Summary
Background/Objective
Plasma-sprayed titanium coating (TC) with rough surfaces has been successfully applied in hip or knee prostheses. This study aimed to investigate the osteoconduction and osseointegration of Type I collagen covalently immobilised on TC (TC-AAC) compared with those of TC.
Methods
In vitro, the migration of human mesenchymal stem cells (hMSCs) on TC and TC-AAC was observed by scanning electron microscopy and visualised fluorescent live/dead assay. In vivo, a rabbit model with femur condyle defect was employed, and implants of TC and TC-AAC were embedded into the femur condyles.
Results
Collagen immobilised on TC could promote hMSCs' migration into the porous structure of the TC. Micro computed tomography images showed that bone trabeculae were significantly more abundant around TC-AAC implants than around TC implants. Fluorescence micrographs indicated more active new-bone formation around implants in the TC-AAC group than in the TC group. The measurement of bone–implant contact on histological sections indicated significantly greater osteointegration around TC-AAC implants than around TC ones.
Conclusion
Immobilised Type I collagen could improve the osteoconduction and osseointegration of TC implants.
Keywords: cell migration, osseointegration, osteoconduction, titanium coatings, type I collagen
Introduction
Plasma-sprayed titanium (Ti) coatings with rough surfaces and macroporous structures have been widely and successfully applied in orthopaedics, such as in hip replacement and knee arthroplasty [1], [2]. Bone tissue could grow into the porous structures and form a mechanical interlock, thereby providing a morphological fixation of implants [3], [4]. However, Ti is not osteoinductive, the early effect of the implants remains unsatisfying, and improved osteointegration is required [5], [6], [7]. To improve osseointegration, the further surface modification of Ti coatings is still one of the most active research areas.
Compared with mechanical and physicochemical methods, biochemical methods—the immobilisation of the main components of the extracellular matrix, enzymes, or peptides on a biomaterial surface—have attracted considerable attention [8], [9], [10], [11]. Type I collagen, the major structural protein in bone, has been most promising because of its well-known role as mediator of osteoblastic cell functions, including adhesion, differentiation, and extracellular-matrix secretion [12], [13]. Therefore, collagen has been used as coating material on metal-based orthopaedic implants, such as magnesium, stainless steel, and Ti [14], [15], [16], [17]. Several strategies have been developed to immobilise Type I collagen onto Ti surfaces, such as adsorptive immobilisation, covalent binding from hydrocarbon plasma followed by acrylic acid grafting, and the use of aminopropylsilane and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) [18], [19], [20]. In our previous study, Type I collagen was immobilised onto plasma-sprayed porous Ti coatings by either adsorptive or covalent immobilisation [21]. The covalently immobilised collagen on Ti coatings showed greater capability to regulate the osteogenic activity of human mesenchymal stem cells (hMSCs) than adsorbed collagen, but whether the stem cells could grow into the pores of the Ti coatings and the osteoconductivity of the covalently immobilised collagen remained to be investigated.
Coating Ti surfaces with collagen stimulates bone formation in vivo. Marra et al [20], [22], [23] found a significant increase of bone–implant contact (BIC) and bone ingrowth on ColTi versus uncoated Ti fixtures in the early phases of healing of the femur trabecular bone, femur cortical bone, and tibia trabecular bone of rabbits. Mueller et al [24] and Sverzut et al [25] also confirmed that Ti-based implant coating with Type I collagen significantly influences peri-implant bone regeneration. However, a significant aspect not completely addressed in existing literature is the effect of the osteoconduction and osseointegration of covalently immobilised Type I collagen on porous Ti coatings in vivo—in other words, whether immobilised Type I collagen could induce new bone and thereby fill the pores of Ti coating. The present study tried to answer this question by providing some in vitro and in vivo results on the evaluation of Type I collagen covalently immobilised on Ti coatings. Particularly, the migration of hMSCs in vitro was observed with scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). For in vivo study in rabbits, peri-implant bone regeneration and the osseointegration of bone–implant interfaces in femur were also investigated.
Materials and methods
Materials
Porous Ti coatings on Ti-6Al-4V substrates (TCs; Φ 10 mm × 2 mm of plates and Φ 2.6 mm × 10 mm of rods) were fabricated by vacuum plasma spraying (F4-VB, Sulzer Metco, Switzerland) as in previous literature [2]. Fine powder (Shanghai, China) with an average size of 28.9 mm was firstly sprayed as a bond layer. Coarse powder with an average size of 98.6 mm was subsequently deposited on the bond layer to form a porous structure with a porosity of approximately 30%. Type I collagen from calf skin was obtained from Sigma-Aldrich (St. Louis, USA). Silane-coupling agent aminopropyltriethoxysilane (APS), toluene, and N-hydroxysuccinimide (NHS) were purchased from Shanghai Sinopharm Chemical Reagent Corporation (Shanghai, China). EDC was purchased from Tokyo Chemical Industry Company, Ltd (Tokyo, Japan).
Preparation of type I collagen-modified Ti coatings
Type I collagen-modified TCs were prepared as previously described [21]. The TC samples were treated in 5M NaOH at 80°C for 12 hours and were then immersed in a boiling APS/toluene solution (APS concentration of 10%) for silanisation. After 12 hours, the APS-coated samples were ultrasonically washed once in methanol and twice in deionised water and then dried prior to further modification. Finally, the samples were immersed in 1 mg/mL Type I collagen/acetic acid (5mM) solution, which included 2.5 mg/mL EDC and 0.63 mg/mL NHS, and were reacted for 6 hours. These Type I collagen covalently immobilised TC samples were denoted as TC-AAC. All samples were sterilised with γ-ray irradiation.
