Summary
Background
Polylactic acid polymer interference screws are commonly used in anterior cruciate ligament (ACL) reconstructions, especially in proximal tibia fixation. However, several concerns have been raised, including the acid products during its degradation in vivo. In recent years, biodegradable magnesium (Mg)-based implants have become attractive because of their favourable mechanical properties, which are more similar to those of natural bone when compared with other degradable materials, such as polymers, apart from their alkaline nature during degradation.
Methods
We developed a pure Mg interference screw for ACL reconstruction. In the present study, 24 fresh cadaver knees were used to compare the mechanical properties of pure Mg interference screws and polylactic acid polymer interference screws for ACL reconstruction via their application on the proximal tibia tested using specific robotics.
Results
Results showed that the pure Mg interference screw group showed similar mechanical stability to the polylactic acid polymer interference screw group, implying comparable postoperative fixation effects.
Conclusion
As there are no commercially available Mg-based interference screws for ACL reconstruction clinically and the in vivo degradation of pure Mg promotes bone formation, our cadaveric study supports its clinical tests for ACL reconstruction.
Keywords: anterior cruciate ligament, biomechanical comparison, cadaveric study, magnesium screw, polylactic acid polymer screw
Introduction
Anterior cruciate ligament (ACL) rupture is one of the most common injuries, which may result in many secondary injuries around knee joints. ACL reconstruction, with a success rate >90% [1], has been the most effective way to treat ACL rupture. The optimal initial graft fixation and the properties of materials are very important in ACL reconstruction. Several years ago, permanent metal interference screws, which provided strong initial fixation, were used in ACL reconstruction [2]. However, the rigid metal interference screws showed several disadvantages, such as the risk of graft damage and accordingly fragility in reconstructions [3], interference with imaging modalities (e.g., magnetic resonance imaging), and requiring an undesirable second operation for implant removal [1]. The interference screws made of permanent metal(s) also increased difficulty in ACL revision [4].
To overcome these limitations of metal screws, biocompatible and biodegradable polylactic acid polymer interference screws were developed for popular ACL reconstruction that provided strong initial fixation and minimal graft damage [5]. A meta-analysis also indicated that there were no clinical difference between the metal interference screws and the polylactic acid polymer interference screws [6]. For regeneration of the soft tissue–bone interface in ACL reconstruction, biodegradable polymer interference screws have become a popular choice as they can be engineered to possess multiphasic properties. However, polymer materials may also have limitations. When poly-l-lactic acid interference screws were used for graft fixation during ACL reconstruction, the devices were reported to be mechanically weaker than metallic devices and often fractured during implantation [7]. Furthermore, according to Johnston et al's [8] study, the interference screws were slowly absorbed over time; 4 years after ACL reconstruction, only 80–90% of screws were completely absorbed. At 5 years follow-up, 29% of patients showed complete ossification of the screw tract in the femur versus 34% in the tibia [8]. Another study also showed poor result that even no bony replacement has taken place up to 24 months postoperatively, and at the same time, after degradation, the bone did not regenerate and the tunnel left was not filled [9]. A long-term follow-up clinical study showed that as the polymer mass reduced, it was replaced by a relatively avascular fibrous tissue containing macrophages, and having an occasional multinucleated giant cell on the implant surface as polymer degradation created an acid local environment [10]. Despite being satisfactory clinically, it would not be an ideal implant material for ACL reconstruction.
In recent years, degradable metals, such as magnesium (Mg) and its alloys, have been intensively investigated preclinically [11], [12], and clinical trials were also conducted to study their potential orthopaedic applications [1], [13], [14], as they possess desirable mechanical properties, good biocompatibility, and biodegradability [15], [16]. The lower moduli compared with permanent metals such as titanium-based materials make the mechanical properties of pure Mg or its alloys closer to those of the cortical bone, which could reduce the level of stress shielding effects during fracture fixation [17]. At the same time, the Mg-based implants developed good mechanical properties; the ultimate loads of the graft were comparable to those when using titanium interference screws on a goat model and supported the use of Mg-based interference screws for fixation of the replacement graft in ACL reconstruction [18].
