Abstract
The presence of indole-3-butyric acid (IBA) as an endogenous auxin in Arabidopsis has been recently demonstrated. However, the in vivo role of IBA remains to be elucidated. We present the characterization of a semi-dominant mutant that is affected in its response to IBA, but shows a wild-type response to indole-3-acetic acid (IAA), the predominant and most studied form of auxin. We have named this mutant rib1 for resistant to IBA. Root elongation assays show that rib1 is specifically resistant to IBA, to the synthetic auxin 2,4-dichlorophenoxyacetic acid, and to auxin transport inhibitors. rib1 does not display increased resistance to IAA, to the synthetic auxin naphthalene acetic acid, or to other classes of plant hormones. rib1 individuals also have other root specific phenotypes including a shortened primary root, an increased number of lateral roots, and a more variable response than wild type to a change in gravitational vector. Adult rib1 plants are morphologically indistinguishable from wild-type plants. These phenotypes suggest that rib1 alters IBA activity in the root, thereby affecting root development and response to environmental stimuli. We propose models in which RIB1 has a function in either IBA transport or response. Our experiments also suggest that IBA does not use the same mechanism to exit cells as does IAA and we propose a model for IBA transport.
Auxins are an important class of plant hormones that have been implicated in all aspects of plant growth and development. Numerous physiological and genetic studies have shown auxins to be involved in phenomena as diverse as tropisms, cell enlargement and division, lateral branching of shoots and roots, vascular differentiation, and early embryonic development (Davies, 1995; Hobbie, 1998). Although indole-3-acetic acid (IAA) is the most studied form of auxin, other auxins are also present in plants. Indole-3-butyric acid (IBA) is a naturally occurring auxin identified in several plant species including Arabidopsis (Epstein and Ludwig-Müller, 1993; Ludwig-Müller et al., 1993). In Arabidopsis and other plants the level of free IBA is comparable with that of free IAA, suggesting that IBA is physiologically relevant. For example, in tobacco leaves and Arabidopsis seedlings, levels of free IBA represent approximately 25% to 30% of the total free auxins (Sutter and Cohen, 1992; Ludwig-Müller et al., 1993). In elongating pea internodes free IBA represents about one-half of the free auxins (Nordström et al., 1991). Although IBA represents a significant proportion of the free auxin pool, its total amount, which includes free and conjugated forms, is generally much lower than that of IAA. In Arabidopsis the amount of conjugated IAA is approximately eight times higher than the level of conjugated IBA. The mode of conjugation also differs between the two auxins; in Arabidopsis the majority of IAA conjugates are linked to amino acids, whereas most of the IBA conjugates are linked to sugars (Ludwig-Müller et al., 1993).
The occurrence of IBA as a natural constituent of plants was recognized as early as 1954 (Blommaert, 1954), but its physiological role is still unknown. Early studies that examined the effects of exogenous auxin application found IBA to be more effective than IAA in promoting the formation of adventitious roots (Zimmerman and Wilcoxon, 1935). Since that time, IBA has become the preferred auxin to induce root formation on cuttings and in tissue culture (Hartmann et al., 1997). This fact may reflect an in vivo role of IBA in root formation. IBA could act directly as a distinct auxin or indirectly through conversion to IAA. Interconversion of IBA and IAA has been demonstrated to occur in maize, Arabidopsis, and several other plant species (Epstein and Ludwig-Müller, 1993). There is no experimental evidence, however, that shows that conversion of IBA to IAA is necessary for IBA action. IBA treatment of peas causes an increase in endogenous levels of IBA and IAA, but only IBA levels increase and remain high in the tissue that form roots (Nordström et al., 1991). This result suggests that IBA is in fact the active auxin in production of roots since its presence, but not that of IAA, is correlated with root initiation. Similar results are obtained in the woody species Populus tremula; no evidence of IBA conversion to IAA is found when radiolabeled IBA is applied to cuttings to induce adventitious root formation (Pythoud and Buchala, 1989). In a recent report Yang and Davies (1999) demonstrate that IBA can also promote stem elongation in peas, and they postulate that IBA is a physiologically active form of auxin in stem elongation in intact plants.
Characterization of mutants in IAA response and of IAA-induced genes over the last 10 to 15 years has contributed extensively to our understanding of IAA transport, signal transduction, and the role of this auxin in plant development. One family of auxin inducible genes, Aux/IAA, contains at least 25 members in Arabidopsis, and these genes encode short-lived nuclear proteins that have been proposed to be transcriptional regulators of downstream genes responsible for mediating auxin-regulated processes (Abel et al., 1994; Kim et al., 1997). A number of IAA-resistant mutants have been isolated in Arabidopsis; they have confirmed the importance of IAA in embryonic development, root formation, cell elongation, gravity response, and apical dominance (Hobbie, 1998; Hobbie et al., 2000). In a few cases the affected genes have been cloned; these examples have shown that auxin resistance can occur as a result of defects in auxin signal transduction or auxin transport (Bennett et al., 1996; Rouse et al., 1998).
IAA undergoes active polar transport from its point of synthesis in the apex of the shoot to its points of action. Transport occurs in a cell-to-cell fashion that is mediated by influx and efflux carriers. Polarity of transport is achieved through localization of the efflux carrier to the basal side of cells (Lomax et al., 1995). Molecular and genetic studies in Arabidopsis have recently confirmed the existence of specific auxin carriers that had been suggested by earlier physiological studies. A single putative influx carrier has been identified; it is encoded by the AUX1 gene and is expressed in root tips (Bennett et al., 1996). AUX1 has homology to amino acid transporters suggesting that it facilitates the uptake of IAA, an amino acid-like molecule. A component of a specific IAA efflux carrier is encoded by the AGR1/EIR1/PIN2/WAV6 gene and has homology to bacterial transmembrane transporters (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998). Protein localization studies have shown that AGR1 functions in a well-defined subset of root epidermal and cortical cells (Müller et al., 1998). Multiple homologs of AGR1 have been found in the Arabidopsis genome, providing evidence for the existence of a family of efflux carriers. Together, these findings suggest that each member may be specific for a certain tissue, developmental stage, or environmental response. For example, the PIN1 gene is one member of this family that has been implicated in auxin transport in stems and in floral development (Okada et al., 1991; Gälweiler et al., 1998).
In contrast with IAA, IBA has been largely ignored in genetic and molecular studies. Limited physiological experiments have been reported in the literature. From these studies we know that IBA, like IAA, undergoes polar transport (Went and White, 1938) and that auxin transport inhibitors can inhibit this transport (Leopold and Lam, 1961). IAA can compete with IBA uptake, suggesting that IAA and IBA most likely utilize the same influx carrier (Ludwig-Müller et al., 1995a). To the best of our knowledge no study has directly addressed the subject of IBA efflux. Conflicting results about the rate of transport of IBA appear in the literature: some report that IBA and 2,4-D (2,4-dichlorophenoxyacetic acid, a synthetic auxin) are transported more slowly than IAA, whereas others conclude that IBA and IAA are transported at the same rate (Epstein and Ludwig-Müller, 1993; Ludwig-Müller et al., 1995a). These discrepancies can be explained by referring to differences in plant species studied, plant organs used, and experimental design.
