Summary
Background/Objective
A biphasic ceramic bone substitute consisting of calcium sulfate and hydroxyapatite has been reported to give good clinical outcome regarding bone regeneration and may serve as a carrier for antibiotics in the treatment of bone infections. Often, the overlying muscle is in direct contact with the synthetic graft. The dissolving bone substitute induces inflammation, which may be harmful to the surrounding soft and muscle tissue. The aim of the present study was to evaluate the surrounding soft tissue reaction and the biodegradation of the biphasic bone substitute.
Methods
Rods (3 mm × 6 mm) were cast and implanted in the rat abdominal rectus muscle. The rods were either soaked or not soaked in autologous bone marrow before insertion to induce bone formation. Thirty-two rats underwent bilateral operation. After 6 weeks and 12 weeks, the bone substitute material and the surrounding muscle were harvested. The right rod was evaluated by histology to study tissue reaction and the left rod was analysed with micro-computed tomography and scanning electron microscopy to study bone substitute degradation.
Results
The muscle tissue around the material was similar at 6 weeks and 12 weeks, with or without prior treatment with bone marrow. The remaining material showed close contact with the muscle, and blood vessels penetrated the material in both groups. Wide bundles of collagen were embedded around the apatite particles, more at the 12-week time point. No bone formation was found, either at 6 weeks or 12 weeks, and scanning electron microscopy showed that the calcium sulfate phase was resorbed after 6 weeks with the calcium phosphate phase remaining intact. Micro-computed tomography showed significantly more hydroxyapatite at 6 weeks than after 12 weeks.
Conclusion
Calcium sulfate hydroxyapatite bone substitute can be used as a carrier for antibiotics or other drugs, without adverse reaction due to the fast resorption of the calcium sulfate. No bone formation was seen despite treating the bone substitute with autologous bone marrow.
Keywords: biodegradation, biphasic calcium ceramics, muscle implantation, tissue reaction
Introduction
Bone grafts are used to augment and enhance healing in a number of surgical procedures, for example in bone tumour resection, eradication in chronic osteomyelitis, nonunion, revision arthroplasty, and spinal fusions. Due to the well described limitations of both autografts and allografts [1], [2], synthetic alternatives have been developed over the past 2 decades. An injectable synthetic bone substitute that contains calcium sulfate (CaS) with embedded hydroxyapatite (HA) has been investigated previously, alone as a void filler in noncritical defects [3] or as a carrier for antibiotics or other drugs. It has been shown to promote bone formation, both in clinical studies and in bone defect animal models [4], [5], [6], [7], [8], [9]. However, few studies have focused on the soft tissue reaction. When a bone substitute is used it is often in direct contact with, or covered by, a muscle. Material leaking from the filled cavity, or during the resorption of the material could cause a harmful tissue reaction.
Clinical data have documented that pure CaS pellets can enhance bone formation in bone defects [10], [11]. CaS pellets seem to function as a conductive scaffold that provides a structural framework for angiogenesis and osteogenesis [12], [13], but lacks any intrinsic osteoinductive or osteogenic capacity [14]. The rapid resorption rate of pure CaS is a clear disadvantage [15], [16] that may affect osteoconduction and cause an inflammatory reaction including short-term drainage from the wound [17], [18], [19], [20]. When CaS is combined with embedded HA, the resorption of the CaS leaves an apatite matrix with porosity that encourages bone ingrowth.
Goodman et al [21], [22], [23], [24] have tested different materials as particles and showed that cobalt, chromium, titanium, and polymer particles as well as polyethylene, all induce an inflammatory reaction. HA has the ability to promote early bone growth [25]. It is a biocompatible material with osteoconductive properties [26], [27] but also has the ability to encourage a host tissue to bond and integrate with an implant. However, with a bone substitute consisting solely of loose particles or granules, the particles may migrate from the implant site before ingrowth of new bone tissue anchors them in place. Particularly in a joint cavity this may increase third body wear [28]. By combining HA particles and calcium sulfate, an injectable material with an initial containment and stability can be obtained since the calcium sulfate will set within 6–12 minutes and bind the apatite particles [8].
