Summary
Background/Objective
The present study investigated the efficacy of poly-d,l-lactic acid (PDLLA) and hyaluronic acid (HyA) on implant fixation when coated onto hydroxyapatite/beta-tri-calcium phosphate (HA/βTCP) granules.
Methods
The effect was assessed in a clinically relevant in vivo gap model in sheep. Thus, four titanium implants combined with either allograft (control), pure HA/βTCP, HyA infiltrated HA/βTCP, or PDLLA reinforced HA/βTCP granules were bilaterally inserted into the trabecular bone of the distal femurs in eight sheep. The insertion created a 2-mm peri-implant gap. After 12 weeks, histomorphometry and push-out test was used for quantification of newly formed bone in the gap, bone-implant contact, and implant fixation.
Results
The histomorphometric analysis revealed the presence of newly formed bone in all groups, though substitute groups showed fragments of nonabsorbed substitute material. A significant larger bone volume was found in the allograft group versus the HA/βTCP-PDLLA group (Zone 1), and in Zone 2 a statistically significantly larger bone volume was found in the allograft compared with the HA/βTCP group. The mechanical properties and the bone-implant contact revealed no statistically significant differences between the groups.
Conclusion
This study demonstrates that HA/βTCP granules coated with PDLLA and HyA have similar bone ingrowth and implant fixation as those with allograft, and with mechanical properties resembling those of allograft in advance, they may be considered as alternative substitute materials for bone formation in sheep.
Keywords: bone ingrowth, HA/βTCP, hyaluronic acid, implant fixation, poly-lactic acid
Introduction
The number of total joint replacement surgeries is increasing each year due to an expanding elderly population. Additionally, a rise in the number of failure rate revision surgeries has been explored, mainly explained by an impaired bone formation around the implants [1]. Damaged bone is often replaced by metal implants, providing the strength and stiffness required for most load-bearing bone sites [2], [3]. However, insertion of implants often also demands supplement of either donor bone or bone substitutes to secure a proper implant fixation [3], [4].
To improve the efficacy and functionality of implants, lots of effort has been put into the development of new bone substitutes used in combination with those metal alloys. An optimal bone substitute has a high biocompatibility, promotes early bone formation at the bone-implant interface, and retains a suitable strength required at the particular skeletal site [5], [6]. All these factors are essential for long-term implant survival, being of both social and economic importance.
Bioceramics, such as beta-tricalcium phosphate (βTCP) and hydroxyapatite (HA), are known to be very compatible with the human body environment inducing a biological response similar to bone [7], [8]. Due to their osteoconductive properties, βTCP and HA are often combined, although their mechanical properties are not entirely comparable to that of bone [3], [4]. Research has shown that the mechanical performance of HA/βTCP-ceramics can be enhanced by reinforcement with polymers, e.g., poly-d,l-lactic acid (PDLLA), poly-glycolic acid, or poly-hydroxy butyrate [3], [9].
PDLLA, a long-chained polymer degraded into lactic acid by the tricarboxylic acid cycle, is ideal due to its biocompatibility, strength, and high solubility [9], [10]. Recent investigation has shown that the addition of 10–15% PDLLA to HA/βTCP scaffolds significantly increases their strength [11], [12]. Additionally, in vivo studies have revealed that HA substitutes combined with PDLLA induce bone formation around titanium implants [12], [13], [14]. Another polymer with favourable bone-stimulating properties is hyaluronic acid (HyA). HyA is a polysaccharide and an important component of the extracellular matrix exhibiting several beneficial chemical properties, i.e., therapeutic agent in arthritis therapy and anti-inflammatory agent in animal models [15]. Further, it promotes osteoblast differentiation, thereby stimulating bone formation, as shown in rat calvarial-derived cell cultures [16] and rat cortical bone [17]. Although the infiltration of HA/βTCP scaffolds with HyA seems not to improve the mechanical properties [11], HyA shows good osteoconductive properties, therefore still considered a resilient composite material. Consequently, it is interesting to evaluate whether HA/βTCP-PDLLA and HA/βTCP-HyA could positively affect the fixation of titanium implants in a clinically relevant large animal model.
The aim of the present study was to investigate the efficacy of HA/βTCP granules reinforced with PDLLA or infiltrated with HyA on the fixation of titanium implants bilaterally inserted into a gap model in sheep. We hypothesized that HA/βTCP substitutes added PDLLA and HyA were able to conduct formation of bone at the bone-implant interphase promoting an appropriate mechanical fixation.
