Summary
Size and location of the lesion, subchondral collapse occurrence, and articular cartilage involvement are general disease progression criteria for direct osteonecrosis of the femoral head (ONFH) classifications. Treatment options for ONFH are usually based on individual factors and lesion characteristics. Although spontaneous repair of ONFH occurs in some cases, untreated ONFH is unlikely to escape the fate of subchondral collapse and usually ends up with total hip arthroplasty. Operations to preserve the femoral head, e.g., core decompression and bone grafting, are usually recommended in younger patients. They are helpful to relieve pain and improve function in the affected femoral head without subchondral collapse, however, poor prognosis after surgical procedures remains the major problem for ONFH. Pharmacological and physical therapies only work in the early stage of ONFH and have also been recommended as a supplement or prevention treatment for osteonecrosis. Following advances in basic science, many new insights focus on bone tissue engineering to optimize therapies and facilitate prognosis of ONFH. In this review, disease classifications, current treatment options, potential therapies, and the relevant translational barriers are reviewed in the context of clinical application and preclinical exploration, which would provide guidance for preferable treatment options and translation into novel therapies.
Keywords: classification, osteonecrosis of the femoral head, potential therapies, translational barriers, treatment options
Introduction
Osteonecrosis of the femoral head (ONFH) is a chronic disease that shows a complicated pathogenesis [1]. Spontaneous repair of ONFH is a slow, discontinuous, and time-dependent process that might only occur in small size lesions without concomitant joint fluid seepage [2], [3]. Naw et al's [4] clinical report revealed that 94% of asymptomatic ONFH will develop to symptomatic ONFH within 5 years. Untreated ONFH is believed to carry a poor outcome and often leads to the occurrence of subchondral collapse within a short period [4], [5]. Various surgical procedures are helpful to relieve pain and improve function of the affected femoral head in the early stages, however, the secondary trauma caused by surgical intervention remains an inevitable clinical problem and surgical procedures may not prevent deformity and collapse in deteriorating ONFH [6]. Therefore, how to reverse the early stage of ONFH and promote reparative bone remodelling becomes the key for maintaining the undestroyed joint adjacent to lesion areas and making available therapies to facilitate a good prognosis. Currently, the attention of surgeons and researchers is focused on: (1) enhancing the sensibility and accuracy of diagnosis to raise the rate of early diagnosis; (2) improving surgical operation technology or developing minimally invasive surgery to avoid the secondary trauma caused by surgical intervention; and (3) exploring drug or grafting products to promote reparative bone remodelling and obtain a good prognosis. This article presents a review of ONFH classification systems, current treatment options, potential therapies, and the relevant translation barriers in the context of clinical application and preclinical exploration. By addressing the relationship between ONFH pathological characteristics and various treatment options, as well as stating potential therapies and its translational barriers, we aim to provide guidance for preferable treatment options and translation into novel therapies.
ONFH classification systems
Patients with suspected ONFH would have one or more of the following criteria: (1) throbbing, deep groin pain, and one or more associated risk factors; and (2) a previous ONFH in another joint [7]. The suspected ONFH needs further validation using image detection before distinguishing lesion progression and choosing treatment options (Figure 1). ONFH classification systems are excellent tools based on imaging data that are widely used to stratify the severity and prognosis, and guide the treatment strategy [8]. There are several staging systems used for classifying ONFH, including Ficat and Arlet classification; the University of Pennsylvania (Steinberg) staging system, Association Research Circulation Osseous (ARCO), and the Japanese Orthopaedic Association (JOA) classification systems (Table 1). There are some denominators among them; the diagnostic data enable easy conversion to any of the four systems so that cross-comparison of results can be made [9].
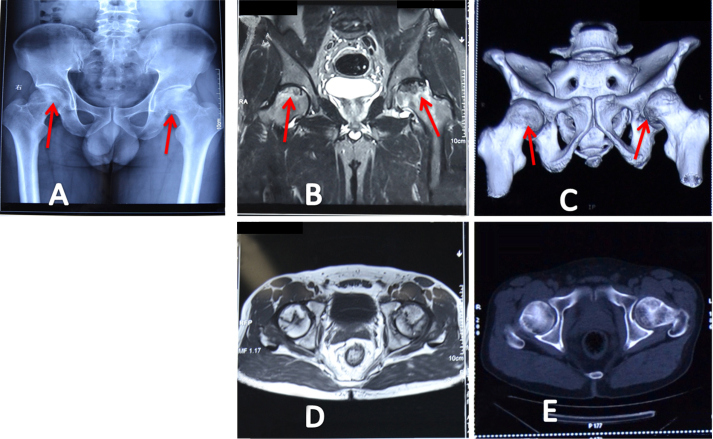
Figure 1.
(A) Dual X-Ray absorptiometry, (B) magnetic resonance imaging, and (C) computed tomography of a 41-year-old man with bilateral osteonecrosis of the femoral head (red arrows), and showing the Ficat and Arlet Stage II and Stage III lesions in the right and left femoral heads, respectively. The volume, angle, and arc of osteonecrotic lesions are detected on (D) magnetic resonance imaging and (E) computed tomography for guiding further treatment.
Table 1.
Classification systems of osteonecrosis of the femoral head.
| Ficat and Arlet [10], [11] (Radiography) |
Steinberg [12], [13] (Radiography; MRI; CT) |
ARCO [14], [15] (Radiography; MRI; CT; scintigraphy) |
JOA [18], [19] (Radiography; MRI; CT; scintigraphy) |
||||
|---|---|---|---|---|---|---|---|
| Stage | Description | Stage | Description | Stage | Description | Stage | Description |
| I | Normal (Patients are asymptomatic) |
0 | Normal physical examination (Patients are asymptomatic) |
0 | None | I | Demarcation line Subdivided by relationship to weight-bearing area: A: medial B: central C: lateral |
| II | Diffuse sclerotic and cystic lesions The integrity structure of hip (Patients have mild intermittent pain in the groin that radiates down the inner aspect of the thigh and a normal gait) |
I | Normal radiography Abnormal CT and MRI (Patients are asymptomatic) |
I | Normal radiography and CT and at least one of the other physical examination methods is positive Area of femoral head involvement: A: minimal < 15% B: moderate 15–30% C: extensive > 30% Length of crescent: A: < 15% B: 15–30% C: > 30% Surface collapse and dome depression: A: < 15% of and < 2 mm B: 15–30% and 2 to 4 mm C: > 30% and > 4 mm (Patients are asymptomatic) |
II | Early flattening without demarcation line around necrosis area |
| III | Subchondral fracture Crescent sign (Patients have increased pain and crepitus during changes in position particularly when arising from sitting) |
II | Diffuse sclerotic Cystic lesions Area of cystic lesions involvement: A: Mild < 15% B: Moderate 15–30% C: Severe: > 30% (Patients are asymptomatic) |
II | Sclerosis Osteolysis Focal porosis (Patients have mild intermittent pain in the groin that radiates down the inner aspect of the thigh and a normal gait) |
III | Cystic lesions Subdivided by site in the femoral head: A: medial B: central C: lateral |
| IV | Femoral head collapse Joint destruction Osteoarthritis Acetabular degeneration (Patients have pain with activity) |
III | Subchondral fracture Crescent sign Area of articular surface involvement: A: Mild < 15% B: Moderate 15–30% C: Severe: > 30% (Patients have mild intermittent pain in the groin that radiates down the inner aspect of the thigh and a normal gait) |
III | Crescent sign Flattening of femoral head (Patients have increased pain and crepitus during changes in position particularly when arising from sitting) |
||
| IV | Flattening of femoral head Area of femoral head involvement: A: Mild < 15% of surface affected and < 2 mm of depression B: Moderate 15–30% surface affected and 2–4 mm of depression C: Severe: > 30% surface affected and > 4 mm depression (Patients have increased pain and crepitus during changes in position particularly when arising from sitting) |
IV | Acetabular changes Joint destruction Osteoarthritis (Patients have pain with activity) |
||||
| V | Joint narrowing or acetabular changes (Patients have pain with activity) |
||||||
| VI | Advanced degeneration changesanges (Patients have pain with rest) |
||||||
ARCO = Association Research Circulation Osseous; CT = computed tomography; JOA = Japanese Orthopaedic Association; MRI = magnetic resonance imaging.
