Abstract
BACKGROUND
The efficacy of vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix has been questioned after publication of the OPPTIMUM study.
OBJECTIVE
To determine whether vaginal progesterone prevents preterm birth and improves perinatal outcomes in asymptomatic women with a singleton gestation and a midtrimester sonographic short cervix.
DATA SOURCES
MEDLINE, EMBASE, LILACS, and CINAHL (from their inception to September 2017), Cochrane databases, bibliographies, and conference proceedings.
STUDY ELIGIBILITY CRITERIA
Randomized controlled trials comparing vaginal progesterone with placebo/no treatment in women with a singleton gestation and a midtrimester sonographic cervical length ≤25 mm.
STUDY APPRAISAL AND SYNTHESIS METHODS
Systematic review and meta-analysis of individual patient data. The primary outcome was preterm birth <33 weeks of gestation. Secondary outcomes included adverse perinatal outcomes and neurodevelopmental and health outcomes at 2 years of age. Individual patient data were analyzed using a two-stage approach. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated. Quality of evidence was assessed using the GRADE methodology.
RESULTS
Data were available from 974 women (498 assigned to vaginal progesterone, 476 assigned to placebo) with a cervical length ≤25 mm participating in five high-quality trials. Vaginal progesterone was associated with a significant reduction in the risk of preterm birth <33 weeks of gestation (RR 0.62, 95% CI 0.47-0.81, P=0.0006; high-quality evidence). Moreover, vaginal progesterone significantly decreased the risk of preterm birth <36, <35, <34, <32, <30 and <28 weeks of gestation, spontaneous preterm birth <33 and <34 weeks of gestation, respiratory distress syndrome, composite neonatal morbidity and mortality, birthweight <1500 and <2500 g, and admission to the neonatal intensive care unit (RRs from 0.47 to 0.82; high-quality evidence for all). There were seven (1.4%) neonatal deaths in the vaginal progesterone group and 15 (3.2%) in the placebo group (RR 0.44, 95% CI 0.18-1.07, P=0.07; low-quality evidence). Maternal adverse events, congenital anomalies, and adverse neurodevelopmental and health outcomes at 2 years of age did not differ between groups.
CONCLUSIONS
Vaginal progesterone decreases the risk of preterm birth and improves perinatal outcomes in singleton gestations with a midtrimester sonographic short cervix, without any demonstrable deleterious effects on childhood neurodevelopment.
Keywords: prematurity, preterm delivery, progestins, progestogens, transvaginal ultrasound, cervical length
INTRODUCTION
Every year, an estimated 15 million babies are born preterm worldwide with rates ranging from 5% in several European countries to 18% in some African countries.1 In 2015, the preterm birth rate in the United States, which had declined over 2007-2014, increased slightly to 9.63%.2 Globally, preterm birth complications are the leading cause of child mortality, responsible for nearly 1 million deaths in 2013.3 In addition, surviving preterm babies are at greater risk for short-term health complications including acute respiratory, gastrointestinal, infectious, central nervous system, hearing, and vision problems, and long- term neurodevelopmental disabilities such as cerebral palsy, impaired learning and visual disorders, as well as chronic diseases in adulthood.4-8
Preterm parturition is a syndrome caused by multiple etiological factors such as intraamniotic infection, extrauterine infections, vascular disorders, decidual senescence, disruption of maternal-fetal tolerance, a decline in progesterone action, uterine overdistension, cervical disease, or maternal stress.9-11 A short cervix, conventionally defined as a transvaginal sonographic cervical length ≤25 mm in the midtrimester of pregnancy, is a powerful risk factor for spontaneous preterm birth and has a high predictive accuracy for spontaneous preterm birth <34 weeks of gestation, and a moderate to low predictive accuracy for spontaneous preterm birth <37 weeks of gestation in both singleton and twin gestations.12-48
In 2012, a systematic review and meta-analysis of individual patient data (IPD) from randomized controlled trials comparing vaginal progesterone with placebo in women with a singleton gestation and a cervical length ≤25 mm in the midtrimester49 reported that the administration of vaginal progesterone was associated with a significant reduction in the risk of preterm birth occurring from <28 weeks of gestation through <35 weeks of gestation. In addition, vaginal progesterone administration was associated with a reduction in the risk of admission to the neonatal intensive care unit (NICU), respiratory distress syndrome (RDS), composite neonatal morbidity and mortality, and birthweight <1500 g. Since the publication of that IPD meta-analysis, vaginal progesterone has been recommended for patients with a singleton gestation and a short cervix by the Society for Maternal-Fetal Medicine (SMFM),50 the American College of Obstetricians and Gynecologists (ACOG),51 the International Federation of Gynecology and Obstetrics (FIGO),52 and the National Institute for Health and Care Excellence (NICE)53, among others.
In 2016, the findings of the OPPTIMUM study were reported. This was a randomized controlled trial comparing vaginal progesterone versus placebo in women at risk of preterm birth because of previous spontaneous preterm birth <34 weeks of gestation, or a cervical length ≤25 mm, or because of a positive fetal fibronectin test combined with other clinical risk factors for preterm birth. 54 The results of that trial showed that vaginal progesterone did not significantly reduce the risk of preterm birth or perinatal morbidity and mortality in the entire population, or in the subgroup of women with a cervical length ≤25 mm. That report created confusion among clinicians and professional/scientific organizations regarding the clinical efficacy of vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix.55, 56 Therefore, we performed a meta-analysis of aggregate data that assessed the effect of vaginal progesterone on the risk of preterm birth ≤34 weeks or fetal death in women with a singleton gestation and a cervical length ≤25 mm, the only outcome measure for which the publication of the OPPTIMUM study reported complete data in this subpopulation of women.57 That meta-analysis showed that vaginal progesterone significantly reduced the risk of preterm birth ≤34 weeks or fetal death by 34%. Subsequently, the lead author of the OPPTIMUM study provided us the individual data for all women with a cervical length ≤25 mm that were included in that trial. Therefore, the objective of this systematic review and IPD meta-analysis was to assess the efficacy of vaginal progesterone in reducing the risk of preterm birth and adverse perinatal outcomes in asymptomatic women with a singleton gestation and a short cervix (cervical length ≤25 mm).
MATERIAL AND METHODS
The study was prospectively registered with the PROSPERO database of systematic reviews (number CRD42017057155) and reported in accordance with the PRISMA-IPD statement.58
Search strategy and selection criteria
We searched MEDLINE, EMBASE, LILACS, CINAHL, the Cochrane Central Register of Controlled Trials, and Research Registers of ongoing trials (all from inception to 30 September 2017), and Google Scholar using the keywords “progesterone” and “preterm birth” to identify all randomized controlled trials comparing vaginal progesterone (any dose) versus placebo/no treatment for the prevention of preterm birth and/or adverse perinatal outcomes in women with singleton gestations. No language restrictions were imposed. We also searched in proceedings of congresses/meetings on maternal-fetal medicine and bibliographies of the retrieved articles, and contacted investigators in the field to locate unpublished studies. Trials were eligible if the primary aim of the study was to prevent preterm birth in women with a “short cervix”, or to prevent preterm birth in women with risk factors other than short cervix but for whom outcomes were available in those with a pre-randomization cervical length ≤25 mm. Quasi-randomized trials, trials that assessed vaginal progesterone in women with threatened or arrested preterm labor, and trials in which vaginal progesterone was administered in the first trimester to prevent miscarriage were excluded from the review. Two authors (RR and AC-A) independently assessed all the potential studies identified in the literature search for eligibility. Disagreements about inclusion were resolved through discussion.
Data collection
The principal investigators of eligible trials were contacted and asked to share their data for this collaborative project. Authors were supplied with a data extraction sheet and requested to supply anonymized data about baseline characteristics, interventions and outcomes for each randomized patient in the trial. Data provided by the investigators were systematically checked for completeness, duplication, consistency, feasibility, and integrity of randomization. In addition, the results from the review’s analysis were cross-checked against the published reports of the trials. Authors were contacted for clarification where discrepancies existed and asked to supply missing data when necessary. Once queries had been resolved, clean data were uploaded to the main study database.