Materials and methods
Eight female sheep of the Merino/Gotland wool mixed breed were used. Their mean age was 4 years (range, 3–5 years) while their mean body weight was 78.0 ± 7.4 kg. The sheep were housed in outdoor paddocks and were fed hay and compound feed throughout the experiment. The animals were housed indoors at the central animal facility 1 week prior to surgery and 2–3 days postoperation. All institutional and national guidelines for the care and use of laboratory animals were followed, and the Danish Animal Experiments Inspectorate approved the study (number: 2011/561-1959).
Study design
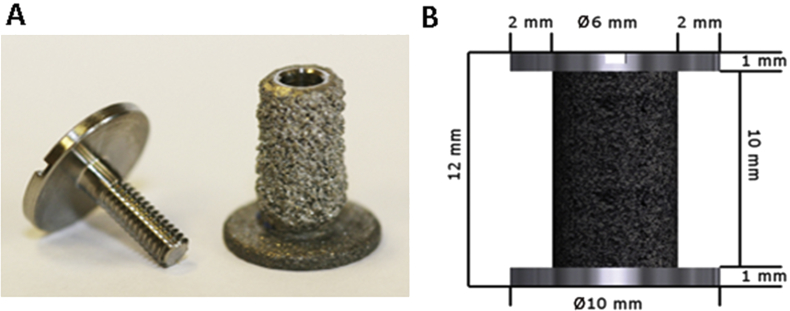
In this study, 32 cylindrical plasma-sprayed titanium alloys (90% titanium, 6% aluminium, 4% vanadium; Biomet, Warsaw, IN, USA; Figure 1A) were bilaterally implanted into a gap model, as previously described [18], [19]. The implants were inserted extra-articularly into trabecular bone of the medial and lateral distal femoral condyles, clearly separated to avoid any potential interference between the implanted substitute materials. The implants were 10 mm high and had a column diameter of 6 mm. The footplate and top washer were 10 mm in diameter, giving a circumferential gap of 2 mm and a volume of 0.5 mL (Figure 1B).
Figure 1.
(A, B) Porous plasma sprayed titanium implants were inserted into the distal femur condyles of eight sheep and the gaps were filled with one of four testing materials.
The gaps were subsequently filled with one of the four materials: allograft (control), pure HA/βTCP, HA/βTCP-HyA, or HA/βTCP-PDLLA granules. Thus, four different graft materials were implanted in each sheep, serving as their own control. To avoid any site-specific differences, implant materials were alternated between the gaps. Allograft is considered the gold standard in many orthopaedic procedures, thus reflecting a relevant control group in this study [20].
Graft materials
Allograft
The allograft was prepared from the distal femurs and proximal tibias of a healthy donor sheep of the same age. After removal of cartilage and soft tissue, the bone was milled in a bone mill (Ossano Scandinavia ApS, Stockholm, Sweden) resulting in 0.5–1.5-mm bone graft particles. The bone graft was packed in sterile 1.5-mL vials and preserved at –80°C until surgery.
Scaffold granules
Porous HA/βTCP granules, consisting of 70% HA and 30% βTCP, were fabricated by the Danish Technological Institute (Taastrup, Denmark). The granules had a particle size of 500–1400 μm and a porosity of approximately 80%. The pore size of the composite graft material was 300–700 μm with an interconnecting pore size of 100–200 μm. HA/βTCP granules infiltrated with the biopolymer HyA [molecular weight (MW) = 650 kDa] provided by Novozymes (Bagsvaerd, Denmark) represented the second group of graft materials. The HyA was coated onto the porous HA/βTCP granules by sterile solvent infiltration based on demineralized water [11]. Briefly, a solution of 3 mL sterile HyA (0.15% w/w) was mixed with 1.5 mL pure HA/βTCP granules and dried under vacuum (room temperature) for 12 hours. The process was repeated until the final concentration of HyA reached 0.15%. The final porosity was approximately 80% [11]. HA/βTCP granules reinforced with an ultrathin layer of 10% PDLLA (50% d-PLA, 50% l-PLA, molecular weight = 308 kDa) to enhance their mechanical strength were provided by PHUSIS (Saint Ismier, France). As previously shown in our lab, they have a porosity of approximately 70% [11], [12].