In vitro study
Cell isolation and culture
hMSCs were isolated and expanded as previously described [26]. Bone marrow aspirates were obtained from three healthy donors (a 26-year-old man, 30-year-old woman, and 35-year-old man) during routine orthopaedic surgical procedures. The Ethical Committee of Shanghai Ninth People's Hospital, Shanghai, China provided ethical approval. Briefly, after being isolated from the bone marrow aspirates, cells were cultured in an α-MEM culture medium supplemented with 10% fetal bovine serum, 1% penicillin (100 U/mL), and streptomycin sulphate (100 mg/mL) (Invitrogen, Carlsbad, CA, USA), and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air, with the growth medium changed every 48 hours. hMSCs passaged up to the fourth generation were used for the experiments described below.
Cell growth evaluation
One millilitre of the cell suspension at a cell density of 5 × 104 viable hMSCs was seeded into a 48-well plate containing two kinds of samples (TC and TC-AAC), and then the mixtures were incubated for 1 day or 5 days in a humidified 37°C/5% CO2 incubator. At each predetermined time point, hMSCs were fixed overnight in 2% glutaraldehyde. After being rinsed twice in PBS, the specimens were dehydrated in a graded series of ethanol (30%, 50%, 70%, 80%, 95%, and 100%) and ethanol/hexamethyldisilazane (HMDS) at various proportions (2:1, 1:1, and 1:2 ethanol/HMDS and 100% HMDS). The samples were then dried in an oven at 37°C overnight. The cells on the sample surface were investigated using SEM.
MTT assay was employed to determine the proliferation of hMSCs on TC and TC-AAC. Briefly, the cells were incubated with the specimens for 1 day or 5 days. At each predetermined time point, 0.1 mL of the MTT solution was added, and the specimens were incubated at 37°C to form formazan, which was then dissolved using 0.5 mL dimethylsulfoxide. Optical density was measured at 570 nm using an automated plate reader (Synergy HT Multi-detection Microplate Reader Shanghai, China).
The cytoskeleton of the hMSCs on the different samples was analyzed using CLSM (A1R, Nikon, Japan). After 24 hours of incubation with the two kinds of specimens described earlier, the cells on the surfaces of the specimens were washed gently with phosphate-buffered saline (PBS) three times and then fixed with 4% paraformaldehyde for 15 minutes at room temperature. The cells were permeabilised with 0.1% Triton X-100 (Shanghai, China) in PBS for 5 minutes and then washed with PBS three times. The cells were incubated with Alexa Fluor 555 phalloidin (Molecular Probes, Sigma-Aldrich, St. Louis, USA) for 1 hour. After washing with PBS again, the cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (Molecular Probes, Sigma-Aldrich, St. Louis, USA). The cell morphologies were visualised using CLSM.
The viability and distribution of cells on the two kinds of coatings after 1 day and 5 days of incubation were evaluated using a live/dead assay kit (Abcam, Cambridge, England) following the standard protocol provided by the manufacturer. Briefly, the cells and scaffold constructs were washed twice with PBS and then incubated in standard working solution at room temperature for 10 minutes. The constructs were then washed twice with PBS and observed under CLSM (A1R, Nikon, Japan).
In vivo study
Surgical procedure
A rabbit model with femur condyle defect was used to evaluate the in vivo function of collagen-modified TCs. Eighteen adult white New Zealand rabbits (male, 2–2.5 kg body weight) obtained from the Laboratory Animal Center of Shanghai Ninth People's Hospital were divided into six groups of three rabbits and six samples each. The use of animals and the experimental protocol were approved by the Institutional Animal Welfare Committee of Shanghai Ninth People's Hospital. The rabbits were anesthetised by injecting 3% Nembutal (30 mg/kg) via the ear vein, and a longitudinal incision was made by scalpel in the rabbit femur under rigorous aseptic conditions. Circular holes 2.9 mm in diameter and 10 mm deep were drilled using a surgical electronic drill and thoroughly rinsed with physiological saline to remove shards of bone. Implants of TC and TC-AAC were used in this study. The wound was sutured with nylon thread. The rabbits were euthanized 1 month, 2 months, or 3 months after implantation.
Micro X-ray computed tomography analysis
After euthanasia, distal femurs with implants were collected under aseptic conditions, fixed in 4% paraformaldehyde for 2 days, and then rinsed with running water for 24 hours. For the assessment of the bone architecture around the different implants after 3 months of implantation, femur condyles with implants were examined using a desktop micro X-ray computed tomography (micro-CT; GE Locus SP, American) machine equipped with an 80 kV X-ray source with a camera pixel size of 15 μm. During scanning, the femur condyles were placed in polyethylene tubes filled with 75 volume% alcohol. Micro-CT images along the transection and midsagittal planes in the region around the implant were obtained. The scans resulted in reconstructed data sets with a voxel size of 28.79 μm. To determine the trabecular volume of interest (VOI) in the axial direction, the region of interest was chosen with its closest edge at 4.0 mm distally from the growth plate. The bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) were calculated as measurements of trabecular bone mass and its distribution [27].
