Abstract
Alstonia scholaris is one of the most important medicinal plants and herein, we present the synthesis of zinc oxide nanoparticles using the bark extract of Alstonia scholaris, and evaluation of their antimicrobial efficacy. Stable ZnO nanoparticles were formed by treating 90 mL of 1 mM zinc nitrate aqueous solution with 10 mL of 10% bark extract. The formation of Alstonia scholaris bark extract mediated zinc oxide nanoparticles was confirmed by UV–visible spectroscopic analysis and recorded the localized surface plasmon resonance (LSPR) at 430 nm. Fourier transform infrared spectroscopic (FT-IR) analysis revealed that primary and secondary amine groups in combination with the proteins present in the bark extract is responsible for the reduction and stabilization of the ZnONPs. The crystalline phase of the nanocrystals was determined by XRD analysis and morphology was studied using transmission electron microscopy (TEM). The hydrodynamic diameter (26.2 nm) and a positive zeta potential (43.0 mV) were measured using the dynamic light scattering technique. The antimicrobial activity of Alstonia scholaris ZnONPs was evaluated (in-vitro) using disc diffusion method against fungi, Gram-negative and Gram-positive bacteria which were isolated from the biofilm formed in drinking water PVC pipelines. The results obtained suggested that ZnO nanoparticles exhibit a good anti-fungal activity than bactericidal effect towards all pathogens tested in in-vitro disc diffusion method (170 ppm, 100 ppm and 50 ppm). Further, the toxicity of biosynthesized ZnONPs was tested against Alstonia scholaris to evaluate the cytotoxic effect that displayed LC50 value of 95% confidence intervals.
Keywords: Zinc oxide nanoparticles, Fungal sp, Alstonia scholaris bark extract, Gram negative and Gram positive bacteria, Brine shrimp assay
Highlights
-
•
It is a first report on the antimicrobial and brine shrimp lethality assay of phytogenic nano zinc oxide particles.
-
•
The results revealed the possible mechanism of antimicrobial activity of ZnO nanoparticles.
1. Introduction
Nanobiotechnology, a branch of nanoscience has been playing a decisive role in 21st century in deciphering diverse tribulations particularly in the fields of farming, medicine and electronics. Nanoscience poses a basic scientific challenge as it requires a control over the connections between atoms. All physiochemical methods of nanoparticles synthesis are having inherent limitations up to a certain extent which impose an important hurdle in the maturation of this science. The possibility of utilizing biological materials for nanoparticles synthesis has appeared as the most efficient and greener approach [1]. Nanomaterials exhibit unique and considerably changed physical, chemical, and biological properties compared to their bulk counterparts [2]. Although physical and chemical methods [3] are more popular for nanoparticle synthesis, the use of toxic compounds limits their applications [4]. Indeed, over the past several years, plants, algae, fungi, bacteria, and viruses have been used for production of metallic nanoparticles [5]. Green synthesis of metallic nanoparticles from plants [6] is been an interesting aspect as the process is ecofriendly and non-toxic. Plant and plant materials have become potential sources for the synthesis of metallic nanoparticles recently. A number of researchers have reported on synthesis of metallic nanoparticles including silver [7], gold [8], titanium dioxide [9], tungsten oxide [10], and copper oxide [11] using different plant materials.
Due to the amenability to biological functionalization, the biological nanoparticles are finding important applications in the field of medicine. The antimicrobial potential of metal based nanoparticles has led to its incorporation in consumer, health-related and industrial products. Use of substances with antimicrobial properties is known to have been common practice for at least 2000 years. The discovery, development and clinical use of antimicrobials during the 20th century have substantially reduced mortality from microbial (bacterial, fungal, viral and parasitic) infections. An antimicrobial kills the microorganisms and inhibits their growth. They are classed according to their function as anti-bacterial, anti-fungal, anti-viral and anti-parasitic. Antimicrobials that kill microbes are called microbicidal and those inhibit their growth are called micro biostatic. The use of higher plants and their preparation to treat infectious and non‐infectious disease is an age old practices and are the only method available in the past. Though the use of natural sources like plant material for curing diverse forms of ailments leads to human civilization, the scientific analysis of different natural sources for their possible medicinal potency is comparatively recent origin. The emergence and spread of antibiotic resistance microorganisms triggered the search of new materials through diverse sources including investigations on plants. Higher plants can serve as both potential antimicrobial crude drugs and a source of new anti-infective agents. Like many other medicinal plants, Alstonia scholaris (L.) R.Br. (Apocynaceae) is an evergreen tropical tree native to Indian subcontinent and South East Asia having grayish rough bark and milky sap rich in poisonous alkaloid. The bark also called dita bark is traditionally used by many ethnic groups of Northeast India and also other parts of the world as an antimicrobial agent against fungal infections, malarial fever, toothache, rheumatism, snake bite, dysentery, bowl disorder, etc., and the latex is used in treating coughs, through sores and fever [12], [13]. Among the several genera of Alstonia, only scholaris species has been studied for antimicrobial potency [14]. Silver has long been recognized as having an inhibitory effect toward many bacterial strains and microorganisms commonly present in medical and industrial processes [15]. Many attempts have been made to use silver nanoparticles as an anticancer agent, and they have all turned up positive [16], [17]. The role of ZnO nanoparticles as an anticancer agent should open new doors in the field of medicine.
However, hardly reports are available on antimicrobial activity of zinc nanoparticles synthesized using the aqueous extract of this plant. There are no reports on antibacterial, antifungal activity and brine shrimp assay of aqueous extract of this Alstonia scholaris plant through Zinc oxide nanoparticles. The present work therefore, attempts to evaluate the antimicrobial activity of the aqueous extract towards pathogenic bacteria and fungi by in-vitro disc diffusion method.
2. Materials and methods
2.1. Chemicals
Zinc nitrate (> 99% pure) was purchased from Sigma Aldrich, India. Potato dextrose broth, Potato dextrose agar, Nutrient broth, Nutrient agar plate, was supplied by Hi-media, India.
2.2. Collection of biofilm formed in poly vinyl chloride (PVC) pipes
The PVC Biofilm samples were collected from four different regions located in and around Tirupati, (Chittoor District) Andhra Pradesh, India. The samples were collected from drinking water PVC pipelines and taken in the sterile container. The collected samples were in amorphous form. These samples were stored in an ice box for further characterization.
