Summary
Background/Objective
Tanshinol is the main active component of Salvia miltiorrhiza Bunge, a significant Traditional Chinese Medicine used to treat cardiovascular disease. We have shown that tanshinol exerts an antiosteoporostic effect via the enhancement of bone formation in vivo and in vitro. However, the mechanism remains unclear. Based on the polyphenol group in the structure of tanshinol, we speculate the protective action on skeletal tissue is related to antioxidative capacity. Our in vitro evidence indicated that tanshinol stimulated osteoblastic differentiation by its antioxidaive capacity. In this study, we aim to further confirm the effect of tanshinol on bone formation and the underlying mechanism in zebrafish in vivo.
Methods
We used a Danio rerio (zebrafish) model, which has a bone formation process similar to humans, and evaluated the relationship between the dose and the effect of tanshinol on bone formation determined using alizarin red S staining or fluorescence intensity analysis in normal and glucocorticoid (GC)-induced inhibition of an osteogenesis model using wild-type zebrafish and cortical bone transgenic zebrafish tg(sp7:egfp). The expression of osteoblast-specific genes and reactive oxygen species (ROS) were tested.
Results
Our data showed that dexamethasone exerts a series of consequences, including the inhibition of bone formation, decrease of bone mass, downregulation of expression of osteoblast-specific genes (runx2a, ALP, osteocalcin, and sp7), as well as the accumulation of ROS generation and decreased capacity of antioxidants. Tanshinol showed a protective effect on promoting bone formation and bone mass both in wild-type larval zebrafish and transgenic zebrafish. Furthermore, tanshinol attenuated the inhibition of osteogenesis elicited by oxidative stress in the zebrafish exposed to dexamethasone.
Conclusion
The present findings suggest that tanshinol prevented decreased osteogenesis in GC-treated larval zebrafish via scavenging ROS and stimulated the expression of osteoblast-specific genes. Tanshinol treatment may be developed as a novel therapeutic approach under recent recognised conditions of GC-induced osteoporosis.
Keywords: glucocorticoid, osteogenesis, polyphenol, tanshinol, zebrafish
Introduction
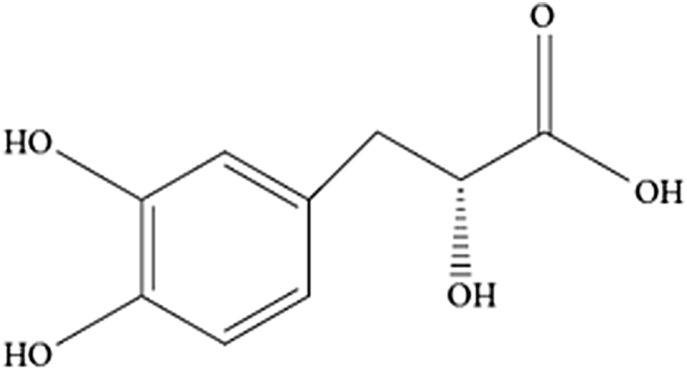
D(+)β-3,4-dihydroxyphenyl lactic acid (tanshinol, also called danshensu), one of the water-soluble components of Salvia miltiorrhiza Bunge, is widely used clinically in China as a principal active ingredient of approved drugs for cardio-cerebral vascular diseases. Literature has highlighted that tanshinol can improve cardiovascular function, promote angiogenesis, enhance microcirculation, and increase blood flow velocity [1]. As a polyphenolic compound, tanshinol shows an antioxidative effect in vivo and in vitro owing to its two phenolic hydroxyl groups (Figure 1), and is thought of as one of the most effective natural product antioxidants [2], [3], [4], [5]. Increasing pharmacological data have indicated that tanshinol protects organisms against oxidative injury [6], [7], [8], [9]. Interestingly, previous studies in our group have indicated that tanshinol ameliorates decreased osteoblasitc function elicited by glucocorticoid (GC) [10]. Our further data demonstrated that the water extract of Salvia miltiorrhiza Bunge consists mainly of tanshinol and salvianolic acid B which are effective in preventing bone loss in rats treated by prednisone [11]. Our recent data further proved that tanshinol attenuates oxidative stress-triggered inhibition of osteoblasitc differentiation [12]. Taken together, as a definite agent to treat cardio-cerebral vascular diseases, tanshinol may be developed as a safe and effective candidate for the treatment of bone disorders including osteoporosis.
Figure 1.
Chemical structure of tanshinol. Tanshinol consists of polyphenolic hydroxyl groups.
Previous evidence reveals a strong relationship between low bone mass and relative risk for cardiovascular disease in postmenopausal women [13]. Further results showed that osteoblasts regulate bone marrow angiogenesis in the process of bone metabolism [14]. Recent data identified a new capillary subtype in the murine skeletal system, coupling angiogenesis with osteogenesis [15]. Interestingly, oxidised lipids accelerate atherogenesis and simutaneously restrain osteoblastic differentiation leading to low bone mineral density [16]. New evidence revealed that oxidative stress elicited by the reaction of the termed advanced-glycation end products and its receptor contributes to the progression of osteoporosis and cardiovascular diseases [17]. Currently, oxidative stress is a widely accepted participant in the pathogenesis of osteoporosis [18]. Therefore, oxidative stress might be developed as a novel therapeutic intervention in osteo-vascular disorders.
Long-term excessive administration of GC is commonly used in the treatment of autoimmune and inflammatory diseases, and often leads to glucorcoticoid-induced osteoporosis (GIO). Previous data highlight that dexamethasone (Dex) can directly or indirectly induce oxidative stress through either inhibition of antioxidant activities or induction of excessive production of reactive oxygen species (ROS) [19]. Oxidative stress hinders osteoblastic differentiation by virtue of the consequence of osteoblastic apoptosis, responsible for the impaired bone formation [20]. Therefore, excessive GC may substantially contribute to bone loss owing to oxidative stress. To date, the medications which control GIO clinically are similar to those used for the treatment of postmenopausal osteoporosis, with different pathological process characterised by the increase in the number and functions of osteoclasts, such as bisphosphonates, raloxifene, parathyroid hormone, and hormone replacement. These agents cannot solve the skeletal problems of the multifactor driven GIO [21], [22], especially under the condition of oxidative stress. Urgently, there is a pressing need to search for an effective therapeutic drug to prevent and treat GIO.