The history of biodegradable Mg-based implants in orthopaedics goes back to the first half of the 20th century. It was Payr who first introduced the use of Mg for joint arthroplasty, fracture fixation with Mg wire, and intramedullary rods [19]. Recently, investigators have reached a consensus that the degradation of Mg in vivo promoted soft tissue repair and new bone formation while being gradually and completely absorbed over time [20].
Magnesium is biodegradable and its degradation products include Mg ions, alkaline environment, and hydrogen [20]. In recent years, biodegradable Mg-based implants developed for orthopaedic applications have become increasingly attractive as magnesium's initial mechanical properties (e.g., Young's elastic modulus) are similar to those of natural bone, with higher stability and Young's modulus compared to other degradable materials such as polymers [21]. There are extensive clinical studies to support its in vivo applications [22], [23]. To date, there have been three clinical trial studies reported in Germany for its indications [1], in Korea for its applications [13], and most recently in China for its application in fixing bony flap in femoral head osteonecrosis [14] based on relevant preclinical experimental models. ACL reconstruction is a clinical routine and has considerable potential for indication of Mg-based interference screws [24], [25]. Accordingly, a few preclinical studies have been conducted on Mg-based interference screws to test their in vivo potential using animal models such as rabbits [26], [27]. We have developed pure Mg interference screw for human application. However, even prior to clinical applications, cadaveric study is essential to confirm its mechanical properties after ACL reconstruction surgery. Accordingly, the aim of the study was to compare the mechanical properties of our pure Mg interference screws and clinically used polylactic acid polymer interference screws in the proximal tibia fixation immediately after ACL reconstruction. As the screw design (diameter, 8 mm; length, 30 mm) and tensile strength (Mg screw ≥ 150 MPa vs. polymer screw 169 MPa [18]) of both Mg screws and polymer screws are similar, we hypothesised that there would be no significant differences in knee stability after ACL reconstruction as compared between the two kinds of screws.
Materials and methods
Specimen preparation
In the study, 24 fresh human cadaveric knees with 15–25 cm proximal and distal to the knee joint were dissected without removal of surrounding soft tissues. All belonged to male individuals whose mean age was 38.5 years (range, 23–54 years). X-ray (Siemens Luminos Select, Siemens, Germany) and magnetic resonance imaging (Philips Intera Achieva 1.5 T, Philips, Holland) were used for scanning the entire knee joints to ensure the absence of osseous and soft tissue abnormalities (such as meniscus, ACL, Posterior cruciate ligament (PCL), Medial collateral ligament (MCL), Lateral collateral ligament (LCL), Medial patella femoral ligament (MPFL)), deformities, or osteoarthritis. The distal tibia and the proximal femur were fixed to the embedding cassette using the polymethyl methacrylate bone cement. To prevent affecting the movement of the knee, the femoral and tibial embedding cassettes were mounted to the mechanical testing robot TX90 (TX90 Bionix; Stäubli Company, Stäubli, Switzerland) using a custom-made clamp. The knee specimens were randomly divided into four groups with six specimens per group, including three ACL reconstruction groups (Group B, ACL-deficient; Group C, grafts of the tibial end were fixed with the pure Mg interference screws; Group D, grafts of the tibial end were fixed with polylactic acid polymer interference screws) and one control group (Group A). At the same time, 36 hamstring tendons were obtained from the same limbs for ACL reconstruction. The hamstring grafts were prepared as the traditional single-bundle technique [28]. Two ends of the graft were woven separately for about 3.5 cm with a diameter of 8 mm and a length of 8.5 cm, and the grafts were left to defrost in isotonic saline (0.9% NaCl solution) for 1 hour prior to the test (Figure 1). A tensile load of 70 N was applied to the graft for 15 minutes (using a specific device, ACL distractor; Stryker Corporation, USA) as an initial graft tension. The specimen preparation protocol had been approved by the ethics committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China.
