Summary
Central dual-energy X-ray absorptiometry (DXA) of the lumbar spine and proximal femur is the preferred method for bone mineral density (BMD) testing. Despite the fracture risk statistics, osteoporosis testing with DXA remains underused. However, BMD can also be assessed with quantitative computed tomography (QCT) that may be available when access to DXA is restricted. For patients undergoing a primary CT study of the abdomen or pelvis, a potential opportunity exists for concurrent BMD screening by QCT without the need for any additional imaging, radiation exposure, or patient time.
Keywords: opportunistic, osteoporosis, quantitative computed tomography, screening
Introduction
Central dual-energy X-ray absorptiometry (DXA) of the lumbar spine and proximal femur is the preferred method for bone mineral density (BMD) testing. Despite the fracture risk statistics, osteoporosis testing with DXA remains underused [1], [2]. However, BMD can also be assessed with other radiologic imaging tools, such as quantitative computed tomography (QCT) that may be available when access to DXA is restricted [3]. Originally, QCT was developed as a methodology using single thick (around 10 mm) CT image slices angled to sample the vertebrae and to avoid the cortical end plates. However, this mode of operation has now been largely superseded by the use of volume images covering regions of interest at the spine or hip.
For patients undergoing screening CT colonography (CTC), a potential opportunity exists for concurrent BMD screening by QCT without the need for any additional imaging, radiation exposure, or patient time [4]. In addition, there are a number of indications for CT imaging for which there is a large overlap between the need for a CT scan and a patient having risk factors for osteoporosis. By utilizing volume-based QCT methodology rather than the older single-slice protocols, use may also be made of these CT images for BMD measurement by QCT [5], [6]. Such dual use of CT images could increase screening rates or, alternatively, preclude the need for DXA screening in some individuals.
Previous studies combining standard CT imaging and QCT have generally focused on BMD measurement at the lumbar spine [7], [8] for which QCT provides a volumetric BMD measure of the trabecular vertebral bone in isolation. This can have an advantage of superior sensitivity due to the higher turnover rate of trabecular bone [9], but QCT T-scores on average are somewhat lower than DXA T-scores for the same age, and the established World Health Organization (WHO) classification of osteoporosis by DXA T-score is not appropriate [10]. By contrast, at the proximal femur, QCT three-dimensional (3D) data may be used to derive a projectional two-dimensional (2D) image of the proximal femur, and this image may be analysed using standard DXA region of interests (ROIs) to determine DXA-equivalent “computed tomography X-ray absorptiometry (CTXA)” areal BMD (aBMD) values in g/cm2 [11]. Using this method, the WHO T-score classifications may be applied and the aBMD measures may be included in FRAX calculations.
The workflow associated with such dual use of CT scans may be improved using “phantom-less” or “asynchronous” calibration methods, so that the BMD measurement does not need to be planned in advance of the CT scan. In addition, using such methods, it is possible to make use of archived CT scans retrospectively [12]. Finally, the use of intravenous (IV) contrast-enhanced CT images for QCT is usually contraindicated and a measurement bias has been shown at the spine [13]. However, some recent studies suggest that for measurements made at the hip, the measurement difference due to contrast enhancement may not be clinically significant, further widening the utility of CT scans for BMD measurement [14].
Standard QCT
QCT may be performed on any CT scanner with the use of a calibration phantom and dedicated analysis software. The patient is usually examined in the supine position, lying on the phantom, usually with a water- or gel-filled cushion between the phantom and patient to avoid CT reconstruction (Figure 1). Calibration phantoms are required to transform the attenuation measured in Hounsfield units into BMD values. When the patient and phantom are examined at the same time, the process may be described as “simultaneous calibration”. Three of the most frequently used calibration phantoms for this purpose are the solid-state Cann–Genant phantom (Mindways Software Inc., Austin, TX, USA) containing five potassium phosphate-equivalent density phases; the five-phase solid-state calcium hydroxyapatite phantoms developed by Image Analysis Inc. (Columbia, KY, USA); and the phantom developed by Kalender et al [15] utilizing two calcium hydroxyapatite phases. This latter phantom is used by Siemens (Siemens Healthcare, Erlangen, Germany) for their commercial QCT product. BMD measurements from different types of calibration phantoms are not interchangeable, unless a cross-calibration calculation is performed.
Figure 1.