Based on a limited donor availability but also the morbidity at the donor site, there will be an increased need for synthetic bone substitute, either alone or in combination with allo- and autografts. In several clinical situations, the bone substitute will come in direct contact with an overlying muscle and may induce an adverse tissue reaction. It is therefore important to study the reaction of a synthetic material in the surrounding tissue, especially the muscles [29], but few studies have focused on the soft tissue reaction. Bone substitute combined with bone marrow may have in vivo osteogenic potential. In a subcutaneous implant rat model, bone marrow in combination with porous HA composites induced bone formation after 4 weeks [30]. In another study, no bone formation was found using a collagen membrane or bone marrow alone, but bone formation was observed when the two were combined [31].
The aim of the present study was to evaluate whether the fast resorption of the CaS phase and the induced inflammation would negatively influence the histologic appearance of the surrounding muscle and soft tissue in a rat rectus abdominal muscle model [32]. Additionally, the effect of soaking the bone substitute in aspirated bone marrow was studied.
Materials and methods
Materials preparation
The injectable bone substitute Cerament (Cerament Bone Void Filler; BoneSupport AB, Lund, Sweden) consists of 60 wt. % CaS and 40 wt. % HA [8], [18]. This ratio results in a mixture with a compressive strength (wet) of 5–8 MPa, comparable to trabecular bone. The HA particles are around 5 μm. The HA is of medical grade and tested according to American standard (ASTM F1185). The bone substitute powder was mixed with iohexol (Cerament C-TRU; 180 mg I/mL), a low osmolar nonionic iodinated contrast agent to form an injectable paste. The ratio of liquid to powder was 0.43. The Cerament was mixed for 30 seconds and then injected into a sterilised Teflon mould with holes that were 3 mm in diameter and 6 mm deep. After 12 hours, the cement was cured and the rods removed and stored in a sterilised glass bottle prior to implantation.
Animals and implantation
A total of 32 female Sprague–Dawley rats with body weight of 190–220 g (age 8 weeks) were operated on. Rats were obtained from Taconic (Ry, Denmark). The rats were housed in a temperature-controlled room (21°C) and fed a standard laboratory diet. Three rats were kept in each cage with free access to food pellets and water. All animal procedures were carried out according to the institutional guidelines and were approved by the Ethic Animal Research Committee (M45-09) at Skåne University Hospital, Lund, Sweden.
The experiment was divided into two groups. In Group 1, 16 rats were implanted with a pair of Cerament rods. In Group 2, 16 rats were implanted with a pair of marrow-treated Cerament rods. Bone marrow was taken from the femoral condyle by making a small incision in the lateral femoral condyle using an 18-gauge needle and a 5-mL syringe. The femoral wound was closed with sutures. Two rods were placed in a Petri dish, and the bone marrow was injected onto the rods and left to soak for 10 minutes prior to implantation.
The rats were anaesthetised by 0.4 mL of a mixture containing 1 mL pentobarbital, 1 ml saline solution and 10 mg/2 mL diazepam. Antibiotics were given (0.5 mL Streptocillin) before the operation. A 10 mm incision was made in the mid line of the abdomen. A muscle pouch (4 mm wide and 8 mm long) was created between the abdominal muscles and then two rods were implanted in each rat, one rod in the left pouch and one rod in the right pouch (Figure 1). The pouches were closed by sutures as was the skin wound. Eight rats from each treatment group were harvested at 6 weeks or 12 weeks after implantation, respectively. One rod from the right side of each animal was taken for histology analysis and the other rod from the left side of the same animal was used for micro-computed tomography (CT) and scanning electron microscope (SEM) analysis.
Figure 1.
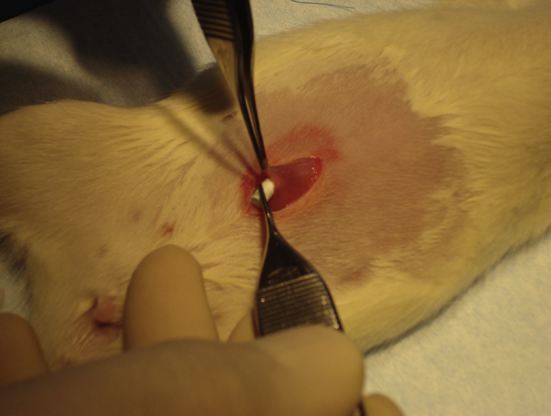
A ceramic rod inserted into a rat abdominal muscle pouch. One incision was used and one Cerament rod was placed on the left side and the other on the right side of the rectus muscle. We implanted two rods in each animal, one for histology and one for micro-computed tomography and scanning electron microscope analysis.
