Summary
Background/Objective
Postmenopausal women may have accelerated disc degeneration due to relative oestrogen deficiency. Two new studies supporting this concept were carried out.
Methods
Study I: The data were from the Osteoporotic Fractures in Men (Hong Kong) and Women (Hong Kong) studies. Both were population-based studies on bone health for elderly Chinese men and women (age ≥ 65 years, n = 2000 for men and n = 2000 for women). Based on lumbar spine radiographs, changes in L1/2–L4/5 disc space height were classified into four categories: 0 = normal; 1 = mild narrowing (< 30% reduction in disc height); 2 = moderate narrowing (30–60% reduction in disc height); and 3 = severe narrowing (> 60% reduction in disc height). Sums of the disc space narrowing scores of each participant were plotted against their age. Study II: 12 healthy individuals and 53 persons who had mild nonspecific low back pain (30 males and 35 females; mean age, 53.4 years; age range, 23–76 years) were recruited. Magnetic resonance imaging was performed on a 3-T system. A multiecho turbo spin echo pulse sequence was used for lumbar disc T2 mapping. Regions of interest were manually drawn over nucleus pulposus on the T2 map of the discs. The means of T2 relaxation times of discs L1/2–L4/5 of the participants were plotted against their age.
Results
Study I: Elderly women had a higher disc space narrowing score than elderly men, and the slope of the plot was steeper for females than for males. When the plots were extrapolated to younger age, they intersected at 59.67 years. Study II: An age-related reduction of T2 value in the nucleus pulposus was demonstrated, which was faster in females than in males. Although females tended to have initial higher T2 value before 50 years, this trend was reversed in elderly persons, with an intersection at 52.4 years.
Conclusion
Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men.
Keywords: disc degeneration, lumbar spine, menopause, magnetic resonance, radiograph
Introduction
Intervertebral disc degeneration begins early in life and is the consequence of a variety of genetic, mechanical, traumatic, and nutritional factors, as well as normal ageing [1], [2], [3]. Disc degeneration can progress to disc herniation, spinal canal stenosis, and, in conjunction with facet joint arthrosis, degenerative spondylolisthesis. Disc degeneration can be associated with low back pain, which decreases the quality of life and increases health care costs. The factors that initiate and influence the progression of disc degeneration are not yet fully understood. Nevertheless, there is a general agreement that spinal mechanical stress accelerates the development of disc degeneration and increases the likelihood of disc herniation [4].
Young men are more susceptible to disc degeneration than young women, most likely due to increased mechanical stress and physical injury. In an analysis of published data of 600 autopsy specimens of young and middle-aged individuals younger than 50 years, intervertebral disc degeneration was observed in men in the 2nd decade of life, occurring at an earlier age than in women; the severity of aged-matched disc degeneration was also generally greater in men [5]. In a later independent histologic study, Łebkowski [6] investigated 308 lumbar intervertebral discs collected during autopsy from 57 women (mean age, 41.8 years) and 79 men (mean age, 42.1 years). Disc degeneration first became readily apparent during the 2nd decade of life, although it was observed to occur in men almost a decade earlier than in women. In a magnetic resonance imaging (MRI)-based survey of young adults aged 20–22 years, lumbar disc degeneration was significantly more frequent in men than in women [7]. These results reinforce the general perception that young men are more susceptible to disc degeneration than young women, most likely due to increased mechanical stress and physical injuries.
However, recent evidence suggests that disc degeneration is common or more severe in elderly women than in elderly men [8], [9]. It was suggested that postmenopausal women might have accelerated disc degeneration due to relative oestrogen deficiency [10]. However, until now there is no direct clinical data to show at what age women start to have as severe disc degeneration as, or more severe disc degeneration than, men. We performed two cross-sectional analyses on two data sets to further test this hypothesis of the effect of menopause on disc degeneration, and seek to understand the average age when Chinese women start to have as severe disc degeneration as that in Chinese men.