2.3. Collection of plant material
Healthy plant of Alstonia scholaris was collected from Mumbai, Maharashtra, India. The identity of the plant was confirmed by Agarkar Research Institute, Pune, India. A voucher specimen (No. AHMA-23537) has been deposited for future reference. From the selected plant bark was collected by scrapping the trunk using neat and clean knife during the month of May, 2016 and collected material was carefully washed and dried at 45 °C to constant weight. The dried bark of plant material were powdered, passed through a BSS no. 85-mesh sieve and stored in air tight container.
2.4. Preparation of aqueous bark extract
The collected Alstonia scholaris bark was allowed to shade dried for 48 h and was ground to get fine powder. Then, 10 g of powder was mixed with 100 mL of distilled water and boiled for 40 min. After that, the extract was filtered by using Whatman No. 1 filter paper and collected the filtrate in plastic bottle and stored at 4 °C for further characterization and experimentation.
2.5. Isolation of fungal and bacterial sp. from drinking water pipeline
Eight fungal species and ten bacterial samples were isolated from drinking water supply PVC pipelines in Tirupati, Chittoor district, AP, India. Through serial dilution pour plate technique, fungal sp. was isolated using potato dextrose agar (PDA) medium and Gram-negative and Gram-positive bacteria were isolated from nutrient agar medium. Further, it is maintained in potato dextrose agar slants (fungi) and nutrient agar slants (bacteria) for onward analysis [18]. Further, it is maintained in potato dextrose agar slants (fungi) and nutrient agar slants (bacteria) for onward analysis. Morphologically dissimilar colonies were selected randomly from all plates and isolated colonies were purified using appropriate medium by streaking methods. Extraction of DNA from Bacterial amplification by using PCR approximately 1300 bp of a consensus 16S rRNA gene: forward primer 63f (51-CAG GCC TAA CAC ATG CAA GTC-31) and reverse primer 1387r (51-GGG CGG WGT GTA CAA GGC-31) Primers 27f and 1392r were also used and Fungal genomic DNA was extracted by using the CTAB fungal amplification by using PCR approximately 200-bp with primer ITS-1f (51-CAACTCCCAAACCCCTGTGA-31) and reverse primer ITS-4r (51-GCGACGATTACCAGTAACGA-31). Then the pure cultures of bacterial and fungal species were sent to sequence analysis, raw sequences were edited and assembled using the Auto Assembler program (V5.2). All the sequences were used to identify the bacteria and fungi with the help of the BLASTn program http://www.ncbi.nlm.nih.gov/BLAST.
2.6. Preparation of Alstonia scholaris zinc oxide nanoparticles
90-mL aqueous solution of 1.0 × 10−3 M Zinc nitrate was mixed with a 10-mL of 10% aqueous solution of Alstonia scholaris bark extract. The samples were then centrifuged using REMI K70 at 12,000 rpm for 15 min to get clear supernatant. The initial concentration of the Alstonia scholaris ZnONPs was measured using inductively coupled plasma optical emission spectrophotometer (ICP-OES) and was found to be 170 ppm. Then, by diluting this solution, each sample of different concentration was used to investigate the concentration dependence of the antibacterial effect of ZnONPs. These Alstonia scholaris Zinc nanoparticles were characterized by using the techniques such as, Fourier transform infrared spectrophotometry (FT-IR), UV–Vis spectrophotometry, Dynamic light scattering (particle size, zeta potential), (XRD) X-ray diffraction and transmission electron microscopy (TEM).
2.7. Measurement of concentration of ZnONPs using inductively coupled plasma optical emission spectrophotometer (ICP-OES)
The concentrations of the Alstonia scholaris bark-extract-mediated ZnONPs were measured using ICP-OES (Prodigy XP, Leeman Labs, USA). The samples were prepared with 10 times dilution after centrifugation at 4000 rpm for 15 min. Then, 20 mL of aliquot was loaded to the racks of automatic sampler and estimated the concentration of ZnONPs thrice.
2.8. Assay for antimicrobial activity of Alstonia scholaris ZnO nanoparticles against microorganisms (Fungi and bacteria)
The antimicrobial activity of Alstonia scholaris ZnONPs was determined on the basis of colony formation (CFU) by in vitro petri dish assays (disc diffusion). Each fungal and bacterial isolates was cultured on growth media that induced prolific conidia and bacterial production. The fungi isolates were grown on potato dextrose agar medium, and bacterial isolates were grown on nutrient agar medium. Conidia were collected from cultures that were incubated at 37 °C for 10 days (fungi), and bacterial cultures were collected from cultures that were incubated at 37 °C for 2 days for (bacteria) and diluted with sterile, deionized water to a concentration of 106 spores mL-1. Aliquots of the conidial suspension and bacterial suspension were mixed with serial concentrations of Zinc preparations to a final volume of 1 mL and were also mixed with sterile, deionized water as control. A 10 µl subsample of the conidia and Alstonia scholaris ZnO mixture stock was taken at 50 ± 0.9, 100 ± 1.1 and 170 ± 1.4 ppm after ZnO treatments and diluted 100-fold with the deionized water. A 10 µl aliquot of the diluted spore suspension was spread on PDA (Becton, Dickson and Company, Sparks, MD) medium. Three PDA plates for fungi and three NA plates for bacteria per each combination of exposure Alstonia scholaris ZnO concentration were tested. The filter paper disc dipped in different ppm and inserted on potato dextrose agar mediums, and then, the plates were incubated at 37 °C for 2–4 days for fungi and bacteria, respectively. The average number of colonies from ZnO-treated spore suspensions (fungi) and (bacteria) was compared with the number on the water control (percent colony formation). The zone size was determined by measuring the diameter of the zone in mm [19], [20], [21].
2.9. Brine shrimp lethality assay (BSLA)
Brine shrimp eggs were obtained from the New Aqua Laboratory in Thampanoor, Thiruvananthapuram. Filtered, artificial seawater was prepared by dissolving 36 g of sea salt in 1 l of distilled water for hatching the shrimp eggs. The seawater was put in a small plastic container (hatching chamber) with a partition for dark (covered) and light areas. Shrimp eggs were added into the dark side of the chamber while the lamp above the other side (light) will attract the hatched shrimp. Two days were allowed for the shrimp to hatch and mature as nauplii (larva). After two days, when the shrimp larvae are ready, 5 mL of the artificial seawater and 5 mL of nanoparticles solution was added to each test tube and 10 brine shrimps were introduced into each tube. Thus, there were a total of 30 shrimps per dilution. The artificial seawater up to 10 mL per test tube is control. The test tubes were left uncovered under the lamp. The number of surviving shrimps were counted and recorded after 24 h. Using probit analysis, the lethality concentration (LC50) was assessed at 95% confidence intervals. LC50 of less than 100 ppm was considered as potent (active). As mentioned by Meyer and others, LC50 value of less than 1000 μg/mL is toxic while LC50 value of greater than 1000 μg/mL is non-toxic. The percentage mortality (%M) was also calculated by dividing the number of dead nauplii by the total number, and then multiplied by 100%. This is to ensure that the death (mortality) of the nauplii is attributed to the compounds present in the nanoparticles.