Histology
The ceramic rod from the right side of each rat with surrounding muscles was harvested and fixed in 4% formalin in buffer, before being decalcified, dehydrated, and embedded in paraffin. The specimens were cut into 5-μm sections and stained with haematoxylin and eosin. The tissue reaction to the material was analysed using optical microscopy by observing inflammatory cells, tissue ingrowth and bone-like tissue in and around the materials.
Micro-CT
The ceramic rod from the left side of each rat was fixed in 70% ethanol. The harvested samples were analysed using micro-CT. Topographic images of the sample was acquired with an isotropic voxel size of 15μm (Skyscan 1172; Bruker Microct, Aartselaar, Belgium) at the energy settings of 100 kV and 100 μA, using a 0.5-mm aluminium filter, and 10 repeated scans. Image reconstruction was performed (Skyscan NRecon, version 1.5.1.4; Bruker Microct) by correcting for ring artefacts and beam hardening (20%). Calibration of bone mineral density was carried out according to the system manufacturer's protocol. A water phantom and two HA phantoms of known densities (0.25 g/cm3 and 0.75 g/cm3) were scanned. To distinguish the remaining fully mineralised tissue, a threshold of 0.6 g/cm3 [33] was used, where the mineralised material was all tissue with a density higher than the threshold. This resulted in greyscale values > 90 (90–255 in 8 bit images) for mineralised material. A quantification of the remaining mineralised material from each specimen at 6 weeks and 12 weeks was performed using custom made Matlab scripts (Matlab, v 7.11.0, MathWorks Inc., Natick, USA). Two nonimplanted rods were imaged with the same settings and used as controls. Thus, the mineralised remaining material was normalised to the mineralised volume of the intact rods. Data were analysed by Student t test.
SEM and energy-dispersive X-ray spectroscopy
One specimen from each treatment and each time point, and one nonimplanted specimen were examined using an SEM equipped with an energy-dispersive X-ray spectroscopy (EDS) system (JSM-6700F; Jeol, Tokyo, Japan). The electron beam energy was 15 kV and the acquisition time 100 seconds. The samples were obtained after micro-CT examination and were dehydrated with ethanol and cut through the material and muscles with an IsoMet Low Speed Diamond Saw (Buehler, Lake Bluff, IL, USA). The samples were coated with carbon in order to identify and evaluate the relative concentrations of chemical elements present in the tissues [34]. The morphology and structure of the tissue integration were analysed. Material elemental analysis with the EDS was performed with interest points in the remaining material clusters and in the tissue. Two different regions of interest were chosen for chemical analysis, one in the remaining material cluster and one containing tissue integrated material. The area of interest was defined with 500× image and a region of approximately 5690 μm2 was used for element analysis in each specimen. The analysed elements included O, P, S, Cl, and Ca [35].
Results
Histology
In the histological samples, there was more remaining material after 6 weeks compared to after 12 weeks. Most of the remaining material consisted of many small aggregated particles around 5 μm in diameter. The material had been invaded by fibrous tissue. Signs of a minor inflammatory reaction were found around the material clusters and, in some sections, giant cells or multinuclear cells were attached to the clusters.
In the haematoxylin and eosin-stained sections, most of the bone substitute was in close contact with the muscle, and muscle fibres penetrated into the material (Figure 2). No muscle necrosis was observed and the formation of granulation tissue was low. A rich network of blood vessels penetrated the material in all groups, i.e., both samples with or without bone marrow treatment, and both after 6 weeks and 12 weeks (Figure 2).
Figure 2.
The interface between the Cerament and muscle (haematoxylin and eosin staining, magnification, ×20). (A) Without bone marrow at 6 weeks; (B) with bone marrow at 6 weeks; (C) without bone marrow at 12 weeks; and (D) with bone marrow at 12 weeks. A close contact can be seen between the remaining material and muscle (black arrows). At both 6 weeks and 12 weeks, a rich network of blood vessels (yellow arrows) has penetrated the material, both the marrow-treated and non-marrow-treated material.