Materials and methods
Study I
The detailed participant information of Study I has been described elsewhere [11]. In brief, 2000 Chinese men and 2000 Chinese women, aged ≥ 65 years, were prospectively recruited from local communities for a prospective cohort study from August 2001 to March 2003, with the aim to follow them up again in 4 years' time. All participants were community dwelling, able to walk without assistance, without bilateral hip replacement, and with the potential to survive the duration of the primary study, as judged by their pre-existing medical status. The study protocol was approved by the Chinese University of Hong Kong Ethics Committee. Written informed consent was obtained from all participants. The baseline results were analysed in this study.
Left lateral lumbar spine radiographs were obtained by adjusting exposure parameters according to participants’ body weight and height. Hard copies of lumbar spine radiographs were analysed in this study. Changes in L1/2–L4/5 intervertebral disc space height were classified into four categories: 0 = normal; 1 = mild narrowing (< 30% reduction in disc height); 2 = moderate narrowing (30–60% reduction in disc height); and 3 = severe narrowing (> 60% reduction in disc height), as described by de Schepper et al [8] and other authors [12], [13], [14] and also applied by the current author in previous studies [11]. The readers were blinded to the clinical characteristics of the participants. Reader 1 was a senior radiologist with > 10 years of experience in reading lumbar radiographs. Reader 2 was a junior radiologist with 5 years of experience in reading radiographs. For discs L1/2–L4/5, the intrareader reproducibility of Reader 1 had a kappa value of 0.81 for Grade 1, 0.912 for Grade 2, and 1 for Grade 3, with an overall kappa value of 0.872. The inter-reader reproducibility between Readers 1 and 2 had a kappa value of 0.72, indicating good agreement. Two readers showed similar results [11], and the results of Reader 1 were further analysed. The current analysis was a retrospective analysis. Sums of the disc space narrowing scores of the study participants were plotted against their age using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Study II
All the data were collected during 2014 prospectively. Twelve healthy individuals and 53 consecutive patients who had mild nonspecific low back pain (30 males and 35 females; mean age, 53.4 ± 13.5 years; age range, 23–76 years) were included in the study; those with lumbar disc protrusion or apparent bulging were not included in the study. To remove the potential confounding role of diurnal disc hydration changes, all participants underwent imaging in the morning. The healthy individuals were all members of the author’s university. The participants with mild nonspecific low back pain who were available to attend a Saturday research MRI session were referred from primary care departments. All participants were confirmed to have no other spine diseases except disc degeneration, based on MRI examination. No participant had a history of intake of hormone medication or spine surgery, according to the electronic history records of the Hong Kong Hospital Authority. The study protocol was approved by the Chinese University of Hong Kong Ethics Committee. Written informed consent was obtained from all participants. MRI acquisition was performed on a 3-T clinical system (Achieva; Philips Healthcare, Best, The Netherlands). A 12-channel receive-only spine coil was used as the signal receiver to cover the lumbar spine, and the built-in body coil was used as a signal transmitter. Volume shimming was employed to minimise B0 heterogeneity.
In addition to the standard T1- and T2-weighted diagnostic anatomical images, a multiecho turbo spin echo pulse sequence was used for T2 mapping. Seven sagittal turbo spin echo images were acquired. Turbo spin echo imaging parameters included the following: field of view (FOV) = 200 mm; pixel = 1.0 mm × 1.0 mm; slice thickness = 4 mm; echo train length = 7; time of echos (TEs) = 16 milliseconds, 32 milliseconds, 48 milliseconds, 64 milliseconds, 80 milliseconds, 96 milliseconds, and 112 milliseconds; time of repetition (TR) = 2300 milliseconds; and number of signal averaging (NSA) = 3; and Sensitivity Encoding (SENSE) factor = 2.
| (1) |
where M0 denote the equilibrium magnetisation and M(TE) denotes the magnetisation acquired with the echo time TE.
The monoexponential equation was linearised by logarithm. T2 maps were generated by fitting each pixel’s intensity as a function of TE using a non-negative least-square fitting algorithm. T2 was calculated as the inverse of the slope of the corresponding straight-line fit [16], [15].