We have isolated an Arabidopsis mutant that is specifically resistant to the auxins IBA and 2,4-D, but not to the auxins IAA or NAA (naphthalene acetic acid, a synthetic auxin). This paper reports the first phenotypic characterization of a mutant that can discriminate between the two endogenous auxins of Arabidopsis, IAA, and IBA. The mutant, designated rib1 for resistant to IBA, displays phenotypes that are consistent with a primary defect in auxin response or transport.
RESULTS
Isolation, Genetic Characterization, and Mapping of rib1
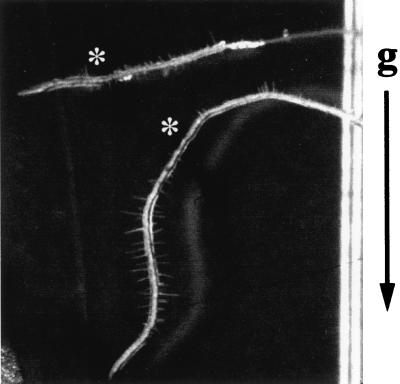
Approximately 1,400 Ds-mutagenized Nossen (No-0) ecotype lines were screened for mutants with defects in root gravitropism. As seen in Figure 1, roots of the rib1 mutant fail to reorient following a change in the direction of the gravitational vector. We recovered a single line with this phenotype during our screening. Molecular and genetic characterization of rib1 indicate that this mutation is not tagged by a Ds element (see “Materials and Methods” for details).
Figure 1.
Isolation of the rib1 mutant. Picture taken during the screen for root gravitropic mutants. Seedlings were grown on vertically oriented plates for 4 d then the plates were rotated on edge 90° and photographed 24 h later. The rib1 root response (top) can be compared with the wild-type root response (bottom). An asterisk indicates the hatch mark showing the position of the root at the time the plate was first rotated. The arrow to the right of the picture indicates the direction of the gravitational vector after rotation. The picture was taken on a stereomicroscope (Leica, Wexlar, Germany) at a magnification of 6.3×.
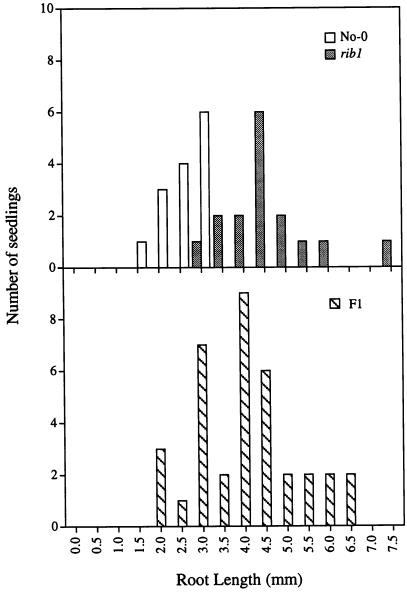
We have taken advantage of the fact that rib1 mutants are 2,4-D resistant (see below) for segregation analysis. rib1 homozygotes were crossed to wild-type No-0 plants. Figure 2 shows the 2,4-D resistance of the F1 population (bottom) compared with the two parental groups (top). In this assay resistance was determined by measuring root elongation on media containing 2,4-D. Homozygous wild-type roots are sensitive to high concentrations of 2,4-D, and were therefore significantly shorter than the rib1 roots that are 2,4-D resistant. Wild-type seedlings had an average root length of 2.8 ± 0.3 mm (se of the mean) compared with an average root length of 4.9 ± 1.1 mm for the homozygous rib1 seedlings. The distribution of root lengths among the F1 heterozygotes appeared intermediate, with an average root length of 4.3 ± 1.2 mm. We conclude from these data and from the analysis of the F2 generation (data not shown) that rib1 is semi-dominant.
Figure 2.
Resistance of rib1 homozygotes and heterozygotes to 2,4-D. Root length distribution of seedlings grown on 6 × 10−8 M 2,4-D for 7 d. A population of F1 seedlings from a No-0 X rib1 cross (bottom) is compared with No-0 and rib1 homozygous populations (top). Statistical analyses (t test) indicate that rib1 is not recessive (P = 4.5 × 10−5).
We mapped rib1 to a single locus at the bottom of chromosome I using cleaved amplified polymorphic sequences (CAPS) and simple sequence length polymorphism (SSLP) markers. rib1 shows significant linkage to nga111 at position 115.5 (25 recombinants/84 chromosomes), to nF22K20 at position 119.5 (21/84), and to g17311 at position 125.4 (18/82). rib1 is not linked to nF5I14 located at position 92.1 (data not shown). These data indicate that rib1 is telomeric to g17311, which maps near the bottom of chromosome I.
Root Slanting and Gravity Response
Although the rib1 mutant was originally identified based on its altered gravitropic response, rib1 roots are not agravitropic. When germinated and grown on vertically oriented plates under our standard growth conditions, rib1 roots are oriented downwards, similar to wild type, and are not randomly oriented as in agravitropic mutants. However, neither wild type nor rib1 grow directly downwards; instead, they slant to the left of vertical (or to the right when viewed through the agar). It is interesting that rib1 mutant roots slant more than wild-type roots on vertically oriented plates. The absolute angle values of this slanting response varies from trial to trial, but rib1 roots usually slant about 20° more to the right than do wild-type roots. For example, in one trial the average angle of rib1 root tips was 52.4°, whereas No-0 roots on the same plates had an average root tip angle of 35.9°. The growth of roots aslant from the gravitational vector is characteristic of certain ecotypes of Arabidopsis, including No-0. This response is surface dependent; i.e. it does not occur when roots are embedded in agar or in other conditions where the interaction of roots with the surface of the agar plate are minimized (Rutherford and Masson, 1996; Mullen et al., 1998). The slanting response is thought to be due to interaction between the root and the agar medium itself (chemotropism, hydrotropism, and thigmotropism), and also to the effects of gravitropism and the endogenous circumnutation movements of Arabidopsis roots (Okada and Shimura, 1990; Simmons et al., 1995b; Mullen et al., 1998). Reduced gravitropism can explain the root slanting phenotype of rib1; roots with a lessened response to gravity would be expected to grow more aslant from the gravitational vector.