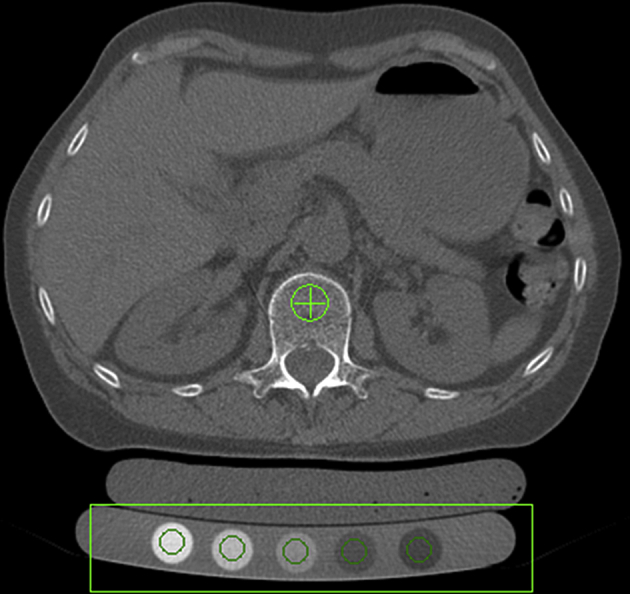
Simultaneous calibration involves the patient lying on top of a calibration phantom, in this case, a five-phase Cann–Genant phantom. A gel-filled bolus bag fills the air gap between the phantom and lumbar region.
Single-slice QCT
Single-slice QCT was the original QCT methodology, which was developed on single-slice CT scanners for trabecular BMD measurements at the lumbar spine. Using the standard methodology, single sections of three to four consecutive vertebrae from T11 to L4 are scanned [16] (Figure 2). A typical acquisition involves 10-mm slice thickness with a gantry tilt used to derive midvertebral sections parallel to the vertebral end plates. The gantry tilt is selected interactively by the technologist from the lateral scout view.
Figure 2.
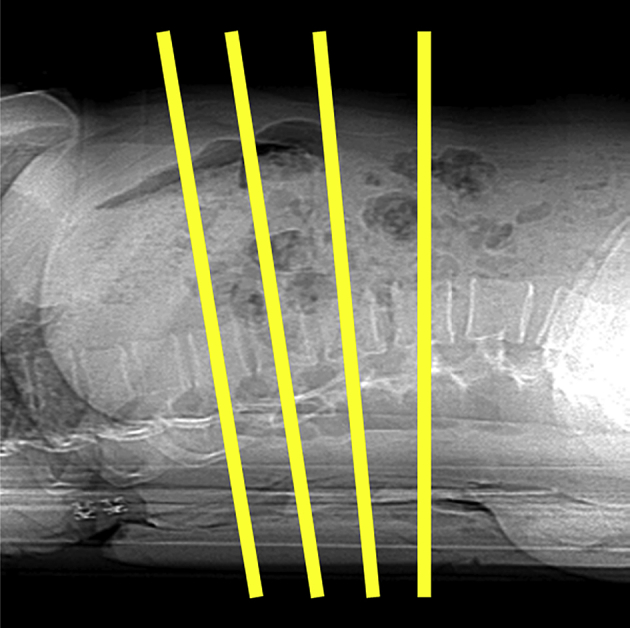
Single-slice quantitative computed tomography protocols typically use a series of three to four 10-mm slices angled parallel to the vertebral end plates.
Single-slice QCT protocols generally have radiation doses that are higher than those of DXA, although these doses are smaller than many other radiographic procedures. Low-dose protocols using 80 kVp (or 120 kVp) and 120 mAs (or 150–200 mAs) result in effective doses of less than 200 μSv [16]. By way of comparison, DXA has radiation doses in the order of 10–15 μSv for the spine and hip. However, QCT has lower exposure doses than many other standard radiology procedures: an anteroposterior lumbar spine radiograph has a dose of 700 μSv and a standard abdominal CT has an exposure dose of the order of 8000 μSv [17].
Although radiation exposure dose can be substantially lower with single-slice QCT compared with volumetric QCT (vQCT), which is described in the next section, a substantial disadvantage with single-slice BMD analysis by 2D QCT is the lower precision compared with that of DXA (1.5–4% vs. 1%), which results in a larger least significant change required to detect significant changes in BMD (6–11% vs. 3%). This, however, is partially offset by the fact that metabolic activity of trabecular bone is higher and that even lower precision single-slice QCT is usually adequate to monitor longitudinal changes that are in the same range as those found with DXA.
Of course, it should be noted that, in the context of opportunistic screening by the dual use of a CT scan, exposure dose is not an issue because a CT scan has already been performed for some other purpose. However, although a single, angled slice could be extracted from a volumetric CT of the abdomen for secondary computation of BMD at the spine, the lower precession means that vQCT is the favoured method.