The central sagittal sections were used for analysis. With T2-weighted images as references, regions of interest were manually drawn over nucleus pulposus on the T2 map of each disc by a radiologist (Figure 1). The region of interest size for nucleus pulposus ranged from 15 mm2 to 45 mm2. The intraclass correlation coefficients for intra- and inter-reader reproducibility were, respectively, 0.928 and 0.914 for nucleus pulposus T2 map [17]. These performances are similar to the findings of one recent report [18]. The means of T2 relaxation times of discs L1/2–L4/5 of the participants were plotted against their age using GraphPad Prism software (GraphPad Software, Inc.).
Figure 1.
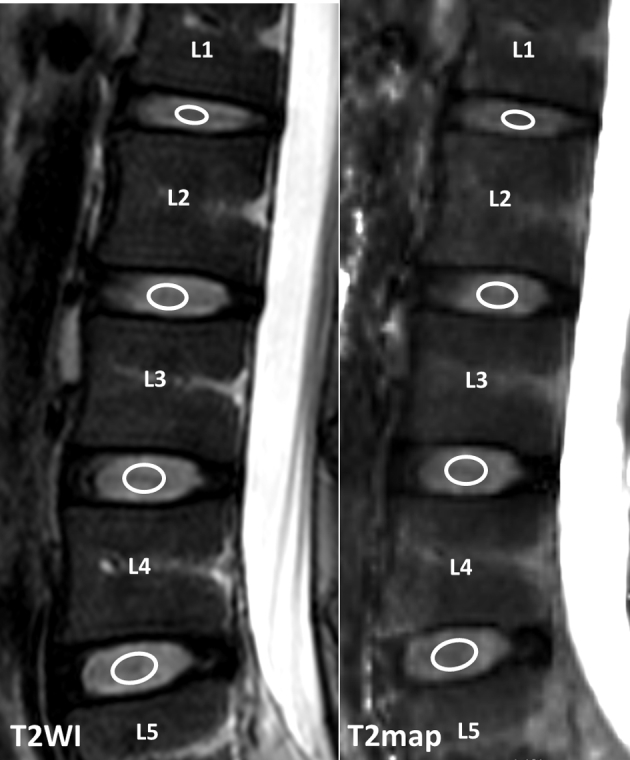
Example of placement of regions of interest over nucleus pulposus in lumbar discs. T2WI = T2-weighted image.
Results
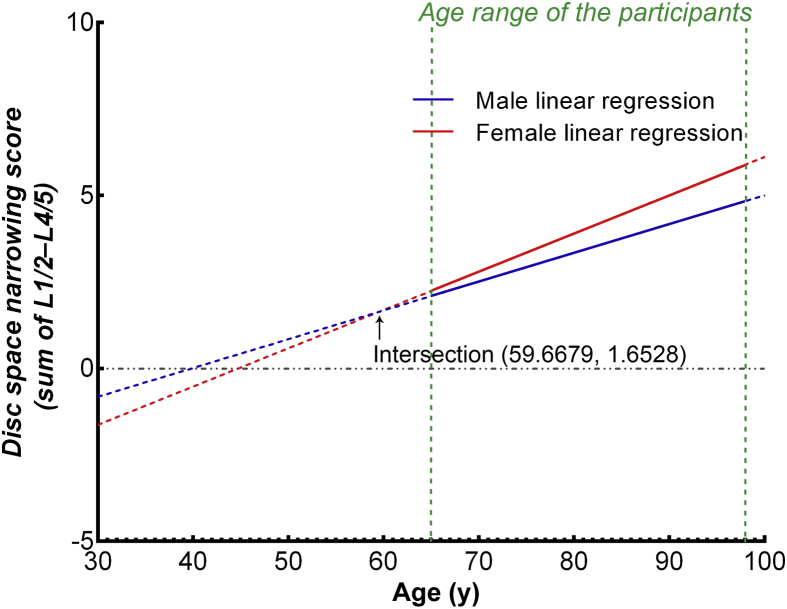
The result of Study I is shown in Figure 2. Female participants in the age range of 65–92 years tended to have a higher disc space narrowing score than the males, and the slope of the plot for females was steeper than that for males. When the slopes for males and females were extrapolated towards younger age, an intersection at 59.67 years with a disc space narrowing score of 1.65 was demonstrated (Figure 2), which was 10.77 years older than the average age of menopause in this cohort of Chinese women, i.e., 48.90 years [19]. This menopause age was similar to that of other reports for Chinese women [21], [20].