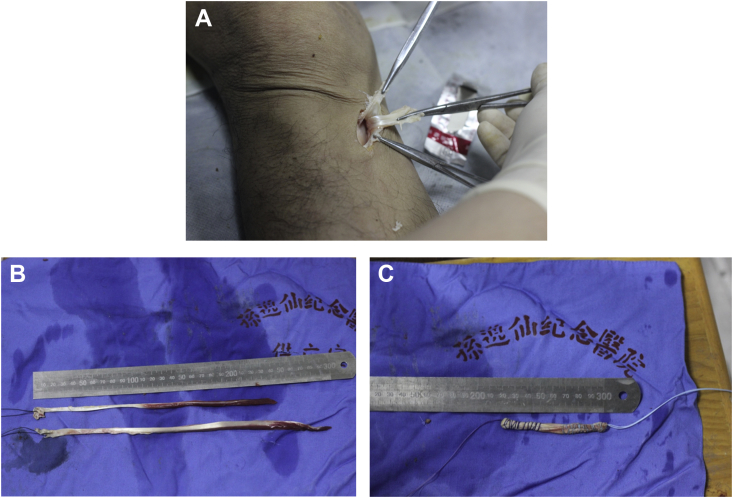
Figure 1.
(A) The hamstring tendon was surgically obtained from the same limbs. (B) Two ends of the graft were woven separately for about 3.5 cm with a diameter of 8 mm and a length of 8.5 cm. (C) The graft was then prepared for ACL reconstruction.
Tunnel preparation
The tunnel was prepared according to the traditional single-bundle technique in Groups B, C, and D, following a published protocol [28]. Using a 55° tibial drill guide, a trained surgeon advanced a guide wire into the centre of the tibial footprint, and an 8-mm tibial tunnel was drilled over the guide wire. On the femoral tunnel side, a tunnel (with an 8-mm diameter) was drilled in the lateral wall of the intercondylar notch, leaving a 2-mm posterior wall within the footprint.
ACL reconstruction
ACL reconstruction was not performed in Group B. In the two remaining groups, the graft was passed through the tibial and femoral tunnels, and the femoral tunnel was fixed with absorbable biointerference screw under tension with the knee at 120° of flexion.
In Group C, the grafts of the tibial end were fixed with the pure Mg interference screws that had the same design as the commercially available polylactic acid polymer interference screws from Smith & Nephew, USA, with a diameter of 8 mm and a length of 30 mm for comparison (Figure 2A).
Figure 2.
(A) Pure magnesium interference screw. (B) Commercially available polylactic acid polymer interference screws from Smith & Nephew, with a diameter of 8 mm and length of 30 mm. The screws have a similar tensile strength: Mg screw, ≥150 MPa; polymer screw, 169 MPa.
In Group D, the grafts of the tibial end were fixed with the polylactic acid polymer interference screws from Smith & Nephew (diameter, 8 mm; length, 30 mm; Figure 2B).
Biomechanical testing
All testing was done using a mechanical testing robot TX90 (TX90 Bionix; Staubil Company; Figure 3) and compared among the four groups.
Figure 3.
The mechanical testing device TX90 (TX90 Bionix; Staubil Company).
The femur and the tibia fixed to the embedding cassette with the polymethyl methacrylate bone cement were mounted onto the tensile tester of the machine. The load consisted of cyclic anterior tibial loads (ATLs) ranging from 0 N to 150 N with a testing speed of 10 N/s at full extension and 15°, 30°, 60°, and 90° of knee flexion. The anterior tibial translation (ATT) during the ATL was measured at above the specified knee flexion angles, respectively.
Statistics
Statistical analysis was conducted using the SPSS 21 software package (SPSS Headquarters, Chicago, IL, USA). In all groups, nonparametric distribution of the data was found (Kolmogorow–Smirnow test). One-way analysis of variance and the Mann–Whitney U Wilcoxon rank-sum test were used to determine the differences in each parameter among the four groups. Statistical significance was set at p < 0.05 (α = 0.05).
Results
ATT at full extension
The results of ATT are summarised in Table 1. Under the 150-N ATL, the ATT of the intact knee was 5.45 ± 0.39 mm at full extension. After the ACL was cut, the translations increased significantly at full extension (8.93 ± 0.71; p < 0.05). For the pure Mg interference screw group, the ATT was 6.29 ± 0.599 mm. For the polylactic acid polymer interference screw group, the ATT was 5.48 ± 0.619 mm at full extension. These values showed no statistical significance among Groups A, C, and D (p > 0.05; Figure 4).