Outcome measures
As in the previous IPD meta-analysis,49 the primary outcome was preterm birth <33 weeks of gestation. Secondary outcomes were preterm birth <37, <36, <35, <34, <32, <30 and <28 weeks of gestation; spontaneous preterm birth <33 and <34 weeks of gestation; mean gestational age at delivery; RDS; necrotizing enterocolitis; intraventricular hemorrhage; proven neonatal sepsis; bronchopulmonary dysplasia; retinopathy of prematurity; fetal death; neonatal death; perinatal death, composite neonatal morbidity and mortality (RDS, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death); Apgar score <7 at 5 minutes; birthweight <1500 and <2500 g; admission to the NICU; use of mechanical ventilation; congenital anomaly, any adverse maternal event, and Bayley-III cognitive composite score, moderate or severe neurodevelopmental impairment, visual or hearing impairment, and disability in renal, gastrointestinal, or respiratory function at 2 years of age.
Risk of bias assessment
Assessments of risk of bias for included trials were done independently by two investigators (RR and AC-A) according to the seven domains outlined in the Cochrane Handbook for Systematic Reviews of Interventions (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias).59 This tool categorizes studies by low, unclear, or high risk of bias in each domain. When the information was not available in the published paper, the trial’s principal investigator was contacted to request clarification or additional information. We resolved any disagreement regarding the risk of bias assessment by consensus.
Data analysis
We analyzed all the data on an intention-to-treat basis. IPD were analyzed using a two-stage approach. In the first stage, estimates of effect were derived from the IPD for each trial, and in the second stage, these were combined using standard methods for meta-analyses of aggregate data.60 We calculated the pooled relative risk (RR) for dichotomous data and mean difference for continuous data with associated 95% confidence interval (CI). Heterogeneity of treatment effect was assessed with the I2 statistic.61 Results from individual studies were pooled using a fixed-effects model if substantial statistical heterogeneity was not present (I2 ≤30%). If I2 values were >30%, a random-effects model was used to pool data across studies, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. We calculated the number needed to treat (NNT) with 95% CI where meta-analysis of dichotomous outcomes revealed a statistically significant beneficial or harmful effect of vaginal progesterone.62
Prespecified subgroup analyses were carried out according to obstetrical history (no previous spontaneous preterm birth and at least one previous spontaneous preterm birth), cervical length (<10, 10-20, and 21-25 mm), maternal age (<20, 20-34, and ≥35 years), race/ethnicity (White, Black, Asian, and Other), body-mass index (<18.5, 18.5-24.9, 25.0-29.9, and ≥30 kg/m2), gestational age at treatment initiation (18-21 and 22-25 weeks), and daily dose of vaginal progesterone (90-100 and 200 mg). Moreover, we performed a post-hoc subgroup analysis according to country in which women were enrolled (United States vs. other countries). A test for interaction between intervention and patient or trial characteristics was calculated to examine whether intervention effects differ between subgroups.63-65 An interaction P value ≥ .05 was considered to indicate that the effect of intervention did not differ significantly between subgroups. We also planned to explore potential sources of heterogeneity and to assess publication and related biases if at least ten studies were included in a meta-analysis, but these analyses were not undertaken due to the limited number of trials included in the review. Subgroup analyses were only performed for the primary outcome of preterm birth <33 weeks of gestation. Prespecified sensitivity analyses to explore the impact of selection, performance and detection biases on results were not carried out because all trials were considered at low risk for these biases. Statistical analyses were performed using Review Manager (RevMan; version 5.3.5; The Nordic Cochrane Centre, Copenhagen, Denmark) and StatsDirect (version 3.0.198; StatsDirect Ltd, Cheshire, UK).
Quality of evidence
The quality of the body of evidence relating to primary and secondary outcomes was assessed using the GRADE approach.66 We used the GRADEpro Guideline Development Tool67 to import data from Review Manager in order to create ‘Summary of findings’ tables. The GRADE approach results in an assessment of the quality of evidence in four grades: (i) high: we are very confident that the true effect lies close to that of the estimate of the effect; (ii) moderate: we are moderately confident in the effect estimate, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; (iii) low: our confidence in the effect estimate is limited, the true effect may be substantially different from the estimate of the effect; and (iv) very low: we have very little confidence in the effect estimate, the true effect is likely to be substantially different from the estimate of effect. The evidence can be downgraded from ’high quality’ by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
This study was exempted from review by the Human Investigation Committee Administration Office of Wayne State University because all included studies were published previously and had each previously received local institutional review board approvals and consent from participants.
RESULTS
Selection, characteristics and risk of bias of studies
Literature searches identified 12 randomized controlled trials that compared vaginal progesterone vs. placebo54, 68-76 or no treatment77, 78 in singleton gestations with the aim of preventing preterm birth and/or adverse perinatal outcomes (Figure 1). Six studies that assessed vaginal progesterone in women at high risk for preterm birth were excluded for the following reasons: cervical length was not measured or data on cervical length were not collected before randomization,68, 73, 77, 78 and inclusion of 27 women with a short cervix (defined as a cervical length ≤28 mm) who underwent cervical cerclage before randomization.75 We requested IPD for women with a cervical length ≤25 mm before randomization from the principal investigators of the remaining six trials.54, 69-72, 74 Data from one trial, which compared vaginal progesterone versus placebo in women with a singleton gestation without previous spontaneous preterm birth and a cervical length ≤30 mm (n=80), could not be obtained.74 We estimated that this trial included approximately 35 patients with a cervical length ≤25 mm. IPD were obtained for 974 women with a cervical length ≤25 mm from five double-blind, placebo-controlled trials; 54, 69-72 498 women were assigned to vaginal progesterone and 476 to placebo. Baseline characteristics were largely balanced between the vaginal progesterone and placebo groups (Table 1).
Figure 1. Summary of evidence search and selection.
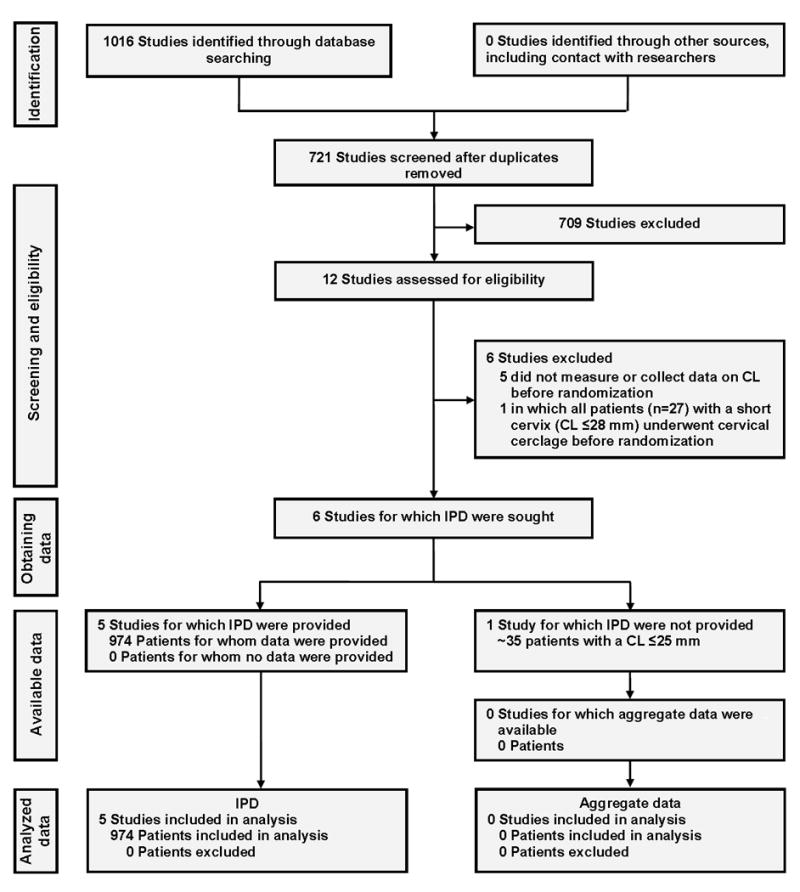
CL, cervical length; IPD, individual patient data
Table 1.