Neither osteoid nor osteoblasts could be found in any specimen. Instead, wide bundles of collagen had formed in the material. More collagen was found around the remaining material clusters after 12 weeks compared to 6 weeks, but no difference was seen when comparing specimens with or without bone marrow treatment (Figure 3).
Figure 3.
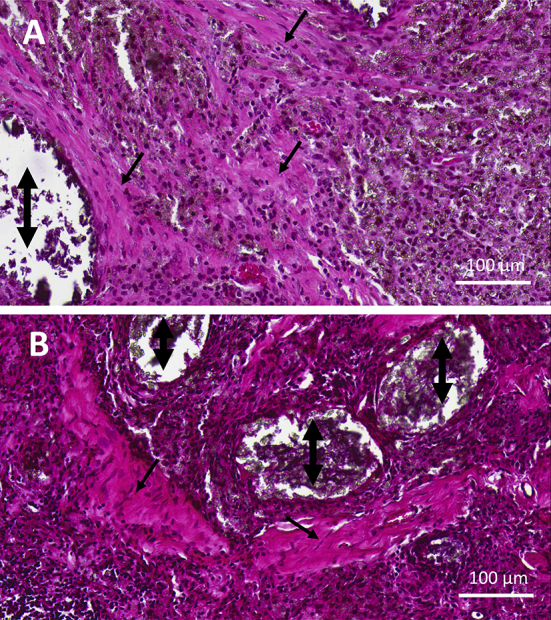
Collagen (black arrows) in close contact with Cerament (double-headed arrows) without bone marrow treatment at (A) 6 weeks and (B) 12 weeks (haematoxylin and eosin staining, magnification, ×20).
Micro-CT
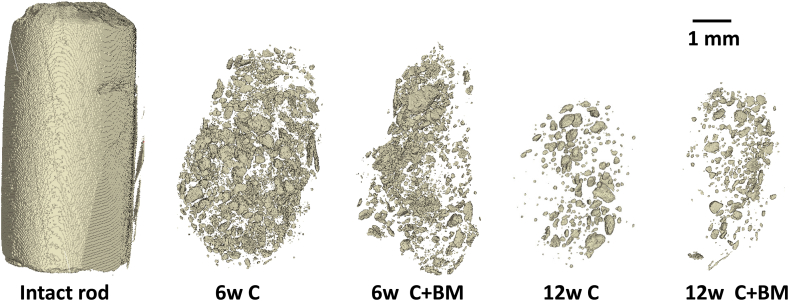
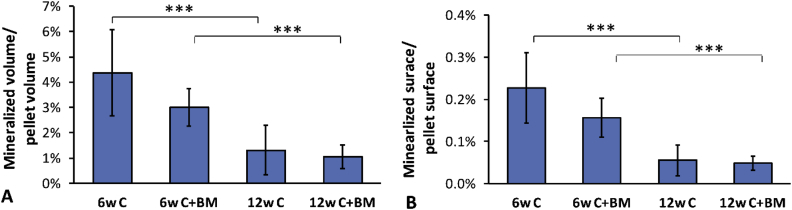
Micro-CT detected clusters of mineral particles within the softer tissue (Figure 4). The remaining mineralised volume was significantly higher after 6 weeks than after 12 weeks (p < 0.001, t test; Figure 5). Also, the mineralised surface was significantly higher after 6 weeks than after 12 weeks (p < 0.001, t test; Figure 5). No statistical differences were observed when comparing samples with or without bone marrow treatment at either time point. Thus, different parts of the material were resorbed during the different time periods (Figure 5).
Figure 4.
Micro-computed tomography showing the remaining Cerament in the harvested muscle and soft tissue. The area between the material clusters is penetrated by cells and collagen which can be seen in the histology panels (see Figure 3).
Figure 5.
Micro-computed tomography evaluation of remaining mineralised volume after 6 weeks and 12 weeks in the Cerament (C) and the Cerament treated with bone marrow (C+BM). (A) The remaining mineralised volume was normalised to the intact pellet volume. The (B) surface of the remaining mineralised material was normalised to the surface of the intact rods. Significant differences are based on Student t test (p < 0.001).