Volumetric QCT
vQCT or 3D QCT has increased precision and is easier to perform compared with single-slice QCT. A contiguous volume with a slice thickness of 1–3 mm with no CT gantry tilt is typically scanned (Figure 3). At the lumbar spine, protocols usually include only two vertebrae between T11 and L4, often L1 and L2, to reduce dose while achieving measurement precision noninferior or superior to that reported for single-slice QCT [16]. Typical values are in the order of 80–120 kVp and between 50 and 200 mAs. Using these parameters, the dose has been estimated using pharmaceutical clinical trials protocols with 1-mm slice width to be as high as 1.5 mSv for the spine and 2.5–3 mSv for the hip [16]. For a fixed noise level, exposure, and consequently dose, decreases inversely to slice thickness. Thus, doses of under 1 mSv for 2.5–3.0-mm slice thicknesses commonly used for clinical QCT are achievable. This is consistent with lower doses on the order of 0.3–0.6 mSv for the spine described by one of the CT manufacturers [18]. The precision for trabecular BMD measurements by vQCT is superior to 2D slice-based QCT, which is comparable with that of DXA [16].
Figure 3.
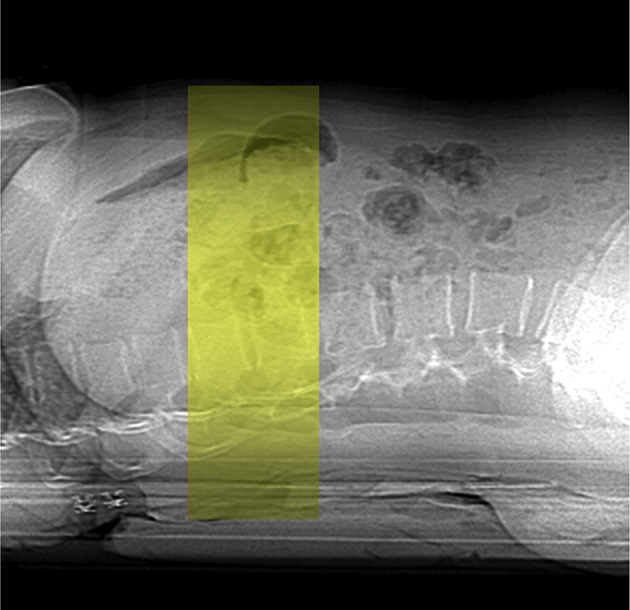
For three-dimensional volumetric quantitative computed tomography protocols, a contiguous volume with a slice thickness of 1–3 mm with no computed tomography gantry tilt is typically scanned.
At the spine, QCT provides a volumetric BMD measure of the trabecular vertebral bone in isolation (Figure 4). This can have an advantage of superior sensitivity because of the higher turnover rate of trabecular bone [9] and can also avoid the confounding effects of joint-space narrowing, osteophytes, aortic calcification, and other extraosseous calcification that can artificially raise a DXA spine BMD measurement [19], [20], [21]. However, the measurement of isolated trabecular bone means that QCT T-scores are somewhat lower, on average, than DXA T-scores for the same age [10], and the established WHO classification of osteoporosis by DXA T-score is not appropriate. To facilitate the interpretation of QCT spine results, the American College of Radiology has in 2008 and 2013 published guidelines for the performance of QCT [22]; based on these guidelines, volumetric trabecular BMD values from 120 mg/cm3 to 80 mg/cm3 are defined as osteopenia and BMD values less than 80 mg/cm3 as osteoporosis.
Figure 4.
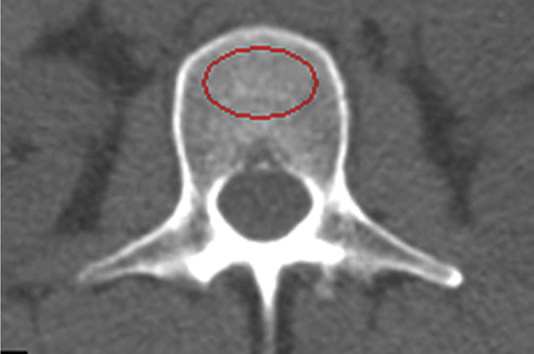
At the spine, a region of interest used measures the trabecular bone mineral density in isolation.