Fluorescence labelling of new-bone formation around implants
To assess the osteogenic activity, the fluorochromic bone marker calcein green (15 mg/kg; Sigma-Aldrich, St. Louis, USA) was administered subcutaneously to all groups one week prior to rabbit euthanasia. The specimens were dehydrated through a series of graded ethanol solutions (50%, 60%, 75%, 85%, 95%, and 100%). The dehydrated specimens were preserved in absolute ethanol for subsequent assay and then embedded in polymethylmethacrylate resin. Undecalcified sections with a thickness of 100 μm were cut using a saw microtome (Leica ST1600, Heidelberg, Germany). The thickness of the sections was polished to 50 mm using P300, P800, and P1200 abrasive paper and then burnished with flannelette and abradum to 20–30 μm. Finally, the fluorescence of calcein in bone tissues surrounding the bone cement was visualised using CLSM (Leica TCS SP2, Leica Microsystems, Heidelberg, Germany).
Measurement of BIC
Sections were prepared as described above. After being dipped in 1% methane acid solution for 3 minutes and 20% methanol solution for 2 hours, the sections were stained with Van Gieson's picrofuchsin and then observed using fluorescence CLSM (Leica TCS SP5, Heidelberg, Germany).
BIC was measured by image analysis software (Bioquant, Nashville, American) according to previously reported methods [28]. BIC levels were defined as the fraction of direct bone apposition at the surface of the implant. The total perimeter observed and the total perimeter attached by bone were measured for each micrograph. Bone apposition was defined as a continuum of material from the surface of the implant to the surrounding bone. The values were the mean of the five samples.
Statistical analysis
All assays of in vitro study were repeated three times. All quantitative data were expressed as the mean ± standard deviation. Statistical differences were determined by an analysis of variance. A p value < 0.05 was considered statistically significant.
Results
Cell growth evaluation
On the 1st day and 5th day after cell culture, the growth and distribution of hMSCs on the surfaces of the two different materials were observed by SEM, and the results are shown in Figure 1. On Day 1, no obvious difference was observed between the cells distributed on the surfaces of TC and TC-AAC (Figures 1A and 1B). After cultivation for 5 days, many cells can be found on the surface of TC, and the cells had obviously proliferated compared with those at Day 1 (Figure 1C). By contrast, few cells were observed on the surface of TC-AAC (Figure 1D), which were not only much fewer than those on the surface of TC at Day 5 but also fewer than those on the surface of TC-AAC at Day 1. The same situation can be found within a larger scope (Figures 1E and 1F).
Figure 1.
SEM images of growth and distribution of hMSCs on TC and TC-AAC after 1 day and 5 days of culture with higher magnification in (A–D) and lower magnification in (E,F). hMSC = human mesenchymal stem cell; SEM = scanning electron microscopy; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
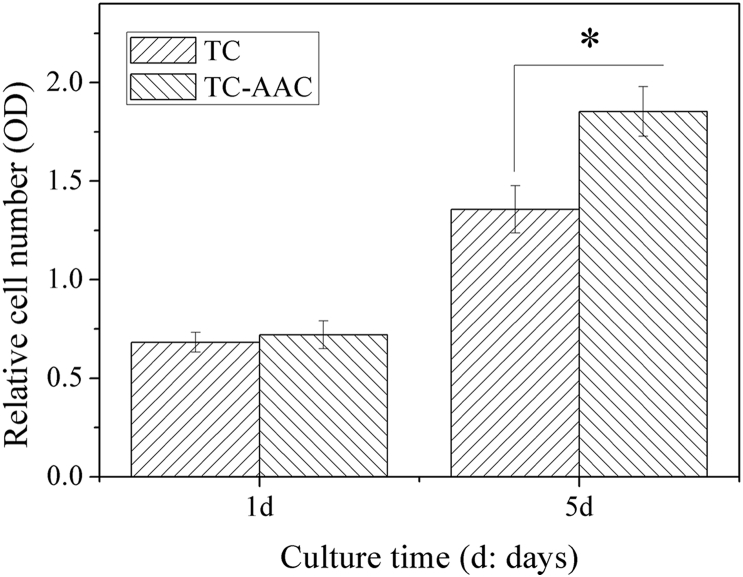
To investigate the proliferation of hMSCs on TC and TC-AAC, MTT assay was employed. The results are shown in Figure 2. On Day 5, the number of cells on TC-AAC was significantly greater than that on TC, indicating that the proliferation of hMSCs on TC-AAC was better than that on TC.
Figure 2.