SEM and EDS
The SEM images and EDS of the intact pellet that had not been implanted showed that both CaS and HA particles were present in the Cerament (Figure 6). The element analysis of the intact Cerament had not been implanted showed a strong sulfur peak of 9.2%. The remaining elements were distributed according to Table 1. SEM observations of the samples implanted in the muscle pouch showed that after 6 weeks the Cerament rod had separated into small clusters and were surrounded by ingrown tissue (Figure 7). After 12 weeks, the clusters were fewer in number and also smaller. The amount, size, and shape of the material particles were similar to those observed after 6 weeks. Based on EDS analysis after both 6 weeks and 12 weeks, no sulfur, and thus no sulfate, was found in the material clusters (Table 1). However, a small amount of sulfur was detected at the tissue surrounding the material particles after both 6 weeks and 12 weeks. All other elements, as well as the Ca/P ratio were similar both after 6 weeks and 12 weeks.
Figure 6.
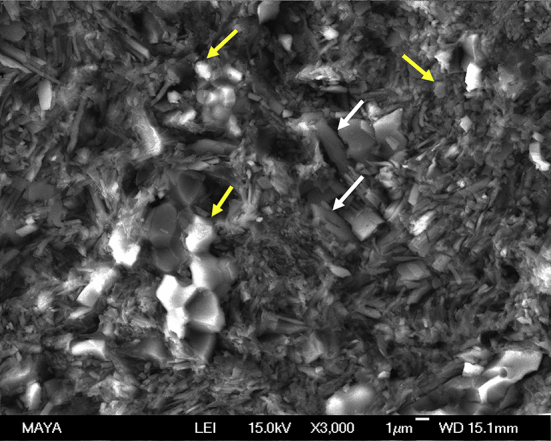
Scanning electron microscopy shows mixed calcium sulfate with long rod shape (white arrows) and hydroxyapatite particles with round shape (yellow arrows) in Cerament that had not been implanted. Energy-dispersive X-ray spectroscopy was performed with the areas of arrow points in the material.
Table 1.
Percentage of elements in the specimens from the intact Cerament rod (control) and after 6 weeks and 12 weeks by energy-dispersive X-ray spectroscopy analysis.
| Specimens | Iron in Cerament/surrounding tissue | Weight (%) |
||||
|---|---|---|---|---|---|---|
| O | P | S | Cl | Ca | ||
| Control | Cerament | 72.6 | 3.7 | 9.2 | 0.4 | 13.6 |
| 6-wk C | Cerament | 63.9 | 13.5 | 0.0 | 0.3 | 21.5 |
| Tissue | 77.2 | 4.1 | 0.5 | 11.9 | 6.4 | |
| 6-wk C+BM | Cerament | 63.8 | 14.8 | 0.0 | 0.4 | 20.2 |
| Tissue | 66.6 | 10.2 | 0.6 | 7.8 | 14.7 | |
| 12-wk C | Cerament | 65.5 | 12.1 | 0.0 | 0.5 | 21.2 |
| Tissue | 79.0 | 4.2 | 0.6 | 10.9 | 5.2 | |
| 12-wk C+BM | Cerament | 62.5 | 11.9 | 0.0 | 3.6 | 21.7 |
| Tissue | 75.0 | 4.3 | 0.7 | 13.5 | 6.3 | |
Data are presented as the mean of two measurements.
BM = bone marrow; C = Cerament.
Figure 7.
Scanning electron microscope pictures show the remaining material cluster or particles (yellow arrows) in the tissue (white arrows). (A) The sample from 6 weeks; (B) particles of the material from the middle part of the material cluster of (A). (C) The sample from 12 weeks; and (D) particles of the material from the middle of the material cluster in (C). Energy-dispersive X-ray spectroscopy was performed with the arrow points in the remaining material clusters and tissue.