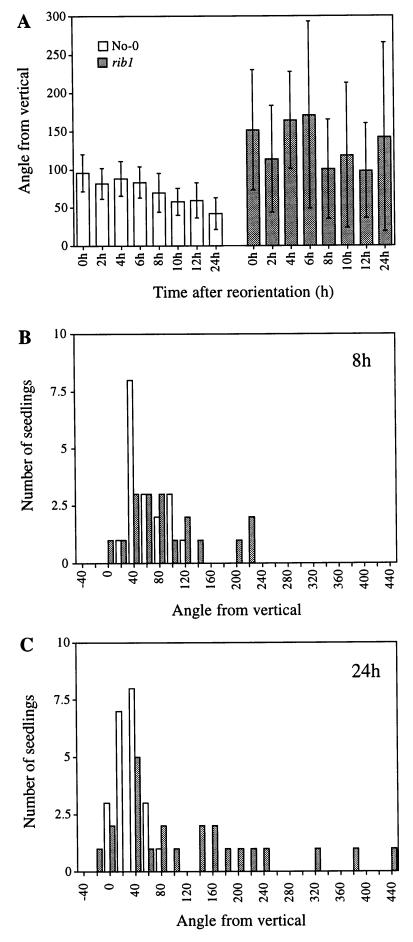
Time course experiments were conducted to characterize the defect in gravitational response of rib1. These reorientation experiments were done in the dark to ensure that phototropism did not affect the results. Vertically grown seedlings were reoriented by 90° and the curvature of the root tips was measured at different time points over a 24-h period. The data in Figure 3A are presented as the average angle of the root tips from vertical, with error bars representing the sd. During the course of the experiments, wild-type roots (white bars) reorient approximately 60° from their starting average of 100° to an angle approaching 40°. In sharp contrast, the response of the rib1 roots (shaded bars) is much more variable over the 24-h time period, as evidenced by the large sd values. The high average root angles are not indicative of a complete failure of rib1 roots to reorient, but rather reflect the variability of the rib1 gravitational response. This point is illustrated in the two representative time points shown in Figure 3, B and C, which show the position of individual roots 8 and 24 h after the plates were rotated, respectively. At 8 h, the majority of wild-type roots are reorienting toward 0°, although a few individuals have not yet responded. In a similar manner, a majority of rib1 roots are also in the process of reorienting at this time point, but they show a much broader distribution. At 24 h (Fig. 3C), wild-type roots are almost fully reoriented, whereas the rib1 response is highly variable. Approximately one-half of the seedlings show a distribution similar to wild type, whereas the remaining one-half are distributed from 140° to 440° from vertical. These results show that rib1 roots are defective in reorientation following a change in the gravitational vector.
Figure 3.
Root gravitropic response. Results of time course of turning experiment. Seedlings were grown on vertically oriented plates for 4 d then the plates were rotated on edge 90°. The angle of the root tip from vertical was measured after the indicated times. Conventions for root tip angle scoring are as follows: 0° indicates roots that grow directly down toward gravity; 90° is perpendicular to the gravity vector; 180° is directly opposite the gravity vector; and >180° represents roots that first turned up and then continued turning to form a loop. Figure 3A represents the average angle from vertical of wild-type (white bars) and rib1 (shaded bars) root tips at different time points. The data for this panel were taken from two combined time course experiments. Error bars represent the sd. B and C show representative time points from one time course experiment. These panels depict a distribution graph of root tip angle at time points 8 h (B) and 24 h (C).
We observed no detectable difference in the level of starch accumulation in rib1 and wild-type roots (data not shown). This observation, in addition to the fact that rib1 seedlings show other phenotypes consistent with a primary defect in auxin response or transport (see below), suggests that rib1 seedlings are most likely affected in gravity response rather than perception.
Morphological Characteristics of rib1
rib1 seedlings have other root-specific phenotypes, as indicated in Table I. rib1 seedlings have a shorter (approximately 20%) primary root than wild type and an increase in the total number of lateral roots (approximately 60%). Both of these differences are highly significant: the P values of t tests are 4.5 × 10−6 and 2.6 × 10−9, respectively. Auxins are known to inhibit root elongation and promote lateral root formation, suggesting that rib1 seedlings could have a heightened auxin response in the absence of exogenous application of IBA, or a change in the distribution or concentration of this auxin.
Table I.
Morphology of wild-type and mutant plants
| Characteristica | Wild Typeb | rib1b | No. of Seedlingsc | t test P Valued |
|---|---|---|---|---|
| Root length (mm) | 22.3 ± 0.6 | 18.7 ± 0.4 | 62–67 | 4.5 × 10−6 d |
| No. lateral roots | 21.0 ± 1.1 | 34.1 ± 1.7 | 74–76 | 2.6 × 10−9 d |
| Plant height (cm) | 23.3 ± 0.7 | 22.2 ± 0.7 | 18–19 | 0.31 |
| No. lateral branches | 3.8 ± 0.2 | 3.5 ± 0.2 | 18–19 | 0.22 |
Root lengths were measured on 7-d-old seedlings, the number of lateral roots was counted on 14-d-old seedlings, and plant height and the number of lateral branches were determined on adult plants.
Values are averages (±se).
Number of seedlings represented by the average.
Marks a highly significant difference.
Soil-grown adult rib1 plants are visually indistinguishable from wild type. No obvious differences were observed in organ development, flowering time, or senescence (data not shown). We determined apical dominance quantitatively by counting the number of primary and secondary inflorescences, and measuring plant height. No differences are seen in these traits between rib1 and wild-type plants (Table I).