Proliferation of hMSCs on surfaces of TC and TC-AAC. *p < 0.05 (comparison between different samples). hMSC = human mesenchymal stem cell; OD = optical density; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
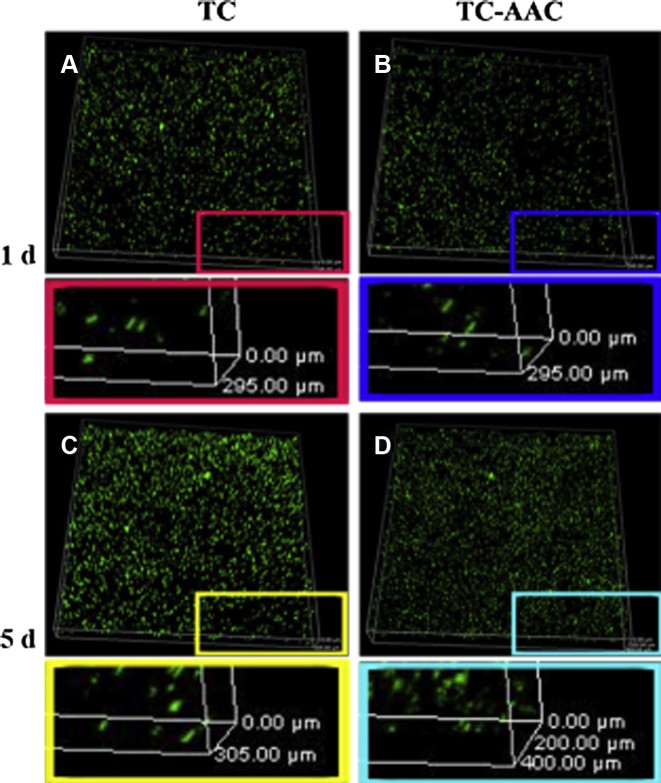
The distribution of cells on the two kinds of coatings after 1 day and 5 days of incubation was evaluated using a live/dead assay kit. As shown in Figure 3, no yellow staining was observed in the two groups, indicating that the Ti, collagen, and hyaluronic acid all have excellent cytocompatibility and noncytotoxicity. After 5 days of incubation, the cell number of TC-AAC and TC was higher than that after 1 day of incubation, and the number of cells on TC-AAC after 5 days of incubation (Figure 3D) was significantly higher than that on TC. On Day 1, the longitudinal distribution of cells on both TC and TC-AAC was about 295 μm. On Day 5, the longitudinal distribution of cells on TC was about 305 μm, which had no significant difference from that on Day 1. The longitudinal distribution of cells on TC-AAC was ∼400 μm, much deeper than that of the other three groups. Furthermore, many cells after 5 days of incubation attached onto the surface of TC, while the majority of cells of TC-AAC were observed deeper in the porosity. The results confirmed that hMSCs migrated more easily into the porous structure of TC-AAC than into that of TC.
Figure 3.
Three dimensional confocal laser scanning micrograph of hMSC distribution on TC and TC-AAC after 1 day and 5 days of incubation evaluated using live/dead assay kit. The live hMSCs were stained green and the dead cells were stained red and were very few on all materials. hMSC = human mesenchymal stem cell; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
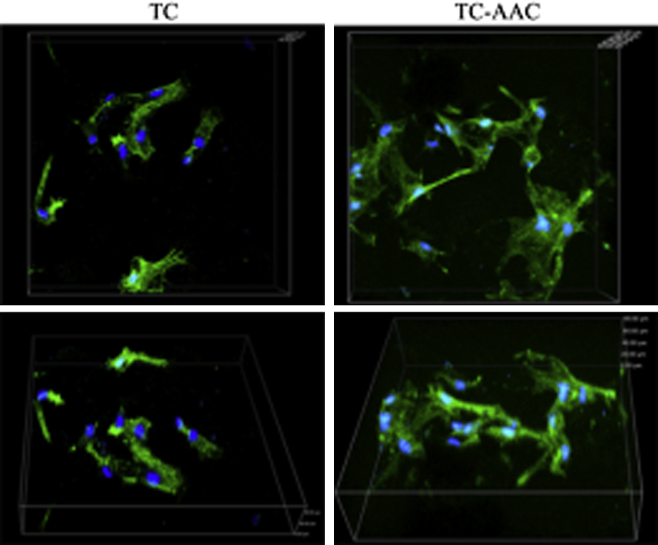
The cytoskeleton of the hMSCs on the two different samples was observed using CLSM (Figure 4). The hMSCs on TC displayed spherical and fusiform morphology, while those on TC-AAC exhibited polygonal and fusiform morphology. The cytoskeleton of the hMSCs on TC-AAC spread better than that on TC.
Figure 4.
Cytoskeletal morphology and spreading of hMSCs on TC and TC-AAC after 24 hours of incubation. Representative images of cells stained with phalloidin for actin filaments (green) and nuclei counterstained with 4′,6′-diamidino-2-phenylindole (blue). hMSC = human mesenchymal stem cell; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
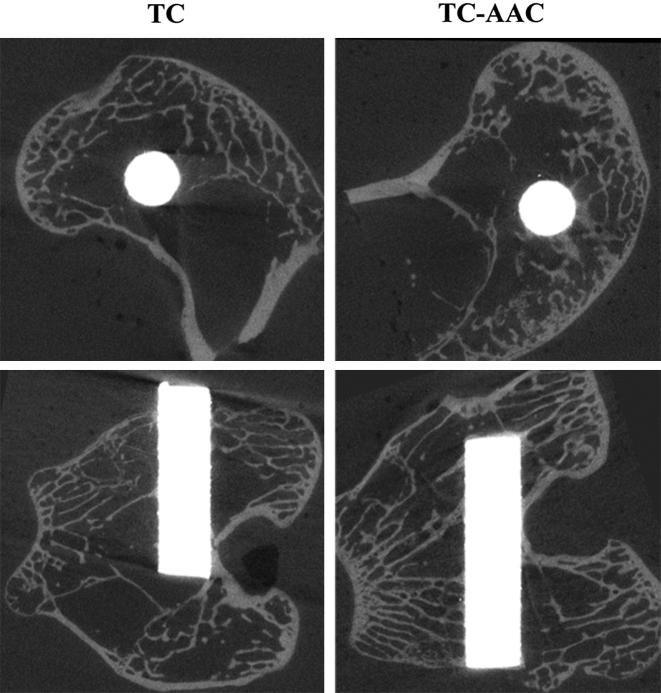
Micro-CT image analysis
Figure 5 shows the micro-CT images along the transection and midsagittal planes in the region around the implants after 3 months of implantation. Trabecular bone around TC-AAC was more abundant than that around TC. Table 1 lists the four parameters of the trabecular VOI (4.0 mm) in the axial direction. The BV/TV, Tb.Th, and Tb.N of TC-AAC were 7.45 ± 0.23%, 0.066 ± 0.002 mm, and 1.11 ± 0.04 mm−3, respectively, all of which were significantly higher than those of TC (6.10 ± 0.22%, 0.062 ± 0.001 mm, and 0.98 ± 0.03 mm−3; p < 0.05). The Tb.Sp of TC-AAC was 0.82 ± 0.04 mm−1, less than that of TC (0.95 ± 0.04 mm−1, p < 0.05).