2.10. Characterization of ZnO nanoparticles (UV-vis, FT-IR, XRD, DLS, TEM)
The bio-reductant nanoparticles were monitored by UV– visible (UV–Vis) spectrum at various time intervals. The UV–Vis spectra of this solution were recorded in spectra 2450, SHIMADZU Spectrophotometer, from 200 to 400 nm. The FT-IR spectrum was taken in the mid-IR region of 400–4000 cm−1. The spectrum was recorded using ATR (attenuated total reflectance) technique. The dried sample was mixed with the KBr (1:200) crystal, and the spectrum was recorded in the transmittance mode. The phyto-reduced ZnO nanoparticles were characterized by XRD. The XRD pattern was recorded using computer controlled XRD-system (JEOL, and Model: JPX-8030 with CuKα radiation (Ni filtered = 13418 Å) in the range of 40 kV, 20 A. The built in software (syn master 7935) program was used for the identification of XRD peaks corresponds to the Bragg's reflections. The aqueous suspension of the synthesized nanoparticles was filtered through a 0.22-lm syringe driven filter unit, and the size and distribution of the nanoparticles were measured using dynamic light scattering technique (Nanopartica, HORIBA, SZ-100). The surface morphology and size of the nanoparticles were studied by transmission electron microscopy (JEOL (JEM- 1010) instrument) with an accelerating voltage of 80 kV. A drop of aqueous ZnONPs on the carbon-coated copper TEM grids was dried and kept under vacuum in desiccators before loading them onto a specimen holder. The particle size and surface morphology of nanoparticles were evaluated using Image J 1.45s software. A Raman spectrophotometer is to detect the maximum sensitivity of the product. The surface defects and sp2hybridization of grapheme sheets were determined by LASER RAMAN spectroscopy (RFS 100/S-Bruker, Inc.,Karlsruhe, Germany, Matlab 6.0 software) at the excitation wavelength of 633 nm with exposure time of 10 s (100%intensity).
2.11. Statistical analysis
All of the data from three independent replicate trials were subjected to analysis using Statistical package for the Social Sciences (SPSS) version 16.0. The data are reported as the mean ± SD and significant differences between mean values were determined with one way analysis of variance (CRD) followed by Duncan's multiple range test (DMRT) (P < 0.05).
3. Results
3.1. Selection of bacteria and fungi present in drinking water pipeline and synthesis of ZnO nanoparticles
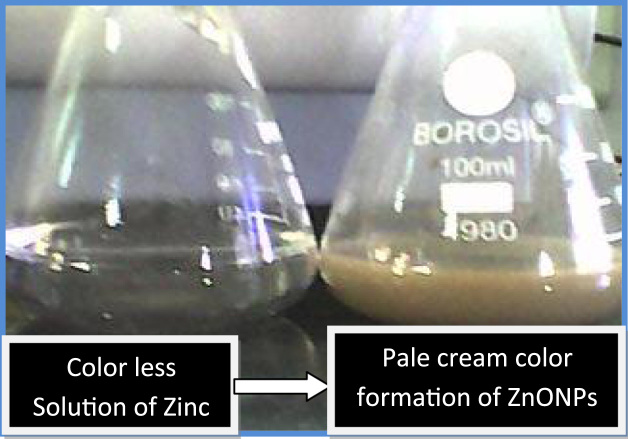
Drinking water pipeline bacteria and fungi species have unusual biological activities depending upon the metabolisms under temperature, pH and pressure. Once the bark (Fig. 1) extract (10 mL) was treated with 90 mL of Zn nitrate solution, the color of zinc nitrate solution is color less when mixing with Alstonia scholaris shown pale cream color (Fig. 2). ZnO nanoparticle was characterized by using the techniques like, Fourier transform infrared spectrophotometry, (FT-IR), UV-Vis spectrophotometry, Dynamic light scattering (Particle size, zeta potential) and transmission electron microscopy (TEM).
Fig. 1.
Photograph showing Alstonia scholaris stem bark.
Fig. 2.
Synthesis of Zinc oxide nanoparticles by Alstonia scholaris stems bark.
3.2. UV-Visible spectral analysis
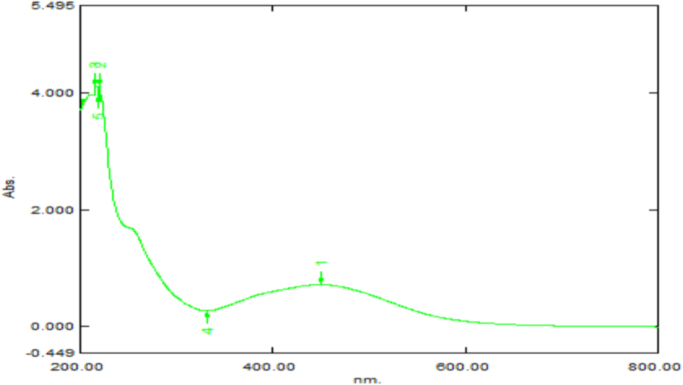
The absorption spectrum was recorded for the sample in the range of 200–500 nm (Fig. 3). The spectrum showed the formation of peak in the wavelength of 430 nm. UV–Vis spectroscopy was used to determine the formation and the stability of the synthesized zinc oxide nanoparticles in aqueous colloidal solution. The bio matrix present in the stem bark extracts Alstonia scholaris may leads to the change in the absorbance and transmittance of UV-Vis spectra [22].
Fig. 3.
UV-visible spectroscopic micrograph showing the localized surface plasmon resonance (LSPR) of ZnO nanoparticles synthesized using Alstonia scholaris bark extract.