Figure 2.
Sums of the disc space narrowing scores of L1/2–L4/5 plotted against age. From the age of 65 years to 92 years, females had faster disc space narrowing than males. The slope of the plot for females is 0.111 (95% CI: 0.091/0.131; r2 = 0.056), steeper than that for males (0.083; 95% CI: 0.064/0.102; r2 = 0.036; p = 0.052; ANOVA). Extrapolation towards younger age shows an intersection at 56.69 years with a disc space narrowing score of 1.65. ANOVA = analysis of variance; CI = confidence interval.
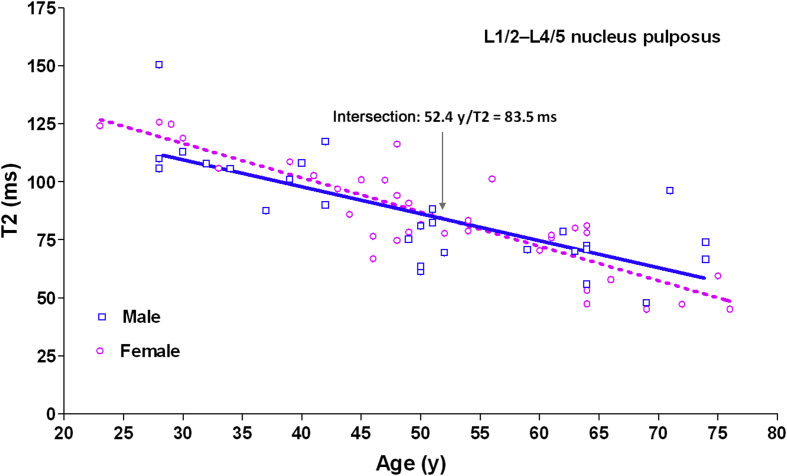
The result of Study II is shown in Figure 3. There was an age-related reduction of T2 value in the nucleus pulposus, as previously reported [15], [22]. This decrease is faster in females. Although females tended to have the initial higher T2 value before 50 years, this trend was reversed in elderly people, with an intersection at 52.4 years (T2 relaxation = 83.5 milliseconds), which was 2.5 years older than the reported average age of menopause for Chinese women (49.0 years or 49.5 years) [21], [20].
Figure 3.
Aging-related reduction of magnetic resonance T2 relaxation time of the nucleus pulposus of L1/2–L4/5 discs for males and females. The slope of the plot for females is −1.48 (95% CI: −1.77/−1.19; r2 = 0.76), steeper than that for males (−1.17; 95% CI: −1.57/−0.76; r2 = 0.57; p = 0.19; ANOVA). Intersection: 52.4 years/T2 relaxation = 83.5 milliseconds. ANOVA = analysis of variance; CI = confidence interval.
Discussion
While young men are more susceptible to disc degeneration than young women, in a cross-sectional radiographic study of individuals aged 55 years and older, de Schepper et al [8] found that disc space narrowing was more prevalent in women than in men. Using an 8-level MR-based disc degeneration grading system to study a cohort of 163 healthy men (mean age, 73.5 years) and 196 healthy women (mean age, 73.2 years), it was shown that elderly female participants had more severe disc degeneration than male participants at all lumbar disc levels [9]. A positive relationship between radiographic lumbar intervertebral disc height and hormone replacement treatment in postmenopausal women has been reported. Untreated menopausal women were found to have the lowest total lumbar disc height, significantly lower than that in both premenopausal women and hormone-treated postmenopausal women [23]. Therefore, a hypothesis was proposed that postmenopausal women may have accelerated disc degeneration due to relative oestrogen deficiency [10], [24].