Surgical procedure
As premedication, the animals received 0.2 mg/kg of Rompun (xylancin hydrochloride, 20 mg/mL; Bayer Animal Health GmbH, Leverkusen, Germany). Anaesthesia was induced with 3 mg/kg of Rapinovent (propofol 10 mg/mL; Schering-Plough Animal Health, Ballerup, Denmark), while the surgical procedures were performed under general anaesthesia (2.0 % isoflurane). Under aseptic conditions, and after iodine disinfection of the lateral femur, the periosteal surface was exposed by an incision through the skin. To prevent any thermal damage of the bone and surrounding tissue, a low-speed drill created a 12-mm deep cylindrical hole with a circumference of 10 mm. To remove residual bone particles, the gap was rinsed with saline before insertion of the implants forming a gap of 2 mm. Subsequently, the concentric gap was randomly filled with one of the four graft materials before the top-washer was tightly placed on the implant. Finally, the wound was sutured in three layers. The procedure was repeated for the medial side as well as the opposite femur. Postoperative analgesia (0.03 mL/mg buprenorphine, Temgesic; Schering-Plough, Ballerup, Denmark) and ampicillin (250 mg/mL ampicillin; Ampivet Vet, Boehringer Ingelheim, Copenhagen, Denmark) was administered daily for 3–4 days [12]. After 12 weeks of observation the sheep were euthanized with an overdose of pentobarbital and both distal femurs were harvested and divided prior to further processing.
Preparation of specimens
The bone implant specimens were cut orthogonally into two parts with an Exakt diamond band saw (Exakt Apparatebau, Norderstedt, Germany). After removal of the top washer, a bone-implant sample of 3.5 mm was prepared and stored at –20°C until assessment of bone-implant fixation by mechanical push-out test. The remaining part of the implant specimen, 5.5 mm, was prepared for histological and histomorphometrical investigations. Briefly, the specimens were dehydrated in graded ethanol series (70–99%) at room temperature, containing 0.4% basic fuchsine, and subsequently embedded in methyl methacrylate (Technovit 9100 NEW; Heraeus Klzer GmbH, Wehrheim, Germany). Using the vertical sectioning method, four sections (approximately 30 μm thick) from each bone specimen (thus 16 sections per sheep) were cut using a microtome (Medeja, Leiden, The Netherlands) and counterstained with 2% light green to visualize mineralized bone [21].
Mechanical testing
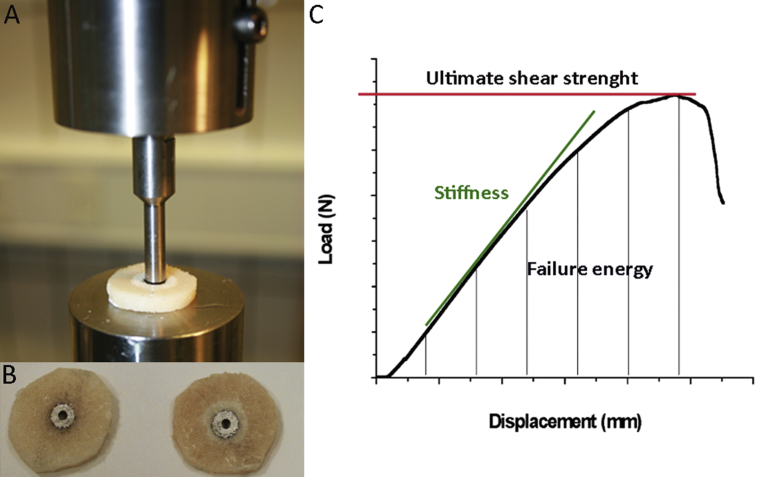
Before assessment of the implant failure by a push-out test performed on an 858 Bionix Material Testing System (hydraulic material testing system; MTS Systems Co., Minneapolis, MN, USA), the bone implant specimens were thawed at room temperature for 2 hours (Figure 2B). The diameter of the gap was 10 mm and the diameter of the test-plate hole under the specimen was 10 mm, while the piston pushing out the implant had a diameter of 6 mm (Figure 2A) [12]. The displacement rate was 5 mm/min. Load versus displacement data were recorded and used for calculation of the mechanical parameters—ultimate shear stiffness (MPa), ultimate shear strength (MPa), and failure energy (kJ/cm2) (Figure 2C).
Figure 2.
(A) The bone implant interface was investigated by push-out test on a 858 Bionix Material Testing System hydraulic; (B) assessing the implant failure of bone specimens; (C) generation of load-displacement curves.
Histology and histomorphometry
We distinguished bone by green/blue surface staining and the presence of osteocytes. Using polarized light, we were able to differentiate between mature lamellar bone with regular parallel alignment of lamellae and immature woven bone, characterized by their randomly oriented collagen fibres and round cell lacunae. Fibrous tissue was stained red and identified by their visible fibril fibres and low cell density, while remnants of substitutes were detected as small grey islets easily identified from the other tissues.