Baseline characteristics of pooled women
| Vaginal progesterone (n=498) | Placebo (n=476) | |
|---|---|---|
| Maternal age (years) | 28.0 (23.6-33.0) | 27.5 (23.5-32.8) |
| Body-mass index (kg/m2) | 24.8 (21.6-29.2)a | 24.8 (21.5-29.4)b |
| Race/ethnicity | ||
| White | 185 (37.2) | 189 (39.7) |
| Black | 181 (36.3) | 176 (37.0) |
| Asian | 100 (20.1) | 89 (18.7) |
| Other | 32 (6.4) | 22 (4.6) |
| Region of enrolment | ||
| Europe | 275 (55.2) | 252 (52.9) |
| North America | 115 (23.1) | 117 (24.6) |
| Asia | 80 (16.1) | 77 (16.2) |
| South America | 15 (3.0) | 17 (3.6) |
| Africa | 13 (2.6) | 13 (2.7) |
| Obstetrical history | ||
| Nulliparous | 225 (45.2) | 215 (45.2) |
| Parous with no previous spontaneous preterm birth | 126 (25.3) | 120 (25.2) |
| Parous with ≥1 previous spontaneous preterm birth | 147 (29.5) | 141 (29.6) |
| Cervical length at randomization | ||
| <10 mm | 48 (9.6) | 57 (12.0) |
| 10-20 mm | 379 (76.1) | 362 (76.0) |
| 21-25 mm | 71 (14.3) | 57 (12.0) |
| Gestational age at randomization (weeks) | 22.6 (21.4-23.6) | 22.6 (21.4-23.4) |
Data are median (interquartile range) or n (%).
n=491
n=470
The main characteristics of the five studies included in the systematic review are depicted in Table 2. Two trials were specifically designed to evaluate the use of vaginal progesterone in women with a short cervix (cervical length ≤15 mm69 and cervical length between 10 and 20 mm72), one tested the effect of vaginal progesterone in women at risk for preterm birth because of previous spontaneous preterm birth, or a sonographic cervical length ≤25 mm, or a positive fetal fibronectin test combined with other clinical risk factors for preterm birth,54 another evaluated the use of vaginal progesterone in women with a history of spontaneous preterm birth,70 and the remaining trial examined the use of vaginal progesterone in women with a previous spontaneous preterm birth, uterine malformations, or twin gestations.71 Three studies54, 69, 72 provided 96% of the total sample size of the IPD meta-analysis. The daily dose of vaginal progesterone used in the trials varied from 90-200 mg and the treatment was administered from 18-25 to 34-36 weeks of gestation. An adequate compliance or adherence to treatment (≥80% of prescribed medication) was reported in >90% of patients participating in four trials. 69-72 In the trial by Norman et al,54 only 66% of patients with a CL ≤25 mm had a compliance ≥80%. Four studies69-72 were considered to be at low risk of selection, performance, detection, attrition and reporting biases (Figure 2). One study54 was considered to be at high risk of attrition bias for the childhood primary outcome because information on the Bayley-III cognitive composite score at two years of age was available for ~70% of surviving children. Moreover, this study was at high risk of compliance bias, which can affect the trial’s statistical power to detect the effects of the intervention.79
Table 2.
Studies included in the individual patient data meta-analysis
| Study, year | Trial enrolment | Participants randomly assigned in original trial | Participants eligible for IPDMA | Treatment groups | Compliance ≥80% |
|---|---|---|---|---|---|
| Fonseca,69 2007 | 8 centers in the UK, Chile, Brazil, and Greece | 250 with a singleton or twin gestation and a cervical length ≤15 mm | 226 | Vaginal progesterone 200 mg/day or placebo from 24-33 6/7 weeks of gestation | 92% for the vaginal progesterone group and 94% for the placebo group |
| O’Brien,70 2007 | 53 centers in US, South Africa, India, Czech Republic, Chile, and El Salvador | 659 with a singleton gestation and previous spontaneous preterm birth | 31 | Vaginal progesterone 90 mg/day or placebo from 18-22 to 37 0/7 weeks of gestation, rupture of membranes or preterm delivery, whichever occurred first | 100% for the vaginal progesterone group and 95% for the placebo group |
| Cetingoz,71 2011 | Single center in Turkey | 160 with twin gestation, or singleton gestation with previous spontaneous preterm birth, or uterine malformation | 8 | Vaginal progesterone suppository 100 mg/day or placebo from 24-34 weeks of gestation | 100% for both study groups |
| Hassan,72 2011 | 44 centers in US, Belarus, Chile, Czech Republic, India, Israel, Italy, Russia, South Africa, and Ukraine | 465 with a singleton gestation and a cervical length between 10-20 mm | 458 | Vaginal progesterone 90 mg/day or placebo from 20-23 6/7 to 36 6/7 weeks of gestation, rupture of membranes or preterm delivery, whichever occurred first | 89% for the vaginal progesterone group and 93% for the placebo group |
| Norman,54 2016 | 66 centers in the UK and Sweden | 1228 with a singleton gestation and previous spontaneous preterm birth, or cervical length ≤25 mm, or a positive fetal fibronectin test combined with other clinical risk factors for preterm birth | 251 | Vaginal progesterone 200 mg/day or placebo from 22-24 to 34 weeks of gestation or preterm delivery, whichever occurred first | 63% for the vaginal progesterone group and 69% for the placebo group |
IPDMA, individual patient data meta-analysis
Figure 2. Risk of bias in each included study.
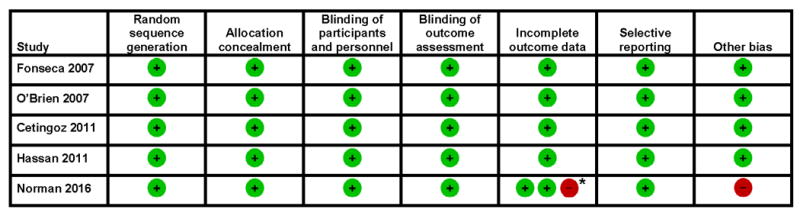
*Low risk of bias for obstetric and neonatal primary outcomes; high risk of bias for childhood primary outcome
Effect of vaginal progesterone on preterm birth
Vaginal progesterone significantly reduced the risk of preterm birth <33 weeks of gestation (14% vs. 22%; RR 0.62, 95% CI 0.47– 0.81; P = .0006; I2 = 0%; NNT 12, 95% CI 8-23; high-quality evidence) (Figure 3). The frequencies of preterm birth <36, <35, <34, <32, <30 and <28 weeks of gestation, and spontaneous preterm birth <33 and <34 weeks of gestation were significantly lower in the vaginal progesterone group (RRs from 0.64 to 0.80; I2 = 0 for all; high-quality evidence for all) (Table 3). Additionally, the mean gestational age at delivery was significantly greater in the vaginal progesterone group than in the placebo group (mean difference 0.74 weeks, 95% CI 0.18-1.30). There was no evidence of an effect of vaginal progesterone on preterm birth <37 weeks of gestation (high-quality evidence).
Figure 3. Effect of vaginal progesterone on preterm birth <33 weeks of gestation.
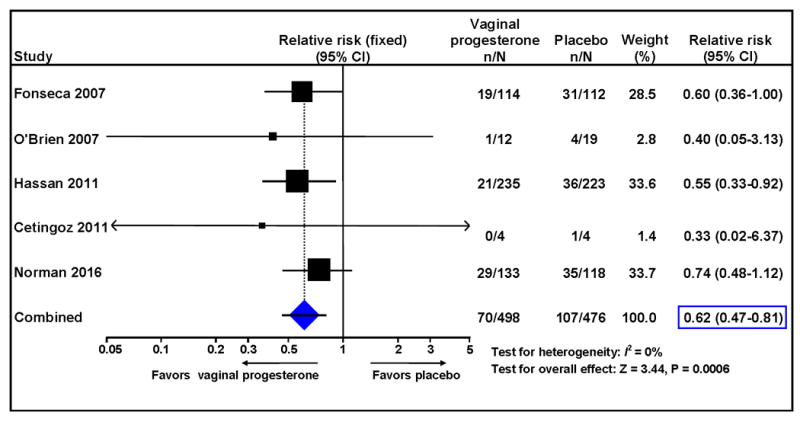
Table 3.