Radiographic disc space is a direct sign of disc degeneration and has been reported to be associated with back pain [25], [26], [27], [28]. Study I represents the largest study on radiographic disc space narrowing in community-dwelling elderly men and women in China [11]. Both men and women from the same community-based population were investigated, thereby enabling a comparison of men and women. Similar to other reports, our data showed a high prevalence of disc space narrowing in elderly individuals, with its severity being higher in women than in men [8], [9]. The current analysis showed that women of >10.77 years after menopause were likely to experience more severe disc space narrowing than men, when on average each participant was likely to have mild degree of disc space narrowing at one level. In Study II, we evaluated 23–76-year-old individuals, an age range in which a broad spectrum of disc degeneration, including early degeneration, is expected. On T2-weighted MR images, disc degeneration is seen as a reduction in signal of the nucleus pulposus and inner fibres of the annulus. T2 relaxation time measurement has been reported to be sensitive to changes in collagen and water content in the intervertebral discs, and T2 relaxation time decreases with disc degeneration [29]. Age-related reduction of T2 value in the disc region of nucleus pulposus and inner annulus fibrosus has been reported [15], [22]. Study II confirmed that the age-related reduction of T2 value in the nucleus pulposus is faster in females. Although female discs tend to have initial higher T2 value before 50 years, this trend is reversed in postmenopausal females.
Oestrogen was originally thought to be exclusively a sexual hormone implicated mainly in the development of the reproductive system. However, the understanding of its physiological functions has considerably improved in recent decades. Oestrogens participate in a variety of biological processes through different molecular mechanisms [30]. Recent evidence suggests that female sex hormones play an important role in the aetiology and pathophysiology of a variety of degenerative diseases [30]. The prevalence of osteoarthritis (OA) is higher among women than among men, and this prevalence increases considerably after menopause [31], [32]. Moreover, with the same degree of radiographic damage, OA is also more symptomatic in women [31], [32]. The dramatic rise in OA prevalence among postmenopausal women, which is associated with the presence of oestrogen receptors (ERs) in joint tissues, suggests a link between OA and the loss of ovarian function [33], [34], [35]. Oestrogens exert their protective effects via ER-alpha and/or ER-beta in the biological body. Animal models have shown that oophorectomy can induce joint damage, and ER-alpha knockout animals develop more and larger osteophytes, as well as a thinner lateral subchondral plate [36], [37]. A lack of oestrogens may increase subchondral bone remodelling, ultimately inducing OA changes [38]. Similar to its effects on articular cartilage, oestrogen may have important effects on intervertebral disc turnover. Song et al [39] reported that expression of both ER-alpha and ER-beta significantly decreased with the aggravation of disc degeneration in males and females. An immunohistochemistry study showed that both ER-alpha and ER-beta proteins in nucleus pulposus of elderly males were significantly higher than that of females. Sex-specific ER expression might be one possible factor for sexual dimorphism of disc degeneration [39], [40].
While both Study I and Study II demonstrated accelerated disc degeneration in women after menopause, Study I demonstrated that Chinese women tend to have a narrower disc space 10.77 years after menopause, and Study II demonstrated that Chinese women have a lower T2 relaxation value 2.5 years after menopause. In 1997, Chow et al [20] reported the mean age of natural menopause was 49.5 years for Chinese women in Taiwan. In 2005, Loh et al [21] reported that the mean age of natural menopause was 49.0 years (95% confidence interval, 48.61–49.43) for Chinese women in Singapore, and there was no significant difference between three ethnic groups of Chinese, Malay, and Indian. The mean age of natural menopause was reported to be 48.9 years in a cohort from southern mainland China [41], and the mean age of perimenopausal women was reported to be 49.3 years in Hong Kong [42]. While recently it was reported that bilateral oophorectomy leads to a rapid decrease in lumbar bone mineral density, an increase in marrow fat content, and a decrease in marrow blood perfusion in lumbar vertebrae, being most substantial during the first 3 months after surgery [43], it is known that the biological changes associated with natural menopause are much slower [44]. Since young and middle-aged men tend to have more severe lumbar disc degeneration than premenopausal women at similar age, and T2 relaxation value is a more sensitive physiological marker than radiograph [45], it is conceivable that morphological disc space narrowing would take longer for women after menopause to reach the same severity of that in men.