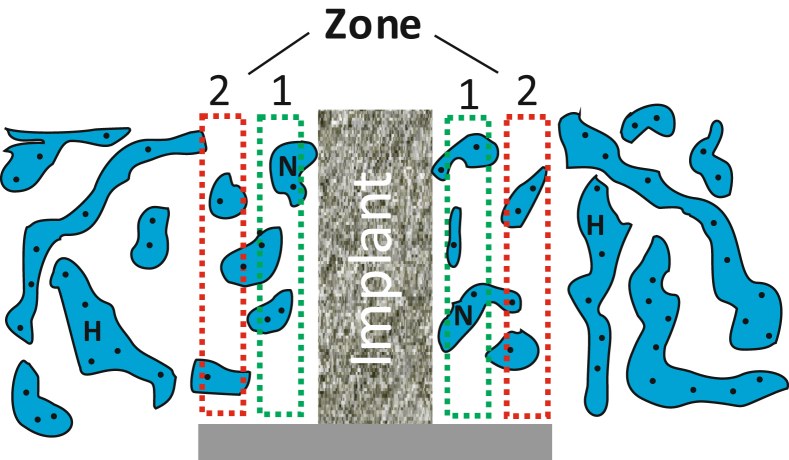
To get unbiased estimates of the anisotropic concentric gaps, four sections from each implant were analysed by point counting (newCAST; Visiopharm, Hørsholm, Denmark) based on the vertical sectioning design [22], [23] to quantify the volume of selected tissues: newly formed bone (BV/TV), bone marrow, fibrous tissue, and remnants of unresorbed HA/βTCP granules according to the American Society for Bone and Mineral Research standards [24]. Tissue volumes were quantified in two predefined zones adjacent to the titanium implant: Zone 1, situated close to the implant surface; and Zone 2, next to host bone. Both zones had a width of approximately 500 μm (Figure 3). Subsequently, by linear interception technique, the bone–implant contact (BIC) was estimated for all groups. Generally, each section was analysed blinded in a random order. However, a complete blinding of the sections was not possible due to easy identification of implant materials, although we were not able to distinguish from the different types of substitute material.
Figure 3.
Schematic view of a titanium implant showing the regions of interest, approximately 500 μm each. Zone 1 is close to the implant and Zone 2 close to host bone (H). N represents the newly formed woven bone.
Statistical analysis
The statistical significance of the differences between the control and the substitute groups was analysed using repeated measurements one-way analysis of variance or a Friedman’s test. Post hoc multiple comparison analysis was done either by Bonferroni or Dunn’s test. D’Agostino and Pearson omnibus normality test was used to assess the normality of the difference between groups. All graphs and statistical analysis were prepared in GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA). Values of p < 0.05 were considered statistically significant.
Results
Observations on animals
All eight sheep were able to walk 3 days after surgery and completed the observation period of 12 weeks without any signs of infection or significant weight change.
Mechanical properties
A destructive push-out test assessing the strength of the bone-implant interface revealed no statistically significant difference in the shear stiffness, shear strength, or failure energy between groups (Table 1).
Table 1.
Mechanical shear properties assessed by push-out test.
| Shear stiffness (MPa) | Max shear strength (MPa) | Failure energy (kJ/cm2) |
|
|---|---|---|---|
| G1: Allograft | 4.8 ± 4.1 | 1.7 ± 2.4 | 612.7 ± 1078 |
| G2: HA/βTCP | 8.2 ± 9.5 | 2.3 ± 1.9 | 635.9 ± 484.8 |
| G3: HA/βTCP-HyA | 6.6 ± 7.3 | 2.3 ± 1.7 | 738.0 ± 562.4 |
| G4: HA/βTCP-PDLLA | 6.2 ± 6.0 | 2.0 ± 2.1 | 742.1 ± 783.9 |
| RM one-way ANOVA (p) | p = 0.86 | p = 0.31 | p = 0.1 |
Data are presented as the mean ± standard deviation.
n = 8 for all groups.
ANOVA = analysis of variance; HA/βTCP = hydroxyapatite/beta-tri-calcium phosphate; HyA = hyaluronic acid; PDLLA = poly-d,l-lactic acid; RM = repeated measures.
Histological observations
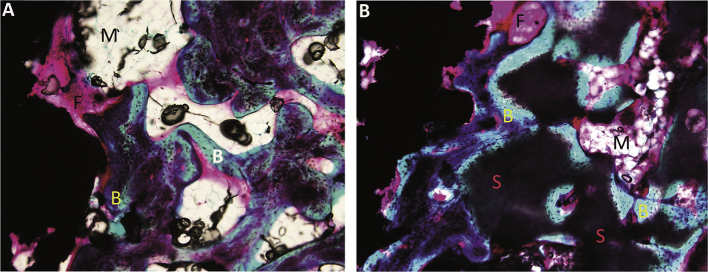
Existence of newly formed bone in the concentric gaps was found in all groups, with remnants of substitute material in the three substitute groups. The remnants were identified as small grey islets surrounded by new bone, fibrous tissue, or bone marrow (Figure 4). The identified bone consisted only of nonlamellar woven bone, and remnants of allograft were not detected. Further, no signs of infection or fibrocartilage were present in any of the groups.