3.3. FT-IR analysis
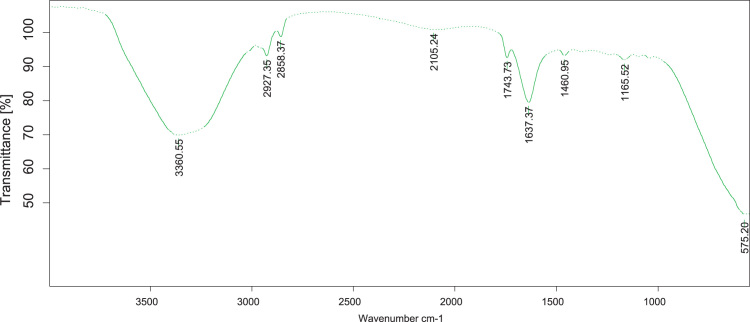
FT-IR Spectrum for nanoparticles synthesized by Alstonia scholaris is represented in Fig. 4. The interface between bio-organic functional groups and metal nanoparticles were illustrated by FT-IR spectrum. The peaks at 3330 cm−1 were assigned to the stretching vibrations of C-H alkynes. These peaks were corresponding to protein enzymes or polysaccharide components from cell biomass. The peak at 2927 and 2898 cm−1 were assigned to C-H stretching vibration of alkanes. The peak at 2106 cm−1was assigned to -C≡C- stretching vibrations of the alkynes. The peak at 1743 and 1637 cm−1were assigned to stretching vibration of –C˭O–carboxylic acids. The peaks at 1480 cm−1 corresponded to C-H stretching vibrations of alkanes. The peak at 1166 cm−1shows that chemical transformation occurs during synthesis of Zinc ions to nanoparticles and C-H stretching vibrations of alkyl halides. In addition to this band at 575 cm−1 corresponds to metal binding carboxylic (M↔C≡O) groups. This functional group May acts template, reducing and capping of nanocrystals. Which with the observation made by FT-IR results.
Fig. 4.
FT-IR spectroscopic micrograph showing the functional groups responsible for the reduction and stabilization of ZnO nanoparticles synthesized using the aqueous extract of Alstonia scholaris bark.
3.4. XRD analysis
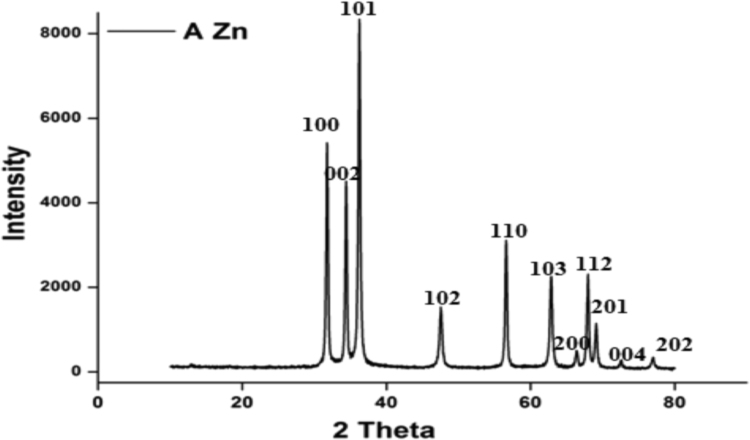
XRD pattern of ZnONPs shows the peaks corresponds to the Bragg's diffraction signals from (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (201) crystal planes. The intensity data were collected over a 2θ range of 20–80°. A definite line broadening of the XRD peaks indicates that the prepared material consists of particles in nanoscale range (Fig. 5). From this XRD patterns analysis, we determined peaks intensity, position and width, full width at half-maximum (FWHM) data. The diffraction peaks located at 31.84°, 34.52°, 36.33°, 47.63°, 56.71°, 62.96°, 68.13°, 69.18°, 70.16°, 73.21° and 78.56° have been indexed as hexagonal wurtzite phase of ZnO (14) and further, it also confirms the synthesized nano powder was free of impurities as it does not contain any characteristic XRD peaks other than ZnO. The synthesized ZnO nanoparticles diameter was calculated using Debye-Scherrer's formula [23].
where 0.89 is Scherrer's constant, λ is the wavelength of X-rays, θ is the Bragg diffraction angle, and β is the full width at half-maximum (FWHM) of the diffraction peak corresponding to plane (101). The average particle size of the sample was found to be 16.2 nm which is derived from the FWHM of more intense peak corresponding to 101 plane located at 36.33° using Scherrer's formula.
Fig. 5.
XRD micrograph showing the Bragg's reflections corresponds to the FCC crystal structure of the Zinc oxide nanoparticles synthesized using bark extract of Alstonia scholaris.
3.5. Dynamic light scattering analysis
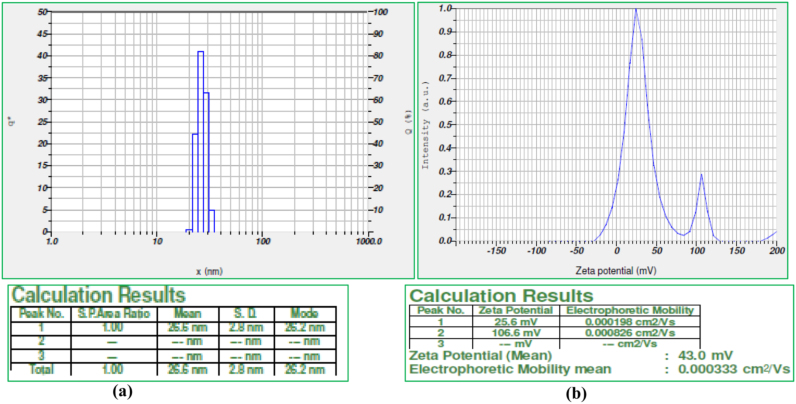
Particle size and zeta potential values were measured using Nanopartica SZ-100. The particle size distribution spectra for the zinc nanoparticles were recorded as diameter (nm) verses frequency (%/nm) spectra with diameter (nm) on x-axis and frequency (%/nm) on y-axis. The zeta potential spectra for the zinc nanoparticles were recorded zeta potential verses intensity spectra with zeta potential (mV) on x-axis and intensity (a.u) on y-axis. Particle size of 26.2 nm with zeta potential of 43.0 mV was recorded for the zinc oxide nanoparticles synthesized from bark extract (Fig. 6a and b) signifies the presence of repulsive electro-static forces among the synthesized ZnO nanoparticles, which leads to the monodispersity of the particles [24].