Projectional QCT: Hip
By contrast, at the proximal femur, 3D QCT data may be used to derive a projectional 2D image of the proximal femur (Figure 5), and this image may be analysed using standard DXA ROIs to determine DXA-equivalent CTXA aBMD values in g/cm2. Because the correlations between these calculated BMD values of the proximal femur and those obtained by DXA are extremely high, the WHO T-score classifications may be applied [23]. The precision of projectional hip BMD values has been found to be slightly better than DXA in the same patients [10], [13], [22], probably because of hip rotation being performed by software rather than at the time of acquisition. Areal CTXA BMD measurements from hip QCT are included in the FRAX tool [24], and hip BMD conversion equations are available between Hologic and Lunar DXA and QCT. In addition, the 2008 and 2013 American College of Radiology QCT Practice Guidelines state that QCT at the hip also provides aBMD with DXA-equivalent T-scores.
Figure 5.
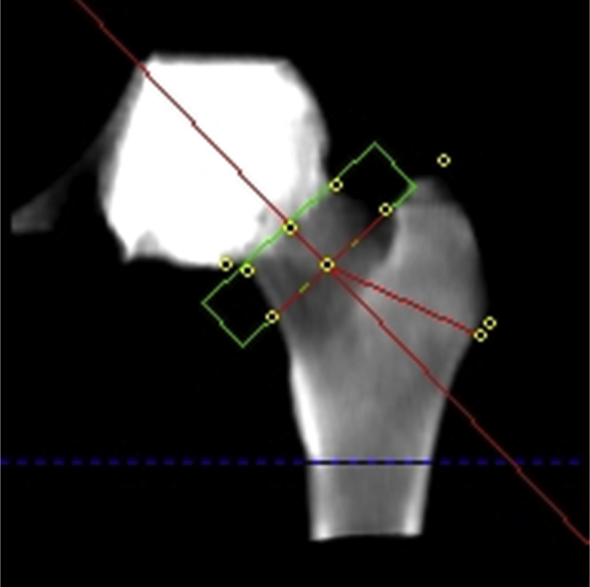
At the hip, computed tomography can produce projectional data that are used with region of interests similar to dual-energy X-ray absorptiometry (DXA), to provide DXA-equivalent areal bone mineral density measurements and T-scores.
Calibration methods
The usual protocol of scanning a calibration phantom with a patient for QCT can be problematic for the dual use of CT images. In particular, the requirement for phantom placement must be known prior to scanning the patient, and the workflow including the patient positioning associated with a regular CT imaging protocol must be altered. There is, therefore, a considerable advantage in not requiring a phantom to be scanned simultaneously with the patient. If a phantom is not scanned simultaneously with the patient, either a separate scan of a calibration phantom (asynchronous calibration) must be performed, or an “internal” or “phantom-less” calibration based on the Hounsfield units of several tissues within the body may be applied.
Although previous studies have indicated that uncalibrated Hounsfield units values from CT scanners may be used for the opportunistic screening of low bone mass [4], the use of a calibration standard means that the derived aBMD results and T-score computations will be consistent across CT scanners from different manufacturers and also consistent at different scanning X-ray energies [25].
Internal BMD calibration
The principal behind QCT utilizing internal calibration is to use CT values of internal tissues as references for the BMD calibration. In general, the method relies on the patient's paraspinal muscle and subcutaneous fat as calibration references and uses the peak of a best-fit Gaussian distribution to the muscle and fat histograms, respectively, for the calibration [26].
Only two technical studies on the use of internal calibration have been published, and both of these were of measurement at the spine [26], [27]. The original method reported by Boden et al [27] was made commercially available by IRIS Inc. (Myersville, MD, USA). This method has been used by Gudmundsdottir et al [28] to investigate age-related vertebral BMD changes in Icelandic women and by Hopper et al [29] to explore the possibility of opportunistic screening using contrast-enhanced and noncontrast abdomen and pelvic scans. The method has been provided as a QCT solution on Philips CT scanners (Philips Healthcare, Cleveland, OH, USA) and has been used in this format by Pickhardt et al [8] for opportunistic screening.
Despite the convenience of using an internal calibration standard, there are some disadvantages to the method. The reproducibility of external phantom systems is generally superior to that of internal calibration systems because of the underlying differences in muscle attenuation related to variations in atrophy, fat infiltration, and hydration [26]. In addition, although Weber et al [14] presented the use of CT scans of inflammatory bowel disease patients for projectional aBMD measurement at the hip, the internal reference standards that were used are not described and no established calibration standards for use at the pelvis for the analysis of BMD at the proximal femur have yet been presented in peer-reviewed literature.