This study has a few limitations. Study I was not primarily designed to assess the effect of oestrogen deficiency on disc generation. Premenopausal females and their male controls were not recruited in this study. However, the large sample size of the cohorts increased our confidence regarding the results. The number of participants for Study II was relatively small, and the information of menopause was not specifically obtained from the female participants. Oestrogen level was not measured in both studies. In the meantime, the recent population-based data consistently reported the average age of natural menopausal Chinese women to be around 49 years. While a linear fit model was used in our study, disc degeneration is not likely to progress linearly with ageing. Another limitation is that the absolute biological age was used for comparison between males and females, and this may not be ideal. Finally, the slope difference between the females and males was close to be significant (p = 0.052) for Study I and not apparently significant for Study II (p = 0.19). Statistical significance depends on the sample size, sample homogeneity (i.e., standard deviation value), and measurement precision. Statistical power could be increased with access to larger sample sizes. Conceptually, this should not affect our conclusion for the study, as two studies from two different cohorts with different methodologies reached similar conclusion.
In conclusion, postmenopausal Chinese women may have accelerated lumbar disc degeneration compared with Chinese men. Oestrogen replacement therapy has been shown to be protective against menopause-associated OA [46], [47]. Evidence also suggests significant protective role of oestrogen against the formation and progression of cerebral aneurysms [48], [49]. Therefore, we postulate that oestrogen replacement therapy may be helpful for patients with symptomatic disc degeneration. However, such an approach remains to be validated in further clinical studies.
Conflicts of interest
The author declares no conflicts of interest.
Funding/support
This study was partially supported by the Research Grants Council of the Hong Kong SAR (Project No.: SEG_CUHK02), and by the National Institute of Health R01 Grant AR049439-01A1 and the Research Grants Council Earmarked Grant CUHK 4101/02 M (via Professor Ping Chung Leung).
Acknowledgements
I thank my colleagues at the Chinese University of Hong Kong for data collection and analysis, particularly Professor Ping Chung Leung, Mr Anthony Kwok, Miss Min Deng, and Dr Xian-Jun Zeng (visiting fellow during 2012 from Nanchang University, Nanchang, China).
References
- 1.Adams M.A., Roughley P.J. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter J.A. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Hangai M., Kaneoka K., Kuno S., Hinotsu S., Sakane M., Mamizuka N. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- 4.Modic M.T., Ross J.S. Lumbar degenerative disk disease. Radiology. 2007;245:43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 5.Miller J.A., Schmatz C., Schultz A.B. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–178. [PubMed] [Google Scholar]
- 6.Łebkowski W.J. Autopsy evaluation of the extent of degeneration of the lumbar intervertebral discs. Pol Merkuriusz Lek. 2002;13:188–190. [In Polish] [PubMed] [Google Scholar]
- 7.Takatalo J., Karppinen J., Niinimäki J., Taimela S., Näyhä S., Järvelin M.R. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine. 2009;34:1716–1721. doi: 10.1097/BRS.0b013e3181ac5fec. [DOI] [PubMed] [Google Scholar]
- 8.de Schepper E.I., Damen J., van Meurs J.B., Ginai A.Z., Popham M., Hofman A. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 2010;35:531–536. doi: 10.1097/BRS.0b013e3181aa5b33. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y.X., Kwok A.W., Griffith J.F., Leung J.C., Ma H.T., Ahuja A.T. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight level MRI-based disc degeneration grading system. Osteoporos Int. 2011;22:91–96. doi: 10.1007/s00198-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y.X., Griffith J.F. Effect of menopause on lumbar disk degeneration: potential etiology. Radiology. 2010;257:318–320. doi: 10.1148/radiol.10100775. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.X., Griffith J.F., Zeng X.J., Deng M., Kwok A.W., Leung J.C. Prevalence and sex difference of lumbar disc space narrowing in elderly Chinese men and women: osteoporotic fractures in men (Hong Kong) and osteoporotic fractures in women (Hong Kong) studies. Arthritis Rheum. 2013;65:1004–1010. doi: 10.1002/art.37857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimura M., Panjabi M.M., Oxland T.R., Crisco J.J., Yamamoto I., Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lane N.E., Nevitt M.C., Genant H.K., Hochberg M.C. Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol. 1993;20:1911–1918. [PubMed] [Google Scholar]