The Ficat and Arlet system was the first classification system for ONFH and this system includes four stages [10], [11]. Stage I is a transitional stage and patients are asymptomatic with a normal radiographic finding, but with increased uptake of tracer on bone scintigraphy [10]. Stage II represents the reparative stage and some diffuse sclerotic and cystic lesions can be observed before flattening of the femoral head occurs. Stage III is characterized by subchondral fracture (crescent sign). Stage IV involves the loss of the femoral head's anatomical sphericity and the occurrence of femoral head collapse and joint destruction. This damage leads to further progressive degeneration such as osteoarthritis and acetabular degeneration. There are some drawbacks in this system. Firstly, the descriptions of various stages are ambiguous and overlapping, and do not allow quantitation of the size of lesions, which makes it impossible to measure subtle degrees of progression. Next, the classification of later stages depends on invasive diagnosis techniques, such as core decompression, which would lead to secondary trauma.
The Steinberg system was established based on the Ficat and Arlet system, and the important modifications included the incorporation of magnetic resonance imaging findings and more clear distinction into seven stages [12]. This system is the first to incorporate the size of lesion measurements as part of a complete system [13]. The ARCO system originates from the Steinberg classification, and several amendments have been made over the years [14], [15]. This system does not provide a method to evaluate either preradiographic lesions or lesions in which the joint line and the acetabulum are involved [14]. The location of ON lesion is detected and the relative information is added to each stage in ARCO system as a supplement, but its specific value is uncertain [16], [17]. The JOA system originated from the Ficat and Arlet system with the location and size of the lesion added to its classification, however, this system only evaluates Ficat Stages II and III and not Stages I and IV [18], [19].
The goals in treatment of ONFH are to relieve pain and preserve the femoral head as long as possible. We believe that an optimal treatment option should be based on an appropriate classification system of ONFH [7]. Currently, for treating ONFH, there is a lack of consensus regarding diagnostic methods, evaluation systems, and indications of various treatment options [8]. Thus, symptoms, imaging and histological data, size and location of lesion, and the indications of articular cartilage involvement and femoral head depression, should be incorporated together to find a preferable treatment option for ONFH.
Current treatment options for ONFH
Nonoperative treatments
Most nonoperative treatment for early stage ONFH involves restricted weight bearing using a cane and activity modification. These methods only work in the early stage, asymptomatic ONFH, but show limited success in preventing disease progression (Table 2) [5], [20]. Restricted weight bearing cannot be recommended as a routine treatment, however, such therapies may have a role for patients with very limited disease or those not fit for further surgery [21], [22]. Other conservative treatments, including the use of pharmacological agents (such as lipid-lowering drugs, anticoagulants, vasodilators, traditional Chinese medicines, and bisphosphonates) and various noninvasive biophysical modalities (such as electromagnetic stimulation, extracorporeal shock-wave therapy, and hyperbaric oxygen) are advised for supplemental treatment of this disease [7], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]. The role of drugs for prevention or treatment is confined to specific aetiological pathways. In the right circumstances, the medical management would be able to arrest ONFH development and induce healing prior to collapse [33]. Meanwhile, these physical therapies are used to address specific physiological factors of ONFH and cannot be recommended as a routine treatment. Clinical studies have further demonstrated that conservative therapies do not achieve satisfactory clinical benefits and are not appropriate for ONFH with subchondral collapse [34].
Table 2.
Treatment options and their advantages and disadvantages.
| Treatment options | Criteria | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Untreated | Asymptomatic ONF | Spontaneous repair in exceptional cases | Poor outcome Developing to symptomatic |
[2], [3], [4], [5] | |
| Nonoperative therapy | Restriction of weight-bearing | The early stage of ONFH (Ficat and Arlet Stage I) | Giving pain relief | Very limited for preventing disease progression | [20], [21], [22] |
| Drugs | With known aetiological pathway | As prevention treatment Supplement treatment for operation |
Very limited benefits Not appropriate for collapse |
[23], [24], [25], [26], [30], [31], [32] | |
| Physical therapy | With known physiological factors | [27], [28], [29] | |||
| Core decompression | Drill a single 8–10 mm core | Ficat and Arlet Stage II Patients have mild intermittent pain Precollapse ONFH |
Giving pain relief Reducing intraosseous pressure Stimulating angiogenesis and osteogenesis |
Lower mechanical strength Secondary trauma |
[35], [36], [37] |
| Drill a single 3.2 mm core | NA | [38] | |||
| Bone grafting | Allo-bone grafting Auto-bone grafting |
Giving pain relief Offering structural support Stimulating angiogenesis and osteogenesis |
Infections Immune response |
[21], [41], [42], [43] | |
| Ceramics Bioglass |
Higher brittleness of implants Slow degradation rates Secondary trauma |
[44], [45], [46] | |||
| Metal implants Stainless steel Titanium Tantalum |
Lower tissue adherence Lower rate of degradation Metal ions toxicity Secondary trauma |
[44], [47], [48], [49] | |||
| Osteotomy | Transtrochanteric rotational osteotomy | Ficat and Arlet Stage II and III Patients have increased pain Patients < 45 years old No long steroids using history A small necrotic angle Minimal osteoarthritic changes Without acetabular involvement |
Giving pain relief Changing biomechanical effect in lesion region Reducing intramedullary pressure |
Ethnic differences Poor fixation Delayed union Secondary deformity Secondary collapse |
[7], [52], [53], [54], [55], [56], [57], [58] |
| Intertrochanteric angular osteotomy | |||||
| Arthroplasty | Limited femoral resurfacing | Ficat and Arlet Stage III Patients have increased pain or crepitus during changes in position Necrotic angle of > 200° Necrotic area >30% Femoral head collapse >2 mm Without acetabular cartilage involvement |
Retaining the viable acetabular cartilage Retaining the bone stock |
Higher failure rates Femoral neck fracture Dislocation of femoral head Secondary trauma |
[59], [60], [61], [67] |
| Full resurfacing | Ficat and Arlet Stage III and IV; Necrotic area <35%; Femoral head neck junction integrity remains preserved; Bone stock providing a stable foundation for other components. |
Best choice for younger patients with end stage arthritis | Dislocation of femoral head Secondary trauma Groin pain Limited implants lifespan |
[22], [62], [63], [64], [65], [66], [67] | |
| Total hip arthroplasty | Ficat and Arlet Stage IV; Femoral head quality is very poor Continuing defective on bone mineral metabolism With acetabular cartilage involvement |
The only choice for degenerated hip joint | Greater mechanical failure rate Limited implants lifespan Dislocation of femoral head Secondary trauma |
[6], [22], [59], [63], [67] | |
ONFH = osteonecrosis of the femoral head.