Discussion
A benign muscle tissue reaction was found around the material, similar at 6 weeks and 12 weeks, and regardless of prior treatment with bone marrow. The remaining material showed close contact with the muscle, and blood vessels penetrated the material in both groups. Wide bundles of collagen were embedded around the apatite particles, in particular at the 12-week time point, in both with or without prior treatment with bone marrow. No bone formation was found in the muscles pouch at 6 weeks or 12 weeks. SEM showed that the CaS was resorbed after 6 weeks, and micro-CT showed that the HA was slowly resorbing between 6 weeks and 12 weeks.
A biphasic in situ setting bone substitute containing CaS and HA has been clinically studied with good outcome, and used both in osteotomies, vertebral bodies, long bones, fracture surgery, and for augmenting cannulated screws [8]. It has also been used in animal defect models showing good biocompatibility and osteoconductivity [4], [5]. We have previously shown good tissue integration and bone ingrowth in a fully loaded joint implant in a rat model using a noncemented tibial prosthesis, treated with the CaS/HA material [9]. Previous studies also demonstrated an increased pull-out strength at 12 weeks in the treated group. Clinical studies have shown good long-term results using a combination of HA particles in combination with allograft in acetabulum [36]. In an earlier study, we were able to show good soft tissue and muscle integrations with a preset of calcium phosphate with CaS material when implanted in the rat muscle pouch model [37]. However, when a bone substitute is used it is often in direct contact with or covered by a muscle and sometimes aspirated bone marrow is added or leaking out from the resected bone area. These types of tissue can give a more severe and different tissue response compared to when using a bone substitute in a contained bone defect. The reports of adverse reactions to surgical-grade CaS used as bone grafting in patients with a so called white drainage were essentially self-limited and appeared benign [20]. However, it may lead to a local inflammatory process at the implantation site. Similarly, a slow-setting calcium phosphate has been shown to evoke significant inflammatory reaction [34]. Large vesicles containing inflammatory effusion formed around a calcium phosphate cement, consisting of an equimolar mixture of tetracalcium phosphate and dicalcium phosphate anhydrous (DCPA). A more rapidly setting calcium phosphate was covered with a layer of granulation tissue and presented an inflammatory cell reaction but did not give any effusion. The analyses in these studies were all carried out in a rat model at 1 week after surgery [35]. Interestingly, DCPA was always found in the slow-setting phosphate cement. However, there was also a small amount of inert DCPA present 1 week after implantation in the fast-setting cement. The absence of similar tissue reactions in our study may be due to the small volume of CaS used and that the HA particles might act as a barrier, retarding the calcium sulfate resorption [8].
In our study, adding bone marrow to the bone substitute rods did not show any effect on the tissue integration and biocompatibility in the soft tissue. A difference between our and previous studies using calcium phosphate cement, is that the material was injected directly into a subcutaneous pouch in the rat abdominal muscle, whereas we used rods that were already pretreated. However, we find it unlikely that this would influence the result, since our CaS/apatite material had a fast setting time of 12 minutes, comparable to the fast-setting phosphate cement in the study reported by Miyamoto et al [35]. We observed a rich network of vessels that had infiltrated the material and, further, thick bundles of collagen around the remaining HA particles at both time points. However, no bone formation was at neither 6 weeks nor 12 weeks. Additionally, from the SEM and EDS, it was clear that there were minimal amounts of sulfate in the remaining material already at 6 weeks. A limitation of the study was that we did not perform immunohistochemistry analysis. This could give more information of the tissue reaction. Furthermore, it is still unclear how the immunological response will be for the specific model compared to humans, but we still believe that these results provide important information and indications for further studies.
Conflicts of interest
L.L. is a board member of Bonesupport AB, Lund, Sweden.
Funding/support
The project was supported by the Faculty of Medicine, Lund University, Stiftelsen för bistånd åt rörelsehindrade i Skåne. The Cerament/Bone Void Filler was supplied by Bonesupport AB, Lund, Sweden.
Acknowledgements
We thank Mea Pelkonen for histology preparation.