Secondary outcomes by intervention group
| Outcome | No of trials | Vaginal progesterone group | Placebo group | RR or mean difference (95% CI) | P value | I2 (%) | NNT (95% CI) |
|---|---|---|---|---|---|---|---|
| Pregnancy outcome | |||||||
| Preterm birth <37 weeks | 554,69-72 | 187/498 (38%) | 199/476 (42%) | 0.90 (0.77-1.05) | 0.19 | 0 | — |
| Preterm birth <36 weeks | 554,69-72 | 139/498 (28%) | 166/476 (35%) | 0.80 (0.67-0.97) | 0.02 | 0 | 14 (9-96) |
| Preterm birth <35 weeks | 554,69-72 | 106/498 (21%) | 141/476 (30%) | 0.72 (0.58-0.89) | 0.003 | 0 | 12 (8-31) |
| Preterm birth <34 weeks | 554,69-72 | 86/498 (17%) | 126/476 (26%) | 0.65 (0.51-0.83) | 0.0006 | 0 | 11 (8-22) |
| Preterm birth <32 weeks | 554,69-72 | 62/498 (12%) | 92/476 (19%) | 0.64 (0.48-0.86) | 0.003 | 0 | 14 (10-37) |
| Preterm birth <30 weeks | 554,69-72 | 49/498 (10%) | 67/476 (14%) | 0.70 (0.49-0.98) | 0.04 | 0 | 24 (14-355) |
| Preterm birth <28 weeks | 554,69-72 | 38/498 (8%) | 54/476 (11%) | 0.67 (0.45-0.99) | 0.04 | 0 | 27(16-881) |
| Spontaneous preterm birth <33 weeks | 554,69-72 | 60/498 (12%) | 82/476 (17%) | 0.70 (0.51-0.95) | 0.02 | 0 | 19 (12-116) |
| Spontaneous preterm birth <34 weeks | 554,69-72 | 73/498 (15%) | 97/476 (20%) | 0.72 (0.55-0.95) | 0.02 | 0 | 18 (11-98) |
| Gestational age at delivery (weeks) | 554,69-72 | 498a | 476a | 0.74 (0.18-1.30) | 0.01 | 0 | NA |
| Any maternal adverse event | 554,69-72 | 51/424 (12%) | 47/422 (11%) | 1.21 (0.87-1.69) | 0.26 | 5 | — |
| Perinatal outcome | |||||||
| Respiratory distress syndrome | 469-72 | 17/365 (5%) | 37/358 (10%) | 0.47 (0.27-0.81) | 0.007 | 0 | 18 (13-51) |
| Necrotizing enterocolitis | 554,69-72 | 11/495 (2%) | 12/475 (3%) | 0.89 (0.41-1.93) | 0.77 | 0 | — |
| Intraventricular hemorrhage | 554,69-72 | 5/494 (1%) | 10/475 (2%) | 0.50 (0.18-1.38) | 0.18 | 0 | — |
| Proven neonatal sepsis | 554,69-72 | 18/494 (4%) | 28/470 (6%) | 0.61 (0.34-1.08) | 0.09 | 0 | — |
| Bronchopulmonary dysplasia | 354,71,72 | 11/367 (3%) | 13/340 (4%) | 0.77 (0.35-1.68) | 0.51 | 0 | — |
| Retinopathy of prematurity | 469-72 | 6/365 (2%) | 3/358 (1%) | 1.78 (0.49-6.47) | 0.38 | 29 | — |
| Fetal death | 554,69-72 | 9/498 (2%) | 8/476 (2%) | 1.06 (0.41-2.72) | 0.91 | 0 | — |
| Neonatal death | 554,69-72 | 7/498 (1%) | 15/476 (3%) | 0.44 (0.18-1.07) | 0.07 | 0 | — |
| Perinatal death | 554,69-72 | 16/498 (3%) | 23/476 (5%) | 0.66 (0.35-1.22) | 0.19 | 0 | — |
| Composite neonatal morbidity/mortalityb | 469-72 | 29/365 (8%) | 49/358 (14%) | 0.59 (0.38-0.91) | 0.02 | 0 | 18 (12-81) |
| Apgar score <7 at 5 min | 554,69-72 | 38/491 (8%) | 43/469 (9%) | 0.83 (0.55-1.26) | 0.39 | 0 | — |
| Birthweight <1500 g | 554,69-72 | 50/497 (10%) | 77/473 (16%) | 0.62 (0.44-0.86) | 0.004 | 0 | 16 (11-44) |
| Birthweight <2500 g | 554,69-72 | 144/497 (29%) | 168/473 (36%) | 0.82 (0.68-0.98) | 0.03 | 0 | 16 (9-141) |
| Admission to NICU | 554,69-72 | 83/496 (17%) | 117/474 (25%) | 0.68 (0.53-0.88) | 0.003 | 0 | 13 (9-34) |
| Mechanical ventilation | 469-72 | 28/365 (8%) | 43/358 (12%) | 0.65 (0.41-1.01) | 0.06 | 0 | — |
| Congenital anomaly | 554,69-72 | 4/491 (1%) | 6/469 (1%) | 0.72 (0.23-2.26) | 0.57 | 0 | — |
| Childhood (2 years of age) outcome | |||||||
| Bayley-III cognitive composite score | 154 | 95.5 (16.1) 88 | 97.7 (16.9) 80 | -2.17 (-7.16 to 2.83) | 0.40 | NA | NA |
| Moderate/severe neurodevelopmental impairment | 154 | 10/81 (12%) | 7/77 (9%) | 1.36 (0.54-3.39) | 0.51 | NA | — |
| Visual or hearing impairment | 154 | 0/100 (0%) | 2/87 (2%) | 0.17 (0.01-3.58) | 0.26 | NA | — |
| Disability in renal, gastrointestinal, or respiratory function | 154 | 1/91 (1%) | 1/84 (1%) | 0.92 (0.06-14.52) | 0.95 | NA | — |
Data are n/N or mean (SD) N unless otherwise indicated. NA, not applicable; NICU, neonatal intensive care unit; NNT, number needed to treat; RR, relative risk.
Total number;
Occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death.
Effect of vaginal progesterone on adverse perinatal and neurodevelopmental outcomes
Treatment with vaginal progesterone was also associated with a significant reduction in the risk of RDS, composite neonatal morbidity and mortality, birthweight <1500 and <2500 g, and admission to the NICU (RRs from 0.47 to 0.82; I2 = 0 for all; high-quality evidence for all). The frequency of neonatal death was 1.4% (7/498) in the vaginal progesterone group and 3.2% (15/476) in the placebo group (RR 0.44, 95% CI 0.18-1.07; P = .07; I2 = 0%; low-quality evidence). There were no significant differences between the study groups in the risk of necrotizing enterocolitis, intraventricular hemorrhage, proven neonatal sepsis, bronchopulmonary dysplasia, retinopathy of prematurity, fetal death, perinatal death, Apgar score less than 7 at 5 min, use of mechanical ventilation, congenital anomalies, and any maternal adverse event (low- to moderate-quality evidence). At two years of age, the Bayley-III cognitive composite scores and the frequencies of moderate/severe neurodevelopmental impairment, visual or hearing impairment, and disability in renal, gastrointestinal, or respiratory function did not differ significantly between the vaginal progesterone and placebo groups (one study;54 low-quality evidence for all).
Subgroup analyses
Subgroup analyses of the primary outcome according to maternal and trial characteristics are shown in Figure 4. There was no evidence of heterogeneity of treatment effect across any of the prespecified variables (all P for interaction ≥ 0.18). The direction of effect favored vaginal progesterone across all strata, although it appeared that the intervention had no effect in women with a cervical length <10 mm. However, the test of interaction among the cervical length groups was not significant (P = 0.22), suggesting that the response to treatment in the cervical length groups was not significantly different. The beneficial effect of vaginal progesterone did not differ significantly between patients with previous spontaneous preterm birth and those with no previous spontaneous preterm birth (P for interaction = 0.74), as well as between US women and non-US women (P for interaction = 0.51). Effects favoring the intervention were statistically significant in several subgroups of particular clinical interest, including patients with no previous spontaneous preterm birth, patients with a history of spontaneous preterm birth, and those receiving either 90-100 or 200 mg/d of vaginal progesterone.
Figure 4. Subgroup analyses of the effect of vaginal progesterone on preterm birth <33 weeks of gestation.