Figure 4.
(A) Histological section representing the allograft group; (B) histological section representing the hydroxyapatite/beta-tri-calcium phosphate group. The presence of newly formed bone (B), fibrous tissue (F), bone marrow (M), and nonabsorbed substitutes (S) are marked on the pictures (magnification ×10).
In four bone specimens, represented by two specimens from the HA/βTCP group, one from the HA/βTCP-PDLLA group, and one from the HA/βTCP-HyA group, the BIC could not be evaluated as no tissues were in direct contact with the implant surface. This could be explained by encapsulation of the implant by fibrous tissue, which may cause a shrinking during preparation of the sections. Consequently, the four specimens were excluded from the BIC analysis.
Histomorphometry
Comparison of the BIC between the groups showed no statistically significant difference (Table 2), while the bone marrow volume was statistically significant, greater in the allograft group (22.1 ± 9.6%) compared with all substitute groups (HA/βTCP: 5.8 ± 8.1%, HA/βTCP-PDLLA: 5.1 ± 4.3%, HA/βTCP-HyA: 5.0 ± 6.4%).
Table 2.
Histomorphometric data of the bone-implant contact in percentage of the total implant surface.
| BV/TV (%) |
Fb.V/TV (%) |
Ma.V/TV (%) |
Remnant substitute (%) |
|
|---|---|---|---|---|
| G1: Allograft | 13.0 ± 10.7 | 61.2 ± 17.0 | 22.1 ± 9.6 | — |
| G2: HA/βTCP | 22.0 ± 12.0 | 69.5 ± 17.4 | 5.8 ± 8.1 | 4.1 ± 6.4 |
| G3: HA/βTCP-HyA | 10.8 ± 9.3 | 77.3 ± 8.1 | 5.1 ± 4.3 | 2.9 ± 3.1 |
| G4: HA/βTCP-PDLLA | 12.6 ± 7.2 | 73.2 ± 15.9 | 5.0 ± 6.4 | 3.6 ± 1.8 |
| RM one-way ANOVA (p) | p = 0.93 | p = 0.73 | p < 0.001 | p = 0.93 |
| Diff. between groups | — | — | G1 > G2, G3, G4 | — |
Data are presented as the mean ± standard deviation.
G1: n = 8, G2: n = 6, G3/G4: n = 7.
ANOVA = analysis of variance; BV/TV = bone volume; Diff. = difference; Fb.V/TV = fibrous tissue volume; HA/βTCP = hydroxyapatite/beta-tri-calcium phosphate; HyA = hyaluronic acid; Ma.V/TV = bone marrow volume; PDLLA = poly-d,l-lactic acid; RM = repeated measures.
The allograft group showed a statistically significantly larger BV/TV in Zone 1 (31.6 ± 13.0 %) compared with the HA/βTCP-PDLLA group (18.5 ± 10.6%), whereas in Zone 2 the BV/TV was significantly larger for allograft (37.7 ± 16.0%) in comparison to the HA/βTCP group (22.2 ± 7.4%; Table 3).
Table 3.
Histomorphometric data showing the percentage of bone volume per tissue volume in Zone 1 and Zone 2.
| BV/TV (%) |
Fb.V/TV (%) |
Ma.V/TV (%) |
Remnant substitute (%) |
|
|---|---|---|---|---|
| Zone 1 | ||||
| G1: Allograft | 31.6 ± 13.0 | 36.9 ± 19.0 | 22.5 ± 16.9 | — |
| G2: HA/βTCP | 22.3 ± 6.7 | 21.5 ± 8.5 | 16.2 ± 10.7 | 35.6 ± 13.7 |
| G3: HA/βTCP-HyA | 24.5 ± 12.8 | 25.6 ± 17.5 | 15.7 ± 17.5 | 30.8 ± 8.6 |
| G4: HA/βTCP-PDLLA | 18.5 ± 10.6 | 24.3 ± 13.2 | 15.1 ± 7.4 | 35.1 ± 13.1 |
| RM one-way ANOVA (p) | p < 0.05 | p < 0.05 | p = 0.67 | p = 0.67 |
| Diff. between groups | G1 > G4 | G1 > G2 | — | — |
| Zone 2 | ||||
| G1: Allograft | 37.7 ± 16.0 | 29.0 ± 19.7 | 27.0 ± 12.8 | — |
| G2: HA/βTCP | 22.2 ± 7.4 | 12.7 ± 4.2 | 24.9 ± 9.5 | 35.1 ± 12.8 |
| G3: HA/βTCP-HyA | 33.0 ± 12.8 | 14.0 ± 9.5 | 18.6 ± 15.0 | 31.4 ± 15.0 |
| G4: HA/βTCP-PDLLA | 24.6 ± 11.8 | 21.0 ± 12.2 | 18.2 ± 9.9 | 30.5 ± 10.9 |
| RM one-way ANOVA (p) | p < 0.05 | p < 0.05 | p = 0.29 | p = 0.46 |
| Diff. between groups | G1 > G2 | G1 > G2 | — | — |
Data are presented as the mean ± standard deviation.