Hormone Response
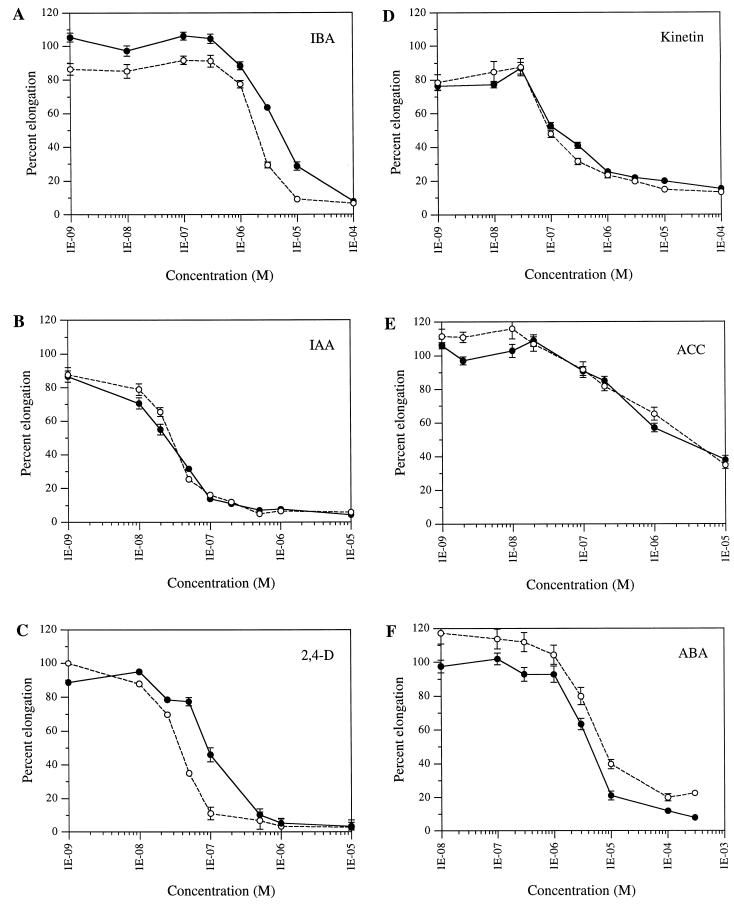
Because many gravitropic response mutants are also auxin resistant, we tested the resistance of rib1 to different auxins using root elongation assays. These assays are based on the fact that exogenous auxin application inhibits wild-type root elongation as a result of a supra-optimal concentration of this hormone. The results of the assays are presented in Figure 4. rib1 is consistently more resistant than wild type to all concentrations of IBA tested. t tests show that these differences are significant in all trials at concentrations that reduce rib1 root elongation by 15% to 90%. The IBA concentration that inhibits root elongation by 50% (IC50) is about 2.5-fold higher in rib1 compared with wild type. In contrast to its response to IBA rib1 exhibits a wild-type response to IAA. The trial depicted in Figure 3 indicates very slight differences between wild type and rib1 response for a few concentrations of IAA. Multiple trials have been done and these small differences are not reproducible. We, therefore, conclude that rib1 does not display an altered response to IAA. Thus, rib1 is able to discriminate between the two endogenous auxins of Arabidopsis, IAA and IBA. rib1 is also more resistant to the synthetic auxin 2,4-D. In the case of 2,4-D the difference in response between rib1 and wild type is significant at concentrations 5 × 10−8 to 10−6 M. rib1 has a wild-type response to NAA (data not shown), another synthetic auxin. This specificity of resistance makes rib1 unique in comparison with other well-characterized auxin-resistant mutants. aux1 and all the axr mutants exhibit resistance to both IAA and 2,4-D (Maher and Martindale, 1980; Estelle and Somerville, 1987; Wilson et al., 1990; Hobbie and Estelle, 1995; Leyser et al., 1996; Hobbie et al., 2000). We examined the IBA dose response of the auxin resistant mutants aux1-7, axr1-3, axr2-1, and axr4-2 and found that they are all IBA resistant (data not shown). Therefore, all these mutants are resistant to both endogenous auxins of Arabidopsis.
Figure 4.
Hormone response of rib1 and wild-type seedlings. Dose-response curves of wild-type (white symbols) and rib1 (black symbols) seedling root elongation on IBA, IAA, 2,4-D, kinetin, ACC, and ABA. Seedlings were grown for 5 d on GM media and then transferred to media containing the indicated amount of hormones or to control media without hormone. New root growth was measured 3 d later. Root elongation is expressed as a percentage of root growth on no hormone for each genotype. Each data point represents the average of 13 to 37 seedlings, and the error bars represent the se of the mean. Errors smaller than the data point symbols are not indicated.
The response of rib1 to growth inhibiting concentrations of other classes of plant hormones was also tested utilizing root elongation assays. rib1 is not more resistant than wild type to the cytokinins, kinetin and benzyladenine (data not shown), to 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor to ethylene, or to ABA (Fig. 4). The rib1 mutants show an increase in sensitivity to ABA. This difference was significant in all trials at concentrations that inhibit rib1 root elongation 10% or more. The results presented in Figure 4 show that rib1 is not resistant to any other class of plant hormones.
Resistance to Auxin Transport Inhibitors
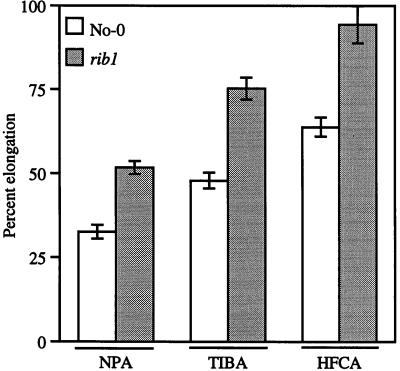
The resistance of rib1 mutants to different auxin transport inhibitors was tested. These compounds all act to block auxin efflux from the cell (Lomax et al., 1995). Figure 5 shows the results of root elongation assays in the presence of three different auxin transport inhibitors: naphthylphthalamic acid (NPA, a phytotropin), 2,3,5-triiodobenzoic acid (TIBA), and 9-hydroxyfluorene-9-carboxylic acid (HFCA, a morphactin). rib1 seedlings are resistant to all three compounds. t tests show that this difference is highly significant. These results are consistent with the fact that auxin transport is thought to be important for lateral root initiation, root elongation, and root gravitropism (Muday and Haworth, 1994; Lomax et al., 1995).
Figure 5.
Response to auxin transport inhibitors. Seedlings were grown for 3 d on GM and then transferred to media containing the indicated amount of inhibitors or to control media without inhibitor. New root growth was measured 5 d later. Elongation of roots on 10−5 M NPA, TIBA, or HFCA is expressed as a percentage of root growth on media containing no inhibitor for both wild type (white bars) and rib1 (shaded bars). Each data point represents the average of 12 to 29 seedlings and the error bars represent the se of the mean.
Root Bending Assay
The auxin resistance profile of rib1 mutants shows a striking similarity to findings by Delbarre et al. (1996). These researchers examined the specificity of efflux carriers for different auxins in tobacco suspension cells and suggested that 2,4-D does not utilize the same efflux carrier as do IAA and NAA. Another physiological assay in Arabidopsis roots was recently developed that shows the same auxin specificity (Utsuno et al., 1998). The findings in both studies support the notion that IAA and NAA exit the cell via a specific efflux carrier. Neither group included IBA in their study. We exploited the physiological assay to investigate the role of rib1 in auxin transport in roots.
The physiological assay as described by Utsuno et al. (1998) uses a clearly visible phenotype (root bending) to study the ability of root cells to efflux auxins. This assay can distinguish wild-type seedlings from agr1 mutant by their different responses to exogenous application of auxin on one side of the root. AGR1 is expressed in a specific subset of cortical and epidermal cells in the meristematic and elongation zone and is thought to be an efflux carrier important for auxin redistribution in the root. In agr1, auxin enters root cells normally, but then accumulates to high levels because of the inability to exit in the absence of a functional efflux carrier. Because the application of auxin in this assay is unilateral, auxin accumulates to inhibitory concentrations on only one side of the root. This results in the root bending phenotype. IAA and NAA elicit the bending response in agr1, whereas 2,4-D does not. This implies that 2,4-D efflux is not dependent on the AGR1-encoded carrier.