Fig. 6.
(a) and (b). Showing the histogram of Zinc nanoparticles (Dynamic light scattering) and zeta potential (43.0 mV) of ZnO nanoparticles synthesized using the bark extract of Alstonia scholaris.
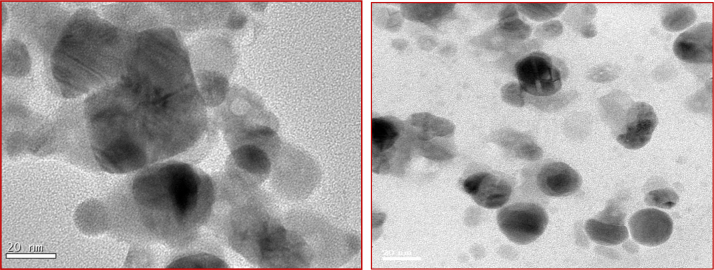
3.6. TEM analysis
The ZnO nanoparticles used in this study were having the mean diameter of 20 nm as shown by TEM micrographs (Fig. 7). The image of nano scale ZnO particles and appears slightly aggregated due to the absence of surface protecting ligands. The particles were crystalline in nature as revealed by the high magnification TEM image of nanoparticles and the lattice of zinc was clearly seen.
Fig. 7.
TEM micrograph of Alstonia scholaris Zinc oxide nanoparticles showing spherical shaped particles with an average size of 20 nm.
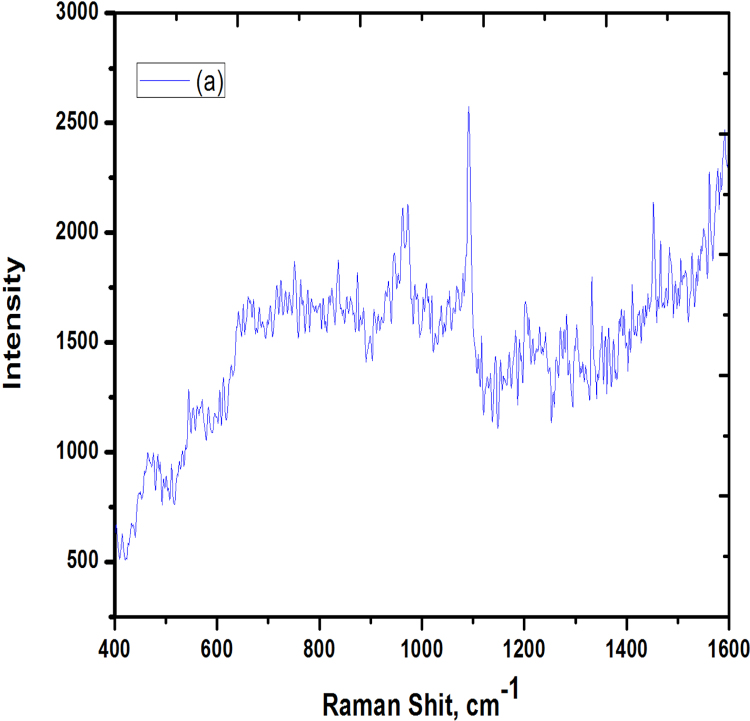
3.7. Laser Raman spectroscopic measurements
The Raman spectrum shows prominent vibrational band related to ZnO sample. Fig. 8 shows the major peak, raised from 1100 cm−1, which shows the presence of ZnO and very small disturbance of the peaks seen around the major peak it is due to the presence of protein compounds present in Alstonia scholaris stem bark. By increasing the concentration of ZnO, the intensity of the peak increases and the linear peak appears without any disturbance peak around it. In the typical spectrum from ZnO sample, the band centered at 1100 cm−1 corresponds to the symmetric stretching vibration of Proteins present in bark.
Fig. 8.
RAMAN spectrum analysis of Zinc oxide nanoparticles synthesized using bark extract of Alstonia scholaris.
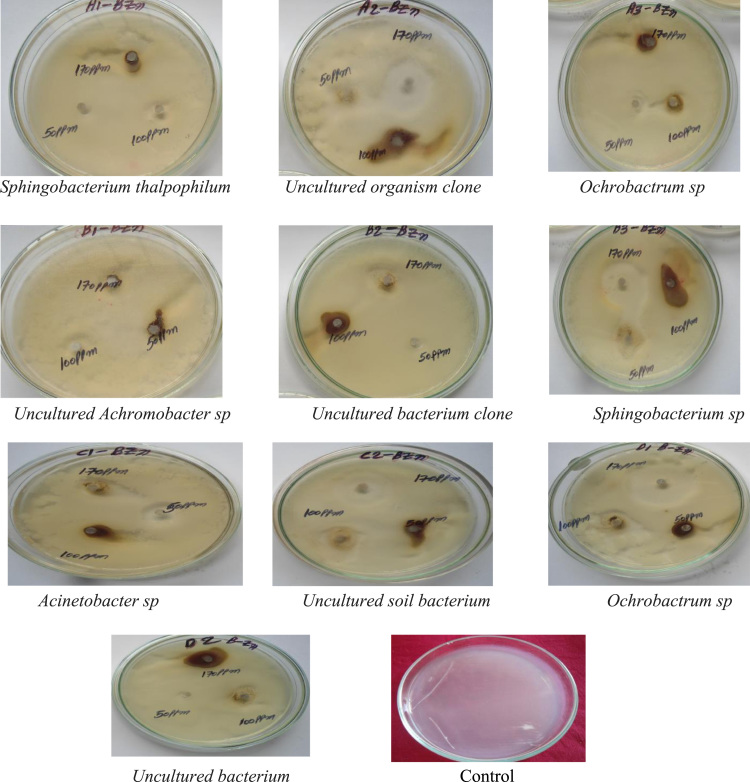
3.8. Antimicrobial efficacy of Alstonia scholaris stem bark-extract mediated ZnO nanoparticles
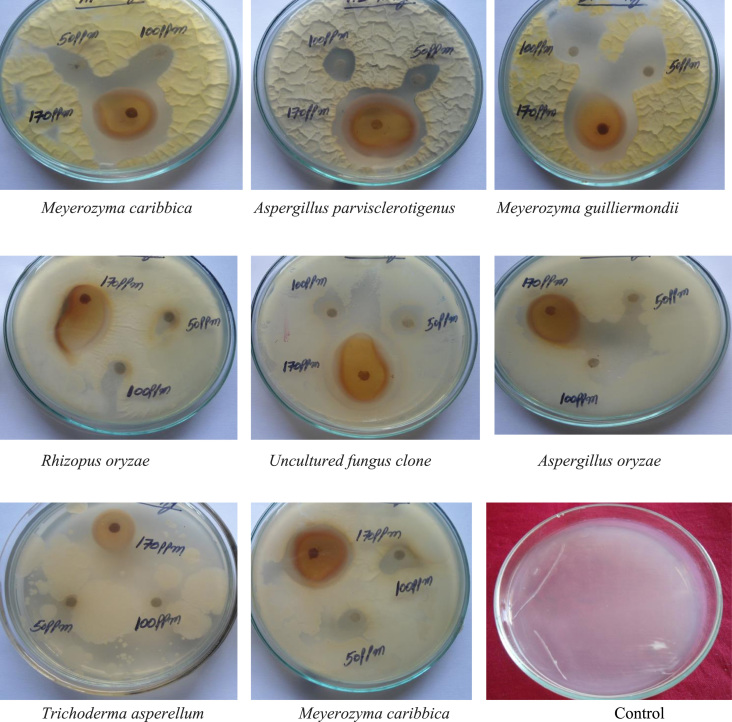
Alstonia scholaris ZnONPs have very strong inhibitory action against fungal sp, Gram-positive and Gram-negative bacteria (Fig. 9, Fig. 10). Three concentrations of ZnONPs (170, 100, 50 ppm) were prepared and were applied against an array of fungal species viz., Meyerozyma caribbica, Aspergillus parvisclerotigenus, Meyerozyma guilliermondii, Rhizopus oryzae, Uncultured fungus clone, Aspergillus oryzae, Trichoderma asperellum and bacterial species viz., Sphingo bacterium thalpophilum, Uncultured organism clone, Ochrobactrum sp, Uncultured Achromo bacter sp, Uncultured bacterium clone, Sphingo bacterium sp, Acinetobacter sp,Uncultured soil bacterium, Ochrobactrum sp. Which were isolated from drinking water PVC pipes. The higher concentration (170 ppm) of ZnONPs showed significant antimicrobial effect (Table 1, Table 2) compared with other concentrations (100, 50 ppm). The higher anti bacterial activity of smaller sized nanoparticles could be due to the large surface area to volume ratio and the surface activity of Zn. The generation of hydrogen peroxide (H2O2) depends strongly on the surface area of Zn which results in more oxygen species on the surface and the higher antifungal and antibacterial activity of the smaller nanoparticles [25], [26]. This could be due to the fact that nanoparticles are more abrasive in nature than bulk Zn, thus contributing to the greater mechanical damage to the cell membrane resulting in enhanced bacterial effect [27].