n = 8 for all groups.
ANOVA = analysis of variance; BV/TV = bone volume; Diff. = difference; Fb.V/TV = fibrous tissue volume; HA/βTCP = hydroxyapatite/beta-tri-calcium phosphate; HyA = hyaluronic acid; Ma.V/TV = bone marrow volume; PDLLA = poly-d,l-lactic acid; RM = repeated measures.
The volume of fibrous tissue was statistically significantly larger in both zones comparing allograft (Zone 1: 36.9 ± 19.0%, Zone 2: 29.0 ± 19.7%) and the HA/βTCP group (Zone 1: 21.5 ± 8.5%, Zone 2: 12.7 ± 4.2%). However, the bone marrow volume revealed no statistically significant difference between the groups, and neither there was any significant difference in the amount of residual substitutes between the substitute groups (Table 3).
Discussion
The purpose of this study was to investigate whether HA/βTCP-HyA or HA/βTCP-PDLLA granules could conduct bone formation for proper implant fixation in a gap model in sheep. According to the histomorphometric evaluation, the allograft group showed a larger BV/TV when compared with the HA/βTCP-PDLLA group (Zone 1). Despite the lowered bone formation in the HA/βTCP-PDLLA group, no statistically significant differences in the mechanical properties as well as the BIC were detected, comparing both HA/βTCP-PDLLA and HA/βTCP-HyA to the allograft. Consequently, the data support our hypothesis that HA/βTCP-PDLLA and HA/βTCP-HyA can conduct an efficient formation of bone and subsequent implant fixation in sheep, demonstrating their continued relevance as synthetic bone substitutes.
The histological investigation revealed the formation of new bone in the gap of all groups. The significantly lower BV/TV in Zone 1 of the HA/βTCP-PDLLA group compared with the allograft group could indicate reduced osteoconductive properties of PDLLA. This is consistent with another study conducted in sheep, showing a delayed formation of bone in gaps filled with PDLLA-coated HA/βTCP substitutes compared with pure substitutes [25]. A lowered porosity of substitute material due to PDLLA coating could explain the reduced bone formation, though the porosity is considered within the limit ensuring optimal bone ingrowth [11], [26], [27]. However, the use of PDLLA has been associated with certain concern due to its acidic degradation and hydrophobic surface, potentially provoking an inflammatory response [28], [29]. Additionally, some have reported on the lack of bone ingrowth for scaffolds consisting purely of PDLLA [14], [30]. However, PDLLA has during the past decades proven to have an excellent biocompatibility without causing systemic or local reactions and are thus considered safe for clinical use. Further, the lowered BV/TV found in the HA/βTCP-PDLLA group was not in reflected in the results from the push-out test or the BIC assessment showing no difference compared with allograft.
Regarding the BIC, numerically higher values were found in the HA/βTCP group compared with the others, although not statistically significant. However, it should be taken into consideration that four of the bone specimens (2 from the HA/βTCP-group, 1 from the HA/βTCP-PDLLA, and 1 from the HA/βTCP-HyA group) were excluded due to the absence of tissue in direct contact with the implant interphase. This exclusion may have influenced the results, but since it was present in all substitute groups it is considered to be a consequence of inappropriate section preparation, and not related to the type of coating.
A high BIC does not necessarily equal a strong implant fixation, dependent on an appropriate quality of bone and structures trabeculae. A push-out test is designed to test the shear mechanical properties of newly formed bone onto the implant surface, and no statistically significant differences in the mechanical properties between the groups were observed. This indicates that HA/βTCP-HyA and HA/βTCP-PDLLA offer similar shear strength, modulus, and failure energy on implant fixation to those of allograft. With mechanical properties and a BIC similar to allograft, PDLLA reinforced HA/βTCP showed promising results as an alternative bone substitute consistent with recent studies, where PDLLA coated HA improved the implant performance in sheep [12], [14], [19] and rabbit [13]. Moreover, HA/βTCP scaffolds reinforced with 10% PDLLA has been shown to attain mechanical properties similar to that of human cancellous bone [11], [13].