Table 1.
Anterior tibial translation at different knee flexion angle.
| Group | N | Knee flexion angle | Mean | Standard deviation | Standard error | 95% Confidence interval for mean |
Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||||
| A | 6 | 15° | 5.63 | 0.5 | 0.2 | 5.1 | 6.16 | 4.68 | 6.17 |
| 30° | 5.63 | 0.5 | 0.2 | 5.1 | 6.16 | 4.68 | 6.17 | ||
| 60° | 5.53 | 0.41 | 0.17 | 5.1 | 5.95 | 4.98 | 6.12 | ||
| 90° | 5.45 | 0.39 | 0.16 | 5.05 | 5.86 | 4.91 | 6.03 | ||
| Full flexion | 5.45 | 0.39 | 0.16 | 5.03 | 5.86 | 4.82 | 5.87 | ||
| B | 6 | 15° | 12.26 | 0.84 | 0.34 | 11.38 | 13.14 | 10.97 | 13.22 |
| 30° | 12.04 | 0.94 | 0.38 | 11.05 | 13.02 | 10.91 | 13.22 | ||
| 60° | 11.47 | 0.83 | 0.34 | 10.6 | 12.34 | 10.23 | 12.58 | ||
| 90° | 11.47 | 0.83 | 0.34 | 10.6 | 12.34 | 10.23 | 12.58 | ||
| Full flexion | 8.93 | 0.71 | 0.29 | 8.18 | 9.68 | 8.19 | 9.98 | ||
| C | 6 | 15° | 5.82 | 0.29 | 0.12 | 5.51 | 6.13 | 5.37 | 6.12 |
| 30° | 5.95 | 0.4 | 0.16 | 5.53 | 6.38 | 5.41 | 6.51 | ||
| 60° | 5.8 | 0.55 | 0.22 | 5.21 | 6.36 | 5.29 | 6.79 | ||
| 90° | 5.46 | 0.36 | 0.15 | 5.07 | 5.84 | 4.98 | 6.02 | ||
| Full flexion | 6.29 | 0.59 | 0.24 | 5.67 | 6.92 | 5.62 | 7.13 | ||
| D | 6 | 15° | 5.78 | 0.41 | 0.17 | 5.35 | 6.21 | 5.33 | 6.56 |
| 30° | 5.84 | 0.5 | 0.21 | 5.31 | 6.37 | 5.02 | 6.58 | ||
| 60° | 5.76 | 0.4 | 0.16 | 5.34 | 6.18 | 5.25 | 6.26 | ||
| 90° | 5.49 | 0.43 | 0.18 | 5.04 | 5.94 | 5.08 | 6.29 | ||
| Full flexion | 5.48 | 0.61 | 0.25 | 4.84 | 6.11 | 4.75 | 6.53 | ||
Group A = intact ACL; Group B = ACL-deficient; Group C = pure magnesium interference screw group; Group D = polylactic acid polymer interference screw group.
Figure 4.
Comparison of anterior tibial translation of each group in different knee angles. These values showed no statistical significance among Groups A, C, and D (p > 0.05). However, there was statistical significance among Groups A, B, C, and D (* p < 0.05). Group A, intact ACL; Group B, ACL-deficient; Group C, pure magnesium interference screw group; Group D, polylactic acid polymer interference screw group.
ATT at 15° flexion
The results of ATT are provided in Table 1. Under the 150-N ATL, the ATT of the intact knee was 5.63 ± 0.50 mm at 15° flexion. After the ACL was cut, these translations increased significantly at 15° flexion angles (12.26 ± 0.84; p < 0.05). For the pure magnesium interference screw group, the ATT was 5.82 ± 0.29 mm. For the polylactic acid polymer interference screw group, the ATT was 5.78 ± 0.41 mm at 15° flexion. These values showed no statistical significance among Groups A, C, and D (p > 0.05; Figure 4).