Operative treatments
Core decompression and bone grafting
Core decompression is the leading surgical treatment for precollapse ONFH and Mont et al's [34] clinical research report showed this operation's success rate reached 70% on follow up of ≥ 5 years without the need for additional surgery. Core decompression involves drilling a single 8–10-mm core into the necrotic lesion that could provide pain relief and reduce intraosseous pressure [35]. Meanwhile, this procedure enhances the process of new bone creeping substitution of the necrotic area by stimulating an angiogenic response in the drill channels, therefore restoring or improving vascular flow to prevent further ischaemic episodes and progressive bone infarction [36]. However, clinical problems still exist, including incomplete reconstructive repair and weakening of the trabecular bone within and adjacent to the necrotic region [37]. Recently, Mont et al [38] described a multiple drill-hole technique using a 3.2-mm pin, and reported an 80% success rate for treatment of early stage ONFH that did not need further surgery within at least 7 years.
Core decompression combined with bone grafting produce an improved effect for ONFH through enhancing bone formation and reducing the risk of proximal femoral fracture, and this treatment has been recommended as a routine treatment for precollapse ONFH [39], [40]. Allo- or auto-bone graft (cortical strut grafts taken from the ilium, fibula, or tibia; cancellous bone graft taken from the greater trochanter and proximal femur) that fill the drill channels not only offer structural support, but also provide scaffolding for repair (Figure 2) [21], [41]. However, these procedures will still have potential risk of viral or bacterial infections and immune response [42], [43]. Ceramic and bioglass implants are also widely used in bone surgical repairs and are able to form bone apatite-like material or carbonate hydroxyapatite on their surfaces, enhancing their osseointegration, however, brittleness and slow degradation rates of these materials are disadvantages for their use [44], [45], [46]. Metals as implant materials (such as stainless steel, titanium and its alloys, and tantalum) have advantages due to their excellent mechanical properties and porous surfaces that serve as delivery systems for special growth factors [44], [47]. However, the lack of tissue adherence and the lower rate of degradation result either in a second surgery to remove the implant or in permanent implantation in the body with the related risks of toxicity due to accumulation of metal ions caused by corrosion [48], [49]. In addition, patients who continue steroid therapy after decompression have a worse prognosis [50]. Recently, pure magnesium and its alloy coated with microarc oxidation and electrophoresis deposition have been shown to reduce the degradation rate of magnesium and have great potential as promising biodegradable implantation materials and internal fixators [51]. Although core decompression seems to be more effective than purely symptomatic treatment, it must be performed in the precollapse stage, because it will not restore femoral head sphericity or remove the collapsed segments from the weight-bearing area [34], [36].
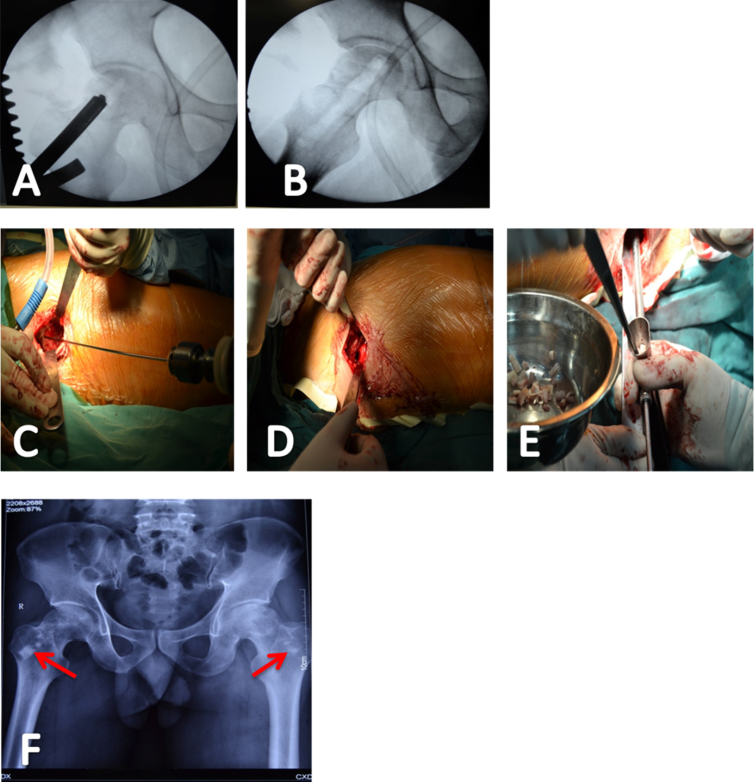
Figure 2.
(A–E) The same patient as described in Figure 1 treated using core decompression and allo-bone grafting. (F) The condition on the first postoperative day was delineated by dual X-ray absorptiometry; allo-bone implants were seen in the tunnel (red arrows).
Osteotomy
Osteotomies are used to rotate the necrotic or collapsing segment of the hip out of the weight-bearing zone or to move the segments of necrotic bone away and replace them with a healthy viable bone [6], [22], [52]. This procedure could change the biomechanical effect, and reduce venous hypertension and intramedullary pressure [52]. Osteotomies are usually recommended to younger patients (aged < 45 years) [53]. These surgical procedures include transtrochanteric rotational osteotomy and intertrochanteric angular osteotomy [54], [55]. However, the prognosis of these two procedures is difficult to compare because there are ethnic differences [7]. Transtrochanteric rotational osteotomy only applied to Asian countries because the posterior capsule of the hip in Asians may be more lax and may allow for better rotation of the anterior portion of the femoral neck. On the contrary, the intertrochanteric angular osteotomy has been more successful in Caucasians due to above anatomic difference [7], [56]. However, poor fixation with screws may cause increased various deformity, delayed union, and even secondary collapse of the femoral head [57]. Osteotomy is used rarely, because this procedure is only suitable for patients with the following criteria: (1) aged < 45 years; (2) not being treated with long-term steroids; (3) with minimal osteoarthritic changes; (4) a small necrotic angle; and (5) without acetabular involvement [53], [58].
Arthroplasty
Limited femoral resurfacing with cement fixation is usually used in younger patients. In this procedure, the damaged cartilage on the femoral side is removed, the viable acetabular cartilage is retained, and bone stock is preserved [59]. However, this limited femoral resurfacing surgery requires years of rigorous training and great skill, because these procedures have high failure rates and are closely related to femoral neck fracture [60], [61]. Mont et al [59] recommend that patients with the following criteria are chosen for limited femoral resurfacing: (1) Ficat and Arlet Stage III; (2) necrotic angle of > 200° or necrotic area > 30%; (3) femoral head collapse of > 2 mm; and (4) the acetabular cartilage has not been damaged.
In addition, full resurfacing arthroplasty has become an increasingly popular choice for younger patients with end-stage arthritis [62], [63]. Parsons and Steele [22] considered that all operations were performed in special patients with the following criteria: (1) necrotic area < 35%; (2) femoral head neck junction integrity remains preserved; and (3) the remains of bone stock could provide a stable foundation for femoral components. This procedure involves replacing a limited portion of the femoral head by a thin cemented polyethylene acetabular component or the renaissance of a large head [64], [65]. The use of these devices for necrotic hips has led to some concerns regarding vascular insult to the femoral head and lower osteointegration rate of fixation components [66], and the design of devices does not address pathology progression at the acetabular surface in the later-stage ONFH, along with many complications including wear, loosening, and groin pain [67]. However, research has found that if good results are seen at an early stage, they would be maintained for a long time.
