., Asokan A. CNS-restricted transduction and CRISPR/Cas9-mediated gene deletion with an engineered AAV vector. Mol. Ther. Nucleic Acids. 2016;5:e338. doi: 10.1038/mtna.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang H., Yang B., Mu X., Ahmed S.S., Su Q., He R., Wang H., Mueller C., Sena-Esteves M., Brown R. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.M., Fyfe J., Moullier P., Colle M.A., Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benkhelifa-Ziyyat S., Besse A., Roda M., Duque S., Astord S., Carcenac R., Marais T., Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther. 2013;21:282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weismann C.M., Ferreira J., Keeler A.M., Su Q., Qui L., Shaffer S.A., Xu Z., Gao G., Sena-Esteves M. Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan. Hum. Mol. Genet. 2015;24:4353–4364. doi: 10.1093/hmg/ddv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Towne C., Schneider B.L., Kieran D., Redmond D.E., Jr., Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- 114.Gray S.J., Matagne V., Bachaboina L., Yadav S., Ojeda S.R., Samulski R.J. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Powell S.K., Rivera-Soto R., Gray S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015;19:49–57. [PMC free article] [PubMed] [Google Scholar]
- 116.Pignataro D., Sucunza D., Vanrell L., Lopez-Franco E., Dopeso-Reyes I.G., Vales A., Hommel M., Rico A.J., Lanciego J.L., Gonzalez-Aseguinolaza G. Adeno-associated viral vectors serotype 8 for cell-specific delivery of therapeutic genes in the central nervous system. Front. Neuroanat. 2017;11:2. doi: 10.3389/fnana.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bender E. Gene therapy: industrial strength. Nature. 2016;537:S57–S59. doi: 10.1038/537S57a. [DOI] [PubMed] [Google Scholar]
- 118.Kwong J.M., Gu L., Nassiri N., Bekerman V., Kumar-Singh R., Rhee K.D., Yang X.J., Hauswirth W.W., Caprioli J., Piri N. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene Ther. 2015;22:138–145. doi: 10.1038/gt.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]