ATT at 30° flexion
The results of ATT are provided in Table 1. Under the 150-N ATL, the ATT of the intact knee was 5.77 ± 0.62 mm at 30° flexion. After the ACL was cut, these translations increased significantly at 30° flexion (12.04 ± 0.94; p < 0.05). For the pure magnesium interference screw group, the ATT was 5.95 ± 0.40 mm. For the polylactic acid polymer interference screw group, the ATT was 5.84 ± 0.50 mm at 30° flexion. These values showed no statistical significance among Groups A, C, and D (p > 0.05; Figure 4).
ATT at 60° flexion
The results of ATT are provided in Table 1. Under the 150-N ATL, the ATT of the intact knee was 5.53 ± 0.41 mm at 60° flexion. After the ACL was cut, these translations increased significantly at 60° flexion (11.47 ± 0.83; p < 0.05). For the pure magnesium interference screw group, the ATT was 5.80 ± 0.55 mm. For the polylactic acid polymer interference screw group, the ATT was 5.76 ± 0.40 mm at 60° flexion. These values showed no statistical significance among Groups A, C, and D (p > 0.05; Figure 4).
ATT at 90° flexion
The results of ATT are provided in Table 1. Under the 150-N ATL, the ATT of the intact knee was 5.53 ± 0.41 mm at 90° flexion. After the ACL was cut, these translations increased significantly at 90° flexion (11.47 ± 0.83; p < 0.05). For the pure magnesium interference screw group, the ATT was 5.80 ± 0.55 mm. For the polylactic acid polymer interference screw group, the ATT was 5.76 ± 0.40 mm at 60° flexion. These values showed no statistical significance among Groups A, C, and D (p > 0.05; Figure 4).
Discussion
In this cadaveric study, we compared the stability of the hamstring tendon ACL reconstruction at the tibia fixation site using either our pure Mg interference screws or the polymer interference screw. The robot-based mechanical testing data showed that the pure Mg interference screw group had a similar mechanical stability to the polylactic acid polymer interference screw group in terms of anterior–posterior translation of the knee joint of the intact knee, after their application in the proximal tibia fixation of the ACL reconstruction.
Because of the Mg effect on promotion of healing and remodelling of the regenerated hard and soft tissue, the Mg-based interference screws were developed in the present study for a relevant clinical indication for ACL reconstruction. As Mg has been shown to promote bone regeneration in many preclinical and clinical studies [1], [11], [12], [13], [14], the use of the interference screws may have potential benefits in healing of the graft in the bone tunnel, as reported in recent animal experimental studies [26].
The current study adopted the testing protocols reported by Farraro et al [29]. In their study, a 67-N ATL was applied to the goat stifle joint at 30°, 60°, and 90° of joint flexion in three states: intact, ACL-deficient, and reconstructed. According to a study, in the intact state, the ATT ranged between 1.8 mm and 2.5 mm at three flexion angles. In the ACL-deficient state, the ATT was between 12.4 mm and 15.8 mm, whereas in the Mg ring repair state, the ATT ranged between 4.3 mm and 5.0 mm at three flexion angles. Other studies [18], [27], which also showed very similar results, supported the use of Mg-based interference screw for fixation of the autograft in ACL reconstruction. All of those studies, however, were animal studies (e.g., quadrupedal goat or rabbit), and the knee joint anatomy and range of motion are obviously different from those of bipedal human beings. In our study, fresh human cadaveric knees were used for surgical reconstruction and testing with preservation of all soft tissues around the knee joint. The intact fresh human cadaveric knees with all the soft tissues preserved for testing can mimic the clinical situation, and the data obtained can be regarded as very essential references or basis together with preclinical biological studies for starting clinical trials. The ATL of 150 N was applied at full extension and at 15°, 30°, 60°, and 90° of knee flexion. For the setting of the maximum load, readers should refer to the study of Christel et al [30]. Our results in the intact state showed that the ATT ranged between 5.45 and 5.77 mm at three flexion angles. In the ACL-deficient state, the ATT was between 8.93 and 12.43 mm, whereas in the reconstruction states with the pure Mg screws and the polylactic acid polymer interference screws, the ATT was back to 5–6 mm. Moreover, the Mg interference screw group demonstrated a similar mechanical stability as compared with that of the intact knee or the clinically approved and also widely used polylactic acid polymer interference screw group.