Once the hip joint has degenerated (the articulation was compromised), total hip arthroplasty (THA) will be needed. However, there is no consensus regarding the utilization of total hip replacement in particular patients with sickle cell disease, systemic lupus erythematosus, postrenal transplant and ongoing steroid use or alcohol abuse [22]. Mont et al [6], [59] and Parsons and Steele [22] also recommend that patients considered for total hip replacement should meet the following criteria: (1) the femoral head quality is very poor; (2) there may be continuing defective bone mineral metabolism; and (3) hip is subjected to continuing insults. However, there is a greater mechanical failure rate in patients aged ≥ 50 years; wearing and loosening are major complications of THA [63], [67]. Currently, the use of contemporary cementing techniques or uncemented components combined with improved bearing surfaces could reduce wear and improve longevity of implants [22]. In addition, coupled with the larger head combinations made possible by hard-on-hard bearing surfaces, this should reduce the loosing or dislocation risk [22]. Recently, metal-on-metal and ceramic-on-ceramic bearing surfaces have become more popular in clinical application. Correctly, the potential advantages of resurfacing over THA are lower dislocation rates, preservation of bone stock, and THA conversion could be performed if necessary [63], [67].
Potential therapies for ONFH and their translational barriers—scaffold-based bone tissue engineering combined with biofactors
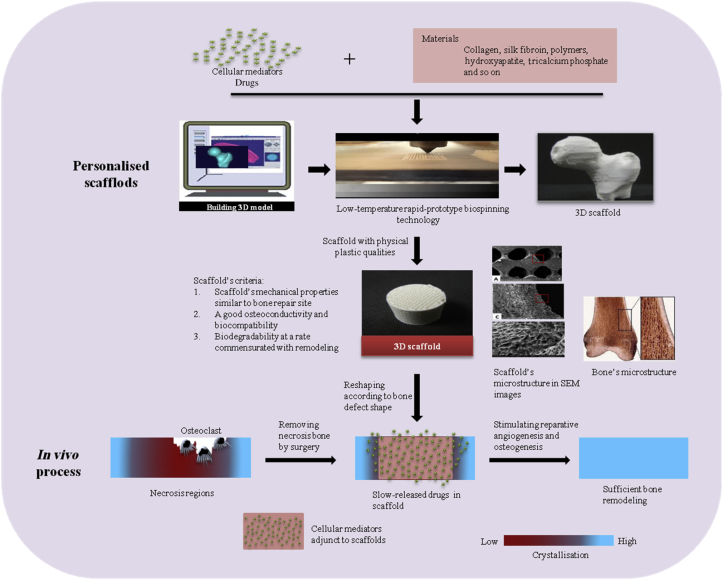
Bone is in a constant state of osteoclasts resorbing the matrix and osteoblasts forming the matrix during the adult stage [68], and this well-balanced state is associated with cellular and vascular events [69], [70]. Various cellular mediators (growth factors, differentiation factors, cytokines, and hormones) are sequestered in vascular and bone matrix, and regulate bone metabolism, function, and regeneration [71], [72]. Currently, evidence implies that the ONFH deterioration is associated with the follow issues [73], [74], [75], [76], [77]: (1) aberrant osteoclastic resorption activity; (2) continuous higher vascular permeability; (3) sluggish reparative angiogenesis and osteogenesis; (4) irreversible connective tissue formation; (5) subsequent lower mechanical properties; (6) necrosis area diffusion; (7) severe joint fluid seepage; and (8) necrosis spreading to joints. Vascular, cellular, and matrix events are all involved in this advanced pathological progression and affect each other [77]. To date, biologics involving antiapoptosis, angiogenesis, and osteogenesis pathways have been screened [78]. Selected biologics (cellular mediators, osteogenic, and angioblastic cells) were injected directly into necrotic regions or seeded in scaffolds and then implanted in the bone defect site where the lesion tissue was removed; these procedures were performed in various preclinical models to test the efficacy [79], [80]. The scaffold combined with biologics serves as a template to facilitate cell interactions and the formation of bone-extracellular matrix that could be more favourable to enhance reparative angiogenesis and osteogenesis compared with signal scaffold implant and cellular mediator injection (Figure 3) [81], [82], [83], [84].
Figure 3.
Potential therapy for osteonecrosis using biodegradable three-dimensional scaffolds with biofactors. 3D = three dimensional; SEM = scanning electron microscopy.
The ideal material or composite used as the component of the scaffold should be nonimmunogenic, nontoxic, controllable, inexpensive, and readily available. A number of scaffold components are currently available [44], [84], [85] and include inorganic materials, organic materials, and biologics. Through control of a variety of different but inter-related parameters, there is the potential to develop novel and increasingly advanced composites [44], [85]. For example, polymers have the advantage of biocompatibility, however, their low mechanical strength and high rates of degradation often affects their use, chemical modification, or other materials participation to improve these polymers implants physical properties and bioactivity. Furthermore, composite scaffolds could also incorporate some biofactors to make a potential bone graft substitute available [44], [86], [87]. In 1998, the first implantation of a porous ceramic seeded with in vitro-expanded autologous osteogenic cells was performed in a bone segmental defect of a patient and it exhibited a good integration and repair process at the interface with the host bone [88], [89]. Since then, a few other similar cases were treated using the same approach [80]. In the last 15 years, bone marrow mesenchymal stem cells, bone marrow cells, periosteal cells, platelet-rich plasma, and/or recombinant human bone morphogenetic proteins 2 and 7 (rhBMP-2 and rhBMP-7) have been incorporated into degradable porous scaffolds as implants for bone defect and necrotic cartilage repair in orthopaedic patients [90], [91], [92], [93], [94], [95]. However, the use of scaffolds combined with biofactors in clinical practice has several major inconveniences [80]. The contribution of the biofactors to the bone formation was difficult to evaluate due to the lack, or inadequacy, of control group patients [96], [97], [98]. The success rate is higher, but complications or nonunions are common, especially in large shaft reconstructions [80], [99]. rhBMP-2 has been the most commercially successful bone tissue engineering product, being utilized in up to 25% of all spinal fusion procedures [99]. However, there have been significant complications with rhBMP use, including patient death, dysphagia, airway compression in spine fusion, and heterotopic bone formation in the spinal canal [100], [101]. In addition, clinical trials of rhBMP-2 incorporation showed risks of cancer, causing the Food and Drug Administration (FDA) to halt its clinical study [102]. The clinical gold standard remains the vascularized free-fibular graft. Although billions of dollars in research funding has been input to explore novel available scaffolds, translation of scaffold-based bone tissue engineering therapies to clinical use is required in the future [103]. Technical and business barriers are critical issues resulting in failed translations and there is a need to address this long-term development.
Dr Scott Hollister [102] provided the 4Fs overview of scaffolds, which served as the fundamental framework for bone tissue engineering, and should be considered in the design of an effective scaffold. The 4Fs are Form, Function, Fixation, and Formation. Form refers to the ability to conform the 3D shape and fill the bone defect site. Function refers to the mechanical properties of the scaffold and requires that scaffolds provide temporary mechanical load bearing within a bone defect. Fixation refers to the ability of the scaffold to integrate and attach to the existing neighbouring bone and soft tissue with good biocompatibility. Formation indicates that scaffolds have a good osteoconductivity, features which are related to porosity, permeability, diffusivity, and delivering osteoinductive factors, including cells, proteins, and genes. Finding a scaffold that addresses all the 4Fs quantitative requirements is an extremely challenging task. There is a common trade-off between the 4Fs during scaffold design, because a strategy priority to promote formation needs special materials or participation of biofactors, which requires a preferable manufacturing technique that intrinsically limits scaffold physical properties related to form, function, and fixation [104]. In general, a scaffold development undergoes two phases [85]: (1) scaffold fabrication needs to entail its physical properties (elasticity, permeability, diffusivity, and degradation) meet functional and formation requirements through regulating the distribution and participation of various materials and micropore structure in three-dimensional space [44], [105], [106], [107], [108]; and (2) innovative biomaterials with controlled biofactor release, combined with a structural device such as surface creating to cater for individual clinical design [109], [110], [111].