Fig. 9.
Synthesized ZnO nanoparticles of Alstonia scholaris showing effective Antibacterial activity towards Gram positive and Gram negative bacteria (Biofilm).
Fig. 10.
Synthesized ZnO nanoparticles of Alstonia scholaris showing effective Antifungal activity towards fungal sp isolated from drinking water PVC pipelines (Biofilm).
Table 1.
In-vitro antibacterial studies of bacteria present in drinking water PVC pipelines using Alstonia scholaris Zinc oxide nanoparticles as inhibitors.
| S.no | Bacteria |
Alstonia scholaris stem bark extract mediated synthesis of zinc oxide nanoparticles Zone of inhibition (mm) |
||
|---|---|---|---|---|
| 170 ± 1.4 ppm | 100 ± 1.1 ppm | 50 ± 0.9 ppm | ||
| 1. | Sphingo bacterium thalpophilum | 1.5 ± 0.04cd | 1.3 ± 0.01cd | 0.9 ± 0.05de |
| 2. | Uncultured organism clone | 1.4 ± 0.05cdef | 1.0 ± 0.06ef | 0.8 ± 0.03def |
| 3. | Ochrobactrum sp | 1.2 ± 0.08f | 1.1 ± 0.05def | 0.7 ± 0.02efg |
| 4. | Uncultured Achromobacter sp | 1.9 ± 0.09b | 1.7 ± 0.14a | 1.2 ± 0.05bc |
| 5. | Uncultured bacterium clone | 1.6 ± 0.04c | 1.4 ± 0.06bc | 0.9 ± 0.02de |
| 6. | Sphingobacterium sp | 1.5 ± 0.05cde | 1.2 ± 0.03cde | 1.0 ± 0.02cd |
| 7. | Acinetobacter sp | 3.0 ± 0.07a | 2.2 ± 0.14a | 1.6 ± 0.18a |
| 8. | Uncultured soil bacterium | 2.2 ± 0.03a | 1.7 ± 0.15a | 1.2 ± 0.16bc |
| 9. | Ochrobactrum sp | 2.6 ± 0.08a | 2.1 ± 0.17a | 1.5 ± 0.24a |
| 10. | Uncultured bacterium | 2.0 ± 0.05ab | 1.6 ± 0.03ab | 0.8 ± 0.05defg |
| C.R.D (P ≤ 0.05) | 0.227 | 0.217 | 0.207 | |
The data presented is ± SE of three measurements.
Data followed by the same letter are not significantly different at P ≤ 0.05, where as those followed by different letters are significantly different at P ≤ 0.05.
Table 2.
In-vitro antifungal studies of fungi present in drinking water PVC pipelines using Alstonia scholaris zinc oxide nanoparticles as inhibitors.
| S. no. | Fungi |
Alstonia scholaris stem bark extract mediated synthesis of zinc oxide nanoparticles zone of inhibition (mm) |
||
|---|---|---|---|---|
| 170 ± 1.4 ppm | 100 ± 1.1 ppm | 50 ± 0.9 ppm | ||
| 1. | Meyerozyma caribbica | 2.5 ± 0.06c | 2.0 ± 0.02a | 1.5 ± 0.06abc |
| 2. | Aspergillus parvisclerotigenus | 2.9 ± 0.01a | 1.8 ± 0.04cd | 1.2 ± 0.08abcde |
| 3. | Meyerozyma guilliermondii | 2.8 ± 0.15ab | 2.0 ± 0.05ab | 1.8 ± 0.03a |
| 4. | Rhizopus oryzae | 2.3 ± 0.06cd | 1.6 ± 0.03de | 1.4 ± 0.02abcd |
| 5. | Uncultured fungus clone | 2.1 ± 0.12de | 1.9 ± 0.06abc | 1.1 ± 0.14abcdeg |
| 6. | Aspergillus oryzae | 2.9 ± 0.03ab | 1.4 ± 0.04ef | 0.9 ± 0.08abcdefg |
| 7. | Trichoderma asperellum | 1.9 ± 0.05ef | 1.1 ± 0.15g | 0.5 ± 0.12cdefghi |
| 8. | Meyerozyma caribbica | 2.1 ± 0.08def | 1.3 ± 0.06fg | 0.8 ± 0.07abcdefgh |
| C.R.D (P ≤ 0.05) | 0.231 | 0.213 | 0.984 | |
The data presented is ± SE of three measurements.