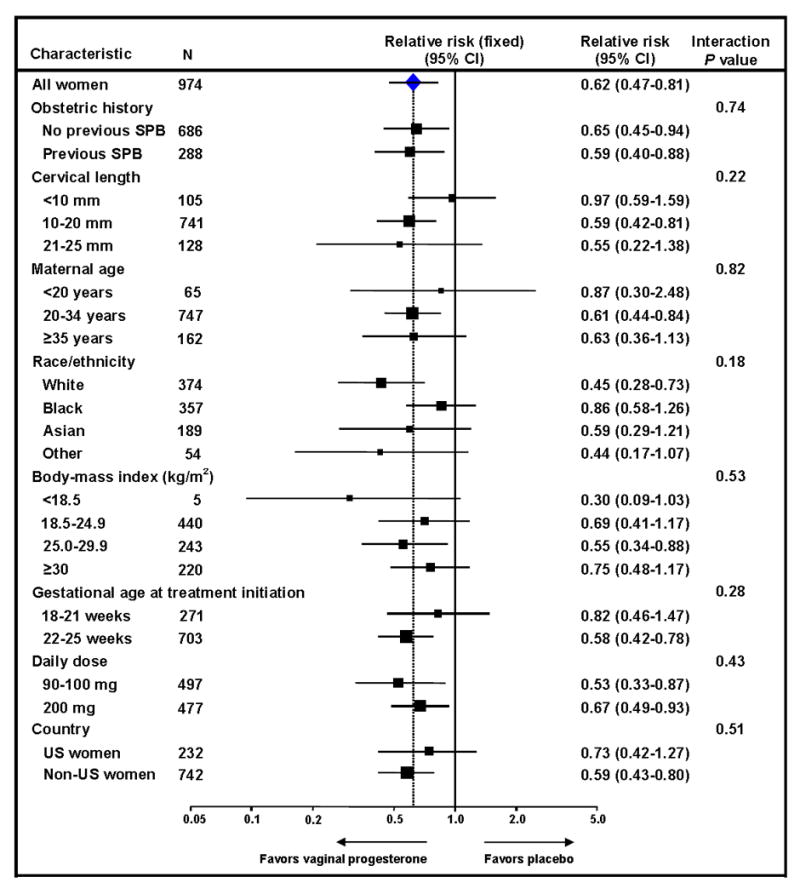
SPB, spontaneous preterm birth
COMMENT
Principal findings of the study
(1) Women with a singleton gestation and a midtrimester short cervix who received vaginal progesterone had a significant reduction in the risk of preterm birth (<28, <30, <32, <33, <34, <35, and <36 weeks of gestation); (2) vaginal progesterone improved neonatal outcome. Indeed, neonates of mothers who received vaginal progesterone had a significantly lower risk of RDS. In addition, vaginal progesterone was also associated with a significant decrease in the risk of composite neonatal morbidity and mortality, low birthweight (<2500 grams), very low birthweight (<1500 grams), and NICU admission; (3) there was a non-significant trend towards reduction of neonatal mortality (by 66%, P = 0.07) and use of mechanical ventilation (by 35%, P = 0.06); (4) evidence from one trial54 showed that, at 2 years of age, there were no significant differences in cognitive scores or the frequency of neurodevelopmental impairment or renal, gastrointestinal, and respiratory morbidity between children exposed prenatally to vaginal progesterone vs. placebo; and (5) there were no significant differences in the frequency of maternal adverse events and congenital anomalies between the vaginal progesterone and placebo groups.
Clinical meaning of the findings
A new finding is that vaginal progesterone administered to women with a mid-trimester short cervix significantly reduces the risk of preterm birth <36 weeks and birthweight <2500 grams. In a previous IPD meta-analysis, vaginal progesterone reduced the rate of preterm birth from <28 to <35 weeks.49 The extended efficacy in reducing the rate of preterm birth to <36 weeks is probably attributable to the larger sample size of the current meta-analysis. This has important implications as late preterm birth (34 to 36 6/7 weeks) represents approximately 72% of all preterm births.80
Vaginal progesterone is expected to reduce neonatal complications by preventing preterm birth. The current IPD meta-analysis shows that vaginal progesterone is significantly associated with a 41% reduction in the frequency of a pre-specified composite outcome of neonatal death combined with the most common neonatal complications affecting preterm neonates, such as RDS, intraventricular hemorrhage, necrotizing enterocolitis, and proven neonatal sepsis, which are important to patients, families, and healthcare providers. This finding is strengthened by the fact that the magnitude of the beneficial effect of vaginal progesterone on the individual components of the composite outcome was consistent with a reduction of about 40-50% for neonatal death, RDS, intraventricular hemorrhage, and proven neonatal sepsis.
The pre-specified composite outcome measure did not restrict the endpoint of morbidity to complications which have a very low prevalence, such as severe intraventricular hemorrhage (grades III/IV), necrotizing enterocolitis (stages II/III), and retinopathy of prematurity (stages III to V). If the composite outcome measure had been restricted to only these severe complications, the risk for a type II error due to limited power could have missed an important clinical effect and mislead physicians and patients.81
In addition, the expectation that vaginal progesterone administered to patients with a short cervix would reduce the frequency of all severe complications of preterm neonates is not realistic, since many morbid events are influenced by postnatal factors, such as barotrauma, oxygen toxicity, systemic and local inflammation, neonatal sepsis, etc. Vaginal progesterone is aimed primarily at preventing preterm birth and may ameliorate some immediate neonatal complications (e.g. RDS); yet, it is unreasonable to expect that it will improve distal outcomes influenced by many other medical and non-medical factors.
Quality of evidence based on GRADE
We assessed primary and secondary outcomes with GRADE methodology, as shown in Table 4. Evidence was graded as “high quality” for all outcomes for which vaginal progesterone significantly reduced their risk. A determination of “high quality” signifies that we are very confident that the true effect lies close to that of the estimate of the effect, and that further research is very unlikely to change this level of confidence.66 Evidence for the remaining outcomes was considered to be moderate to low quality.
Table 4.
Summary of Findings table on the quality of evidence for each outcome measure
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with placebo | Risk with vaginal progesterone | ||||
| Preterm birth <33 weeks | Study population | RR 0.62 (0.47 to 0.81) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 225 per 1000 | 139 per 1000 (106 to 182) | ||||
| Preterm birth <37 weeks | Study population | RR 0.90 (0.77 to 1.05) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 418 per 1000 | 376 per 1000 (322 to 439) | ||||
| Preterm birth <36 weeks | Study population | RR 0.80 (0.67 to 0.97) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 349 per 1000 | 279 per 1000 (234 to 338) | ||||
| Preterm birth <35 weeks | Study population | RR 0.72 (0.58 to 0.89) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 296 per 1000 | 213 per 1000 (172 to 264) | ||||
| Preterm birth <34 weeks | Study population | RR 0.65 (0.51 to 0.83) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 265 per 1000 | 172 per 1000 (135 to 220) | ||||
| Preterm birth <32 weeks | Study population | RR 0.64 (0.48 to 0.86) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 193 per 1000 | 124 per 1000 (93 to 166) | ||||
| Preterm birth <30 weeks | Study population | RR 0.70 (0.49 to 0.98) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 141 per 1000 | 99 per 1000 (69 to 138) | ||||
| Preterm birth <28 weeks | Study population | RR 0.67 (0.45 to 0.99) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 113 per 1000 | 76 per 1000 (51 to 112) | ||||
| Spontaneous preterm birth <33 weeks | Study population | RR 0.70 (0.51 to 0.95) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 172 per 1000 | 121 per 1000 (88 to 164) | ||||
| Spontaneous preterm birth <34 weeks | Study population | RR 0.72 (0.55 to 0.95) | 974 (5 studies) | ⊕⊕⊕⊕ High | |
| 204 per 1000 | 147 per 1000 (112 to 194) | ||||
| Gestational age at delivery (weeks) | The mean gestational age at delivery (weeks) in the intervention groups was 0.74 higher (0.18 to 1.3 higher) | 974 (5 studies) | ⊕⊕⊕⊕ High | ||
| Respiratory distress syndrome | Study population | RR 0.47 (0.27 to 0.81) | 723 (4 studies) | ⊕⊕⊕⊕ High | |
| 103 per 1000 | 49 per 1000 (28 to 84) | ||||
| Necrotizing enterocolitis | Study population | RR 0.89 (0.41 to 1.93) | 970 (5 studies) | ⊕⊕⊖⊖ low1 | |
| 25 per 1000 | 22 per 1000 (10 to 49) | ||||
| Intraventricular hemorrhage | Study population | RR 0.50 (0.18 to 1.38) | 969 (5 studies) | ⊕⊕⊖⊖ low1 | |
| 21 per 1000 | 11 per 1000 (4 to 29) | ||||
| Proven neonatal sepsis | Study population | RR 0.61 (0.34 to 1.08) | 964 (5 studies) | ⊕⊕⊕⊖ Moderate2 | |
| 60 per 1000 | 36 per 1000 (20 to 64) | ||||
| Bronchopulmonary dysplasia | Study population | RR 0.77 (0.35 to 1.68) | 707 (3 studies) | ⊕⊕⊖⊖ low1 | |
| 38 per 1000 | 29 per 1000 (13 to 64) | ||||
| Retinopathy of prematurity | Study population | RR 1.78 (0.49 to 6.47) | 723 (4 studies) | ⊕⊕⊖⊖ low1 | |
| 8 per 1000 | 15 per 1000 (4 to 54) | ||||
| Fetal death | Study population | RR 1.06 (0.41 to 2.72) | 974 (5 studies) | ⊕⊕⊖⊖ low1 | |
| 17 per 1000 | 18 per 1000 (7 to 46) | ||||
| Neonatal death | Study population | RR 0.44 (0.18 to 1.07) | 974 (5 studies) | ⊕⊕⊖⊖ low3 | |
| 32 per 1000 | 14 per 1000 (6 to 34) | ||||