Karageorgiou and Kaplan [44] systematically analysed potential porous 3D biomaterial scaffolds according to their physical properties and biological functions and provided guidance regarding design choices of available bone grafts. However, many successful scaffolds in preclinical models may be implausible in some clinical applications due to technical barriers. Firstly, the sequential fabrication technology needed to address all the 4Fs is difficult to achieve due to the limitation of engineering and materials science domain development [112], [113]. Secondly, the safety and efficacy of key parameter allocation involving trade-off of the 4Fs are difficult to validate before a wide application in clinics, because the assessment of patient responses is a more complex task for heterogeneity of genetic background and dissimilarity of bone defect types and bone loss patterns [114]. Finally, the controlled biologic release system in a scaffold makes it difficult to match desirable delivery dose and time scale; existing biologic carriers use a very higher dose delivered over a relatively short timescale, which has led to many side effects such as oedema, heterotopic bone and vascular formation, and increased cancer risk [101], [115]. The above issues require more investigation in preclinical and clinical trials.
In addition, the business barrier is another critical issue that needs to be addressed in scaffold-based bone tissue engineering translation. The business challenges to translation include regulatory approval, obtaining external funding support, obtaining surgeon acceptance, and obtaining approval for insurance reimbursement [102]. This procedure is very long and uncontrollable, with a need to invest a lot of time, energy, and money. The first step is to establish a quality system covering the scaffold design and manufacturing, as well as a biofactor attachment [116], [117]. This would further assure scaffold biosafety and reduce the investment risk to extend the scaffold product to a combination product. The second step in conducting a scaffold from discovery to the clinic is to assure the material components used in the scaffold have been approved by the FDA or China FDA. In parallel, the preclinical studies required in a Class II or Pre-Market Approval application, and the preclinical trials performed, should match International Standards Organization 10993 guidelines [117], [118]. The last but the most important part is clinical trials. In general, the clinical trial consists of 4 difference research phases (Phase I, II, III, IV), and a commercially scaffold need to require approvals from phase I to phase IV. Unfortunately, many scaffolds fail to pass Clinical Trial Phase II approval and then the relevant study is halted by the FDA [102]. The failed cases imply that the successful translation of scaffold-based bone tissue engineering still requires more innovation techniques and preferable biofactors.
Future perspectives
Despite the challenges in bone tissue engineering being very frustrating, there remains tremendous optimism concerning the potential to replace damaged and degenerated structures and tissue. The integration of multiple disciplines, such as cell biology, molecular biology, biomechanics science, immunology, structure engineering science, computer science, three-dimensional printing technology, and translational science, may accelerate bone tissue engineering development and product translation. These advances might improve bone healing by alternative approaches in surgery and facilitate the prognosis of ONFH.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Funding/support
This work was supported by National Science Foundation of China (No. 81171771, 81302782 and 51203178) and NSFC-DG-RTD Joint Scheme (No. 51361130034) and the European Union's 7th Framework Program under grant agreement (No. NMP3-SL-2013-604517), and the Opening Project of Shanghai Key Laboratory of Orthopaedic Implants (No. KFKT2014001).
References
- 1.Seamon J., Keller T., Saleh J., Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng E.Y., Thongtrangan I., Laorr A., Saleh K.J. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86A:2594–2599. doi: 10.2106/00004623-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura J., Harada Y., Oinuma K., Iida S., Kishida S., Takahashi K. Spontaneous repair of asymptomatic osteonecrosis associated with corticosteroid therapy in systemic lupus erythematosus: 10-year minimum follow-up with MRI. Lupus. 2010;19:1307–1314. doi: 10.1177/0961203310372951. [DOI] [PubMed] [Google Scholar]
- 4.Nam K.W., Kim Y.L., Yoo J.J., Koo K.H., Yoon K.S., Kim H.J. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90A:477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 5.Hernigou P., Poignard A., Nogier A., Manicom O. Fate of very small asymptomatic stage-1 osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86A:2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mont M.A., Jones L.C., Hungerford D.S. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 7.Marker D.R., Seyler T.M., McGrath M.S., Delanois R.E., Ulrich S.D., Mont M.A. Treatment of early stage osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(Suppl. 4):175–187. doi: 10.2106/JBJS.H.00671. [DOI] [PubMed] [Google Scholar]
- 8.Mont M.A., Marulanda G.A., Jones L.C., Saleh K.J., Gordon N., Hungerford D.S. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl. 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt-Sody M., Kirchhoff C., Mayer W., Goebel M., Jansson V. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop. 2008;32:283–287. doi: 10.1007/s00264-007-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficat R.P. Idiopathic bone necrosis of the femoral-head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 11.Jawad M.U., Haleem A.A., Scully S.P. In brief: Ficat classification: avascular necrosis of the femoral head. Clin Orthop Relat Res. 2012;470:2636–2639. doi: 10.1007/s11999-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 13.Steinberg M.E., Bands R.E., Parry S., Hoffman E., Chan T., Hartman K.M. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271. [PubMed] [Google Scholar]
- 14.Gardeniers J.W.M. The ARCO perspective for reaching one uniform staging system of osteonecrosis. NATO Adv Sci Inst Series. 1993;247:375–380. [Google Scholar]
- 15.Stulberg B.N., Gardeniers J.W.M. Methodologic problems in staging and evaluating osteonecrosis. NATO Adv Sci Inst Series. 1993;247:373–374. [Google Scholar]
- 16.Ito H., Matsuno T., Omizu N., Aoki Y., Minami A. Mid-term prognosis of non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 2003;85B:796–801. [PubMed] [Google Scholar]
- 17.Nishii T., Sugano N., Ohzono K., Sakai T., Haraguchi K., Yoshikawa H. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–157. doi: 10.1097/00003086-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto Y. The role of the JOA in the globalization of orthopedics. J Orthop Sci. 2009;14 doi: 10.1007/s00776-009-1319-x. 131–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohzono K., Saito M., Sugano N., Takaoka K., Ono K. The fate of nontraumatic avascular necrosis of the femoral head. A radiologic classification to formulate prognosis. Clin Orthop Relat Res. 1992;277:73–78. [PubMed] [Google Scholar]