Asynchronous BMD calibration
An alternative to either simultaneous calibration or the use of internal calibration standards is the method of using an external calibration standard but scanned in an “asynchronous” mode at a different time from the patient scan [30]. In a recent study on asynchronous calibration [31], T-scores derived from CTXA were compared with T-scores obtained from DXA. Correlation between DXA and CTXA T-scores was as high as the correlation of T-scores between different DXA scanners, indicating that asynchronous calibration of the CT images was very successful in that study. The period between the CT scans of the patients and those of the external calibration phantom were reported as being approximately 4 years, which indicates good CT scanner stability over this period.
Dual use of CT scans
The usual procedure for a producing a dedicated QCT hip BMD acquisition uses an anterior–posterior computed radiograph or localizer obtained by the scanner from the iliac crest to a few centimetres past the lesser trochanter. At the spine, a lateral localizer is used to define a volume covering two contiguous vertebral levels. A contiguous series of CT images are then acquired from the superior femoral head to approximately 1 cm below the lesser trochanter with a 2.5–3.0-mm slice thickness and spacing between images. For opportunistic screening or dual use of CT images, CT volume images may be sampled using software tools such as SlicePick (Mindways Software Inc., Austin, TX, USA) to produce a simulated projection anterior–posterior image to locate the femoral head and lesser trochanter or to produce a simulated projection lateral image to locate vertebral levels in the same way as using a localizer. A contiguous set of slices is then chosen covering this region for BMD analysis (Figure 6).
Figure 6.
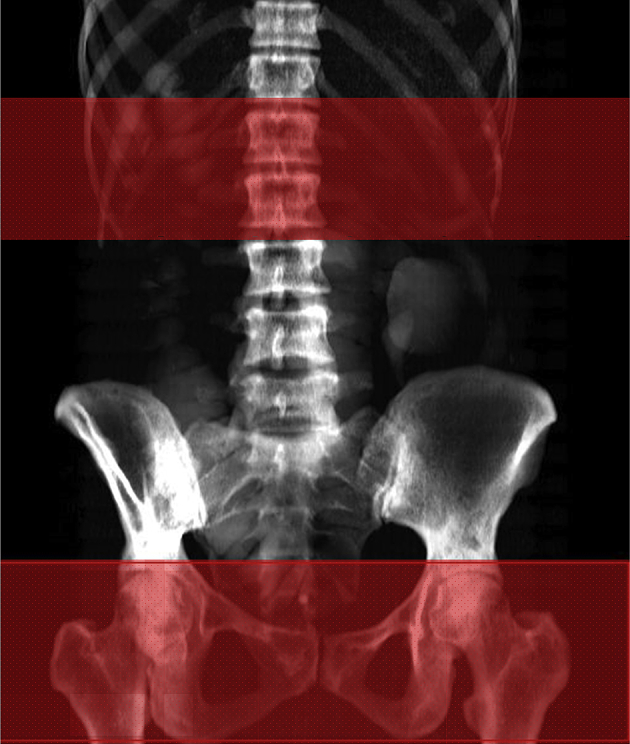
A volume at the hip or spine may be manually segmented from standard computed tomography scans covering a region from the top of the acetabulum to below the lesser trochanter, or a pair of contiguous vertebral levels, using the SlicePick tool from Mindways.
In the context of CTC screening, for which there is an obvious overlap in the screened population due to age [12], the changes required to normalize CTC workflow to facilitate QCT of the hip are easily accommodated and comprise asynchronous calibration scans performed periodically (e.g., monthly), ensuring that the CTC scan volume appropriately includes the entire anorectal junction so that the lesser trochanters are imaged, which is often a standard protocol in CTC imaging.
Influence of contrast media
Many of the existing CT abdominal and pelvic CT scans that might be used for the diagnosis of low BMD have been acquired after IV contrast administration. Because contrast media increase X-ray absorption, it would be expected that BMD values in perfused areas such as the trabecular compartment of the vertebrae will increase, and QCT bone density measurement is contraindicated for patients undergoing IV contrast CT scans. While trabecular bone is measured at the spine, projectional measurement of BMD at the hip incorporates both cortical and trabecular bone. Thus, it is generally expected that vBMD measurement by QCT at the spine should be more affected than aBMD measured by QCT at the hip.