Our study had several limitations. First, we did not test the reconstructed knee to failure, i.e., without providing ultimate load, because the maximum load of the TX90 device (TX90 Bionix; Staubil Company) used in the study was 150 N. However, the pull-out strength test is very important; when we were testing the new biodegradable screws for ACL reconstruction, we would test the pull-out strength along with the degradation rate in the following study. Second, the Mg-based interference screw has no opening at the screw head as compared with clinically used ones, and this increased the difficulty of screw insertion. We need to further improve or modify the design of the Mg-based interference screws to address this issue.
In conclusion, the present study investigated an innovative biodegradable metal as an orthopaedic implant, i.e., pure Mg interference screws for fixation of hamstring autograft in ACL reconstruction. Our unique knee biomechanical testing demonstrated enough initial mechanical properties in the proximal tibia fixation of ACL reconstruction compared with the currently and clinically used polylactic acid polymer interference screw, implying its effects in achieving immediate postoperative outcome. Long-term stability, degradation rate, and safety of Mg-based interference screws will be further discussed in future studies. In addition, the design of Mg screws will be modified to satisfy the clinical requirements in our next step.
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgments
We thank the staff involved in this study for their time. This study was supported by research grants from the Industry University Research Project in Guangdong Province, Science, and Technology Department of Guangdong Province (Reference No. 2011A090100004).
Contributor Information
Ling Qin, Email: b664730@mailserv.cuhk.edu.hk.
Yue Ding, Email: dingyue36@126.com.
References
- 1.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber F.A., Elrod B.F., McGuire D.A., Paulos L.E. Preliminary results of an absorbable interference screw. Arthroscopy. 1995;11:537–548. doi: 10.1016/0749-8063(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 3.Halewood C., Hirschmann M.T., Newman S., Hleihil J., Chaimski G., Amis A.A. The fixation strength of a novel ACL soft-tissue graft fixation device compared with conventional interference screws: a biomechanical study in vitro. Knee Surg Sports Traumatol Arthrosc. 2011;19:559–567. doi: 10.1007/s00167-010-1255-5. [DOI] [PubMed] [Google Scholar]
- 4.Emond C.E., Woelber E.B., Kurd S.K., Ciccotti M.G., Cohen S.B. A comparison of the results of anterior cruciate ligament reconstruction using bioabsorbable versus metal interference screws: a meta-analysis. J Bone Joint Surg Am. 2011;93:572–580. doi: 10.2106/JBJS.J.00269. [DOI] [PubMed] [Google Scholar]
- 5.Piltz S., Strunk P., Meyer L., Plitz W., Lob G. Fixation strength of a novel bioabsorbable expansion bolt for patellar tendon bone graft fixation: an experimental study in calf tibial bone. Knee Surg Sports Traumatol Arthrosc. 2004;12:376–383. doi: 10.1007/s00167-003-0463-7. [DOI] [PubMed] [Google Scholar]
- 6.Shen C., Jiang S.-D., Jiang L.-S., Dai L.-Y. Bioabsorbable versus metallic interference screw fixation in anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials. Arthroscopy. 2010;26:705–713. doi: 10.1016/j.arthro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Smith C.A., Tennent T.D., Pearson S.E., Beach W.R. Fracture of Bilok interference screws on insertion during anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:E115–E117. doi: 10.1016/j.arthro.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Johnston M., Morse A., Arrington J., Pliner M., Gasser S. Resorption and remodeling of hydroxyapatite-poly-l-lactic acid composite anterior cruciate ligament interference screws. Arthroscopy. 2011;27:1671–1678. doi: 10.1016/j.arthro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Tecklenburg K., Burkart P., Hoser C., Rieger M., Fink C. Prospective evaluation of patellar tendon graft fixation in anterior cruciate ligament reconstruction comparing composite bioabsorbable and allograft interference screws. Arthroscopy. 2006;22:993–999. doi: 10.1016/j.arthro.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Walton M., Cotton N.J. Long-term in vivo degradation of poly-l-lactic acid (PLLA) in bone. J Biomater Appl. 2007;21:395–411. doi: 10.1177/0885328206065125. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10:2834–2842. doi: 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Witte F., Xi T., Zheng Y., Yang K., Yang Y. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015;21:237–249. doi: 10.1016/j.actbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Yang G.-F., Kim Y.-C., Han H.-S., Lee G.-C., Seok H.-K., Lee J.-C. In vitro dynamic degradation behavior of new magnesium alloy for orthopedic applications. J Biomed Mater Res Part B Appl Biomater. 2015;103:807–815. doi: 10.1002/jbm.b.33259. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D., Huang S., Lu F., Wang B., Yang L., Qin L. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Waizy H., Diekmann J., Weizbauer A., Reifenrath J., Bartsch I., Neubert V. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J Biomater Appl. 2014;28:667–675. doi: 10.1177/0885328212472215. [DOI] [PubMed] [Google Scholar]
- 16.Böse D., Eggebrecht H., Haude M., Schmermund A., Erbel R. First absorbable metal stent implantation in human coronary arteries. Am Heart Hosp J. 2006;4:128–130. doi: 10.1111/j.1527-5299.2006.04668.x. [DOI] [PubMed] [Google Scholar]
- 17.Hort N., Huang Y., Fechner D., Störmer M., Blawert C., Witte F. Magnesium alloys as implant materials—principles of property design for Mg-RE alloys. Acta Biomater. 2010;6:1714–1725. doi: 10.1016/j.actbio.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Farraro K.F., Kim K.E., Woo S.L.-Y., Flowers J.R., McCullough M.B. Revolutionizing orthopaedic biomaterials: the potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J Biomech. 2014;47:1979–1986. doi: 10.1016/j.jbiomech.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Ding W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen Biomater. 2016;3:79–86. doi: 10.1093/rb/rbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolters L., Angrisani N., Seitz J., Helmecke P., Weizbauer A., Reifenrath J. Applicability of degradable magnesium LAE442 alloy plate-screw-systems in a rabbit model. Biomed Tech (Berl) 2013 doi: 10.1515/bmt-2013-4059. [DOI] [PubMed] [Google Scholar]
- 22.Nayak S., Bhushan B., Jayaganthan R., Gopinath P., Agarwal R.D., Lahiri D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J Mech Behav Biomed Mater. 2015;59:57–70. doi: 10.1016/j.jmbbm.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Zhao S., Liu B., Chen M., Mao J., He H. Corrosion-controlling and osteo-compatible Mg ion-integrated phytic acid (Mg-PA) coating on magnesium substrate for biodegradable implants application. ACS Appl Mater Interfaces. 2014;6:19531–19543. doi: 10.1021/am506741d. [DOI] [PubMed] [Google Scholar]
- 24.Sanders T.L., Maradit Kremers H., Bryan A.J., Larson D.R., Dahm D.L., Levy B.A. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016 doi: 10.1177/0363546516629944. [DOI] [PubMed] [Google Scholar]
- 25.Stanley L.E., Kerr Z.Y., Dompier T.P., Padua D.A. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009–2010 through 2013–2014. Am J Sports Med. 2016 doi: 10.1177/0363546516630927. [DOI] [PubMed] [Google Scholar]
- 26.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Diekmann J., Bauer S., Weizbauer A., Willbold E., Windhagen H., Helmecke P. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: a pilot in vivo study in rabbits. Mater Sci Eng C Mater Biol Appl. 2016;59:1100–1109. doi: 10.1016/j.msec.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Kilinc B.E., Kara A., Oc Y., Celik H., Camur S., Bilgin E. Transtibial vs anatomical single bundle technique for anterior cruciate ligament reconstruction: a retrospective cohort study. Int J Surg. 2016;29:62–69. doi: 10.1016/j.ijsu.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Farraro K.F., Sasaki N., Woo S.L.-Y., Kim K.E., Tei M.M., Speziali A. A magnesium ring device to restore function of a transected anterior cruciate ligament in the goat stifle joint. J Orthop Res. 2016 doi: 10.1002/jor.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christel P.S., Akgun U., Yasar T., Karahan M., Demirel B. The contribution of each anterior cruciate ligament bundle to the Lachman test: a cadaver investigation. J Bone Joint Surg Br. 2012;94:68–74. doi: 10.1302/0301-620X.94B1.26562. [DOI] [PubMed] [Google Scholar]