Notwithstanding murine animal models of GIO applied for many years, there are a dozen experimental shortcomings for the research and development of new drugs, including long cycles, large expense, high labour-intensity, etc. In addition, according to the evidence of our laboratory, very distinct bone loss was not detectable in GIO rats [23]. Danio rerio (zebrafish), however, has recently been developed as an ideal animal model characterised by small size and short generation time. In particular, it is suitable for the screening of antiosteoporotic agents because of features similar to humans in terms of bone architecture, bone metabolism, and signalling pathways [24], [25]. For the past decade, a significant delay in the early process of mineralisation was observed in the zebrafish embryo of AB wild-type strains exposed to prednisolone [26]. Hereafter, there are now increasing documents focused on bone formation in zebrafish [27], [28], [29]. To monitor directly the bone formation status, our team adopted a transgenic zebrafish, named tg(sp7:egfp) zebrafish, in which bone tissue expresses green fluorescent protein (GFP) under the control of promoters for the preosteoblast intermediate marker osterix (sp7) [29], [30].
Based on the lines of evidence above, we used juvenile wild-type zebrafish and tg(sp7:egfp) zebrafish as in vivo models to investigate the effects of GC on the dysfunction of bone formation, and to determine the relationship between the dose of tanshinol and its efficacy to intervene abnormal bone metabolism triggered by GC. Meanwhile, we will testify the hypothesis that tanshinol can enhance bone formation via the regulation of gene expression associated with bone metabolism, and attenuate GC-induced inhibition of osteogenesis via the protection of skeletal tissue from oxidative stress. Tanshinol may be developed as a potential candidate for the prevention and/or treatment of osteoporosis.
Materials and methods
Animal husbandry
Wild type zebrafish (AB strain) and osterix:nlsGFP transgenic zebrafish [tg(sp7:egfp)] were treated under standard procedures [31]. Adult zebrafish mate and spawn naturally under the condition of day to night-time controlled at 14:10 hours. The zebrafish embryos and larvae of AB strain and tg(sp7:egfp) were cultured in egg water (5mM NaCl, 0.17mM KCl, 0.4mM CaCl2, 0.16mM MgSO4, and 10 ppm methylene blue) containing 30 ppm N-phenylthiourea and in egg water without N-phenylthiourea under isothermal conditions at 28.5°C, respectively.
Preparations of agent solution
Dex (MP, USA) and Calcitriol Soft Capsule (Rocalirol; Roche, Switzerland) were dissolved in dimethyl sulphoxide (DMSO; Sigma-Aldrich, Japan) as stock solution at a concentration of 10mM and 6 × 10−6 μg/mL, respectively, and were stored at −20°C. Tanshinol (Tianjingshilang, China) was identified and its content was determined using high performance liquid chromatography (HPLC; Agilent, USA; tanshinol with content of 90%) with the standard reference (National Institutes for Food and Drug Control, Beijing, China). Tanshinol was diluted to a final concentration of 50μM in egg water before use.
Experimental designs
At 3 days' post-fertilization (dpf), zebrafish larvae were raised in egg water in 24-well plates and randomly divided into the following groups (n = 12 larvae/2 wells/group). For detection of the role of tanshinol in the process of bone formation, the following concentrations were used: (1) control; (2) 0.5μM tanshinol (T0.5); (3) 1μM tanshinol (T1); (4) 2.5μM tanshinol (T2.5); and (5) 5μM tanshinol (T5). For the investigation of the role of tanshinol in osteogenesis in zebrafish larvae exposed to Dex, the following treatments were executed: (1) vehicle control (0.1% DMSO); (2) 10μM Dex; (3) Dex + 1μM tanshinol (D+T1); (4) Dex + 2.5μM tanshinol (D+T2.5); (5) Dex + 5μM tanshinol (D+T5); (6) Dex + 10μM tanshinol (D+T10); (7) Dex + 50μM tanshinol (D + T50); and (8) Dex + 6 × 10−6 μg/mL rocalirol (D+R). During 3-dpf and 9-dpf, the stock solution of agents were diluted in egg water as indicated. The final volumes of medium/well were adjusted to 1 mL, and 50% of the medium of each well was replaced every day. At 9-dpf, zebrafish larvae were collected. The AB strains were stained with alizarin red (MP, USA), and tg(sp7:egfp) were measured using green fluorescence of head bone, respectively. For quantitative real-time polymerase chain reaction (qRT-PCR) analysis and oxidative stress assay, 3-dpf zebrafish larvae were transferred into six-well plates and randomly divided into three groups (n = 90 larvae/3 well/group) as follows: (1) vehicle control (0.1% DMSO); (2) 10μM Dex; and (3) Dex + 5μM tanshinol. Determinations were performed at 9-dpf after the treatment outlined above.
Alizarin red staining
Bone mineralised matrix deposition, an important indicator of bone formation, was evaluated using alizarin red staining. Alizarin red, a dye which can attach specifically to calcium salts, is widely used to observe and measure mineralisation of bone. At 9-dpf, zebrafish larvae AB strain were collected and fixed for 2 hours in a 4% parformaldehyde solution, then stained with 0.1% alizarin red (MP, USA) in 0.5% potassium hydroxide as described in previous papers [32]. Larval heads were placed on a slide as previously reported [26], and images of the dorsal aspect head bone of zebrafish larvae was photographed using a M205 FA stereo microscope (Leica, Germany) equipped with a DFC310 FX camera (Leica, Germany). The area and integral optical density (IOD) of alizarin red staining were determined using Image-Pro Plus image analysis software version 6.0 (Media Cybernetics, USA). More than nine pieces of zebrafish were used in each group.