We have performed the bending assay using the agr1 allele, eir1-1, and have obtained the same results reported by Utsuno et al. (1998) for the auxins IAA, NAA, and 2,4-D (Table II). The majority of eir1 seedlings bend their roots into the medium when transferred to plates containing IAA or NAA; 2,4-D or media lacking hormone does not elicit a bending response. Neither wild type nor aux1 individuals consistently undergo root bending in response to any of the tested auxins. rib1 seedlings respond like wild type in this assay on all tested auxins (Table II). A wild-type response on NAA and IAA suggests that rib1 is not affected in the same efflux pathway as is eir1. Likewise, a wild-type response on IBA and 2,4-D suggests that rib1 is not altering IBA or 2,4-D efflux in the root epidermal and cortical cells in which AGR1 mediates IAA redistribution. However, this does not preclude a role of RIB1 for IBA transport in other root cell types.
Table II.
Root-bending assay results
| Genotype | No Auxin | IAA | NAA | 2,4-D | IBA |
|---|---|---|---|---|---|
| eir1-1a | 0 /21 | 16 /22 | 21 /22 | 0 /21 | 0 /25 |
| WT (Col-O) | 0 /24 | 6 /20 | 0 /22 | 0 /24 | 0 /28 |
| aux1-7a | 0 /20 | 1 /19 | 0 /20 | 0 /16 | 0 /21 |
| WT (No-O) | 0 /18 | 6 /21 | 0 /20 | 0 /17 | 0 /24 |
| rib1 | 0 /22 | 4 /23 | 0 /23 | 0 /23 | 0 /26 |
Data expressed as no. of turned roots/total no. of roots.
eir1-1 and aux1-7 are in the Columbia (Col-0) background.
It is interesting that IBA fails to elicit root bending in eir1 seedlings; this suggests that IBA, like 2,4-D, does not use the EIR1-encoded efflux carrier. Results of the root-bending assay mirror the rib1 resistance pattern: rib1 is resistant to IBA and 2,4-D, but not to IAA or NAA, and eir1 roots bend in response to IAA and NAA, but not to IBA or 2,4-D. These results support the idea that 2,4-D and IBA could behave similarly in terms of transport, in a manner that is distinct from the transport of IAA and NAA. Perhaps another member of the EIR1 family functions as an efflux carrier specific for IBA and 2,4-D transport.
DISCUSSION
We have isolated and characterized a novel Arabidopsis mutant that exhibits resistance to the natural auxin IBA and to the synthetic auxin 2,4-D, but not to IAA or NAA. This specificity of auxin response is unique among published auxin resistant mutants; it demonstrates that rib1 can discriminate between the two known endogenous auxins of Arabidopsis. Although still considered to be a synthetic auxin by many, IBA was first identified in potato in the 1950s (Blommaert, 1954). In the last decade investigators have shown that IBA is also present in Arabidopsis and many other species at physiologically relevant concentrations. Despite this, most studies of auxins have focused on IAA as the only natural auxin in plants. The isolation and characterization of the rib1 mutant provides a genetic tool that can be used to dissect the in vivo role of IBA in plants.
Conversion of IBA to IAA has been shown to occur in many different species (Epstein and Ludwig-Müller, 1993). A mutant defective in this process would be resistant to IBA if IBA response is mediated through the conversion of IBA to IAA. Although this could be the case for the rib1 mutant, this model cannot account for the 2,4-D resistance displayed by rib1. 2,4-D is an active auxin that does not require modification for activity. In addition, rib1 seedlings have alterations in root growth, which are observed in the absence of IBA treatment. These phenotypes suggest an elevated auxin response and are inconsistent with the reduced production of IAA from IBA predicted by a defect in conversion. rib1 does not appear to be defective in β-oxidation, which is the probable mechanism by which IBA is converted to IAA (Normanly et al., 1995). β-oxidation is an enzymatic process that results in shortening of carbon chains by two carbons at a time. Because β-oxidation is also required to mobilize fatty acid reserves during seed germination, mutants defective in β-oxidation grow much more poorly on media lacking Suc (Hayashi et al., 1998; Richmond and Bleecker, 1999). rib1 has no requirement for Suc to promote germination and growth of seedlings in the light or in the dark (data not shown). We conclude from these data that the rib1 defect does not lie in conversion of IBA to IAA. Rather, it is likely that the rib1 mutant phenotypes reflect a change in the ability of seedlings to respond specifically to IBA.
In addition to being resistant to IBA, rib1 also shows resistance to all auxin transport inhibitors tested by root elongation assays: NPA, TIBA, and HFCA. These compounds act principally by blocking carrier-mediated auxin efflux from cells. This inhibition of efflux results in auxin levels that are supra-optimal for root elongation (Muday and Haworth, 1994). For this reason many auxin response mutants are also resistant to auxin transport inhibitors (Simmons et al., 1995a; Fujita and Syono, 1996; Ruegger et al., 1997). In a similar manner, rib1 resistance to auxin transport inhibitors is likely to be a secondary consequence of IBA resistance. Early experiments by Leopold and Lam (1961) report that IBA transport in stem segments of sunflower was inhibited by TIBA. Our results support the idea that other IAA transport inhibitors also inhibit IBA transport, resulting in IBA accumulation in cells.
Hormone resistance of rib1 is limited to specific auxins. The response of rib1 to cytokinins and ethylene is similar to the wild-type response. However, rib1 displays a slight increase in sensitivity to ABA compared with wild type. Interaction between different classes of plant hormones is a theme often encountered in plant physiology (Davies, 1995). Ludwig-Müller et al. (1995b) report that either ABA or water stress induces IBA synthetase activity and accumulation of free IBA in maize. The authors propose that the increased IBA levels could mediate the changes in root growth that allow plants to adapt to water stress conditions. It is, therefore, likely that crosstalk between IBA and ABA signaling accounts for the higher ABA sensitivity of rib1.
In addition to its altered response to specific hormones and to auxin transport inhibitors, rib1 also shows other phenotypes in roots; it has a shorter primary root, more lateral roots, and a highly variable response to a change in the direction of the gravitational vector. The aerial portion of adult rib1 plants is indistinguishable from wild type. The phenotypic differences between rib1 and wild type are most consistent with a primary defect in auxin response or transport in the root.
The rib1 mutation could affect some component of IBA perception or signal transduction in the roots. rib1 mutant phenotypes are consistent with a heightened response to endogenous IBA levels. An increase in lateral root number and a decrease in primary root length can be phenocopied by IBA application to wild-type seedlings (data not shown; Fig. 4). This model can also explain the IBA resistance phenotype of rib1; rib1 roots are shorter than wild type because of an increased response to endogenous IBA, and a higher level of applied IBA is therefore required for further inhibition. Similar hypotheses have been proposed to explain auxin resistance caused by semi-dominant mutations in AXR3/IAA17 and SHY2/IAA3, two members of the Aux/IAA family of auxin inducible genes (Leyser et al., 1996; Rouse et al., 1998; Tian and Reed, 1999). Both mutants have a shorter primary root in the absence of auxin and are resistant to further root elongation inhibition by auxin. By analogy to the shy2 and axr3 mutants, rib1 could be a gain-of-function mutation in an IBA-inducible gene. We predict that such a gene would be induced by IBA, but not by IAA, treatment. To date, no studies have been done to identify genes specifically induced by IBA. Another possibility is that rib1 could affect IBA perception or another step in IBA signal transduction. This putative IBA signaling pathway would contribute to the regulation of lateral root formation, root elongation, and root gravitropism, phenotypes that are affected in the rib1 mutant.