We decided to investigate the effect of high molecular weight (MW) HyA, because this form of HyA is reported to stimulate the formation of bone as well as supporting an osteogenic differentiation of bone marrow stem cells [16], [31]. Its presence in callus during fracture healing in rabbits further supports its potential as a bone ingrowth stimulator [32]. Our results showed that HyA infiltrated HA/βTCP granules could conduct a bone formation and implant fixation comparable to that of allograft. This is in consistence with previous studies showing bone ingrowth around HyA-coated titanium implants in rabbit femurs [33], [34]. Other studies have reported increased osteoblastic activity [16] and more bone formation in rats when applied to bone wounds [17]. Borsari and coworkers [35] were, nevertheless, not able to detect any significant differences in bone ingrowth between HyA-coated or uncoated titanium implants inserted in young, aged, and ovariectomized sheep, respectively.
An apparent limitation of the present study is the small number of animals included, reducing the power of the study. However, the gaps were systematically filled with allograft or substitute, and each animal served as their own control reducing the biological variation among the individuals. Insertion of the implants into a nonweight-bearing position and the fact that the load on the skeletal sites in sheep is different from humans points out another weakness of the study prohibiting a direct extrapolation to patients. However, the design enables an investigation of the selected bone substitutes in a more controlled milieu avoiding the influence of, for example, synovial fluids. Overall, HA/βTCP-PDLLA and HA/βTCP-HyA exhibited an osteoconductive potential correspondent to allograft, signifying their promising properties as bone graft substitutes in a large animal model.
Conclusion
This study has demonstrated that HA/βTCP granules reinforced with PDLLA or infiltrated with HyA had similar bone ingrowth and implant fixation as those with allograft.
In perspective, mechanical properties resembling those of allograft in advance, the bone substitutes may be considered as alternatives to allograft for bone healing in this sheep model.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Funding/support
Odense University Hospital Free Research Fund (numbers: 700-701-003-316, 1021-1912), and University of Southern Denmark (number: 2010-11202; 1-year PhD fellowship for CMA) supported the study.
Acknowledgments
We are thankful to Gitte Højlund Reinberg for her excellent technical assistance, Maria Vinther Juhl (Danish Technological Institute) for assisting with the HyA-coating procedure, Birgitte Mølholm Malle (Novozymes A/S) for kindly donating the HyA solution, and the staff of the Biomedical Laboratory for taking good care of the animals.
References
- 1.Dickson G., Buchanan F., Marsh D., Harkin-Jones E., Little U., McCaigue M. Orthopaedic tissue engineering and bone regeneration. Technol Health Care. 2007;15:57–67. [PubMed] [Google Scholar]
- 2.Carson J.S., Bostrom M.P. Synthetic bone scaffolds and fracture repair. Injury. 2007;38(Suppl. 1):S33–S37. doi: 10.1016/j.injury.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133–2151. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 4.Brydone A.S., Meek D., Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–1343. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- 5.Gazdag A.R., Lane J.M., Glaser D., Forster R.A. Alternatives to autogenous bone graft: Efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Nandi S.K., Roy S., Mukherjee P., Kundu B., De D.K., Basu D. Orthopaedic applications of bone graft and graft substitutes: a review. Indian J Med Res. 2010;132:15–30. [PubMed] [Google Scholar]
- 7.Hannink G., Arts J.J. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011;42(Suppl. 2):S22–S25. doi: 10.1016/j.injury.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Dorozhkin S.V. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–1485. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 9.Gunatillake P.A., Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 10.Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen S.S., Ding M., Juhl M.V., Theilgaard N., Overgaard S. Mechanical strength of ceramic scaffolds reinforced with biopolymers is comparable to that of human bone. J Mater Sci Mater Med. 2011;22:1111–1118. doi: 10.1007/s10856-011-4290-y. [DOI] [PubMed] [Google Scholar]
- 12.Ding M., Røjskjaer J., Cheng L., Overgaard S. The effects of a novel-reinforced bone substitute and Colloss(R)E on bone defect healing in sheep. J Biomed Mater Res B Appl Biomater. 2012;100:1826–1835. doi: 10.1002/jbm.b.32750. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa S., Tamura J., Neo M., Goto K., Shikinami Y., Saito M. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for use as a bone substitute. J Biomed Mater Res A. 2005;75:567–579. doi: 10.1002/jbm.a.30460. [DOI] [PubMed] [Google Scholar]