Fluorescence imaging
Because the osteoblasts of tg(sp7:egfp) zebrafish express GFP, osteoblast differentiation and bone formation can be directly observed and assessed via fluorescence microscopy. After being fixed in 4% parformaldehyde solution for 2 hours, the bodies of 9-dpf tg(sp7:egfp) zebrafish were divided into two sections. The head was placed in a confocal laser special glass-bottom dish, wrapped in 1% low-melting agarose gel. The fluorescence of GFP in the dorsal aspect head bone tissues of larvae was visualised using a TCS SP5Ⅱconfocal lasers canning microscopy (LSCM, Leica, Germany). High-resolution confocal fluorescence images were analysed using Image-Pro Plus image analysis software version 6 (Media Cybernetics, USA). Fluorescence images were converted to greyscale images, and the fluorescence intensity was converted to grey value. An intensity range was set to include all grey localisations, and the area and the density of this grey localisation were calculated. The same intensity range was applied to all images of larvae. More than nine pieces of zebrafish were used in each group.
qRT-PCR assay
Total RNA from each group of zebrafish larvae (n = 10 larvae/group) was extracted using Trizol reagent (Abcam, USA) according to the manufacturer's instructions. Complementary DNA was synthesised using SuperScript II reverse transcriptase (Invitrogen, USA). qRT-PCR was performed using Roche LightCycler 480 QPCR System (Roche, Switzerland). Paired primers of osteoblast-specific genes (runx2a, ALP, OC, and sp7) are listed in Table 1. The data generated were analysed for cycle threshold (Ct) values using the accompanying Opticon Monitor software. Relative mRNA expression was quantified by subtracting the β-actin Ct value from the Ct value of the genes of interest and expressed as 2−△Ct, as described by the protocol of the manufacturer (n = 3).
Table 1.
Primer sequences for quantitative real-time polymerase chain reaction.
| mRNA | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
|---|---|---|
| runx2a | GACTCCGACCTCACGACAA | CGTCCCGTCAGGAACATC |
| sp7 | AAGAAACCTGTCCACAGCTG | GAGGCTTTACCGTACACCTT |
| ALP | CAGTGGGAATCGTCACAACAA | CCACACAGTGGGCATAAGCA |
| OC | TGGCCTCTATCATCATGAGACAGA | CTCTCGAGCTGAAATGGAGTCA |
Oxidative stress assay
To express the action of tanshinol on rescued osteogenesis under oxidative stress elicited by GC, the indices of oxidative stress including ROS, superoxide dismutase (SOD), malonic dialdehyde (MDA), glutathione peroxidase (GSH), and hydrogen peroxidase (catalase, CAT) were measured using chemical colourimetry according to the manufacturer's instructions (Beyotime Institute of Biotechnology, or Nanjin Jianchen Bioengineering Institute, China). Briefly, 9-dpf zebrafish larvae were washed twice using ultrapure water, and homogenised in 500 μL ultrapure water with a glass homogeniser. The homogenate was centrifuged and the supernatant was collected and stored at −20°C until the assay was performed.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (IBM Corp, NY, USA). Data are presented as mean ± standard deviation, or mean ± standard error of the mean. The statistical differences among various treatments were tested using one-way analysis of variance with Fisher's Least Significant Difference test if the data were normally distributed and had equivalency of variances. Otherwise, Dunn's method for post hoc test was used to perform pairwise comparisons of the treatment groups. If probabilities were less than 0.05 (p < 0.05), statistical differences were considered significant.
Results
Effect of tanshinol on bone mineralisation in intact zebrafish larvae AB strain
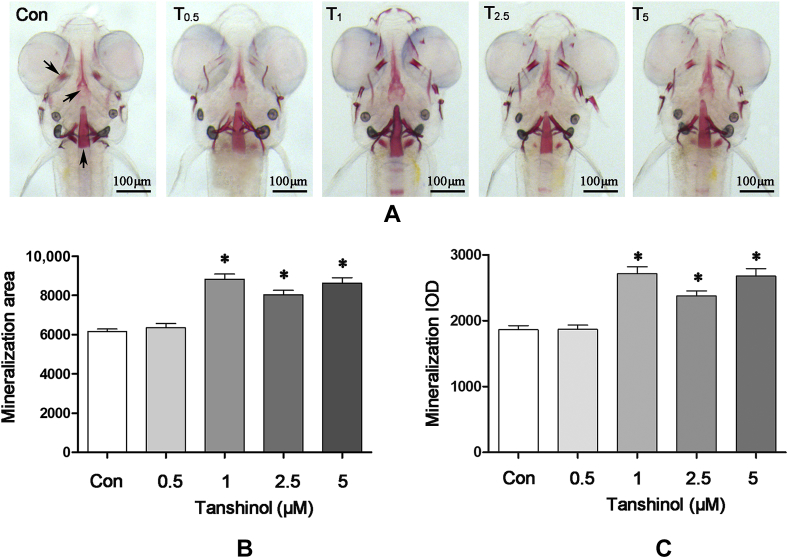
In order to test the effects of tanshinol on zebrafish larval bone formation, zebrafish larvae AB strain were exposed to tanshinol in the increasing concentrations (0.5μM, 1μM, 2.5μM, and 5μM) during 3-dpf and 9-dpf. As shown in Figure 2, the area and IOD of head bone stained with alizarin red was increased in intact zebrafish larvae treated with tanshinol (>0.5μM) compared with the control (p < 0.05). However, tanshinol at the concentrations of 1μM, 2.5μM, and 5μM exerted the same action to promote the increase of the area and IOD of head bone in zebrafish larvae. The data suggests that tanshinol can promote bone formation of cranial bones in zebrafish larval.
Figure 2.
Effects of tanshinol on bone mineralisation of wild-type AB strains zebrafish larval skull. Images of (A) the dorsal aspect head bone stained with alizarin red in wild-type AB strains zebrafish larvae at 9 days' postfertilisation (dpf) with or without exposure to tanshinol (0.5μM, 1μM, 2.5μM, 5μM); (B) the effect of tanshinol on bone mineralisation area in 9-dpf zebrafish; and (C) the effect of tanshinol on bone mineralisation integral optical density in 9-dpf zebrafish. Con = control, egg water; IOD = integral optical density; T0.5 = tanshinol 0.5μM; T1 = tanshinol 1μM; T2.5 = tanshinol 2.5μM; T5 = tanshinol 5μM. Data are given as mean ± standard deviation (n ≥ 9). *p < 0.05 versus control.