- 20.Kaushik A.P., Das A., Cui Q. Osteonecrosis of the femoral head: an update in year 2012. World J Orthop. 2012;3:49–57. doi: 10.5312/wjo.v3.i5.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Peng J., Lu S.B. Summary of the various treatments for osteonecrosis of the femoral head by mechanism: a review. Exp Ther Med. 2014;8:700–706. doi: 10.3892/etm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons S.J., Steele N. Osteonecrosis of the femoral head: Part 2. Options for treatment. Curr Orthopaed. 2008;22:349–358. [Google Scholar]
- 23.Pritchett J.W. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Meizer R., Radda C., Stolz G., Kotsaris S., Petje G., Krasny C. MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint localizations treated with the prostacyclin analogue iloprost. Wien Klin Wochenschr. 2005;117:278–286. doi: 10.1007/s00508-005-0326-y. [DOI] [PubMed] [Google Scholar]
- 25.te Winkel M.L.T., Appel I.M., Pieters R., van den Heuvel-Eibrink M.M. Impaired dexamethasone-related increase of anticoagulants is associated with development of osteonecrosis in childhood acute lymphoblastic leukaemia. Haematol-Hematol J. 2008;93:1570–1574. doi: 10.3324/haematol.12956. [DOI] [PubMed] [Google Scholar]
- 26.Hofstaetter J.G., Wang J., Glimcher M.J. The use of alendronate in the treatment of osteonecrosis of the femoral head to reduce degenerative osteoarthritis of the hip. J Bone Miner Res. 2004;19:S324. [Google Scholar]
- 27.Massari L., Fini M., Cadossi R., Setti S., Traina G.C. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88A:56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 28.Vulpiani M.C., Vetrano M., Trischitta D., Scarcello L., Chizzi F., Argento G. Extracorporeal shock wave therapy in early osteonecrosis of the femoral head: prospective clinical study with long-term follow-up. Arch Orthop Trauma Surg. 2012;132:499–508. doi: 10.1007/s00402-011-1444-9. [DOI] [PubMed] [Google Scholar]
- 29.Uzun G., Mutluoglu M., Ozdemir Y. Osteonecrosis of femoral head and hyperbaric oxygen therapy. Arch Orthop Trauma Surg. 2009;129:1583–1584. [Google Scholar]
- 30.Zhang G., Qin L., Sheng H., Yeung K.W., Yeung H.Y., Cheung W.H. Epimedium-derived phytoestrogen exert beneficial effect on preventing steroid-associated osteonecrosis in rabbits with inhibition of both thrombosis and lipid-deposition. Bone. 2007;40:685–692. doi: 10.1016/j.bone.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G., Wang X.L., Sheng H., Xie X.H., He Y.X., Yao X.S. Constitutional flavonoids derived from Epimedium dose-dependently reduce incidence of steroid-associated osteonecrosis not via direct action by themselves on potential cellular targets. PLoS One. 2009;4:e6419. doi: 10.1371/journal.pone.0006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G., Qin L., Sheng H., Wang X.L., Wang Y.X., Yeung D.K. A novel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone. 2009;44:345–356. doi: 10.1016/j.bone.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland J.C., Brennan O., Kennedy O.D., Rackard S., O'Brien F.J., Lee T.C. Subchondral osteopenia and accelerated bone remodelling post-ovariectomy—a possible mechanism for subchondral microfractures in the aetiology of spontaneous osteonecrosis of the knee? J Anat. 2013;222:231–238. doi: 10.1111/joa.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mont M.A., Carbone J.J., Fairbank A.C. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman J.R. Core decompression for osteonecrosis of the hip. Clin Orthop Relat Res. 2004;418:29–33. doi: 10.1097/00003086-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Floerkemeier T., Lutz A., Nackenhorst U., Thorey F., Waizy H., Windhagen H. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35:1461–1466. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei B.F., Ge X.H. Treatment of osteonecrosis of the femoral head with core decompression and bone grafting. Hip Int. 2011;21:206–210. doi: 10.5301/HIP.2011.6525. [DOI] [PubMed] [Google Scholar]
- 38.Mont M.A., Ragland P.S., Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res. 2004;429:131–138. doi: 10.1097/01.blo.0000150128.57777.8e. [DOI] [PubMed] [Google Scholar]
- 39.Rosenwasser M.P., Garino J.P., Kiernan H.A., Michelsen C.B. Long-term follow-up of thorough debridement and cancellous bone-grafting of the femoral-head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17–27. [PubMed] [Google Scholar]
- 40.Pater A.N., Mittal S., Vina R.F., Benetti F., Trehan N. Long term follow-up of coronary artery bypass grafting with autologous bone marrow cell therapy. Cytotherapy. 2014;16:S39. [Google Scholar]
- 41.He W., Li Y., Zhang Q., Wang H., Fang B., Pang Z. Primary outcome of impacting bone graft and fibular autograft or allograft in treating osteonecrosis of femoral head. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:530–533. [In Chinese] [PubMed] [Google Scholar]
- 42.Stevenson S., Shaffer J.W., Goldberg V.M. The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res. 1996:86–95. doi: 10.1097/00003086-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Lord C.F., Gebhardt M.C., Tomford W.W., Mankin H.J. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70A:369–376. [PubMed] [Google Scholar]
- 44.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 45.López-Alvarez M., Rodríguez-Valencia C., Serra J., González P. Bio-inspired ceramics: promising scaffolds for bone tissue engineering. Procedia Engineer. 2013;59:51–58. [Google Scholar]
- 46.Han X., Li X.F., Lin H.M., Ma J., Chen X., Bian C.H. Hierarchical meso-macroporous bioglass for bone tissue engineering. J Sol-Gel Sci Techn. 2014;70:33–39. [Google Scholar]
- 47.Svehla M., Morberg P., Zicat B., Bruce W., Sonnabend D., Walsh W.R. Morphometric and mechanical evaluation of titanium implant integration: comparison of five surface structures. J Biomed Mater Res. 2000;51:15–22. doi: 10.1002/(sici)1097-4636(200007)51:1<15::aid-jbm3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Matusiewicz H. Potential release of in vivo trace metals from metallic medical implants in the human body: from ions to nanoparticles—a systematic analytical review. Acta Biomater. 2014;10:2379–2403. doi: 10.1016/j.actbio.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 49.Jakobsen S.S., Lidén C., Søballe K., Johansen J.D., Menné T., Lundgren L. Failure of total hip implants: metals and metal release in 52 cases. Contact Dermatitis. 2014;71:319–325. doi: 10.1111/cod.12275. [DOI] [PubMed] [Google Scholar]
- 50.Wang G.J., Dughman S.S., Reger S.I., Stamp W.G. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985;67A:121–124. [PubMed] [Google Scholar]
- 51.Tang J., Wang J.L., Xie X.H., Zhang P., Lai Y.X., Li Y.D. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J Orthop Transl. 2013;1:41–48. [Google Scholar]
- 52.Shannon B.D., Trousdale R.T. Femoral osteotomies for avascular necrosis of the femoral head. Clin Orthop Relat Res. 2004;418:34–40. doi: 10.1097/00003086-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Ha Y.C., Kim H.J., Kim S.Y., Kim K.C., Lee Y.K., Koo K.H. Effects of age and body mass index on the results of transtrochanteric rotational osteotomy for femoral head osteonecrosis. J Bone Joint Surg Am. 2011;93A:75–84. doi: 10.2106/JBJS.J.01215. [DOI] [PubMed] [Google Scholar]