- 14.Jensen T., Jakobsen T., Baas J., Nygaard J.V., Dolatshahi-Pirouz A., Hovgaard M.B. Hydroxyapatite nanoparticles in poly-d,l-lactic acid coatings on porous titanium implants conducts bone formation. J Biomed Mater Res A. 2010;95:665–672. doi: 10.1002/jbm.a.32863. [DOI] [PubMed] [Google Scholar]
- 15.Volpi N., Schiller J., Stern R., Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16:1718–1745. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 16.Huang L., Cheng Y.Y., Koo P.L., Lee K.M., Qin L., Cheng J.C. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J Biomed Mater Res A. 2003;66:880–884. doi: 10.1002/jbm.a.10535. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki T., Watanabe C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone. 1995;16:9–15. doi: 10.1016/s8756-3282(94)00001-8. [DOI] [PubMed] [Google Scholar]
- 18.Babiker H., Ding M., Sandri M., Tampieri A., Overgaard S. The effects of bone marrow aspirate, bone graft, and collagen composites on fixation of titanium implants. J Biomed Mater Res B Appl Biomater. 2012;100:759–766. doi: 10.1002/jbm.b.32509. [DOI] [PubMed] [Google Scholar]
- 19.Ding M., Andreasen C.M., Dencker M.L., Jensen A.E., Theilgaard N., Overgaard S. Efficacy of a small cell-binding peptide coated hydroxyapatite substitute on bone formation and implant fixation in sheep. J Biomed Mater Res A. 2015;103:1357–1365. doi: 10.1002/jbm.a.35281. [DOI] [PubMed] [Google Scholar]
- 20.Hing K.A. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Trans A Math Phys Eng Sci. 2004;362:2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard S., Søballe K., Jørgen H., Gundersen G. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: Optimizing the stereological vertical-section design. J Orthop Res. 2000;18:313–321. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 22.Gundersen H.J., Bagger P., Bendtsen T.F., Evans S.M., Korbo L., Marcussen N. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard S. Calcium phosphate coatings for fixation of bone implants. Evaluated mechanically and histologically by stereological methods. Acta Orthop Scand. 2000;71:1–74. [Google Scholar]
- 24.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habibovic P., Kruyt M.C., Juhl M.V., Clyens S., Martinetti R., Dolcini L. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J Orthop Res. 2008;26:1363–1370. doi: 10.1002/jor.20648. [DOI] [PubMed] [Google Scholar]
- 26.Syed F.A., Ng A.C. The pathophysiology of the aging skeleton. Curr Osteoporos Rep. 2010;8:235–240. doi: 10.1007/s11914-010-0035-y. [DOI] [PubMed] [Google Scholar]
- 27.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Taylor M.S., Daniels A.U., Andriano K.P., Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994;5:151–157. doi: 10.1002/jab.770050208. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L., Wang B., Yang G., Gauthier M. Poly(lactic acid)-based biomaterials: synthesis, modification and applications. In: Ghista Dhanjoo N., editor. Biomedical science, engineering and technology. InTech; Croatia: 2012. p. 902. Published: January 20, 2012. [Google Scholar]
- 30.Rosen C.J., Ackert-Bicknell C., Rodriguez J.P., Pino A.M. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou L., Zou X., Chen L., Li H., Mygind T., Kassem M. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J Orthop Res. 2008;26:713–720. doi: 10.1002/jor.20539. [DOI] [PubMed] [Google Scholar]
- 32.Maurer P.H., Hudack S.S. The isolation of hyaluronic acid from callus tissue of early healing. Arch Biochem Biophys. 1952;38:49–53. doi: 10.1016/0003-9861(52)90008-8. [DOI] [PubMed] [Google Scholar]
- 33.Morra M., Cassinelli C., Cascardo G., Fini M., Giavaresi G., Giardino R. Covalently-linked hyaluronan promotes bone formation around Ti implants in a rabbit model. J Orthop Res. 2009;27:657–663. doi: 10.1002/jor.20797. [DOI] [PubMed] [Google Scholar]
- 34.Aguado E., Pascaretti-Grizon F., Gaudin-Audrain C., Goyenvalle E., Chappard D. Beta-TCP granules mixed with reticulated hyaluronic acid induce an increase in bone apposition. Biomed Mater. 2014;9:015001. doi: 10.1088/1748-6041/9/1/015001. [DOI] [PubMed] [Google Scholar]
- 35.Borsari V., Fini M., Giavaresi G., Rimondini L., Consolo U., Chiusoli L. Osteointegration of titanium and hydroxyapatite rough surfaces in healthy and compromised cortical and trabecular bone: in vivo comparative study on young, aged, and estrogen-deficient sheep. J Orthop Res. 2007;25:1250–1260. doi: 10.1002/jor.20413. [DOI] [PubMed] [Google Scholar]