- 14.Kettler A., Wilke H.J. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J. 2006;15:705–718. doi: 10.1007/s00586-005-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y.X., Griffith J.F., Leung J.C., Yuan J. Age related reduction of T1rho and T2 magnetic resonance relaxation times of lumbar intervertebral disc. Quant Imaging Med Surg. 2014;4:259–264. doi: 10.3978/j.issn.2223-4292.2014.07.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y.X., Zhao F., Griffith J.F., Mok G.S., Leung J.C., Ahuja A.T. T1rho and T2 relaxation times for lumbar disc degeneration: an in vivo comparative study at 3.0-Tesla MRI. Eur Radiol. 2013;23:228–234. doi: 10.1007/s00330-012-2591-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.X., Mok G.S.P., Zhang D., Chen S.Z., Yuan J. A comparison of three approaches for defining nucleus pulposus and annulus fibrosus on sagittal MR images. Proc Int Soc Magn Reson Med. 2015;23:1217. [Google Scholar]
- 18.Menezes-Reis R., Salmon C.E., Carvalho C.S., Bonugli G.P., Chung C.B., Nogueira-Barbosa M.H. T1ρ and T2 mapping of the intervertebral disk: comparison of different methods of segmentation. AJNR Am J Neuroradiol. 2015;36:606–611. doi: 10.3174/ajnr.A4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L.C., Wang Y.X., Gong J.S., Griffith J.F., Zeng X.J., Kwok A.W. Prevalence and risk factors of lumbar spondylolisthesis in elderly Chinese men and women. Eur Radiol. 2014;24:441–448. doi: 10.1007/s00330-013-3041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow S.N., Huang C.C., Lee Y.T. Demographic characteristics and medical aspects of menopausal women in Taiwan. J Formos Med Assoc. 1997;96:806–811. [PubMed] [Google Scholar]
- 21.Loh F.H., Khin L.W., Saw S.M., Lee J.J., Gu K. The age of menopause and the menopause transition in a multiracial population: a nation-wide Singapore study. Maturitas. 2005;52:169–180. doi: 10.1016/j.maturitas.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Niu G., Yang J., Wang R., Dang S., Wu E.X., Guo Y. MR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: apparent diffusion coefficient versus T2 quantitation. AJNR Am J Neuroradiol. 2011;32:1617–1623. doi: 10.3174/ajnr.A2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron Y.M., Brincat M.P., Galea R., Calleja N. Intervertebral disc height in treated and untreated overweight post-menopausal women. Hum Reprod. 2005;20:3566–3570. doi: 10.1093/humrep/dei251. [DOI] [PubMed] [Google Scholar]
- 24.Wáng Y.X. Advance modern medicine with clinical case reports. Quant Imaging Med Surg. 2014;4:439–443. doi: 10.3978/j.issn.2223-4292.2014.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raastad J., Reiman M., Coeytaux R., Ledbetter L., Goode A.P. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44:571–585. doi: 10.1016/j.semarthrit.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Andersson G.B. Epidemiology of low back pain. Acta Orthop Scand Suppl. 1998;281:28–31. doi: 10.1080/17453674.1998.11744790. [DOI] [PubMed] [Google Scholar]
- 27.Peterson C.K., Bolton J.E., Wood A.R. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine. 2000;25:218–223. doi: 10.1097/00007632-200001150-00013. [DOI] [PubMed] [Google Scholar]
- 28.Borenstein D.G., O'Mara J.W., Jr., Boden S.D., Lauerman W.C., Jacobson A., Platenberg C. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am. 2001;83-A:1306–1311. doi: 10.2106/00004623-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Marinelli N.L., Haughton V.M., Muñoz A., Anderson P.A. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine. 2009;34:520–524. doi: 10.1097/BRS.0b013e318195dd44. [DOI] [PubMed] [Google Scholar]