| Perinatal death | Study population | RR 0.66 (0.35 to 1.22) | 974 (5 studies) | ⊕⊕⊕⊖ Moderate2 | |
| 48 per 1000 | 32 per 1000 (17 to 59) | ||||
| Composite neonatal morbidity/mortality | Study population | RR 0.59 (0.38 to 0.91) | 723 (4 studies) | ⊕⊕⊕⊕ High | |
| 137 per 1000 | 81 per 1000 (52 to 125) | ||||
| Apgar score <7 at 5 min | Study population | RR 0.83 (0.55 to 1.26) | 960 (5 studies) | ⊕⊕⊕⊖ Moderate4 | |
| 92 per 1000 | 76 per 1000 (50 to 116) | ||||
| Birthweight <1500 g | Study population | RR 0.62 (0.44 to 0.86) | 970 (5 studies) | ⊕⊕⊕⊕ High | |
| 163 per 1000 | 101 per 1000 (72 to 140) | ||||
| Birthweight <2500 g | Study population | RR 0.82 (0.68 to 0.98) | 970 (5 studies) | ⊕⊕⊕⊕ High | |
| 355 per 1000 | 291 per 1000 (242 to 348) | ||||
| Admission to NICU | Study population | RR 0.68 (0.53 to 0.88) | 970 (5 studies) | ⊕⊕⊕⊕ High | |
| 247 per 1000 | 168 per 1000 (131 to 217) | ||||
| Mechanical ventilation | Study population | RR 0.65 (0.41 to 1.01) | 723 (4 studies) | ⊕⊕⊕⊖ Moderate2 | |
| 120 per 1000 | 78 per 1000 (49 to 121) | ||||
| Congenital anomaly | Study population | RR 0.72 (0.23 to 2.26) | 960 (5 studies) | ⊕⊕⊖⊖ low1 | |
| 13 per 1000 | 9 per 1000 (3 to 29) | ||||
| Bayley-III cognitive composite score at 2 years of age | The mean Bayley-III cognitive composite score at 2 years of age in the intervention groups was 2.17 lower (7.16 lower to 2.83 higher) | 168 (1 study) | ⊕⊕⊖⊖ low5 | ||
| Moderate/severe neurodevelopmental impairment at 2 years of age | Study population | RR 1.36 (0.54 to 3.39) | 158 (1 study) | ⊕⊕⊖⊖ low6 | |
| 91 per 1000 | 124 per 1000 (49 to 308) | ||||
| Visual or hearing Impairment at 2 years of age | Study population | RR 0.17 (0.01 to 3.58) | 187 (1 study) | ⊕⊕⊖⊖ low6 | |
| 23 per 1000 | 4 per 1000 (0 to 82) | ||||
| Disability in renal, gastrointestinal, or respiratory function at 2 years of age | Study population | RR 0.92 (0.06 to 14.52) | 175 (1 study) | ⊕⊕⊖⊖ Low6 | |
| 12 per 1000 | 11 per 1000 (1 to 173) | ||||
| Any maternal adverse event | Study population | RR 1.21 (0.87 to 1.69) | 846 (5 studies) | ⊕⊕⊕⊖ Moderate7 | |
| 111 per 1000 | 135 per 1000 (97 to 188) | ||||
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI).
CI, confidence interval; NICU, neonatal intensive care unit; RR, relative risk
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Few events; 95% CI does not include effect and is imprecise (lower and upper bounds <0.75 and >1.25, respectively)
95% CI does not include effect and is imprecise (lower bound <0.75)
Few events; 95% CI does not include effect and is imprecise (lower bound <0.75)
95% CI does not include effect and is imprecise (lower and upper bounds <0.75 and >1.25, respectively)
Small sample size; 95% CI does not include effect and is imprecise
Small sample size and few events; 95% CI does not include effect and is imprecise (lower and upper bounds <0.75 and >1.25, respectively
95% CI does not include effect and is imprecise (upper bound >1.25)
Subgroup analyses according to history of spontaneous preterm birth
This meta-analysis also shows a beneficial effect of vaginal progesterone across a range of subgroups, including patients with or without a previous spontaneous preterm birth.
The results of an indirect comparison meta-analysis concluded that vaginal progesterone and cerclage have a similar efficacy to prevent preterm birth and perinatal morbidity and mortality in patients with a short cervix and a history of preterm birth.82 The findings reported herein reaffirm that vaginal progesterone should be offered as an alternative to cerclage in patients with a singleton gestation, previous spontaneous preterm birth and a cervical length ≤25 mm.82
Subgroup analysis according to country of enrollment (USA vs. non-USA)
In 2012, the PREGNANT trial72 was reviewed by the U.S. Food and Drug Administration (FDA) for a New Drug Application for the treatment of women with a singleton gestation and a midtrimester sonographic short cervix with vaginal progesterone. The application filed by a pharmaceutical company was not approved by the FDA. One of the reasons posited by the FDA was an alleged lack of statistically significant efficacy of vaginal progesterone in women enrolled in the USA.
Recently, Yusuf and Wittes analyzed several examples of regional differences in the results of randomized clinical trials in medicine, and provided their assessment as to whether or not such differences are likely to be due to chance.83 The PREGNANT trial,72 was one of the examples of variations in results among countries assessed by Yusuf and Wittes (who also examined the post-hoc analysis of the FDA). These investigators concluded that “geography does not trump biology in this case, and we would have applied the overall results of the trial to the U.S”. Consistent with this conclusion by Yusuf and Wittes, a subgroup analysis in the current IPD meta-analysis showed that the beneficial effects of vaginal progesterone on preterm birth <33 weeks of gestation did not differ significantly between women enrolled in the U.S. (RR 0.73, 95% CI 0.42-1.27) and women enrolled outside the U.S. (RR 0.59, 95% CI 0.43-0.80), as the interaction test for subgroup differences was non-significant (P = 0.51).
Subgroup Analysis according to Vaginal Progesterone Dose and Cervical Length
There was no difference in efficacy in the prevention of preterm birth when either 90-100 or 200 mg per day of vaginal progesterone was used. Therefore, either regimen can be used in practice.
Insofar as cervical length, vaginal progesterone appeared to have no effect on the risk of preterm birth <33 weeks in patients with a cervical length <10 mm. Whether this lack of efficacy has a biological basis, or is a chance finding, is unclear. Although the interaction test for subgroup differences was not significant (P = 0.22), suggesting that vaginal progesterone has no differential efficacy in the pre-specified cervical length groups, it is possible that women with a very short cervix are more likely to have intra-amniotic inflammation and may be less responsive to vaginal progesterone.84-87 However, we performed a post-hoc subgroup analysis examining the effect of vaginal progesterone on the risk of composite neonatal morbidity and mortality according to cervical length, which showed that the beneficial effect of vaginal progesterone did not differ significantly between women with a cervical length <10 mm (RR 0.68, 95% CI 0.33-1.41) and those with a cervical length between 10-25 mm (RR 0.59, 95% CI 0.35-0.99) with a non-significant interaction P value of 0.75. Further trials assessing the efficacy of vaginal progesterone in women with a cervical length <10 mm are warranted.
Long-term effects of prenatal exposure to vaginal progesterone
Current evidence suggests that in-utero exposure to vaginal progesterone does not have an effect on neurodevelopmental outcomes at least until 2 years of age and, possibly, until 6 years of age. Overall, the OPPTIMUM study54 found that there were no significant differences in neurodevelopmental outcomes at 2 years of age between children exposed in-utero to vaginal progesterone and those exposed to placebo. O’Brien et al.88 assessed neurodevelopmental outcomes at 6, 12 and 24 months of age in children born to women enrolled in their trial,70 and found similar frequencies of suspected developmental delay in the vaginal progesterone and placebo groups. Similar findings have been reported in children born to mothers participating in trials that compared vaginal progesterone and placebo in unselected twin gestations,89, 90 at a mean age of ~56 months.91, 92 Therefore, there is no evidence that vaginal progesterone has adverse effects on childhood neurodevelopmental outcomes.