Of the studies to date that investigated the feasibility of dual use of CT images [4], [7], [8], [13], [14], [26], [29], [31], [32], [33], around a half have addressed the issue of dealing with the potential for IV contrast to increase BMD measurement [13], [14], [26], [32], [33]. Most of these previous studies on the effect of IV contrast on QCT measurement have focused on BMD measurement at the lumbar spine, and to date only a few studies have addressed this issue at the hip [13], [14], [33]. In the study by Gruber et al [33], the measurement made was volumetric BMD at the hip and this was compared with DXA measurement. No projection method was employed to measure a DXA-equivalent aBMD from QCT data. Although both the other studies [13], [14] did produce aBMD measurements by projection from vQCT, Weber et al [14] used an internal calibration standard, and Bauer et al [13] used an external calibration standard within the patient scan for simultaneous calibration.
The previous study by Bauer et al [13] used BMD measurements obtained from vQCT of the spine and hip and correlated them with those derived from nondedicated contrast-enhanced standard CT data sets to derive a calibration factor for vQCT. Based on linear regression, a correlation coefficient of r = 0.98 was calculated for lumbar BMD with the equation BMD (QCT) = 0.96 × BMD (MD–CT) – 20.9 mg/cm3. The investigators concluded that with the conversion factors, reliable vBMD measurements can be calculated for the spine from routine abdominal and pelvic CT data sets. However, the absolute numbers show considerable divergence because of the presence of the contrast in the vertebral bone marrow.
Habashy et al [30] reported a similar additive bias of 20 mg/cm3 to 23 mg/cm3 in volumetric spine BMD estimates for a paediatric population. This group reported a cross-calibration equation including compensation for IV contrast volume: BMD (QCT) = BMD (with contrast) – 35.3 mg/cm3 + 0.14 mg/cm3/mL × (IV contrast volume in millilitre). A standard error of the estimate of 10.79 mg/cm3 was reported for the prediction of standard externally calibrated QCT BMD estimates from QCT BMD estimates derived in the presence of IV contrast agent. Assuming a standard deviation of 26 mg/cm3 for a 1 T-score unit change implies a standard error of about 0.4 T-score units for this standard error. This degree of uncertainty compares favourably to a precision of 0.4 T-score units reported by Kiebzak et al [36] for femoral neck T-scores obtained across DXA units from different manufacturers. While evidence exists supporting the notion of screening for low bone mass based on lumbar spine QCT BMD estimates obtained in the presence of IV contrast agent, this finding is tempered by results from Acu et al [34] that show a significant time dependency to such BMD measurements relative to the time since IV contrast agent injection.
At the hip, Bauer et al [13] showed a coefficient of r = 0.99 with the equation BMD (QCT) = 0.99 × BMD (MDCT) – 12 mg/cm2 (p < 0.01). In their study, Weber et al [14] found that DXA- and contrast-enhanced CT enterography-generated T-scores for femoral neck BMD were highly correlated (R2 = 0.84, p < 0.0001), with a linear agreement and no significant offset from the origin. Interestingly, the large study by Pickhardt et al. [4] based on the use of CT values without any calibration stated that IV contrast did not affect the ability of CT to distinguish patients with low BMD from those with normal BMD.
Conclusion
The potential clinical implications of extracting BMD data from routine CT examinations are very wide ranging as virtually any unenhanced abdominopelvic CT scan could presumably be employed. Given the enormous patient volume of body CT scanning currently performed in older adults for a wide variety of clinical indications, this represents a unique opportunity to expand osteoporosis screening. Importantly, this opportunistic screening requires no additional patient time or radiation exposure, and very little time from radiology staff, further enhancing the clinical yield of the CT scan; and one clear advantage of CT over DXA BMD screening is its ability to accurately identify unsuspected osteoporotic compression fractures, which clearly diagnose osteoporosis independent of the patient's DXA T-score [35].
Classically, QCT has utilized an in-scan phantom for simultaneous calibration because of instabilities in CT equipment. In particular, differences in table height from scan to scan of a given patient can at least partly be compensated with a simultaneous calibration. In the last decade or so, CT scanner stability has improved a great deal, which has led to the possibility of asynchronous calibration methods. There are a number of potential advantages in avoiding the need for the placement of a calibration device beneath the patient by allowing for calibration using a phantom to take place asynchronously. Asynchronous calibration can achieve the following:
-
•
Simplify radiology facility workflow by avoiding the need for CT calibration phantom and table pad placement.
-
•
Reduce the risk of needing to rescan a patient due to improper placement of a CT calibration phantom.
-
•
Reduce overall patient exposure to ionizing radiation by combining a medically appropriate bone density study with another medically necessary CT study utilizing the same CT scan covering the abdomen (spine), pelvis (proximal femur), or abdomen and pelvis.