Effect of tanshinol on bone mineralisation in zebrafish larvae AB strain treated by Dex
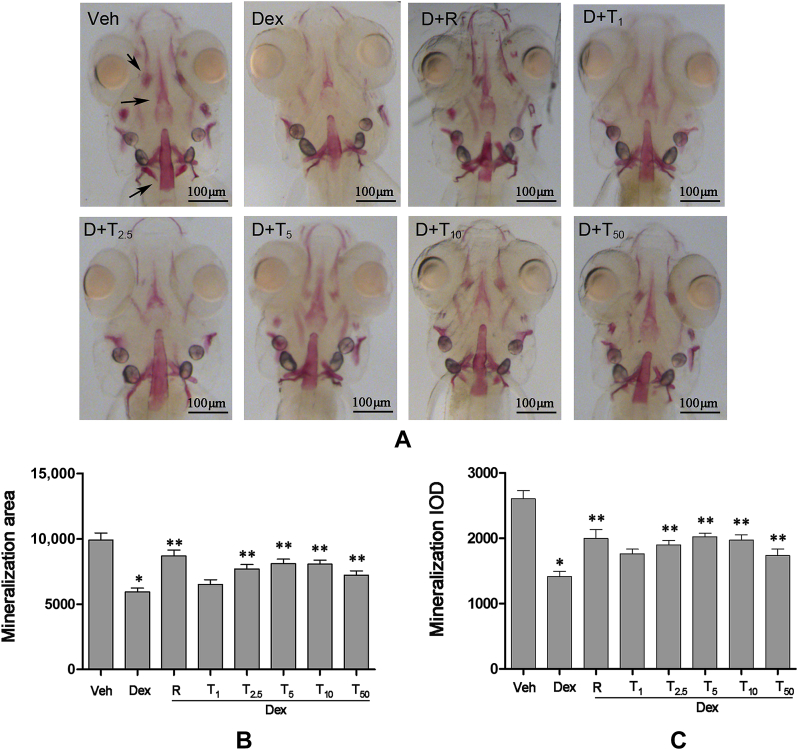
To investigate whether tanshinol can prevent GC-induced inhibition of osteogenesis, zebrafish larvae AB strain during 3-dpf and 9-dpf were treated with tanshinol (1μM, 2.5μM, 5μM, 10μM, and 50μM) in the presence of 10μM Dex. According to the results shown in Figure 3, 10μM Dex elicited a significant decrease of the area and IOD stained with alizarin red in larval skull compared with the control, rocalirol, as a positive control reversed the decrease of the stained area and IOD. Mostly, tanshinol in dosages ranging from 2.5μM to 50μM hampered the inhibition of Dex on the area and IOD of the cranial bone in zebrafish larvae, reaching the climax of the impact at the concentration of 5μM. The result indicates that tanshinol attenuates the Dex-induced inhibition of bone mineralisation in zebrafish larvae.
Figure 3.
Protective effects of tanshinol on head bone mineralisation of wild-type AB strains zebrafish larval exposed to dexamethasone (Dex). Images of (A) the dorsal aspect head bone stained with alizarin red in wild-type AB strains zebrafish larvae at 9 days' postfertilisation (dpf) exposed to Dex (10μM) in the presence or absence of tanshinol; (B) the effect of tanshinol on bone mineralisation area in 9-dpf zebrafish exposed to Dex; and (C) the effect of tanshinol on bone mineralisation integral optical density in 9-dpf zebrafish exposed to Dex. Dex = Dex 10μM; D+R = Dex + rocalirol 6 × 10−6 μg/mL); D+T1 = Dex + tanshinol 1μM; D+T2.5 = Dex + tanshinol 2.5μM; D+T5 = Dex + tanshinol 5μM; D+T10 = Dex + tanshinol 10μM; D+T50 = Dex + tanshinol 50μM; IOD = integral optical density; Veh = vehicle control, 0.1% dimethyl sulphoxide. Data are given as mean ± standard deviation (n ≥ 9). *p < 0.05 versus vehicle control. **p < 0.05 versus Dex treatment.
Effect of tanshinol on osteoblastic differentiation in tg(sp7:egfp) zebrafish
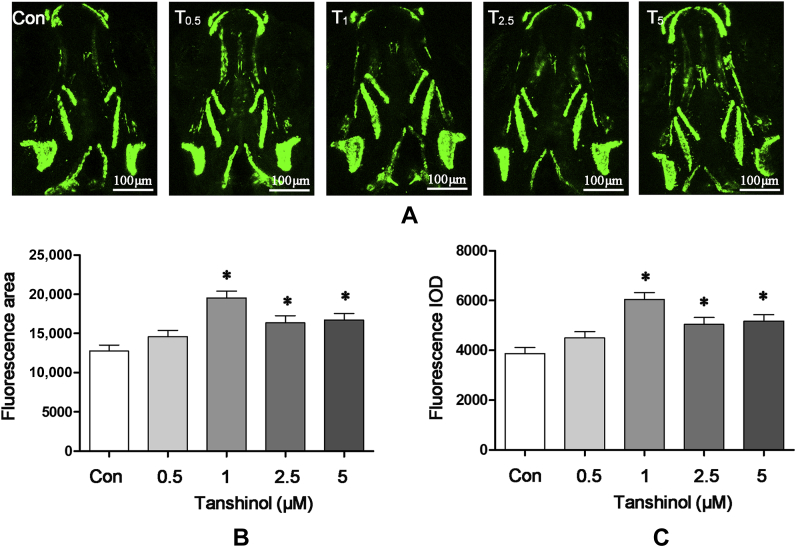
Encouraged by the findings above, we proceeded to explore the action of tanshinol on osteoblastic differentiation in tg(sp7:egfp) zebrafish larvae expressing fluorescent proteins under control of the promoter osterix (sp7), a transcription factor of osteoblastic differentiation. As is shown in Figure 4, tanshinol at the concentrations of 1μM, 2.5μM, and 5μM exerts a significant positive effect on the fluorescence area and intensity of skull in tg(sp7:egfp) zebrafish larvae at 9-dpf treated as zebrafish AB above, using confocal lasers canning microscopy. Results of quantitative analysis further indicated that treatment with 1μM tanshinol lead to the maximal intensity of green fluorescence area and IOD of head bone in tg(sp7:egfp) zebrafish larvae. The data suggest that tanshinol stimulates osteoblasts differentiation in tg(sp7:egfp) zebrafish larvae.
Figure 4.
Effects of tanshinol on osteoblasts differentiation in tg(sp7:egfp) zebrafish larvae. Fluorescence images of (A) the dorsal aspect measured using laser scanning confocal microscopy in tg(sp7:egfp) zebrafish larvae at 9 days' postfertilisation (dpf) with or without tanshinol (0.5μM, 1μM, 2.5μM, 5μM). (B) Effect of tanshinol on green fluorescence area of 9-dpf tg (sp7:egfp) zebrafish. (C) Effect of tanshinol on green fluorescence integral optical density of 9-dpf tg (sp7:egfp) zebrafish. Con = control, egg water; IOD = integral optical density; T0.5 = tanshinol 0.5μM; T1 = tanshinol 1μM; T2.5 = tanshinol 2.5μM; T5 = tanshinol 5μM. Data are given as mean ± standard deviation (n ≥ 9). ∗p < 0.05 versus vehicle control.