- 54.Jingushi S. Osteotomy for osteonecrosis of the femoral head: knowledge from our long-term treatment experience at Kyushu University. In: Sofue Muroto, Endo Naoto., editors. Treatment of osteoarthritic change in the hip: joint preservation or joint replacement? Springer Japan; Tokyo: 2007. pp. 79–88. [Google Scholar]
- 55.Yasunaga Y., Hisatome T., Ikuta Y., Nakamura S. A histological study of the necrotic area after transtrochanteric anterior rotational osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Br. 2001;83B:167–170. doi: 10.1302/0301-620x.83b2.11503. [DOI] [PubMed] [Google Scholar]
- 56.Dean M.T., Cabanela M.E. Transtrochanteric anterior rotational osteotomy for avascular necrosis of the femoral-head. Long-term results. J Bone Joint Surg Br. 1993;75:597–601. doi: 10.1302/0301-620X.75B4.8331115. [DOI] [PubMed] [Google Scholar]
- 57.Hiranuma Y., Atsumi T., Kajiwara T., Tamaoki S., Asakura Y. Evaluation of instability after transtrochanteric anterior rotational osteotomy for nontraumatic osteonecrosis of the femoral head. J Orthop Sci. 2009;14:535–542. doi: 10.1007/s00776-009-1363-6. [DOI] [PubMed] [Google Scholar]
- 58.Lee M.S., Tai C.L., Senan V., Shih C.H., Lo S.W., Chen W.P. The effect of necrotic lesion size and rotational degree on the stress reduction in transtrochanteric rotational osteotomy for femoral head osteonecrosis—a three-dimensional finite-element simulation. Clin Biomech. 2006;21:969–976. doi: 10.1016/j.clinbiomech.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Mont M.A., Rajadhyaksha A.D., Hungerford D.S. Outcomes of limited femoral resurfacing arthroplasty compared with total hip arthroplasty for osteonecrosis of the femoral head. J Arthroplasty. 2001;16:134–139. doi: 10.1054/arth.2001.28722. [DOI] [PubMed] [Google Scholar]
- 60.Squire M., Fehring T.K., Odum S., Griffin W.L., Bohannon Mason J. Failure of femoral surface replacement for femoral head avascular necrosis. J Arthroplasty. 2005;20:108–114. doi: 10.1016/j.arth.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 61.Bose V.C., Baruah B.D. Resurfacing arthroplasty of the hip for avascular necrosis of the femoral head a minimum follow-up of four years. J Bone Joint Surg Br. 2010;92B:922–928. doi: 10.1302/0301-620X.92B7.23639. [DOI] [PubMed] [Google Scholar]
- 62.Daniel J., Pynsent P.B., McMinn D.J.W. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86B:177–184. doi: 10.1302/0301-620x.86b2.14600. [DOI] [PubMed] [Google Scholar]
- 63.Gustilo R.B., Mendoza R.M., Burnham W.H. Long-term results of total hip-arthroplasty in younger age group: comparative-analysis with young resurfacing arthroplasty patients. Orthopedics. 1983;6:60–69. doi: 10.3928/0147-7447-19830101-06. [DOI] [PubMed] [Google Scholar]
- 64.Reito A., Eskelinen A., Puolakka T., Pajamaki J. Results of metal-on-metal hip resurfacing in patients 40 years old and younger. Arch Orthop Trauma Surg. 2013;133:267–273. doi: 10.1007/s00402-012-1640-2. [DOI] [PubMed] [Google Scholar]
- 65.Hammond L.C., Lin E.C., Harwood D.P., Juhan T.W., Gochanour E., Klosterman E.L. Clinical outcomes of hemiarthroplasty and biological resurfacing in patients aged younger than 50 years. J Shoulder Elbow Surg. 2013;22:1345–1351. doi: 10.1016/j.jse.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Beaulé P.E., Campbell P.A., Hoke R., Dorey F. Notching of the femoral neck during resurfacing arthroplasty of the hip: a vascular study. J Bone Joint Surg Br. 2006;88B:35–39. doi: 10.1302/0301-620X.88B1.16682. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.B., Sugano N., Nakata K., Matsui M., Ohzono K. Comparison between bipolar hemiarthroplasty and THA for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2004:161–165. doi: 10.1097/01.blo.0000128217.18356.87. [DOI] [PubMed] [Google Scholar]
- 68.Shea J.E., Miller S.C. Skeletal function and structure: implications for tissue-targeted therapeutics. Adv Drug Deliv Rev. 2005;57:945–957. doi: 10.1016/j.addr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Assouline-Dayan Y., Chang C., Greenspan A., Shoenfeld Y., Gershwin M.E. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheu. 2002;32:94–124. [PubMed] [Google Scholar]
- 70.Fondi C., Franchi A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab. 2007;4:21–26. [PMC free article] [PubMed] [Google Scholar]
- 71.Mountziaris P.M., Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roodman G.D. Treatment strategies for bone disease. Bone Marrow Transpl. 2007;40:1139–1146. doi: 10.1038/sj.bmt.1705802. [DOI] [PubMed] [Google Scholar]
- 73.Plenk H., Hofmann S., Breitenseher M., Urban M. Pathomorphological aspects and repair mechanisms of femoral head osteonecrosis. Orthopade. 2000;29:389–402. doi: 10.1007/s001320050460. [In German] [DOI] [PubMed] [Google Scholar]
- 74.Zhang G., Sheng H., He Y.X., Xie X.H., Wang Y.X., Lee K.M. Continuous occurrence of both insufficient neovascularization and elevated vascular permeability in rabbit proximal femur during inadequate repair of steroid-associated osteonecrotic lesions. Arthritis Rheum. 2009;60:2966–2977. doi: 10.1002/art.24847. [DOI] [PubMed] [Google Scholar]
- 75.Glimcher M.J., Kenzora J.E. Nicolas Andry award. The biology of osteonecrosis of the human femoral-head and its clinical implications. 1. Tissue biology. Clin Orthop Relat Res. 1979;183:284–309. [PubMed] [Google Scholar]
- 76.Glimcher M.J., Kenzora J.E. The biology of osteonecrosis of the human femoral head and its clinical implications. III. Discussion of the etiology and genesis of the pathological sequelae; comments on treatment. Clin Orthop Relat Res. 1979;140:273–312. [PubMed] [Google Scholar]
- 77.Glimcher M.J., Kenzora J.E. The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;139:283–312. [PubMed] [Google Scholar]
- 78.Jimi E., Hirata S., Osawa K., Terashita M., Kitamura C., Fukushima H. The current and future therapies of bone regeneration to repair bone defects. Int J Dent. 2012;2012:148261. doi: 10.1155/2012/148261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Keefe R.J., Mao J. Bone tissue engineering and regeneration: from discovery to the clinic-an overview introduction. Tissue Eng Part B Rev. 2011;17:389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cancedda R., Giannoni P., Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240–4250. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 81.Cui Q.J., Botchwey E.A. Emerging ideas: treatment of precollapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat R. 2011;469:2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie X.H., Wang X.L., Zhang G., Liu Z., Yao D., Hung L.K. Impaired bone healing in rabbits with steroid-induced osteonecrosis. J Bone Joint Surg Br. 2011;93B:558–565. doi: 10.1302/0301-620X.93B4.25442. [DOI] [PubMed] [Google Scholar]
- 83.Chen S.H., Wang X.L., Xie X.H., Zheng L.Z., Yao D., Wang D.P. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: an in vitro efficacy study. Acta Biomater. 2012;8:3128–3137. doi: 10.1016/j.actbio.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 84.Cordonnier T., Sohier J., Rosset P., Layrolle P. Biomimetic materials for bone tissue engineering: state of the art and future trends. Adv Eng Mater. 2011;13:B135–B150. [Google Scholar]