- 30.Martín-Millán M., Castañeda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine. 2013;80:368–373. doi: 10.1016/j.jbspin.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence R.C., Helmick C.G., Arnett F.C., Deyo R.A., Felson D.T., Giannini E.H. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Ushiyama T., Ueyama H., Inoue K., Ohkubo I., Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7:560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich W., Haitel A., Holzer G., Huber J.C., Kolbus A., Tschugguel W. Estrogen receptor beta is the predominant estrogen receptor subtype in normal human synovia. J Soc Gynecol Investig. 2006;13:512–517. doi: 10.1016/j.jsgi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Sciore P., Frank C.B., Hart D.A. Identification of sex hormone receptors in human and rabbit ligaments of the knee by reverse transcription-polymerase chain reaction: evidence that receptors are present in tissue from both male and female subjects. J Orthop Res. 1998;16:604–610. doi: 10.1002/jor.1100160513. [DOI] [PubMed] [Google Scholar]
- 36.Ma H.L., Blanchet T.J., Peluso D., Hopkins B., Morris E.A., Glasson S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Sniekers Y.H., van Osch G.J., Ederveen A.G., Inzunza J., Gustafsson J.A., van Leeuwen J.P. Development of osteoarthritic features in estrogen receptor knockout mice. Osteoarthritis Cartilage. 2009;17:1356–1361. doi: 10.1016/j.joca.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Bellido M., Lugo L., Roman-Blas J.A., Castañeda S., Caeiro J.R., Dapia S. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010;12:R152. doi: 10.1186/ar3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X.X., Yu Y.J., Li X.F., Liu Z.D., Yu B.W., Zhen Guo Z. Estrogen receptor expression in lumbar intervertebral disc of the elderly: gender- and degeneration degree-related variations. Joint Bone Spine. 2014;81:250–253. doi: 10.1016/j.jbspin.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Wáng Y.X. Continued progression of lumbar disc degeneration in postmenopausal women. Climacteric. 2015;18:435. doi: 10.3109/13697137.2014.999662. [DOI] [PubMed] [Google Scholar]
- 41.Yang D., Haines C.J., Pan P., Zhang Q., Sun Y., Hong S. Menopausal symptoms in mid-life women in southern China. Climacteric. 2008;11:329–336. doi: 10.1080/13697130802239075. [DOI] [PubMed] [Google Scholar]
- 42.Ho S.C., Chan S.G., Yip Y.B., Cheng A., Yi Q., Chan C. Menopausal symptoms and symptom clustering in Chinese women. Maturitas. 1999;33:219–227. doi: 10.1016/s0378-5122(99)00056-0. [DOI] [PubMed] [Google Scholar]
- 43.Wáng Y.X., Griffith J.F., Deng M., Yeung D.K., Yuan J. Rapid increase in marrow fat content and decrease in marrow perfusion in lumbar vertebra following bilateral oophorectomy: an MR imaging-based prospective longitudinal study. Korean J Radiol. 2015;16:154–159. doi: 10.3348/kjr.2015.16.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pansini F., Bagni B., Bonaccorsi G., Albertazzi P., Zanotti L., Farina A. Oophorectomy and spine bone density: evidence of a higher rate of bone loss in surgical compared with spontaneous menopause. Menopause. 1995;2:109–115. [Google Scholar]
- 45.Griffith J.F. Functional imaging of the musculoskeletal system. Quant Imaging Med Surg. 2015;5:323–331. doi: 10.3978/j.issn.2223-4292.2015.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wluka A.E., Davis S.R., Bailey M., Stuckey S.L., Cicuttini F.M. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis. 2001;60:332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna F.S., Wluka A.E., Bell R.J., Davis S.R., Cicuttini F.M. Osteoarthritis and the postmenopausal woman: epidemiological, magnetic resonance imaging, and radiological findings. Semin Arthritis Rheum. 2004;34:631–636. doi: 10.1016/j.semarthrit.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Stober T., Sen S., Anstätt T., Freier G., Schimrigk K. Direct evidence of hypertension and the possible role of post-menopause oestrogen deficiency in the pathogenesis of berry aneurysms. J Neurol. 1985;232:67–72. doi: 10.1007/BF00313903. [DOI] [PubMed] [Google Scholar]
- 49.Jamous M.A., Nagahiro S., Kitazato K.T., Tamura T., Kuwayama K., Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part II: experimental study of the effects of hormone replacement therapy in rats. J Neurosurg. 2005;103:1052–1057. doi: 10.3171/jns.2005.103.6.1052. [DOI] [PubMed] [Google Scholar]