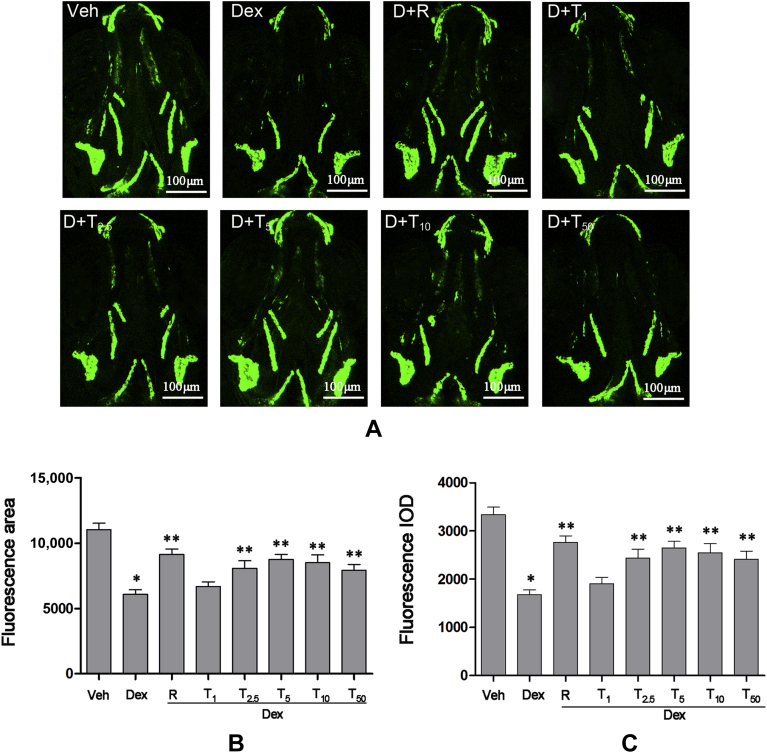
Effect of tanshinol on osteoblastic differentiation in tg(sp7:egfp) zebrafish treated by Dex
Cellular responses induced by Dex may contribute to the suppression of osteoblastic differentiation in zebrafish. Therefore, we examined the protective action of tanshinol (1μM, 2.5μM, 5μM, 10μM, and 50μM) on osteoblastic differentiation measured by the area and intensity of Sp7 green fluorescence in tg(sp7:egfp) zebrafish at 9-dpf. As is shown in Figure 5, tg(sp7:egfp) zebrafish treated with 10μM Dex exhibited a reduction in the fluorescence area and IOD, which was blocked by rocalirol. In line with the evidence achieved from zebrafish larvae AB strain, tanshinol (2.5–50μM) hampered the inhibition of Dex on the fluorescence area and IOD in zebrafish larvae, especially at the concentration of 5μM. The results suggest that tanshinol ameliorates Dex-elicited inhibition of osteoblastic differentiation in tg(sp7:egfp) zebrafish.
Figure 5.
Protective effects of tanshinol counteracting dexamethasone (Dex)-induced inhibition of osteoblasts differentiation in tg(sp7:egfp) zebrafish larvae. (A) Fluorescence images of the dorsal aspect measured with laser scanning confocal microscopy in tg(sp7:egfp) zebrafish larvae at 9 days' post fertilisation (dpf) exposure to Dex (10μM) in the presence or absence of tanshinol. (B) Effect of tanshinol on fluorescence area in 9-dpf tg (sp7:egfp) zebrafish exposed to Dex. (C) Effect of tanshinol on fluorescence integral optical density in 9-dpf tg (sp7:egfp) zebrafish exposed to Dex. Dex = Dexamethasone 10μM; D+R = Dex + rocalirol 6 × 10−6 μg/mL; D+T1 = Dex + tanshinol 1μM; D+T2.5 = Dex + tanshinol 2.5μM; D+T5 = Dex + tanshinol 5μM; D+T10 = Dex + tanshinol 10μM; D+T50 = Dex + tanshinol 50μM; IOD = integral optical density; Veh = vehicle control, 0.1% dimethyl sulphoxide. Data are given as mean ± standard deviation (n ≥ 9). *p < 0.05 versus vehicle control. **p < 0.05 versus Dex treatment.
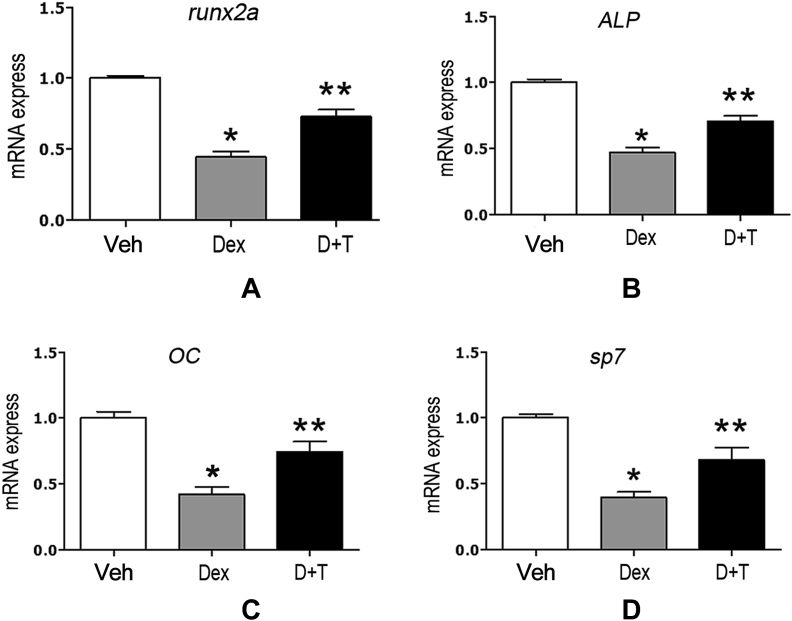
Tanshinol attenuates Dex-elicited downregulation of osteoblast-specific genes expression in zebrafish
To further investigate the protective influence of tanshinol on the process of bone formation under conditions of Dex treatment, the alteration of expressions of osteoblast-specific genes was determined using qRT-PCR assay in zebrafish larvae exposed to 10μM Dex. As shown in Figure 6, zebrafish larvae treated with Dex showed a trend toward reduction of the expression of osteoblast-specific genes, including runx2a, osteocalcin (OC), ALP, and osterix (sp7), compared with the corresponding control. Importantly, tanshinol counteracted the downregulation of osteoblast-specific genes elicited by Dex. The evidence suggests that tanshinol rescues the decreased bone formation triggered by Dex.