Figure 5.
Micro-CT images along transection and midsagittal planes in region around implants after 3 months of implantation. CT = computed tomography; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
Table 1.
Microstructural parameters of trabecular volume of interest in axial direction.
| BV/TV (%) | Tb.Th (mm) | Tb.N (mm−3) | Tb.Sp (mm−1) | |
|---|---|---|---|---|
| TC | 6.10 ± 0.22 | 0.062 ± 0.001 | 0.98 ± 0.03 | 0.95 ± 0.04 |
| TC-AAC | 7.45 ± 0.23 | 0.066 ± 0.002 | 1.11 ± 0.04 | 0.82 ± 0.04 |
Data are presented as mean ± standard deviation.
BV/TV = bone volume fraction; Tb.N = trabecular number; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
Fluorescence micrograph of new-bone formation around implants
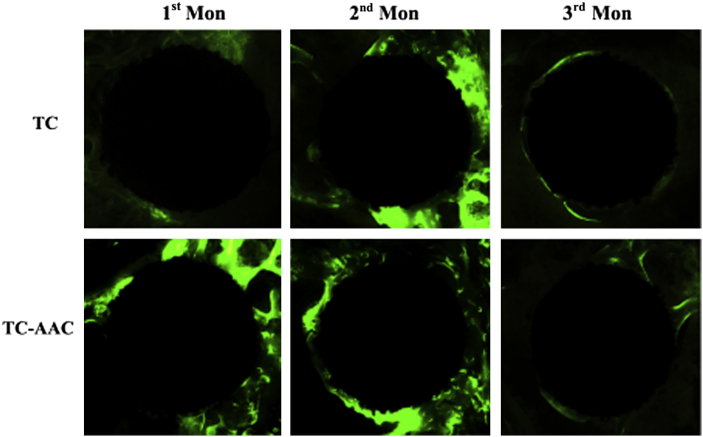
The bright-green fluorescence labelling of calcein in the newly formed bone surrounding the two kinds of implants is shown in Figure 6. As for TC, little green fluorescence was detected around it after 1 month of implantation, indicating that not much new bone had formed around the surface of TC. New bone around TC became more obvious after 2 months of implantation, and this growth continued to the end of the 3rd month after implantation—about half of the region surrounding TC was covered with green fluorescence in both the 2nd month and 3rd month. By contrast, much green fluorescence can be seen around TC-AAC after 1 month and 2 months of implantation. However, new bone was almost not found in the 3rd month.
Figure 6.
Confocal laser scanning micrograph showing green fluorescence labelling of calcein in newly formed bone around implant in two groups. TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
Histological observation and BIC
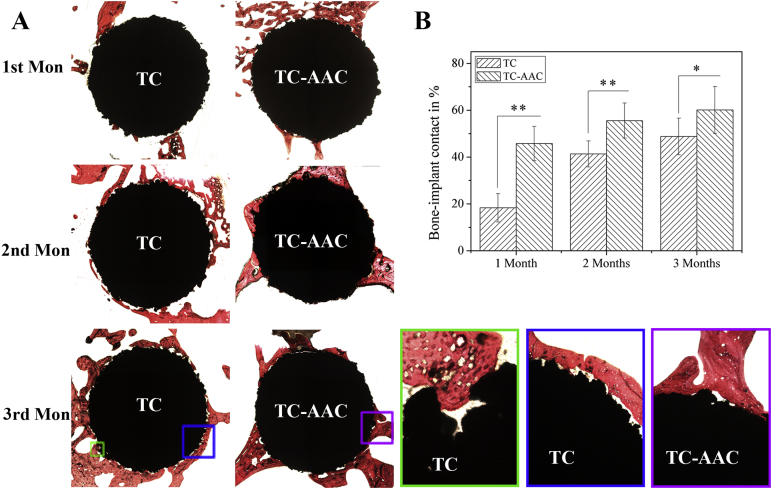
Figure 7A shows the histological appearance of implants and bone after 1 month, 2 months, and 3 months of implantation. The histological sections of implants and bone after 1 month of implantation showed that only a small number of trabeculae attached to the surfaces of the TC implants. By contrast, much new bone can be found on the TC-AAC surfaces and had close contact with the TC-AAC implants. In the 2nd month, new bone was found on the surface of TC. After 3 months of growth, new bone filled the surfaces of the TC implants. Close observation showed that new bone tissues had just grown along the periphery of the porous structure and few of them had come into the pores. Sometimes, new bone grew into the pores, but they were very hard to fill. New bone was also found around TC-AAC. Unlike those in TC, the vast majority of bone tissues closely combined with TC-AAC.