- 85.Navarro M., Michiardi A., Castano O., Planell J.A. Biomaterials in orthopaedics. J R Soc Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S.H., Lei M., Xie X.H., Zheng L.Z., Yao D., Wang X.L. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013;9:6711–6722. doi: 10.1016/j.actbio.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 87.Vanluyn M.J.A., Vanwachem P.B., Damink L.O., Dijkstra P.J., Feijen J., Nieuwenhuis P. Relations between in vitro cytotoxicity and cross-linked dermal sheep collagens. J Biomed Mater Res. 1992;26:1091–1110. doi: 10.1002/jbm.820260810. [DOI] [PubMed] [Google Scholar]
- 88.Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S.M., Mukhachev V., Lavroukov A. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 89.Marcacci M., Kon E., Moukhachev V., Lavroukov A., Kutepov S., Quarto R. Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 90.Anderson J.A., Little D., Toth A.P., Moorman C.T., Tucker B.S., Ciccotti M.G. Stem cell therapies for knee cartilage repair the current status of preclinical and clinical studies. Am J Sport Med. 2014;42:2253–2261. doi: 10.1177/0363546513508744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vacanti C.A., Bonassar L.J., Vacanti M.P., Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–1514. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 92.Morishita T., Honoki K., Ohgushi H., Kotobuki N., Matsushima A., Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients' mesenchymal stem cells. Artif Organs. 2006;30:115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 93.Moghaddam A., Elleser C., Biglari B., Wentzensen A., Zimmermann G. Clinical application of BMP 7 in long bone non-unions. Arch Orthop Trauma Surg. 2010;130:71–76. doi: 10.1007/s00402-009-0982-x. [DOI] [PubMed] [Google Scholar]
- 94.Arrabal P.M., Visser R., Santos-Ruiz L., Becerra J., Cifuentes M. Osteogenic molecules for clinical applications: improving the BMP-collagen system. Biol Res. 2013;46:421–429. doi: 10.4067/S0716-97602013000400013. [DOI] [PubMed] [Google Scholar]
- 95.Skogh A.C.D., Kihlström L., Neovius E., Persson C., Beckman M.O., Engstrand T. Variation in calvarial bone healing capacity: a clinical study on the effects of BMP-2-hydrogel or bone autograft treatments at different cranial locations. J Craniofac Surg. 2013;24:339–343. doi: 10.1097/SCS.0b013e31827ff2b6. [DOI] [PubMed] [Google Scholar]
- 96.Kon E., Filardo G., Perdisa F., Di Martino A., Busacca M., Balboni F. A one-step treatment for chondral and osteochondral knee defects: clinical results of a biomimetic scaffold implantation at 2 years of follow-up. J Mater Sci Mater Med. 2014;25:2437–2444. doi: 10.1007/s10856-014-5188-2. [DOI] [PubMed] [Google Scholar]
- 97.Enea D., Cecconi S., Calcagno S., Busilacchi A., Manzotti S., Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30–35. doi: 10.1016/j.knee.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Riedel G., Valentin-Opran A. Selection of a control group in BMP clinical studies. J Bone Joint Surg Am. 2001;83A:S159–S160. [PubMed] [Google Scholar]
- 99.Cahill K.S., Chi J.H., Day A., Claus E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 100.Vaidya R., Carp J., Sethi A., Bartol S., Craig J., Les C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh K., Ahmadinia K., Park D.K., Nandyala S.V., Marquez-Lara A., Patel A.A. Complications of spinal fusion with utilization of bone morphogenetic protein. Spine. 2014;39:91–101. doi: 10.1097/BRS.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 102.Hollister S.J., Murphy W.L. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17:459–474. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., Dickinson I.C. Bone regeneration based on tissue engineering conceptions—a 21st century perspective. Bone Res. 2013;3:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coelho P.G., Hollister S.J., Flanagan C.L., Fernandes P.R. Bioresorbable scaffolds for bone tissue engineering: optimal design, fabrication, mechanical testing and scale-size effects analysis. Med Eng Phys. 2015;37:287–296. doi: 10.1016/j.medengphy.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Santana B.P., Paganotto G.F., Nedel F., Piva E., de Carvalho R.V., Nor J.E. Nano-/microfiber scaffold for tissue engineering: physical and biological properties. J Biomed Mater Res A. 2012;100:3051–3058. doi: 10.1002/jbm.a.34242. [DOI] [PubMed] [Google Scholar]
- 106.Rubenstein D.A., Lu H., Mahadik S.S., Leventis N., Yin W. Characterization of the physical properties and biocompatibility of polybenzoxazine-based aerogels for use as a novel hard-tissue scaffold. J Biomater Sci Polym Ed. 2011;23:1171–1184. doi: 10.1163/092050611X576954. [DOI] [PubMed] [Google Scholar]
- 107.Zhao H., Ma L., Gao C., Shen J. A composite scaffold of PLGA microspheres/fibrin gel for cartilage tissue engineering: fabrication, physical properties, and cell responsiveness. J Biomed Mater Res B Appl Biomater. 2009;88:240–249. doi: 10.1002/jbm.b.31174. [DOI] [PubMed] [Google Scholar]
- 108.Han X.Y., Zhang F.Q., Fu Y.F., Chen D.M. Fabrication and physical properties of porous individual beta-TCP scaffold to reconstruct residual alveolar ridge in dog. Shanghai Kou Qiang Yi Xue. 2008;17:51–54. [In Chinese] [PubMed] [Google Scholar]
- 109.Ionita D., Grecu M., Ungureanu C., Demetrescu I. Antimicrobial activity of the surface coatings on TiAlZr implant biomaterial. J Biosci Bioeng. 2011;112:630–634. doi: 10.1016/j.jbiosc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 110.Li J.S., Mak A.F.T. Hydroxyapatite nano-particles coating on the pore surface within poly(DL-lactic-co-glycolic acid) scaffold. Key Eng Mater. 2007;334–335:1237–1240. [Google Scholar]
- 111.Spiller K.L., Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv Transl Re. 2015;5:101–115. doi: 10.1007/s13346-013-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu T.M.G., Hollister S.J., Halloran J.W., Feinberg S.E., Orton D.G. Manufacturing and characterization of 3-D hydroxyapatite bone tissue engineering scaffolds. Ann N Y Acad Sci. 2002;961:114–117. doi: 10.1111/j.1749-6632.2002.tb03061.x. [DOI] [PubMed] [Google Scholar]
- 113.Dias M.R., Guedes J.M., Flanagan C.L., Hollister S.J., Fernandes P.R. Optimization of scaffold design for bone tissue engineering: a computational and experimental study. Med Eng Phys. 2014;36:448–457. doi: 10.1016/j.medengphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Bernstein P., Bornhauser M., Gunther K.P., Stiehler M. Bone tissue engineering in clinical application: assessment of the current situation. Orthopade. 2009;38:1029–1037. doi: 10.1007/s00132-009-1493-8. [In German] [DOI] [PubMed] [Google Scholar]
- 115.Shields L.B.E., Raque G.H., Glassman S.D., Campbell M., Vitaz T., Harpring J. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 116.Hollister S.J., Flanagan C.L., Zopf D.A., Morrison R.J., Nasser H., Patel J.J. Design control for clinical translation of 3D printed modular scaffolds. Ann Biomed Eng. 2015;43:774–786. doi: 10.1007/s10439-015-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.May-Newman K., Cornwall G.B. Teaching medical device design using design control. Expert Rev Med Devices. 2012;9:7–14. doi: 10.1586/erd.11.63. [DOI] [PubMed] [Google Scholar]
- 118.Kinsel D. Design control requirements for medical device development. World J Pediatr Congenit Heart Surg. 2012;3:77–81. doi: 10.1177/2150135111422720. [DOI] [PubMed] [Google Scholar]