Figure 6.
Effect of tanshinol on the expressions of osteoblast-specific genes in zebrafish larvae exposed to dexamethasone (Dex). mRNA levels of (A) runx2a; (B) alp; (C) OC; and (D) sp7 genes in experimental groups were determined with quantitative real-time polymerase chain reaction. Dex = dexamethasone 10μM; D+T = Dex + tanshinol 5μM; Veh = vehicle control, 0.1% dimethyl sulfoxide. Date are given as mean ± standard error of the mean of three independent experiments. *p < 0.05 versus vehicle control. **p < 0.05 versus Dex treatment.
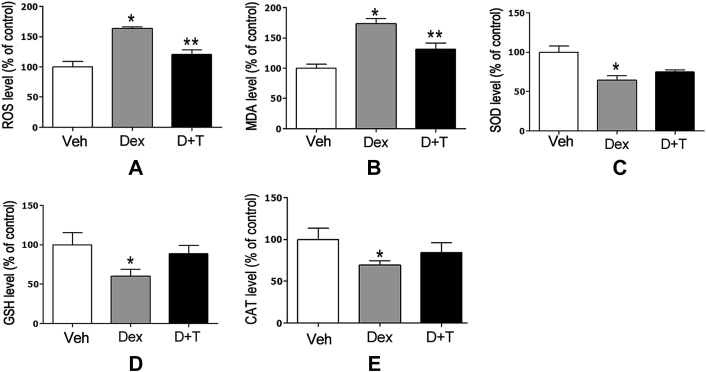
Effect of tanshinol on oxidative stress in zebrafish
Intracellular ROS products are thought of widely as an early response to oxidant stimuli, following a series of deleterious molecule events for oxidative stress. Notwithstanding the inhibitory impact of Dex on osteoblasitc differentiation and bone formation of cranial bones in zebrafish larvae, we next ask whether Dex induced oxidative stress in zebrafish larvae contributes to decreased bone metabolism. As expected, zebrafish larvae exposed to Dex exhibited a remarkable increase of ROS accumulation measured using an oxidation-sensitive fluorescent probe dye, DCFH-DA (Beyotime institute of Biotechnology, China), in line with an increase of MDA level (Figure 7A and B). Meanwhile, the activities of SOD, glutathione peroxidase, and CAT showed a significant trend toward reduction in zebrafish larvae treated by Dex. By contrast, tanshinol hampered these alterations of ROS generation and MDA level initiated by Dex. However, the protective effect of tanshinol on antioxidants mentioned above had no statistical difference compared with the control (Figure 7C, D, and E). Based on the results of our present study, tanshinol diminishes oxidative stress stimulated by Dex in zebrafish larvae.
Figure 7.
Protective effects of tanshinol against dexamethasone-induced oxidative stress level in zebrafish larvae. (A) The accumulation of reactive oxygen species; (B) malondialdehyde level; and the activities of (C) superoxide dismutase; (D) glutathione; and (E) catalase were determined as methods described in oxidative stress assay section of Materials and methods. CAT = catalase; Dex = dexamethasone 10μM; D+T = Dex+tanshinol 5μM; GSH = glutathione; MDA = malondialdehyde; SOD = superoxide dismutase; Veh = vehicle control, 0.1% dimethyl sulphoxide. Data are given as mean ± standard error of the mean of three independent experiments.*p < 0.05 versus vehicle control. **p < 0.05 versus Dex treatment.
Discussion
Extensive studies highlight that the increase of oxidative stress in skeletal tissue impairs osteoblastic differentiation and bone formation, contributing to osteoporosis, which might be hampered by antioxidants [12]. Tanshinol, as a natural antioxidant, is the main effective chemical composition of many clinical therapeutic agents for cardio-cerebro vascular diseases. Previous evidence in our team demonstrated that tanshinol in vitro can stimulate the function of differentiation, proliferation, and mineralisation in osteoblasts, and ameliorate GC-induced bone marrow stromal cell adipogenesis [10], [12]. In the present work, further evidence demonstrates that tanshinol exerts a positive effect on the function of osteoblasitc differentiation and mineralisation both in intact zebrafish larvae AB strain and in tg(sp7:egfp) zebrafish. Meanwhile, tanshinol rescues impaired osteogenesis elicited by Dex, involved in hampering the downregulation of gene expression related to bone formation in zebrafish exposed to Dex, contributing to the protective impact on skull formation. Additionally, tanshinol attenuates accumulated ROS products and increased MDA levels, resulting in antioxidative stress influence under the conditions of Dex in zebrafish. The findings, herein, reveal that tanshinol diminishes the deleterious effects of GC on skeletal tissue during bone formation in zebrafish owing to antioxidative effect.
Based on the present data, we found that the effective concentration of tanshinol to stimulate osteogenesis in zebrafish treated by a vehicle control differs from zebrafish exposed to Dex. In accordance with our previous finding in vitro [12], tanshinol at a concentration of 1μM is beneficial to the stimulation of osteogenesis in zebrafish; however, it exerts no significant action on osteoblastic differentiation and bone formation in either wild-type zebrafish or tg(sp7:egfp) zebrafish in the presence of Dex, whereas 5μM tanshinol is the optimal concentration. The reasons for this difference may involve more molecules of tanshinol being needed to deal with the complicated situation in the two types of zebrafish exposed to Dex, however, it should be investigated in detail. Moreover, we found that zebrafish larvae treated with Dex (5–20μM) show a dose-dependent inhibition of skull formation, and 10μM Dex is more appropriate without death (the data not shown). According to these finding, 5μM tanshinol was taken as a feasible experimental dose to explore the gene expression related to osteogenesis and antioxidative effect in zebrafish larvae treated with Dex at the optimal concentration of 10μM.