Figure 7.
(A) Histological morphology of interface between implants and bone tissue after 1 month, 2 months, and 3 months of implantation in rabbit femur condyles; (B) percentages of measured bone–implant contact of titanium coating-based implants. *p > 0.05 (n = 5). **p < 0.05 (n = 5). TC = titanium coating; TC-AAC = type I collagen covalently immobilised on TC.
The results of the BIC measurement are summarised in Figure 7B. The value was 42.7 ± 7.3% for the TC-AAC implants after 1 month of implantation, significantly higher than that for the TC implants (18.3 ± 6.0%, p = 0.00075). Direct BIC increases with an increase in time of implantation. After 3 months of implantation, the value for the TC-AAC implants (60.1 ± 9.8%) was also higher than that for the TC implants (48.7 ± 7.8%), although the difference between them was not significant (p = 0.011).
Discussion
Given their macroporous structures and high binding strength with substrates, plasma-sprayed Ti coatings as orthopaedic implants have attracted increasing attention. Cell attachment, migration, proliferation, and differentiation in the porous structure determine the suitability of implants [29]. Both in vitro and in vivo studies confirm that coating biomolecules, such as Type I collagen, on the surface of Ti-based implants is one of the most effective strategies to improve the bioactivity of Ti and accelerate early osteogenesis [30], [31], [32], [33]. In our previous study, Type I collagen was immobilised covalently on the surface of plasma-sprayed porous Ti coatings, whose surfaces consequently demonstrated improved hMSC attachment, proliferation, and differentiation. In the present work, the biological functions of collagen-modified Ti coatings were further investigated in vitro and in vivo.
hMSCs have multidirectional differentiation potency and play an important role in osseointegration [34]. The distribution and proliferation of cells observed using SEM, MTT assay, and three dimensional cell live/dead assay were employed to investigate cell migration on the surfaces of TC and TC-AAC. The number of cells on the surface of TC-AAC after 5 days of cultivation was so low that it could not reflect cell proliferation, which was demonstrated by MTT assay (Figure 2). It was speculated that the cells had migrated into the pores of TC-AAC, which was confirmed by the results of three dimensional live/dead cell assay. The results indicated that immobilised collagen could be helpful to cell migration. Although showing good biocompatibility, Ti is bioinert and thus affects cell migration [35]. Furthermore, the rough surface of Ti coating affects cell adhesion. As shown in Figure 4, the hMSCs on TC displayed spherical and fusiform morphology, and cells cannot closely attach to TC, as shown in the SEM morphology of hMSCs cultured on the specimens in our previous work [21]. The proliferated cells with poor adhesive morphology mostly stay in the same place and have difficulty migrating elsewhere, as shown in Figure 1C. Type I collagen is considered the basic initial bone matrix protein in bone formation [36]. It forms a scaffold for the attachment and migration of hMSCs and modulates various aspects of cell behaviour by mediating the flux of chemical and mechanical stimuli [37]. With the help of immobilised collagen, the hMSCs on TC-AAC displayed polygonal and fusiform morphology (Figure 4), and they closely attached to TC-AAC. Excellent cell migration may be helpful for osteoconduction and osseointegration.
To evaluate the osteoconduction and osseointegration of collagen-immobilised Ti coatings, a rabbit model with femur condyle defect was used. Both micro-CT scanning and histological analysis were employed. The micro-CT scans indicated that the osseointegration of TC-AAC implants is better than that of TC implants after 3 months of implantation. The fluorescent marker calcein was used to label new-bone formation around the implants. Following injection into the body, these active fluorescent markers can bind with inorganic salt and then deposit into the calcified bone matrix [38], [39]. Therefore, new bone formed after the injection of calcein is dyed green, which can reflect the status of each stage of osseointegration. After 1 month of implantation, little green fluorescence is observed around TC, while much green fluorescence is observed around TC-AAC, indicating that at early stages, the osseointegration of TC implants is poor and the osteogenic activity of TC-AAC is stronger than that of TC. Similar results were obtained in the histological observation. More bone tissues closely combined with TC-AAC than with TC, and the BIC of TC-AAC implants was significantly higher than that of TC implants, especially at the 1st month and 2nd month. Previous literature [20], [24] has also confirmed that covalently immobilised Type I collagen can improve the BIC of Ti-based implants. This improvement may be attributed to the bioinertness of Ti, which prevents osteogenesis [40]. Our previous in vitro experimental results [21] demonstrated that the introduction of Type I collagen can improve osteoblast differentiation, which may promote bone formation. All results indicated that immobilised collagen can improve the early osseointegration of TC-based implants.
The early stabilisation of TC-based implants may be improved by increased BIC. When bone tissue grows into the pores, the mechanical interlock between implant and bone may occur, and it may strengthen with more bone embedded into the pores. Xue et al [40] confirmed by push-out tests that alkali-modified Ti coating improves shear strength and BIC. Push-out tests can enable the direct measurement of the bonding strength between implant and bone and will thus be performed in our future experiments.
Conclusion
In this study, the osteoconduction and osseointegration of Type I collagen covalently immobilised on Ti coatings were investigated. The results of in vitro assay showed that immobilised collagen can induce hMSCs' migration into the pores of Ti coating. Accordingly, in vivo study demonstrated that immobilised collagen can improve the osteoconduction and osseointegration of TC-based implants and may thus be helpful to the early stabilisation of hip and knee prostheses in orthopaedic clinics.