Data followed by the same letter are not significantly different at P ≤ 0.05, where as those followed by different letters are significantly different at P ≤ 0.05.
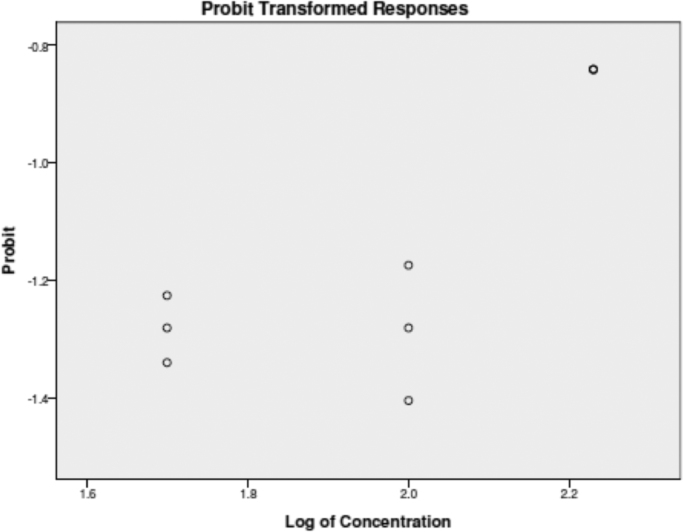
3.9. Brine shrimp lethality assay (BSLA)
The Zinc oxide nanoparticles tested showed poor brine shrimp larvicidal activity. The lethality concentration (LC50) of Zinc Oxide nanoparticles were 50 ppm (μg/mL), 100 ppm, and 170 ppm respectively (Fig. 11). The degree of lethality was directly proportional to the concentration of the nanoparticles. Minimum mortalities (30%) were observed at a concentration of 50 ppm while that of 100 and 170 ppm there were no mortality. Based on the results, the brine shrimp lethality of the three different ppm of ZnO was found to be concentration-dependent. The observed lethality of the ZnO to brine shrimps indicated the presence of potent cytotoxicity. According to these results the ZnONPs are slightly toxic (active) if it has an LC50 value of less than 1000 μg/mL while non-toxic (inactive) if it is greater than 1000 μg/mL [28]
Fig. 11.
LC50 (Median Lethal Concentration) values were calculated using the regression line obtained by plotting the concentration against the death percentage on a probit scale, and the results were evaluated with probit analysis (SPSS 13.0).
4. Discussion
Green-synthesized metallic nanoparticles have a potential role in the form of nano-drugs and nano-fertilizers in the current scenario, this work may help in future development of nano-medicines for plant growth and development and the effective control of disease causing pathogens.
In the present study the presence of phytochemicals in the bark extract itself helps in the synthesis of metal oxide nanoparticle by inducing oxidation and reduction reaction. The functional groups of phytochemicals induce the nanoparticle synthesis were alkynes and alkanes that are widely seen in secondary metabolites such as terpenoids, carboxylic acids, alkaloids, etc. Proposed that the proteins can bind with nanoparticles either through free amine groups or crystalline residues in the proteins. The carboxylic groups are known to coordinate with metal ions which may act as a nucleation site for nanoparticles formation. The overall peak from FT-IR observation confirms the presence of protein moiety in the samples of Zinc oxide nanoparticles. It can be assumed that protein molecules or peptide chains may act as template nucleation site to reduce Zinc ions to form ZnO nanoparticles.
As a preliminary confirmation, the rapid synthesis of ZnONPs was measured using the UV- Visible spectroscopy at a maximum absorbance of 290 nm. The bio-reduction of zinc oxide nanoparticles is extracellular and promises the vast development of green synthesis of metallic nanoparticles using the plants and plant bark sources.
Further the XRD analysis proved the crystalline nature of the ZnONPs, The size of the particles and zeta potential was determined by DLS (Dynamic light scattering analyzer) here, If the hydrosols have a large negative or positive zeta potential then they will tend to repel with each other and there will be no tendency of the particles to agglomerate. For the zinc nanoparticles synthesized from Alstonia scholaris bark extract, least particle size and high zeta potential values were recorded. However, if the particles have low Zeta potential values then there will be no force to prevent the particles coming together and flocculating. The reported zeta potential result can then be used as an indicator of the dispersion stability.
The TEM analysis of ZnONPs demonstrated the size approximately in the range of 30–20 nm. TEM image has shown individual ZnO nanoparticles as well as a number of aggregates, spherical-shaped nanoparticles. This TEM result coincides with results already reported, which shows formation of spherical shaped nanoparticles and aggregated molecules in Alstonia scholaris bark extract, The Raman spectra also shown the ZnO present at 1100 cm−1.