The present evidence confirms that tanshinol with polyphenolic hydroxyl groups in the structure exerts the inhibitory action on oxidative stress, in line with our previous finding in C2C12 cells treated with H2O2 [12]. Actually, GC has been described as an oxidant stimulus in previous publications, to which oxidative stress is a susceptive response in skeletal tissue [19], [33], [34] Our evidence demonstrates that Dex initiates excessive ROS accumulation and increased MDA, and simultaneously leads to inhibition of the activity of the anxioxdants including SOD, GSH, and CAT in zebrafish larvae. However, the present data shows that tanshinol diminishes the increase of ROS products and MDA levels, and it exerts no effect statistically on the activities of these antioxidants. The finding suggests that tanshinol, as a strong antioxidant, may fight directly against oxidative stress by itself in zebrafish, instead of the upregulation of the endogenous antioxidants. The mechanism remains to be elucidated. Our previous data revealed that water extract of danshen consists mainly of tanshinol and salvianolic acid B, and helps to promote the expressions of runx2 and β-catenin mRNA and block Dickkopf 1 and peroxisome proliferator-activated receptor mRNA, which contributes to the beneficial influence on osteoblastic differentiation in vitro [11]. Moreover, our new finding showed that tanshinol attenuates oxidative stress via the downregulation of FoxO3a signalling, and rescues the decrease of osteoblastic differentiation through the upregulation of Wnt signal under oxidative stress in C2C12 cells [12]. In this study, we confirmed that tanshinol hampers Dex-initiated downregulation of expression of osteoblast-specific genes, including of runx2a, osteocalcin, ALP, and osterix in zebrafish larva. Collectively, tanshinol rescues decreased bone formation via diminishing the dysregulation of osteoblast-specific genes and attenuates oxidative stress elicited by Dex.
Zebrafish is a new type of ideal model which possesses several advantages, including extrauterine development, small size, short generation time, optically transparent embryos, strong regeneration ability, and genomic conservation between zebrafish and humans [35]. By contrast, rodential animals such as rats, mice, and rabbits have too many limitations including long cycles, large expense, high labour-intensity, limited sensitivity, and unsuitable testing for trace ingredients [23], [36]. In particular, zebrafish had a high similarity with humans in terms of bone architecture, bone cells, matrix proteins, and molecular signalling, suitable for the screening of agents to prevent and treat osteoporosis [24], [25], [26], [27], [28], [29]. Moreover, the cranial bone of zebrafish larvae develops in approximately 1 week from 3-dpf to 9-dpf with two kinds of osteogenesis similar to humans, including endochondral and intramembranous ossification [37]. Interestingly, literature reported previously that there are few tartrate-resistance acid phosphatase (a specificity marker enzyme of differentiation and bone resorption of osteoclast)-positive osteoclasts observed in zebrafish larvae until 20-dpf [38], [39], which indicates that there is osteogenesis or bone formation, however, there is few bone remodelling in zebrafish larvae. Therefore, zebrafish larvae might be more suitable for the research and development of anabolic agents for GIO than other models. Despite the numerous advantages listed above, bone formation of skull cannot be directly observed in the model of wild-type zebrafish larvae, and it is a fine and complicated procedure to stain in a tiny model. In transgenic zebrafish tg(sp7:egfp), sp7 gene is expressed accompanying GFP expression in the process of osteoblast differentiation, and GFP-positive osteoblasts are visible in zebrafish. Therefore, tg(sp7:egfp) can be used to monitor bone formation directly without the shortcomings of wild type zebrafish mentioned above [32]. We have investigated the capacity of osteogenesis of cranial bones in the two types of zebrafish larvae in the study.
In summary, the present evidence indicates that tanshinol protects organisms against oxidative stress elicited by Dex via scavenging excessive accumulation of ROS generation, and simultaneously attenuates the inhibitory effect of Dex on osteoblastic differentiation and mineral bone formation of cranial bone via hampering of the dysregulation of osteoblastic-specific genes in wild-type zebrafish larvae and in tg (sp7:egfp) zebrafish larvae. In fact, tanshinol with the phenolic hydroxyl group exerts therapeutic efficacy in cardio-cerebral vascular diseases for 10 years as reported in clinical practice of China, owing to strong antioxidative and antiapoptotic actions on cells and tissues [11], [12], [40]. Based on our previous evidence, tanshinol exhibits a significant protective influence on skeletal tissue, and may be developed as a safe and effective candidate for the prevention of and therapeutic applications in bone disorders including osteoporosis. The action of tanshinol in relation to the stimulation of bone formation is taken as an acceptable anabolic pharmacological benefit, exhibiting an inspiring trend in the research and development of therapeutic agents for GIO.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Funding/support
This research was supported with grants from the National Natural Science Foundation of China (81273518), the Science and Technology Planning Project of Guangdong Province (2012B060300027), and Scientific Research Subject of Traditional Chinese Medicine Bureau of Guangdong Province (20152152), China.
Acknowledgements
The authors are thankful to Dr Chung-Der Hsiao's group from Chung Yuan Christian University, Taiwan for use of the tg (sp7:egfp) zebrafish.
Contributor Information
Jingjing Zhang, Email: gdmccrc@163.com.
Liao Cui, Email: cuiliao@163.com.