Another explanation for the rib1 root phenotypes is that this mutation causes an increase in endogenous IBA levels. Although a formal possibility, the phenotypes of rib1 are not fully consistent with this model. The dominant Trp biosynthetic mutant amt-1/trp5-1 accumulates more Trp (Kreps and Town, 1992), and has elevated levels of free IBA (3.5-fold), conjugated IBA (2.5-fold), and conjugated IAA (3.3-fold; Ludwig-Müller et al., 1993). Like rib1, the adult growth pattern of this mutant is reported to be the same as wild type (Kreps and Town, 1992). However, trp5-1 does not show increased resistance to IBA (data not shown). The root gravitropic defect is also not consistent with an elevated level of auxin; the sur1 mutant has elevated levels of IAA, but displays no defect in root gravitropism (Boerjan et al., 1995). On the other hand a defect in the control of IBA levels in specific cells or tissues, as opposed to an overall increase in auxin levels as in trp5 and sur1, could result in the rib1 phenotypes, including altered root gravitropism. Changes in IBA biosynthesis, degradation, conjugation, or subcellular compartmentalization could result in localized elevations of IBA levels. Changes in IBA transport could also have the same result.
There is growing evidence that mutations affecting IAA transport have differential responses to various auxins. For example, the eir1 efflux mutant behaves differently in root bending assays in response to the auxins IAA and NAA compared with 2,4-D. Likewise, the IAA influx mutant, aux1, also displays specificity in response to auxins; it is resistant to IAA and 2,4-D, but not to NAA (Yamamoto and Yamamoto, 1998; Marchant et al., 1999). Another example is the pis1 mutant, a putative regulator of auxin transport, which is specifically hypersensitive to 2,4-D, but not to IAA or NAA (Fujita and Syono, 1997). rib1, like eir1, aux1, and pis1, has a specific auxin response profile. It is therefore tempting to speculate that rib1 affects polar auxin transport. In addition, polar auxin transport has been shown to be important for lateral root formation, root gravitropic response, and root elongation, which are processes affected in rib1 (Lomax et al., 1995).
At this time, we cannot define at what step in IBA transport RIB1 might function. A semi-dominant phenotype is consistent with defects in a regulatory or a structural component of the transport machinery. We are investigating this model further by constructing double mutant lines between rib1 and the known auxin carrier mutants aux1 and eir1, and by direct assessment of auxin transport in rib1.
The recent discovery of multiple homologs of genes encoding auxin efflux and influx carriers suggests that many such carriers exist in plants, each potentially having its own specificity in terms of expression pattern and function (Bennett et al., 1996; Gälweiler et al., 1998). Our root bending assays show that the EIR1 carrier appears specific for IAA and NAA transport and is not required for IBA and 2,4-D transport. This is also consistent with the results of Delbarre et al. (1996), which show that 2,4-D does not use the IAA/NAA-specific efflux carrier expressed in tobacco cell cultures. We suggest that efflux carriers also exist for IBA transport. The differential expression of this hypothetical IBA carrier, in conjunction with that of the IAA carriers, would provide exquisite control over the levels of both endogenous auxins throughout the plant.
This hypothesis might appear to contradict another conclusion of Delbarre et al. (1996), who suggested that 2,4-D efflux would be mostly carrier independent. However, carrier independent efflux is inconsistent with the fact that 2,4-D undergoes polar transport, since it is the basal localization of the efflux carrier that determines the polarity of transport. To explain the results of Delbarre et al., we suggest that the hypothetical IBA/2,4-D efflux carrier is not present in tobacco cell cultures such as those used in their study. However, the carrier would be expressed in specific, differentiated tissues such as the root.
We have isolated other IBA resistant mutants that are currently being characterized (J. Poupart and C.S. Waddell, unpublished data). Other researchers have also recently isolated IBA-resistant mutants (Zolman et al., 2000). Among these, two mutants were recovered that like rib1, are resistant to IBA and 2,4-D, but not to IAA. These mutants map to separate loci from rib1. Therefore, the specificity of auxin resistance demonstrated by these mutants may represent a new class of auxin-resistant mutants. The small number of these mutants recovered to date, together with the fact that no allelic mutations have been recovered, indicate that the genome has not yet been saturated for this class of mutants. Characterization of new IBA-specific mutants will allow us to further understand the activity of this endogenous auxin in plant development. The phenotypes displayed by rib1 imply an in vivo role of IBA in roots and seedlings. Phenotypic analysis of new alleles of rib1, as well as cloning and sequencing of the gene, should provide insights into the function of the RIB1 protein in the plant.
MATERIALS AND METHODS
rib1 is in the No-0 ecotype of Arabidopsis. All phenotypic characterization of the mutant was done with rib1 homozygotes derived from the selfed progeny of the original rib1 isolate. Other mutants used in this paper were obtained from the Arabidopsis Biological Resource Center at Ohio State University.
Plant Growth Conditions
Seeds were surface sterilized in a solution of 1.5% (w/v) sodium hypochlorite and 0.02% (w/v) SDS for 5 min, and rinsed four or five times with sterile water. Seeds were stratified 4 to 7 d in the dark at 4°C before being germinated on Petri dishes containing germination medium (GM) containing 0.7% (w/v) Difco agar. GM consists of 1× Murashige and Skoog basal salts, 1% (w/v) Suc, 0.5g/L MES [2-(N-morpholino)ethanesulfonic acid], 1 mg/L thiamine, 0.5 mg/L pyridoxin, 0.5 mg/L nicotinic acid, and 100 mg/L myo-inositol, with pH adjusted to 5.7 with 1 n KOH (Valvekens et al., 1988). Plated seeds were placed in a growth chamber under the following conditions, unless otherwise stated: 24°C, with a 16-h light cycle at a light intensity of 80 to 90 μmol m−2 s−1.
For determination of adult phenotypes, crosses, and seed production, 7- to 10-d-old seedlings were transferred from GM plates to pots containing a 1:1:1 mixture of perlite:vermiculite: Sunshine Mix # 1 (Sun Gro Horticulture Inc., Bellevue, WA). Plants were grown at 24°C under continuous white fluorescent light, and were fertilized twice during their growth period with 0.25× Hoagland solution. Light intensity was approximately 90 μmol m−2 s−1.