-
•
Allow for retrospective extraction of bone density information from CT scans of the abdomen and/or pelvis acquired for other purposes, again resulting in an overall reduction in exposure of a patient to ionizing radiation.
However, retrospective analysis of existing CT scans must still be treated with some caution without some knowledge of the scanner stability between patient and phantom scans. Further investigation is required to assess the impact that IV contrast might have on BMD measurement by QCT evaluation at both the spine and the hip. However, if the use of postcontrast CT scans can be validated for BMD evaluation, particularly for the use of projectional aBMD at the hip—for which there appears to be little or no significant change—the clinical impact would be accentuated even further.
Conflicts of interest
Both authors are employees and shareholders of Mindways Software Inc., Austin, TX, USA.
References
- 1.Curtis J.R., Carbone L., Cheng H., Hayes B., Laster A., Matthews R. Longitudinal trends in use of bone mass measurement among older americans, 1999–2005. J Bone Miner Res. 2008;23:1061–1067. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warriner A.H., Outman R.C., Feldstein A.C., Roblin D.W., Allison J.J., Curtis J.R. Effect of self-referral on bone mineral density testing and osteoporosis treatment. Med Care. 2014;52:743–750. doi: 10.1097/MLR.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Link T. Axial and peripheral QCT. In: Guglielmi G., editor. Osteoporosis and bone densitometry measurements. Springer; Berlin: 2013. pp. 123–132. [Google Scholar]
- 4.Pickhardt P.J., Pooler B.D., Lauder T., del Rio A.M., Bruce R.J., Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genant H.K., Cann C.E., Ettinger B., Gordan G.S. Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Ann Intern Med. 1982;97:699–705. doi: 10.7326/0003-4819-97-5-699. [DOI] [PubMed] [Google Scholar]
- 6.Kopperdahl D.L., Aspelund T., Hoffmann P.F., Sigurdsson S., Siggeirsdottir K., Harris T.B. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570–580. doi: 10.1002/jbmr.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers R.M., Baecher N., Yao J., Liu J., Pickhardt P.J., Choi J.R. Feasibility of simultaneous computed tomographic colonography and fully automated bone mineral densitometry in a single examination. J Comput Assist Tomogr. 2011;35:212–216. doi: 10.1097/RCT.0b013e3182032537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickhardt P.J., Lee L.J., del Rio A.M., Lauder T., Bruce R.J., Summers R.M. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011;26:2194–2203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams J.E. Quantitative computed tomography. Eur J Radiol. 2009;71:415–424. doi: 10.1016/j.ejrad.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner K.G., von Stetten E., Miller P. Discordance in patient classification using T-scores. J Clin Densitom. 1999;2:343–350. doi: 10.1385/jcd:2:3:343. [DOI] [PubMed] [Google Scholar]
- 11.Cann C.E., Adams J.E., Brown J.K., Brett A.D. CTXA hip—an extension of classical DXA measurements using quantitative CT. PLoS One. 2014;9:e91904. doi: 10.1371/journal.pone.0091904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickhardt P., Bodeen G., Brett A., Brown J.K., Binkley N. Comparison of Lunar DXA and QCT at the femoral neck using asynchronous calibration of CT colonography exams. J Clin Densitom. 2013;16:273–274. doi: 10.1016/j.jocd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Bauer J.S., Henning T.D., Müeller D., Lu Y., Majumdar S., Link T.M. Volumetric quantitative CT of the spine and hip derived from contrast-enhanced MDCT: conversion factors. AJR Am J Roentgenol. 2007;188:1294–1301. doi: 10.2214/AJR.06.1006. [DOI] [PubMed] [Google Scholar]
- 14.Weber N.K., Fidler J.L., Keaveny T.M., Clarke B.L., Khosla S., Fletcher J.G. Validation of a CT-derived method for osteoporosis screening in IBD patients undergoing contrast-enhanced CT enterography. Am J Gastroenterol. 2014;109:401–408. doi: 10.1038/ajg.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalender W.A., Klotz E., Suess C. Vertebral bone mineral analysis: an integrated approach with CT. Radiology. 1987;164:419–423. doi: 10.1148/radiology.164.2.3602380. [DOI] [PubMed] [Google Scholar]