References
- 1.Summers B.N., Eisenstein S.M. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 2.Younger E.M., Chapman M.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Abramo A., Geijer M., Kopylov P., Tägil M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J Biomed Mater Res B Appl Biomater. 2010;92:281–286. doi: 10.1002/jbm.b.31524. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson M., Wang J.S., Wielanek L., Tanner K.E., Lidgren L. Biodegradation and biocompatibility of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg (Br) 2004;86-B:120–125. [PubMed] [Google Scholar]
- 5.Wang J.S., Tanner K.E., Abdulghani S., Lidgren L. Indentation testing of a bone defect filled with two different injectable bone substitutes. Bioceramics. 2005;17:89–92. [Google Scholar]
- 6.Hatten H.P., Jr., Voor M.J. Bone healing using a bi-phasic ceramic bone substitute demonstrated in human vertebroplasty and with histology in a rabbit cancellous bone defect model. Interv Neuroradiol. 2012;18:105–113. doi: 10.1177/159101991201800114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcia S., Boi C., Dragani M., Marini S., Marras M., Piras E. Effectiveness of a bone substitute (CERAMENT) as an alternative to PMMA in percutaneous vertebroplasty: 1-year follow-up on clinical outcome. Eur Spine J. 2012;21(Suppl. 1):S228–S229. doi: 10.1007/s00586-012-2228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson M., Zheng M.H., Tägil M. The composite of hydroxyapatite and calcium sulphate: a review of preclinical evaluation and clinical applications. Expert Rev Med Devices. 2013;10:675–684. doi: 10.1586/17434440.2013.827529. [DOI] [PubMed] [Google Scholar]
- 9.Zampelis V., Tägil M., Lidgren L., Isaksson H., Atroshi I., Wang J.S. The effect of a biphasic injectable bone substitute on the interface strength in a rabbit knee prosthesis model. J Orthop Surg Res. 2013;8:25. doi: 10.1186/1749-799X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltier L.F. The use of plaster of Paris to fill defects in bone. Clin Orthop. 1961;21:1–31. [PubMed] [Google Scholar]
- 11.Peltier L.F., Jones R.H. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J Bone Joint Surg Am. 1978;60:820–822. [PubMed] [Google Scholar]
- 12.Snyders R.V., Jr., Eppley B.L., Krukowski M., Delfino J.J. Enhancement of repair in experimental calvarial bone defects using calcium sulfate and dextran beads. J Oral Maxillofac Surg. 1993;51:517–524. doi: 10.1016/s0278-2391(10)80506-3. [DOI] [PubMed] [Google Scholar]
- 13.von Rechenberg B., Génot O.R., Nuss K., Galuppo L., Fulmer M., Jacobson E. Evaluation of four biodegradable, injectable bone cements in an experimental drill hole model in sheep. Eur J Pharm Biopharm. 2013;85:130–138. doi: 10.1016/j.ejpb.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Beuerlein M.J., McKee M.D. Calcium sulfates: what is the evidence? J Orthop Trauma. 2010;24(Suppl. 1):S46–S51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- 15.Hak D.J. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg. 2007;15:525–536. doi: 10.5435/00124635-200709000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Slater N., Dasmah A., Sennerby L., Hallman M., Piattelli A., Sammons R. Back-scattered electron imaging and elemental microanalysis of retrieved bone tissue following maxillary sinus floor augmentation with calcium sulphate. Clin Oral Implants Res. 2008;19:814–822. doi: 10.1111/j.1600-0501.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 17.Coetzee A.S. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol. 1980;106:405–409. doi: 10.1001/archotol.1980.00790310029007. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D., Alk D., Sandbank J., Farber R., Halperin N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transpl. 1999;4:91–97. [PubMed] [Google Scholar]