Isolation of the rib1 Mutant
The Ac/Ds transposon-tagging system described in Honma et al. (1993) was used to generate a population of 1,419 lines containing independent Ds excision events (C.S. Waddell, unpublished data). The selfed progeny of these lines was germinated and grown on vertically oriented GM plates containing 1.2% (w/v) Noble agar (Sigma, St. Louis). After 4 d, the plates were rotated 90° on edge. Twenty-four hours later, the plates were scored visually for defects in downward reorientation of the roots. rib1 was isolated because its root failed to reorient downward (see Fig. 1). Selection on chlorsulfuron demonstrated the absence of the csr1-1 transgene, the selectable marker present in the Ds element. Southern analysis with Ds-specific probes confirmed that rib1 mutants do not contain a reintegrated Ds element (data not shown).
Genetic Characterization and Mapping of rib1
rib1 homozygotes were crossed to wild-type No-0 plants to determine the genetic basis of the rib1 mutation. The resulting F1 populations were plated on vertically oriented GM plates containing 0.8% (w/v) Difco agar and 6 × 10−8 M 2,4-D, and scored for resistance by measuring root elongation after 7 d of growth. Wild-type and homozygous mutant seedlings were always included on the same plate with the F1 population being analyzed. Reciprocal crosses were done with rib1 as the female (three crosses) or the male (six crosses) parent, and all crosses gave the same results; data is presented from the F1 population of a single cross in which rib1 was the male parent.
F2 mapping populations were generated by crossing rib1 homozygotes to Columbia wild-type plants. Linkage was examined in 2,4-D-resistant and 2,4-D-sensitive individuals. The genotypes of F2 individuals were verified by generating a selfed F3 population for each individual and scoring the 2,4-D resistance of the F3 population. DNA was isolated from pools of 50 F3 seedlings using the protocol of Dellaporta et al. (1983). This DNA was used for PCR reactions to determine linkage of rib1 to CAPS and SSLP markers covering the Arabidopsis genome. Primers were obtained from Biocorp Inc. (Montreal) or from Research Genetics (Huntsville, AL). Standard SSLP and CAPS PCR conditions were used (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). Map positions of the markers were taken from the Lister and Dean RI map (http://nasc.nott.ac.uk/new_ri_map.html).
Gravity and Slanting Response
Seeds were exposed to white light for 1 to 2 h to induce germination and were grown for 4 d in the dark on vertically oriented plates on GM containing 1.2% (w/v) Noble agar (Sigma). One plate was scored to determine the root angle at time 0 h. The other plates were rotated on edge 90° clockwise (when seedlings are viewed through the lid). Individual plates were removed after 2, 4, 6, 8, 10, 12, or 24 h, digitally scanned, and the angle of each root tip from vertical was measured using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the internet at http://rsb.info.nih.gov/nih-image/). No-0 and rib1 seedlings were plated together to ensure that they were exposed to the same gravity conditions. Similar results were obtained in two separate trials in the dark (data presented), and in three separate trials in the light (data not shown).
Root slanting was measured as the angle from vertical of roots grown for 4 d on vertically oriented GM plates containing 1.2% (w/v) Noble agar under standard growth chamber conditions. Slanting was scored by viewing the seedlings through the lids of the plates.
Starch Determination
Starch accumulation in root tips was examined using the protocol described by Bullen et al. (1990). Iodine and potassium iodide were obtained from Fisher (Nepean, ON). The starch mutants pgm-1 and adg1-1 were used as negative controls. Wild-type and rib1 seedlings had clearly visible starch granules in their root tips, whereas none were detectable in pgm-1 and adg1-1 root tips.
Hormone and Inhibitor Response
All hormones and inhibitors were purchased from Sigma with the exception of NPA, which was purchased from Chem Service (West Chester, PA). MilliQ water, 1 n NaOH, absolute ethanol, or dimethylsulfoxide were used to make the appropriate stocks of these compounds. ABA, IAA, IBA, and NAA were dissolved in 1 n NaOH and diluted in water to a final stock concentration of 1 to 2 mg/mL. A 1-mg/mL stock of ACC was made in water. All stocks diluted in water were filter-sterilized. 2,4-D, HFCA, and TIBA stocks were made in ethanol at concentrations of 2, 10, and 0.5 mg/mL, respectively. A 20-mg/mL NPA stock was made in dimethylsulfoxide. Solvent-only controls were included for the NPA, HFCA, and TIBA assays. Under our conditions we found that the addition of ethanol up to a concentration of 0.25% did not affect root elongation. Appropriate amounts of the sterile stocks were added to media after autoclaving to obtain the different concentrations required.
Root elongation assays for hormone response were performed as described in Wilson et al. (1990) and for auxin transport inhibitor response as described in Ruegger et al. (1997). In both cases GM containing 0.8% (w/v) Difco agar was used instead of solidified nutrient solution. IAA and IBA response assays were done under yellow long-pass filters (Acrylic yellow-2208, 3.18 mm thick, Commercial Plastics, Montreal) to prevent photodegradation of the auxins (Stasinopoulos and Hangarter, 1990). Wild-type and rib1 seedlings were transferred to each of the two halves of the same plate to ensure that they were being exposed to exactly the same conditions. Data are expressed as the percentage of root growth on no hormone or on solvent-only control plates. Similar results were obtained in two to five separate trials. Data from a single representative trial are presented for hormone resistance. A single data point from a representative trial is presented for auxin transport inhibitor resistance.
Root-Bending Assays
Root-bending assays were performed as described in Utsuno et al. (1998) except that GM with 1.5% (w/v) Noble agar was used instead of solidified nutrient solution. In brief, mutant and wild-type seedlings were germinated on vertically oriented media containing no hormone for 3 d. Mutant and wild-type seedlings were then transferred to vertical plates containing different auxins: 3.3 × 10−8 M IAA, 1 × 10−8 M 2,4-D, 4 × 10−6 M IBA, and 6.6 × 10−7 M NAA or to control plates containing no hormone. All plates were then placed under yellow long-pass filters and the root response was scored 2 d later. Concentrations of IAA, 2,4-D, and NAA are those reported by Utsuno et al. (1998). We tested three different concentrations of IBA (1 × 10−6 M, 2 × 10−6 M, and 4 × 10−6 M) that were physiologically similar to the concentrations of IAA used; i.e. these concentrations inhibited roots to about the same level in root elongation assays. All three concentrations gave similar results, and we therefore chose to use only the highest concentration for repeat experiments. Mutants and their wild-type parental lines were placed on the same plates to ensure that they were exposed to exactly the same concentration of hormone and conditions. The responses of roots to the auxins are either continued growth along the surface of the plate or a sharp 90° turn into the media. This bending phenotype is easily scorable, and the sharp angle of the turn makes it distinct from a normal growth pattern in which a root might penetrate the test medium.