- 16.Engelke K., Adams J.E., Armbrecht G., Augat P., Bogado C.E., Bouxsein M.L. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Damilakis J., Adams J.E., Guglielmi G., Link T.M. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010;20:2707–2714. doi: 10.1007/s00330-010-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer J.S., Virmani S., Mueller D.K. Quantitative CT to assess bone mineral density as a diagnostic tool for osteoporosis and related fractures. MedicaMundi. 2010;54:31–37. [Google Scholar]
- 19.Yu E.W., Thomas B.J., Brown J.K., Finkelstein J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guglielmi G., Floriani I., Torri V., Li J., van Kuijk C., Genant H.K. Effect of spinal degenerative changes on volumetric bone mineral density of the central skeleton as measured by quantitative computed tomography. Acta Radiol. 2005;46:269–275. doi: 10.1080/02841850510012661. [DOI] [PubMed] [Google Scholar]
- 21.Smith J.A., Vento J.A., Spencer R.P., Tendler B.E. Aortic calcification contributing to bone densitometry measurement. J Clin Densitom. 1999;2:181–183. doi: 10.1385/jcd:2:2:181. [DOI] [PubMed] [Google Scholar]
- 22.American College of Radiology . American College of Radiology; Reston, VA: 2013. ACR–SPR–SSR practice guideline for the performance of quantitative computed tomography (QCT) bone. [Google Scholar]
- 23.Khoo B.C., Brown K., Cann C., Zhu K., Henzell S., Low V. Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporos Int. 2009;20:1539–1545. doi: 10.1007/s00198-008-0820-y. [DOI] [PubMed] [Google Scholar]
- 24.Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cann C.E. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166:509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 26.Mueller D.K., Kutscherenko A., Bartel H., Vlassenbroek A., Ourednicek P., Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol. 2011;79:375–381. doi: 10.1016/j.ejrad.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Boden S.D., Goodenough D.J., Stockham C.D., Jacobs E., Dina T., Allman R.M. Precise measurement of vertebral bone density using computed tomography without the use of an external reference phantom. J Digit Imaging. 1989;2:31–38. doi: 10.1007/BF03168013. [DOI] [PubMed] [Google Scholar]
- 28.Gudmundsdottir H., Jonsdottir B., Kristinsson S., Johannesson A., Goodenough D., Sigurdsson G. Vertebral bone density in Icelandic women using quantitative computed tomography without an external reference phantom. Osteoporos Int. 1993;3:84–89. doi: 10.1007/BF01623378. [DOI] [PubMed] [Google Scholar]
- 29.Hopper K.D., Wang M.P., Kunselman A.R. The use of clinical CT for baseline bone density assessment. J Comput Assist Tomogr. 2000;24:896–899. doi: 10.1097/00004728-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Habashy A.H., Yan X., Brown J.K., Xiong X., Kaste S.C. Estimation of bone mineral density in children from diagnostic CT images: a comparison of methods with and without an internal calibration standard. Bone. 2011;48:1087–1094. doi: 10.1016/j.bone.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickhardt P.J., Bodeen G., Brett A., Brown J.K., Binkley N. Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography. J Clin Densitom. 2015;18:5–12. doi: 10.1016/j.jocd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Link T.M., Koppers B.B., Licht T., Bauer J., Lu Y., Rummeny E.J. In vitro and in vivo spiral CT to determine bone mineral density: initial experience in patients at risk for osteoporosis. Radiology. 2004;231:805–811. doi: 10.1148/radiol.2313030325. [DOI] [PubMed] [Google Scholar]
- 33.Gruber M., Bauer J.S., Dobritz M., Beer A.J., Wolf P., Woertler K. Bone mineral density measurements of the proximal femur from routine contrast-enhanced MDCT data sets correlate with dual-energy X-ray absorptiometry. Eur Radiol. 2013;23:505–512. doi: 10.1007/s00330-012-2629-5. [DOI] [PubMed] [Google Scholar]
- 34.Acu K., Scheel M., Issever A.S. Time dependency of bone density estimation from computed tomography with intravenous contrast agent administration. Osteoporos Int. 2014;25:535–542. doi: 10.1007/s00198-013-2440-4. [DOI] [PubMed] [Google Scholar]
- 35.Raisz L.G. Clinical practice. Screening for osteoporosis. N Engl J Med. 2005;353:164–171. doi: 10.1056/NEJMcp042092. [DOI] [PubMed] [Google Scholar]
- 36.Kiebzak G.M., Faulkner K.G., Wacker W., Hamdy R., Seier E., Watts N.B. Effect of precision error on T-scores and the diagnostic classification of bone status. J Clin Densitom. 2007;10(3):239–243. doi: 10.1016/j.jocd.2007.03.002. [DOI] [PubMed] [Google Scholar]