- 19.Kelly C.M., Wilkins R.M., Gitelis S., Hartjen C., Watson J.T., Kim P.T. The use of a surgical grade calcium sulphate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001;382:42–50. doi: 10.1097/00003086-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Lee G.H., Khoury J.G., Bell J.E., Buckwalter J.A. Adverse reactions to OsteoSet bone graft substitute, the incidence in a consecutive series. Iowa Orthop J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman S., Aspenberg P., Song Y., Knoblich G., Huie P., Regula D. Tissue ingrowth and differentiation in the bone-harvest chamber in the presence of cobalt-chromium-alloy and high-density-polyethylene particles. J Bone Joint Surg. 1995;77:1025–1035. doi: 10.2106/00004623-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Goodman S., Aspenberg P., Song Y., Doshi A., Regula D., Lidgren L. Effects of particulate high-density polyethylene and titanium alloy on tissue ingrowth into bone harvest chamber in rabbits. J Appl Biomater. 1995;6:27–33. doi: 10.1002/jab.770060105. [DOI] [PubMed] [Google Scholar]
- 23.Goodman S., Aspenberg P., Wang J.S., Song Y., Doshi A., Regula D. Cement particles inhibit bone growth into titanium chambers implanted in the rabbit. Acta Orthop Scand. 1993;64:627–633. doi: 10.3109/17453679308994585. [DOI] [PubMed] [Google Scholar]
- 24.Goodman S., Aspenberg P., Song Y., Regula D., Lidgren L. Polyethylene and titanium alloy particles reduce bone formation. Dose dependence in bone harvest chamber experiments in rabbits. Acta Orthop Scand. 1996;67:599–605. doi: 10.3109/17453679608997764. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.S., Goodman S., Aspenberg P. Bone formation in the presence of phagocytosable hydroxyapatite particles. Clin Orthop. 1994;304:272–279. [PubMed] [Google Scholar]
- 26.Stevenson S. Biology of bone grafts. Orthop Clin North Am. 1999;30:543–552. doi: 10.1016/s0030-5898(05)70107-3. [DOI] [PubMed] [Google Scholar]
- 27.Keating J.F., McQueen M.M. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br. 2001;83:3–8. doi: 10.1302/0301-620x.83b1.11952. [DOI] [PubMed] [Google Scholar]
- 28.Morscher E.W., Hefti A., Aebi U. Severe osteolysis after third/body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg (Br) 1998;80:267–272. doi: 10.1302/0301-620x.80b2.8316. [DOI] [PubMed] [Google Scholar]
- 29.Myginda T., Stiehler M., Baatrup A., Li H., Zou X., Flyvbjerg A. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036–1047. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Noshi T., Yoshikawa T., Ikeuchi M., Dohi Y., Ohgushi H., Horiuchi K. Enhancement of the in vivo osteogenic potential of marrow/hydroxyapatite composites by bovine bone morphogenetic protein. J Biomed Mater Res. 2000;52:621–630. doi: 10.1002/1097-4636(20001215)52:4<621::aid-jbm6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Alvis M., Lalor P., Brown M.K., Thorn M.R., Block J.E., Hornby S. Osteoinduction by a collagen mineral composite combined with isologous bone marrow in a subcutaneous rat model. Orthopedics. 2003;26:77–80. doi: 10.3928/0147-7447-20030101-19. [DOI] [PubMed] [Google Scholar]
- 32.Wang J.S., Aspenberg P. Basic fibroblast growth factor and bone induction in rats. Acta Orthop Scand. 1993;64:557–561. doi: 10.3109/17453679308993692. [DOI] [PubMed] [Google Scholar]
- 33.Isaksson H., Harjula T., Koistinen A., Iivarinen J., Seppänen K., Arokoski J.P. Collagen and mineral deposition in rabbit cortical bone during maturation and growth: effects on tissue properties. J Orthop Res. 2010;12:1626–1633. doi: 10.1002/jor.21186. [DOI] [PubMed] [Google Scholar]
- 34.Perdikouri C., Tägil M., Isaksson H. Characterizing the composition of bone formed during fracture healing using scanning electron microscopy techniques. Calcif Tissue Int. 2015;96:11–17. doi: 10.1007/s00223-014-9930-z. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto Y., Ishikawa K., Takechi M., Toh T., Yuasa T., Nagayama M. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J Biomed Mater Res. 1999;48:36–42. doi: 10.1002/(sici)1097-4636(1999)48:1<36::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Whitehouse M.R., Dacombe P.J., Webb J.C.J., Blom A.W. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: high survivorship in 43 patients with a median follow-up of 7 years. A follow-up report. Acta Orthop. 2013;84:365–370. doi: 10.3109/17453674.2013.792031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.S., Zheng M., Hessle L., Lidgren L. Proceedings of the 8th World Biomaterial Congress, Amsterdam: The Netherlands. 2008. Bone induction of biphasic calcium phosphate cement with multiple growth factors in rat. [Google Scholar]