Conflicts of interest
The authors declare no conflicts of interest in this work.
Funding/support
This work was funded by the National Natural Science Foundation of China (Nos. 51232007, 51172264, and 31200718), the Shanghai Science and Technology Development Fund (13DZ2294000), and the Opening Project of the Key Laboratory of Inorganic Coating Materials, Chinese Academy of Sciences (KLICM-2014-04).
Contributor Information
Xue-Bin Zheng, Email: xbzheng@mail.sic.ac.cn.
Ting-Ting Tang, Email: ttt@sjtu.edu.cn.
References
- 1.Xie Y., Liu X., Zheng X., Ding C. Bioconductivity of plasma sprayed dicalcium silicate/titanium composite coatings on Ti–6Al–4V alloy. Surface Coatings Technol. 2005;199:105–111. [Google Scholar]
- 2.Shi J., Ding C., Wu Y. Biomimetic apatite layers on plasma-sprayed titanium coatings after surface modification. Surface Coatings Technol. 2001;137:97–103. [Google Scholar]
- 3.Jaeggi C., Mooser R., Frauchiger V., Wyss P. 3D characterization of open porous vacuum plasma sprayed titanium coatings by means of high resolution micro computer tomography. Mater Lett. 2009;63:2643–2645. [Google Scholar]
- 4.Chen Y., Zheng X., Ji H., Ding C. Effect of Ti–OH formation on bioactivity of vacuum plasma sprayed titanium coating after chemical treatment. Surface Coatings Technol. 2007;202:494–498. [Google Scholar]
- 5.Treves C., Martinesi M., Stio M., Gutierrez A., Jimenez J.A., Lopez M.F. In vitro biocompatibility evaluation of surface-modified titanium alloys. J Biomed Mater Res Part A. 2010;92A:1623–1634. doi: 10.1002/jbm.a.32507. [DOI] [PubMed] [Google Scholar]
- 6.Lin L., Wang H., Ni M., Rui Y., Cheng T.Y., Cheng C.K. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J Orthop Translation. 2014;2:35–42. [Google Scholar]
- 7.Yoon I.K., Hwang J.Y., Jang W.C., Kim H.W., Shin U.S. Natural bone-like biomimetic surface modification of titanium. Appl Surface Sci. 2014;301:401–409. [Google Scholar]
- 8.Puleo D.A., Nanci A. Understanding and controlling the bone–implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 9.Geißler U., Hempel U., Wolf C., Scharnweber D., Worch H., Wenzel K.W. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J Biomed Mater Res. 2000;51:752–760. doi: 10.1002/1097-4636(20000915)51:4<752::aid-jbm25>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.L., Li Q.L., Chen J.Y., Chen C., Huang N. Improving blood-compatibility of titanium by coating collagen–heparin multilayers. Appl Surface Sci. 2009;255:6894–6900. [Google Scholar]
- 11.Ao H., Xie Y., Qin A., Ji H., Yang S., Huang L. Immobilization of hyaluronic acid on plasma-sprayed porous titanium coatings for improving biological properties. J Biomater Sci. 2014;25:1211–1224. doi: 10.1080/09205063.2014.926577. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno M., Fujisawa R., Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Maghdouri-White Y., Bowlin G.L., Lemmon C.A., Dréau D. Mammary epithelial cell adhesion, viability, and infiltration on blended or coated silk fibroin–collagen type I electrospun scaffolds. Mater Sci Eng: C. 2014;43:37–44. doi: 10.1016/j.msec.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Ma J., Thompson M., Zhao N., Zhu D. Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. J Orthop Translation. 2014;2:118–130. doi: 10.1016/j.jot.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller R., Abke J., Schnell E., Macionczyk F., Gbureck U., Mehrl R. Surface engineering of stainless steel materials by covalent collagen immobilization to improve implant biocompatibility. Biomaterials. 2005;26:6962–6972. doi: 10.1016/j.biomaterials.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Uezono M., Takakuda K., Kikuchi M., Suzuki S., Moriyama K. Hydroxyapatite/collagen nanocomposite-coated titanium rod for achieving rapid osseointegration onto bone surface. J Biomed Mater Res Part B: Appl Biomater. 2013;101B:1031–1038. doi: 10.1002/jbm.b.32913. [DOI] [PubMed] [Google Scholar]
- 17.Ao H., Xie Y., Tan H., Yang S., Li K., Wu X. Fabrication and in vitro evaluation of stable collagen/hyaluronic acid biomimetic multilayer on titanium coatings. J Royal Soc Interface. 2013;10:20130070. doi: 10.1098/rsif.2013.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker D., Geißler U., Hempel U., Bierbaum S., Scharnweber D., Worch H. Proliferation and differentiation of rat calvarial osteoblasts on type I collagen-coated titanium alloy. J Biomed Mater Res. 2002;59:516–527. doi: 10.1002/jbm.1265. [DOI] [PubMed] [Google Scholar]
- 19.Müller R., Abke J., Schnell E., Scharnweber D., Kujat R., Englert C. Influence of surface pretreatment of titanium- and cobalt-based biomaterials on covalent immobilization of fibrillar collagen. Biomaterials. 2006;27:4059–4068. doi: 10.1016/j.biomaterials.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Morra M., Cassinelli C., Cascardo G., Cahalan P., Cahalan L., Fini M. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials. 2003;24:4639–4654. doi: 10.1016/s0142-9612(03)00360-0. [DOI] [PubMed] [Google Scholar]