Strengths and limitations
A major strength of this study was the inclusion of individual data for most patients (97%) with a singleton gestation and a short cervix who have been randomized to receive vaginal progesterone or placebo in trials that assessed this intervention with the aim of preventing preterm birth. Individual data for approximately 35 patients with a cervical length ≤25 mm who participated in a trial stopped early due to low enrollment could not be obtained from the investigators.74 In this trial, vaginal progesterone was associated with a non-significant reduction in the risk of composite neonatal morbidity and mortality and preterm birth <32 and <34 weeks of gestation. We performed several simulated meta-analyses by including the results for women with a cervical length ≤30 mm reported in this study. After assuming the worst-case scenario (all adverse outcomes among patients with a cervical length ≤25 mm receiving vaginal progesterone and none among patients with a cervical length ≤25 mm receiving placebo), we found that the inclusion of data from this study in the meta-analyses resulted in minimal changes in the overall estimates of effect size, whereas the beneficial effects of vaginal progesterone on the risk of preterm birth and neonatal morbidity and mortality remained statistically significant. Other strengths of the present study are the absence of clinical and statistical heterogeneity in almost all meta-analyses, and the balance in prognostic factors between the vaginal progesterone and placebo groups at baseline, which reduces the possibility of introducing biases in the estimates of intervention effects.
The main limitation of our study was the lack of data on the outcome measure RDS and the use of mechanical ventilation, because this information was not collected in the OPPTIMUM study.54 The net effect was a reduction in the sample size of meta-analyses for these outcomes and for the composite outcome of neonatal morbidity and mortality. A second limitation was that some subgroup analyses included a small number of patients, which limits the statistical power to estimate the effects within these subgroups.
RDS is the most common complication of preterm birth, and therefore, it is an appropriate endpoint when assessing neonatal morbidity. Similarly, the requirement for mechanical ventilation is an important endpoint, given that it reflects the severity of RDS, and complications may arise during or after mechanical ventilation. Most trials designed to study the effects of interventions in the prevention of preterm birth have also included RDS as a main endpoint. Indeed, even the PROGRESS trial, aimed at determining the effect of vaginal progesterone in patients with a history of preterm birth, used RDS as a primary endpoint.76.
Cost-effectiveness of mid-trimester sonographic cervical length and vaginal progesterone in women with a short cervix
Several cost-effectiveness studies have shown that the combination of universal transvaginal cervical length screening and vaginal progesterone administration to women with a short cervix is a cost-effective intervention that reduces preterm birth and associated perinatal morbidity and mortality, regardless of the cutoff used to define a short cervix in the decision and economic analyses. Cahill et al.93 compared four strategies and found that universal cervical length screening to identify women with a cervical length ≤15 mm and subsequent treatment with vaginal progesterone was the most cost-effective strategy and the dominant choice over the other three alternatives: cervical length screening for women at increased risk for preterm birth and treatment with vaginal progesterone; risk-based treatment with 17-OHPC without screening; and no screening or treatment93.
Werner et al94 found that universal cervical length screening followed by treatment with vaginal progesterone if cervical length <15 mm could prevent 22 cases of neonatal death or long-term neurologic deficits and save approximately $19.6 million for every 100,000 women screened. In 2015, Werner et al95 reevaluated the cost-effectiveness of universal transvaginal cervical length screening and vaginal progesterone administration to women with a singleton gestation, no previous spontaneous preterm birth and a cervical length ≤20 mm. Despite using a low prevalence of cervical length ≤20 mm in the model (0.83%), this intervention continued to be cost-effective when compared to routine care.
In 2016, Einerson et al96 reported that universal transvaginal cervical length screening to women with no previous spontaneous preterm birth and treatment with vaginal progesterone to those with a cervical length ≤20 mm was more cost-effective in comparison to both risk-based screening and no screening of transvaginal cervical length. Crosby et al97 reported that universal cervical length screening and treatment with vaginal progesterone to women with a cervical length ≤15 mm in a population at low risk of preterm birth in Ireland would reduce the rate of preterm birth <34 weeks of gestation by 28% and would be cost-effective. Pizzi et al98 performed an economic analysis of the PREGNANT trial72 and found that vaginal progesterone was both cost-saving and cost-effective as compared with placebo. A cost-effectiveness analysis of universal cervical length screening in women without a previous spontaneous preterm birth and treatment with vaginal progesterone to those with a short cervix (cervical length ≤20 mm), reported that this intervention would be cost-effective if vaginal progesterone reduces the risk of preterm birth <33 weeks of gestation by more than 36%.99 In our IPD meta-analysis, vaginal progesterone decreased the risk of preterm birth <33 weeks of gestation by 38%. Finally, five cost-effectiveness and decision analyses published only in abstract form also reported that vaginal progesterone administration was a cost-effective strategy for preventing preterm birth in women with a short cervix.100-104
Implementation of universal cervical length screening and vaginal progesterone administration to patients with a sonographic short cervix
Several authors have critically assessed if cervical length screening meets the criteria outlined by the World Health Organization of a good screening test. Combs105 as well as Khalifeh and Berghella106 have concluded that universal midtrimester transvaginal cervical length screening for women with a singleton gestation, followed by treatment with vaginal progesterone for those with a short cervix meets all 10 criteria outlined by the World Health Organization for endorsing the implementation of a screening test in clinical medicine.107 Based on the totality of evidence, we and others have recommended universal transvaginal cervical length screening at 18-24 weeks of gestation in women with a singleton gestation and the administration of vaginal progesterone for those with a sonographic short cervix.52, 57, 105, 106, 108-118
In 2016, Son et al119 reported on the results of introducing a universal transvaginal cervical length screening program in women with a singleton gestation without a previous preterm birth and treatment with vaginal progesterone to those with a cervical length ≤20 mm at Northwestern Memorial Hospital in Chicago, IL (46,598 women in the prescreening group and 17,609 in the screened group). The implementation of this program was associated with a significant reduction in the rates of preterm birth <37, <34 and <32 weeks of gestation when compared with preterm birth rates before implementation of the program. These significant differences were driven by a reduction in spontaneous preterm births. Furthermore, these reductions were similar in both nulliparous and parous women.
Similarly, Temming et al120 evaluated the implementation of a universal transvaginal cervical length screening program in women with a singleton gestation followed by treatment with vaginal progesterone to those with a cervical length ≤20 mm in St Louis, MO. The rates of preterm birth <24 and <28 weeks of gestation were significantly lower among women who underwent cervical length screening (N=9731) than those patients who did not participate in the screening program (N=1661). There was also a non-significant reduction in the rate of preterm birth <34 weeks of gestation among screened women.
A smaller study that assessed a similar program in women with a singleton gestation without a history of spontaneous preterm birth at a single institution in Philadelphia, PA reported that the rate of spontaneous preterm birth was similar between women undergoing transvaginal cervical length screening (N=1569) and those not screened (N=602).121 However, this study was underpowered to detect differences in spontaneous preterm birth rates between the study groups. Schoen et al122 assessed the reasons behind the decrease in preterm birth rates in the US in the last seven years and suggested that the use of vaginal progesterone in pregnant women with a short cervix is one of the interventions that contributed to this reduction.
Recently, Newnham et al123 reported the results of a prospective population-based cohort study that evaluated the effects of implementation of a statewide multifaceted program on the preterm birth rates in Western Australia before and after the first full year of operation. One of the key interventions of the program was the universal cervical length measurement at 18-20 weeks of gestation in women with a singleton gestation and treatment with vaginal progesterone to those with a cervical length ≤25 mm. The implementation of the program in 2014 was followed by a statistically significant 7.6% reduction in the rate of preterm birth in 2015, which was lower than in any of the preceding 6 years. The effect extended from the 28-31 week gestational age group onward. Further studies are required to elucidate the precise contribution of the different elements of the program to the reduction in preterm birth.