References
- 1.Adams J.D., Wang R., Yang J., Lien E.J. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med. 2006;1:3–7. doi: 10.1186/1749-8546-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B.L., Jiang W., Zhao Y., Hou J.W., Xin W.J. Scavenging effects of salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int. 1996;38:1171–1182. [PubMed] [Google Scholar]
- 3.Liu J., Yang C.F., Lee B.L., Shen H.M., Ang S.G., Ong C.N. Effect of Salvia miltiorrhiza on aflatoxin B1-induced oxidative stress in cultured rat hepatocytes. Free Radic Res. 1999;31:559–568. doi: 10.1080/10715769900301131. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X., Cheung C.M., Yang J.M., Or P.M., Lee W.Y., Yeung J.H. Danshen (Salvia miltiorrhiza) water extract inhibits paracetamol-induced toxicity in primary rat hepatocytes via reducing CYP2E1 activity and oxidative stress. J Pharm Pharmacol. 2015;67:980–989. doi: 10.1111/jphp.12381. [DOI] [PubMed] [Google Scholar]
- 5.Chong C.M., Zhou Z.Y., Razmovski-Naumovski V., Cui G.Z., Zhang L.Q., Sa F. Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci Lett. 2013;543:121–125. doi: 10.1016/j.neulet.2013.02.069. [DOI] [PubMed] [Google Scholar]
- 6.Wu L., Qiao H., Li Y., Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–658. doi: 10.1016/j.phymed.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Hu J., Li Y.L., Li Z.L., Li H., Zhou X.X., Qiu P.C. Chronic supplementation of paeonol combined with danshensu for the improvement of vascular reactivity in the cerebral basilar artery of diabetic rats. Int J Mol Sci. 2012;13:14565–14578. doi: 10.3390/ijms131114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Wang J.W., Xu Y., Zhang K., Yi B., Sun J. Effect of beta-(3,4-dihydroxyphenyl)lactic acid on oxidative stress stimulated by high glucose levels in human peritoneal mesothelial cells. J Int Med Res. 2012;40:943–953. doi: 10.1177/147323001204000313. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y., Wang M., Le X., Meng J., Huang L., Yu P. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011;18:1024–1030. doi: 10.1016/j.phymed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Cui L., Liu Y.Y., Wu T., Ai C.M., Chen H.Q. Osteogenic effects of D+beta-3,4-dihydroxyphenyl lactic acid (salvianic acid A, SAA) on osteoblasts and bone marrow stromal cells of intact and prednisone-treated rats. Acta Pharmacol Sin. 2009;30:321–332. doi: 10.1038/aps.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui L., Li T., Liu Y., Zhou L., Li P., Xu B. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One. 2012;7:e34647. doi: 10.1371/journal.pone.0034647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y.J., Su Y.J., Wang D., Chen Y.H., Wu T., Li G. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signalling. Oxid Med Cell Longev. 2013;2013:1–18. doi: 10.1155/2013/351895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanko L.B., Christiansen C., Cox D.A., Geiger M.J., McNabb M.A., Cummings S.R. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 14.Schipani E., Wu C., Rankin E.B., Giaccia A.J. Regulation of bone marrow angiogenesis by osteoblasts during bone development and homeostasis. Front Endocrinol (Lausanne) 2013;85:1–6. doi: 10.3389/fendo.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parhami F., Morrow A.D., Balucan J., Leitinger N., Watson A.D., Tintut Y. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S.I. Bone metabolism and cardiovascular function update. Impairment of osteo-vascular interaction by glyco-oxidative stress. Clin Calcium. 2014;24:85–91. [PubMed] [Google Scholar]
- 18.Manolagas S.C. From oestrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida M., Han L., Ambrogini E., Weinstein R.S., Manolagas S.C. Glucocorticoids and tumour necrosis factor alpha increase oxidative stress and suppress Wnt protein signalling in osteoblasts. J Biol Chem. 2011;286:44326–44335. doi: 10.1074/jbc.M111.283481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein R.S. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010;46:564–570. doi: 10.1016/j.bone.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S., Huang J., Zheng L., Liu Y., Liu G., Li N. Glucocorticoid-induced osteoporosis in growing rats. Calcif Tissue Int. 2014;95:362–373. doi: 10.1007/s00223-014-9899-7. [DOI] [PubMed] [Google Scholar]
- 24.Barbazuk W.B., Korf I., Kadavi C., Heyen J., Tate S., Wun E. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000;10:1351–1358. doi: 10.1101/gr.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barut B.A., Zon L.I. Realizing the potential of zebrafish as a model for human disease. Physiol Genomics. 2000;2:49–51. doi: 10.1152/physiolgenomics.2000.2.2.49. [DOI] [PubMed] [Google Scholar]
- 26.Barrett R., Chappell C., Quick M., Fleming A. A rapid, high content, in vivo model of glucocorticoid-induced osteoporosis. Biotechnol J. 2006;1:651–655. doi: 10.1002/biot.200600043. [DOI] [PubMed] [Google Scholar]
- 27.Fleming A., Sato M., Goldsmith P. High-throughput in vivo screening for bone anabolic compounds with zebrafish. J Biomol Screen. 2005;10:823–831. doi: 10.1177/1087057105279952. [DOI] [PubMed] [Google Scholar]
- 28.Yu P.B., Hong C.C., Sachidanandan C., Babitt J.L., Deng D.Y., Hoyng S.A. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N., Felber K., Elks P., Croucher P., Roehl H.H. Tracking gene expression during zebrafish osteoblast differentiation. Dev Dyn. 2009;238:459–466. doi: 10.1002/dvdy.21838. [DOI] [PubMed] [Google Scholar]
- 30.DeLaurier A., Eames B.F., Blanco-Sanchez B., Peng G., He X., Swartz M.E. Zebrafish sp7:EGFP: a transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. Genesis. 2010;48:505–511. doi: 10.1002/dvg.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerfield M. 4th ed. University of Oregon Press; Oregon: 2000. The zebrafish book. [Google Scholar]
- 32.Walker M.B., Kimmel C.B. A two-colour acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- 33.Hurson C.J., Butler J.S., Keating D.T., Murray D.W., Sadlier D.M., O'Byrne Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord. 2007;8:1–12. doi: 10.1186/1471-2474-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camm E.J., Tijsseling D., Richter H.G., Adler A., Hansell J.A., Derks J.B. Oxidative stress in the developing brain: effects of postnatal glucocorticoid therapy and antioxidants in the rat. PLoS One. 2011;6:e21142. doi: 10.1371/journal.pone.0021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldsmith P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol. 2004;4:504–512. doi: 10.1016/j.coph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo K. Glucocorticoid and bone. Osteocytic osteolysis: potential modulation by glucocorticoids. Clin Calcium. 2014;24:1337–1342. [PubMed] [Google Scholar]
- 37.Haga Y., Dominique V.R., Du S.J. Analysing notochord segmentation and intervertebral disc formation using the twhh:gfp transgenic zebrafish model. Transgenic Res. 2009;18:669–683. doi: 10.1007/s11248-009-9259-y. [DOI] [PubMed] [Google Scholar]
- 38.Witten P.E., Hansen A., Hall B.K. Features of mono- and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol. 2001;250:197–207. doi: 10.1002/jmor.1065. [DOI] [PubMed] [Google Scholar]
- 39.Chen B., Yan Y.L., Liu C., Bo L., Li G.F., Wang H. Therapeutic effect of deferoxamine on iron overload-induced inhibition of osteogenesis in a zebrafish model. Calcif Tissue Int. 2014;94:353–360. doi: 10.1007/s00223-013-9817-4. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Xie Y.H., Yang Q., Wang S.W., Zhang B.L., Wang J.B. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7:e48872. doi: 10.1371/journal.pone.0048872. [DOI] [PMC free article] [PubMed] [Google Scholar]