Root Length Determination
Root length was determined on 7-d-old seedlings grown under our standard conditions. Roots were measured by tracing magnified seedlings using an overhead projector. A transparent ruler placed beside the roots was also traced for use as a scale bar. The tracings were then digitally scanned and measured using the NIH Image program.
ACKNOWLEDGMENTS
The technical assistance of Sharon Chen in the initial stages of this work is gratefully acknowledged. We wish to thank Marc Trudel for help with statistical analysis, Dr. G.K. Muday, A.V. Babwah, and A.J. Windsor for helpful comments on the manuscript, and Dr. B. Bartel for communicating results prior to publication. Mutant seeds stocks were obtained from the Arabidopsis Biological Resource Center at Ohio State University.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant to C.S.W.). J.P. was funded by postgraduate scholarships from the Fonds pour la Formation des Chercheurs et l'Aide à la Recherche and the Natural Sciences and Engineering Research Council of Canada.
LITERATURE CITED
- Abel S, Oeller PW, Theologis A. Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA. 1994;91:326–330. doi: 10.1073/pnas.91.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Blommaert KLJ. Growth and inhibiting substances in relation to the rest period of the potato tuber. Nature. 1954;174:970–972. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen BL, Best TR, Gregg MM, Barsel SE, Poff KL. A direct screening procedure for gravitropic mutants in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1990;93:525–531. doi: 10.1104/pp.93.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence and functions. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–12. [Google Scholar]
- Delbarre A, Müller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Epstein E, Ludwig-Müller J. Indole-3-butyric acid in plants: occurrence, synthesis, metabolism and transport. Physiol Plant. 1993;88:382–389. [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Fujita H, Syono K. Genetic analysis of the effects of polar transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol. 1996;37:1094–1101. doi: 10.1093/oxfordjournals.pcp.a029059. [DOI] [PubMed] [Google Scholar]
- Fujita H, Syono K. PIS1, a negative regulator of the action of auxin transport inhibitors in Arabidopsis thaliana. Plant J. 1997;12:583–595. doi: 10.1046/j.1365-313x.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Hartmann HT, Kester DE, Davies FT, Jr, Geneve RL. Plant Propagation: Principles and Practices. Ed 6. Upper Saddle River, NJ: Prentice Hall; 1997. The biology of propagation by cuttings; pp. 276–328. [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell. 1998;10:183–195. doi: 10.1105/tpc.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M. The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development. 2000;127:22–32. doi: 10.1242/dev.127.1.23. [DOI] [PubMed] [Google Scholar]
- Hobbie LJ. Auxin: molecular genetic approaches in Arabidopsis. Plant Physiol Biochem. 1998;36:91–102. [Google Scholar]
- Honma MA, Baker BJ, Waddell CS. High-frequency germinal transposition of DsALS in Arabidopsis. Proc Natl Acad Sci USA. 1993;90:6242–6246. doi: 10.1073/pnas.90.13.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux-IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using codominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Town CD. Isolation and characterization of a mutant of Arabidopsis resistant to α-methyltryptophan. Plant Physiol. 1992;99:269–275. doi: 10.1104/pp.99.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A, Lam S. Polar transport of three auxins. In: Klein RM, editor. Plant Growth Regulation: Fourth International Conference on Plant Growth Regulation. Ames: The Iowa University Press; 1961. pp. 411–418. [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Ludwig-Müller J, Raisig A, Hilgenberg W. Uptake and transport of indole-3-butyric acid in Arabidopsis thaliana: comparison with other natural and synthetic auxins. J Plant Physiol. 1995a;147:351–354. [Google Scholar]
- Ludwig-Müller J, Sass S, Sutter EG, Wodner M, Epstein E. Indole-3-butyric acid in Arabidopsis thaliana: I. Identification and quantification. Plant Growth Regul. 1993;13:179–187. [Google Scholar]
- Ludwig-Müller J, Schubert B, Pieper K. Regulation of IBA synthetase from maize (Zea mays L.) by drought stress and ABA. J Exp Bot. 1995b;46:423–432. [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EP, Martindale SJB. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Müller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Haworth P. Tomato root growth, gravitropism and lateral development: correlation with auxin transport. Plant Physiol Biochem. 1994;33:193–203. [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML. Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol. 1998;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPin2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A-C, Jacobs FA, Eliasson L. Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol. 1991;96:856–861. doi: 10.1104/pp.96.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Slovin JP, Cohen JD. Rethinking auxin biosynthesis and metabolism. Plant Physiol. 1995;107:323–329. doi: 10.1104/pp.107.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pythoud F, Buchala AJ. The fate of vitamin D3 and indolylbutyric acid applied to cuttings of Populus tremula L. during adventitious root formation. Plant Cell Environ. 1989;12:489–494. [Google Scholar]
- Richmond T, Bleecker A. A defect in beta-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell. 1999;11:1911–1923. doi: 10.1105/tpc.11.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. Changes in auxin response from mutations in an AUX/IAA gene. Science. 1998;279:1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday GK, Estelle M. Reduced naphtylphtalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R, Masson PH. Arabidopsis thaliana sku mutant seedlings show exaggerated surface dependent alteration in root growth vector. Plant Physiol. 1996;111:987–998. doi: 10.1104/pp.111.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Migliaccio F, Masson P, Caspar T, Söll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995a;93:790–798. [PubMed] [Google Scholar]
- Simmons C, Söll D, Migliaccio F. Circumnutation and gravitropism cause root waving in Arabidopsis thaliana. J Exp Bot. 1995b;46:143–150. [Google Scholar]
- Stasinopoulos TC, Hangarter RP. Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol. 1990;93:1365–1369. doi: 10.1104/pp.93.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter EG, Cohen JD. Measurement of indole-3-butyric acid in plant tissues by isotope dilution gas chromatography-mass spectrometry analysis. Plant Physiol. 1992;99:1719–1722. doi: 10.1104/pp.99.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Utsuno K, Shikanai T, Yamada Y, Hashimoto T. AGR, an agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol. 1998;39:1111–1118. doi: 10.1093/oxfordjournals.pcp.a029310. [DOI] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW, White R. Experiments on the transport of auxin. Bot Gaz. 1938;100:465–484. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto KT. Differential effects of 1-naphtaleneacetic acid, indole-3-acetic acid and 2, 4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;39:660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- Yang T, Davies PJ. Promotion of stem elongation by indole-3-butyric acid in intact plants of Pisum sativum L. Plant Growth Regul. 1999;27:157–160. [Google Scholar]
- Zimmerman PW, Wilcoxon F. Several chemical growth substances which cause initiation of roots and other responses in plants. Contrib Boyce Thompson Inst. 1935;7:209–228. [Google Scholar]
- Zolman BK, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156 [DOI] [PMC free article] [PubMed]