The anti-microbial activity of ZnONPs has proved that these can be used as potent anti-bacterial and anti-fungal agents, Results clearly demonstrate that the nanoparticles showed anti-microbial effect in a dose-dependent manner Maximum zone of inhibition was observed in all fungal species at 170 ppm than 100 and 50 ppm, Minimum zone of inhibition was observed in all bacterial species at 50 ppm. Different mechanism of action of nanoparticle against gram + and gram – bacteria has been already reported in previous literature because of difference in structural composition. The zinc oxide nanoparticles are inhibiting the microbial growth in in-vitro antimicrobial activities. Where, as Brine Shrimp Lethality Assay shown very less cytotoxic results based on this we can the bio synthesized metal zinc oxide nanoparticles has emerged as a low cost, simpler and better choice than physical and chemical methods as it is fast, clean and friendly with environment this type of nanoparticles can be used for pharmaceuticals industries, agricultural industries and Textile industries
5. Conclusion
The synthesis of ZnONPs using Alstonia scholaris has been demonstrated and these phytogenic ZnONPs exhibited significant antimicrobial activity against fungal sp., Gram positive and Gram negative bacteria. The present evaluation of the antibacterial and antifungal property therefore offers a scientific basis for the use of this plant as suitable anti bacterial agent against a range of pathogens but further investigation against the broader range of pathogens is certainly required to identify the active ingredients. But to understand the mechanisms of action of these agents, more detailed chemical structure elucidation of the bioactive components followed by therapeutic investigations and toxicological assessment are required. The Alstonia scholaris mediated synthesis of ZnO nanoparticles shown effective antimicrobial activity against fungal sp, Gram positive and Gram negative bacteria and points to the commercial use of these biological synthesized nanoparticles in biomedical and agriculture as fungicides for the effective control of disease causing pathogens. While brine shrimp lethality assay elucidates their importance in pharmacological industry. Further, the study of active compounds present in Alstonia scholaris after the synthesis of ZnONPs can develop novel drugs for human welfare in near future.
Acknowledgments
Authors are thankful to Acharya N G Ranga Agricultural University for providing research facilities at Institute of Frontier Technology, Regional Agricultural Research Station, Tirupati to carryout this research work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.04.004.
Appendix A. Transparency document
Supplementary material
References
- 1.Bhattacharya D., Gupta R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005;25:199. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 2.Singh R.P., Shukla K.V., Yadav S.R., Sharma K.P., Singh K.P., Pandey C.A. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011;2:313–317. [Google Scholar]
- 3.Prasad T.N.V.K.V., Sudhakar P., Sreenivasulu Y., Latha P., Munaswamy V., Raja Reddy K., Sreeprasad T.S., Sajanlal P.R., Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. [Google Scholar]
- 4.Salam H.A., Rajiv P., Kamaraj M., Jagadeeswaran P. Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012;1:85–90. [Google Scholar]
- 5.Kaushik N., Thakkar M.S., Snehit S., Mhatre M.S., Rasesh Y. MS biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Girija S., Balachandran Y.L., Kandakumar J. Plants as green nanofactories: application of plant biotechnology in nanoparticle synthesis. Plant Cell Biotechnol. Mol. Biol. 2009;10:79–86. [Google Scholar]
- 7.Prabha S., Supraja N., Garud M., Prasad T.N.V.K.V. Synthesis, characterization and antimicrobial activity of Alstonia scholaris bark-extract-mediated silver nanoparticles. J. Nanostruct. Chem. 2014 [Google Scholar]
- 8.Sreekanth T.V.M., Nagajyothi P.C., Supraja N., Prasad T.N.V.K.V. Evaluation of the antimicrobial activity and cytotoxicity of phytogenic gold nanoparticles. Appl. Nanosci. 2014 [Google Scholar]
- 9.Sundrarajan M., Gowri S. Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011;8:447–451. [Google Scholar]
- 10.Gunalan S., Rajeshwari S., Venkatesh R. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012;22:693–700. [Google Scholar]
- 11.Padil V.V.T., Cernik M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013;8:889–898. doi: 10.2147/IJN.S40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S. Medicinal Plants of North-east Region. 1st ed. Scientific Publishers; Rajasthan, India: 2002. [Google Scholar]
- 13.G. Khanikar, GharooaSikitsher Nidan, 3rd ed. Puthiteertha Publication, Assam, 2007.
- 14.Khan M.R., Omoloso A.D., Kihara M. Fitoterapia. 2003;74:736–740. doi: 10.1016/s0367-326x(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 15.Mostafa A., Oudadesse H., Legal Y., Foad E., Cathelineau G. Characteristics of silver- hydroxyapatite/PVP nanocomposite. Bioceram. Dev. Appl. 2011;1:1–3. [Google Scholar]
- 16.Murphy C.J. Sustainability as a design criterion in nanoparticle synthesis and applications. J. Mater. Chem. 2008;18:2173–2176. [Google Scholar]
- 17.Vaidyanathan R., Kalishwaralal K., Gopalram S., Gurunathan S. Nanosilver the burgeoning therapeutic molecule and its green synthesis. Biotechnol. Adv. 2009;27(6):924–937. doi: 10.1016/j.biotechadv.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya S., Zaman M.K. Antibacterial activity of root of Indian zanthoxylum nitidum. Asian J. Pharm. Clin. Res. 2005;25:199. [Google Scholar]
- 19.Cynthia H., Callaghan O. Assessment of a new antibiotic. In: Hugo W.B., Russel A.D., editors. Pharmaceutical Microbiology. 3rd ed. Blackwell Scientific Publications; Oxford: 1983. pp. 122–134. [Google Scholar]
- 20.Yamac M., Bilgili F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006;44:660–667. [Google Scholar]
- 21.Reeves W., White A. Oxford University Press; New York, USA: 1999. Clinical Antimicrobial Assay. [Google Scholar]
- 22.Supraja N., Prasad T.N.V.K.V., Giridhara Krishna T., David E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2015 [Google Scholar]
- 23.Khoshhesab Z.M., Sarfaraz M., Asadabad M.A. Preparation of ZnO nanostructures by chemical precipitation method. Synth. React. Inorg. Metal-org. Nano-met. Chem. 2011;41:814–819. [Google Scholar]
- 24.Roy S., Triparna M., Shatarupa T., Das P. Biosynthesis, characterization and antifungal activity of silver nanoparticles synthesized by the fungus Aspergillus foetidus MTCC8876. Dig. J. Nanomater. Biostruct. 2013;8:197–205. [Google Scholar]
- 25.Yamamoton O., Sawai J., Sasamoto T. Effect of lattice constant of zinc oxide on antibacterial characteristics. Int. J. Inorg. Mater. 2000;2:451–454. doi: 10.1023/B:JMSM.0000036271.35440.36. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto O., Komatsu M., Sawai J., Nakagaw Z. Antibacterial activity of ZnO powder with crystallographic orientation. J. Mater. Sci. Mater. Med. 2008;19(3):1407–1412. doi: 10.1007/s10856-007-3246-8. [DOI] [PubMed] [Google Scholar]
- 27.Padmavathy N., Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles – an antibacterial study. Sci. Technol. Adv. Mater. 2008;9:1–7. doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olowa F. Lilybeth, Nunezan M. Olga. Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City. Philipp. Int. Res. J. Biol. Sci. 2013;2(11):74–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material