- 21.Ao H., Xie Y., Tan H., Wu X., Liu G., Qin A. Improved hMSC functions on titanium coatings by type I collagen immobilization. J Biomed Mater Res Part A. 2014;102:204–214. doi: 10.1002/jbm.a.34682. [DOI] [PubMed] [Google Scholar]
- 22.Morra M., Cassinelli C., Cascardo G., Mazzucco L., Borzini P., Fini M. Collagen I-coated titanium surfaces: mesenchymal cell adhesion and in vivo evaluation in trabecular bone implants. J Biomed Mater Res Part A. 2006;78A:449–458. doi: 10.1002/jbm.a.30783. [DOI] [PubMed] [Google Scholar]
- 23.Morra M., Cassinelli C., Cascardo G., Bollati D., Rodriguez y Baena R. Multifunctional implant surfaces: surface characterization and bone response to acid-etched Ti implants surface-modified by fibrillar collagen I. J Biomed Mater Res Part A. 2010;94A:271–279. doi: 10.1002/jbm.a.32702. [DOI] [PubMed] [Google Scholar]
- 24.Mueller C.K., Thorwarth M., Schmidt M., Schlegel K.A., Schultze-Mosgau S. Comparative analysis of osseointegration of titanium implants with acid-etched surfaces and different biomolecular coatings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:726–736. doi: 10.1016/j.tripleo.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sverzut A.T., Crippa G.E., Morra M., de Oliveira P.T., Beloti M.M., Rosa A.L. Effects of type I collagen coating on titanium osseointegration: histomorphometric, cellular and molecular analyses. Biomed Mater. 2012;7:035007. doi: 10.1088/1748-6041/7/3/035007. [DOI] [PubMed] [Google Scholar]
- 26.Tang T.T., Lu H., Dai K.R. Osteogenesis of freeze-dried cancellous bone allograft loaded with autologous marrow-derived mesenchymal cells. Mater Sci Eng C. 2002;20:57–61. [Google Scholar]
- 27.Qin L., Yao D., Zheng L., Liu W.C., Liu Z., Lei M. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials. 2015;59:125–143. doi: 10.1016/j.biomaterials.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X.W., Xie X.H., Yu Z.F., Tang T.T. Augmentation of screw fixation with injectable calcium sulfate bone cement in ovariectomized rats. J Biomed Mater Res Part B: Appl Biomater. 2009;89B:36–44. doi: 10.1002/jbm.b.31184. [DOI] [PubMed] [Google Scholar]
- 29.Mohd Daud N., Sing N.B., Yusop A.H., Abdul Majid F.A., Hermawan H. Degradation and in vitro cell–material interaction studies on hydroxyapatite-coated biodegradable porous iron for hard tissue scaffolds. J Orthop Translation. 2014;2:177–184. [Google Scholar]
- 30.Scharnweber D., Born R., Flade K., Roessler S., Stoelzel M., Worch H. Mineralization behaviour of collagen type I immobilized on different substrates. Biomaterials. 2004;25:2371–2380. doi: 10.1016/j.biomaterials.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Morra M., Cassinelli C., Cascardo G., Bollati D., Baena R.R.Y. Gene expression of markers of osteogenic differentiation of human mesenchymal cells on collagen I-modified microrough titanium surfaces. J Biomed Mater Res Part A. 2011;96A:449–455. doi: 10.1002/jbm.a.32948. [DOI] [PubMed] [Google Scholar]
- 32.Kung S., Devlin H., Fu E., Ho K.Y., Liang S.Y., Hsieh Y.D. The osteoinductive effect of chitosan-collagen composites around pure titanium implant surfaces in rats. J Periodontal Res. 2011;46:126–133. doi: 10.1111/j.1600-0765.2010.01322.x. [DOI] [PubMed] [Google Scholar]
- 33.Korn P., Schulz M.C., Hintze V., Range U., Mai R., Eckelt U. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. J Biomed Mater Res Part A. 2014;102:2334–2344. doi: 10.1002/jbm.a.34913. [DOI] [PubMed] [Google Scholar]
- 34.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y., Cai K., Luo Z., Zhang Y., Li L., Lai M. Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces. Biomaterials. 2012;33:3515–3528. doi: 10.1016/j.biomaterials.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Christenson R.H. Biochemical markers of bone metabolism: an overview. Clin Biochem. 1997;30:572–593. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 37.Bhatnagar R.S., Qian J.J., Wedrychowska A., Sadeghi M., Wu Y.M., Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5:53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 38.Ruhé P.Q., Kroese-Deutman H.C., Wolke J.G., Spauwen P.H., Jansen J.A. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials. 2004;25:2123–2132. doi: 10.1016/j.biomaterials.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Tan H., Ao H., Ma R., Tang T. Quaternised chitosan-loaded polymethylmethacrylate bone cement: biomechanical and histological evaluations. J Orthop Translation. 2013;1:57–66. [Google Scholar]
- 40.Xue W., Liu X., Zheng X., Ding C. In vivo evaluation of plasma-sprayed titanium coating after alkali modification. Biomaterials. 2005;26:3029–3037. doi: 10.1016/j.biomaterials.2004.09.003. [DOI] [PubMed] [Google Scholar]