Based on current national vital statistics2 and results of our IPD meta-analysis, we have estimated that the implementation of universal transvaginal cervical length screening in women with a singleton gestation in the United States and treatment with vaginal progesterone to those with a short cervix (cervical length ≤25 mm) would result in an annual reduction of approximately 31,800 preterm births <34 weeks of gestation and of 19,800 cases of major neonatal morbidity or neonatal mortality if the overall prevalence of a short cervix is 9%,13 and of approximately 7000 preterm births <34 weeks of gestation and of 4400 cases of major neonatal morbidity or neonatal mortality if the overall prevalence of a short cervix is 2%.116
The effects of progesterone on the uterine cervix
Progesterone is critical for pregnancy maintenance and a withdrawal of progesterone action is believed to be central to the initiation of parturition in most mammalian species, including primates.124-131 Progesterone exerts biological effects in the myometrium132-136, chorioamniotic membranes137, and the uterine cervix (i.e. control of cervical remodeling).138, 139 Progesterone withdrawal (in rats, rabbits and sheep) or a decline in progesterone action (in guinea pigs and primates)129 has been proposed as a key control mechanism for cervical ripening by Elovitz et al.140, 141, Mahendroo et al.142, 143 Word et al.144 Yellon et al.145-147, Chwalisz et al.148-150 Thus, a large body of evidence supports a role for progesterone in cervical remodeling151-158. For example: (1) administration of antiprogestins to women in the mid-trimester and at term induces cervical ripening151-158; and (2) administration of progesterone-receptor antagonists such as mifepristone (RU486) or onapristone to pregnant guinea pigs159, old-world monkeys160 and Tupaja belangeri induces cervical ripening.144 It is interesting that cervical responsiveness to antiprogestins increases with advancing gestational age 144 and that their effects on the cervix are not always accompanied by changes in myometrial activity.144 Indeed, Stys et al.161 demonstrated a functional dissociation between the effects of progesterone in the myometrium and those in the cervix. Collectively, the evidence indicates that a major site of progesterone action is the uterine cervix.
A decline in progesterone action probably causes cervical changes by inducing changes in extracellular matrix metabolism, and perhaps inflammation (leukocyte infiltration and production of chemokines162 such as interleukin-8139, nitric oxide150, 157, prostaglandins139 and matrix-degrading enzymes.163, 164 It is also possible that cervical remodeling is influenced by NF-kB (nuclear factor-kappa B), a transcription factor which mediates the effect of certain pro-inflammatory cytokines such as interleukin-1β165-168 and tumor necrosis factor-α.169-171 This is potentially relevant because NF-kB can oppose progesterone action.132, 167, 172-174 Thus, NFkB could provide a link between inflammation, a decline in progesterone action and cervical remodeling.
The traditional understanding of the mechanisms of action of progesterone is that this hormone acts through nuclear receptors to induce genomic actions.175-182 However, it is now clear that some of the actions of progesterone are induced through membrane receptors and non-genomic mechanisms.183-187 The precise role of progesterone receptors, deoxyribonucleic acid-binding properties and/or transcriptional activity in determining progesterone action on the cervix remains to be elucidated.
Another unresolved issue is why progesterone administration to pregnant women, who already have a very high concentration of circulating progesterone,144 would result in a therapeutic effect. In fact, it has been argued that the circulating concentration of progesterone in pregnant women is in excess of that required to saturate progesterone receptors.144 However, these biochemical considerations were developed before the realization that some actions of progesterone are independent of its nuclear receptors188, 189. It is possible that the change in progesterone concentrations at the time of spontaneous parturition in the human occurs locally and not in the systemic circulation.190, 191 Recently, the laboratories of Lye and Mesiano have provided evidence in support of a novel mechanism whereby a functional progesterone withdrawal could occur in the myometrium, independent of progesterone concentrations in the peripheral circulation192-194. Whether this specific mechanism is operational in the uterine cervix remains to be determined.
Recent studies195 about the mechanisms of action of progestogens in vivo have shown that vaginal progesterone has local anti-inflammatory effects at the maternal fetal interface. Specifically, when vaginal progesterone is administered to pregnant mice, it fosters an anti-inflammatory microenvironment at the maternal-fetal interface by increasing CD4+ Tregs and reducing CD8+CD25+Foxp3+ T cells, macrophages, and Interferon γ+ neutrophils.195 In addition, the administration of vaginal progesterone decreases the infiltration of active matrix metalloproteinase-9-positive neutrophils and monocytes in the cervix, reduces the plasma concentration of interleukin-1β, and reduces the frequency of endotoxin-induced preterm birth.195
In summary, progesterone has anti-inflammatory effects and also modulates other biological processes implicated in cervical ripening.
Conclusions
There is persuasive evidence that vaginal progesterone reduces the risk of preterm birth and adverse perinatal outcomes in patients with a singleton gestation and a midtrimester short cervix, regardless of the history of spontaneous preterm birth, without any demonstrable deleterious effects on childhood neurodevelopment or maternal health. The findings of our meta-analysis of individual patient data should reassure clinicians and professional/scientific organizations that vaginal progesterone is efficacious and safe for reducing preterm birth and neonatal morbidity and mortality in these women. In addition, recent evidence assessing the implementation of universal cervical length screening in women with a singleton gestation and treatment with vaginal progesterone to those with a short cervix suggests that this intervention could contribute to a reduction in the rate of preterm birth and associated neonatal morbidity and mortality in the United States.
Acknowledgments
We are grateful to Professor Jane E. Norman and the investigators of the OPPTIMUM trial for providing the individual data for the 251 patients with a cervical length of 25 mm or less. Professor Jane Norman is Principal Investigator at the Tommy’s Centre for Maternal and Fetal Health, MRC, University of Edinburgh Centre for Reproductive Health, University of Edinburgh, Edinburgh, UK. The OPPTIMUM study was funded by the Efficacy and Mechanism Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute of Health Research (NIHR) partnership, award number G0700452, revised to 09/800/27. The EME Programme is funded by the MRC and NIHR, with contributions from the Chief Scientist Office in Scotland and National Institute for Social Care and Research in Wales.
Financial support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services
Role of the funding source: The funder had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication
Footnotes
Disclosure: RR, AC-A, EDF, EC, SSH, and KHN declare no conflict of interest. JMO’B was involved in studies of progesterone gel treatment for preterm birth prevention sponsored by a maker of progesterone gel. He served on advisory boards and as a consultant for Watson Pharmaceuticals, a company with a financial interest in marketing vaginal progesterone gel for preterm birth prevention; he and others are listed in a patent on the use of progesterone compounds to prevent preterm birth (USA Patent Number 7884093: progesterone for the treatment and prevention of spontaneous preterm birth). He has received no royalty payments. GWC was an Employee of Columbia Laboratories, Inc. when the previous meta-analysis of individual patient data was conducted in 2011.
Professor Jane Norman has no conflict of interest in relation with our meta-analysis of individual patient data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1. [PubMed] [Google Scholar]
- 3.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Institute Of Medicine Committee On Understanding Premature B, Assuring Healthy O. The National Academies Collection: Reports funded by National Institutes of Health. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US), National Academy of Sciences; 2007. [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 6.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131:e1240–63. doi: 10.1542/peds.2012-2177. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15:804–11. doi: 10.1111/obr.12214. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–67. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 13.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal- Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047–53. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 15.Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal ultrasonographic cervical measurement at 24 to 26 weeks’ gestation. Am J Obstet Gynecol. 1997;177:1149–55. doi: 10.1016/s0002-9378(97)70032-2. [DOI] [PubMed] [Google Scholar]
- 16.Wennerholm UB, Holm B, Mattsby-Baltzer I, et al. Fetal fibronectin, endotoxin, bacterial vaginosis and cervical length as predictors of preterm birth and neonatal morbidity in twin pregnancies. Br J Obstet Gynaecol. 1997;104:1398–404. doi: 10.1111/j.1471-0528.1997.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 17.Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol. 1997;177:723–30. doi: 10.1016/s0002-9378(97)70259-x. [DOI] [PubMed] [Google Scholar]
- 18.Grisaru-Granovsky S, Farine D, Barrett J, et al. Is a single ultrasound measurement of cervical length a predictor of the risk of preterm delivery in multifetal pregnancy? American Journal of Obstetrics and Gynecology. 1998;178:191S. [Google Scholar]
- 19.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 20.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet Gynecol. 2000;15:288–91. doi: 10.1046/j.1469-0705.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 21.Guzman ER, Walters C, O’Reilly-Green C, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103–7. doi: 10.1067/mob.2000.108896. [DOI] [PubMed] [Google Scholar]
- 22.Soriano D, Weisz B, Seidman DS, et al. The role of sonographic assessment of cervical length in the prediction of preterm birth in primigravidae with twin gestation conceived after infertility treatment. Acta Obstet Gynecol Scand. 2002;81:39–43. doi: 10.1046/j.0001-6349.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 23.Vayssiere C, Favre R, Audibert F, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596–604. doi: 10.1067/mob.2002.127380. [DOI] [PubMed] [Google Scholar]
- 24.Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol. 2003;22:305–22. doi: 10.1002/uog.202. [DOI] [PubMed] [Google Scholar]
- 25.Owen J, Yost N, Berghella V, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561–6. doi: 10.1002/uog.1048. [DOI] [PubMed] [Google Scholar]
- 27.Sperling L, Kiil C, Larsen LU, et al. How to identify twins at low risk of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2005;26:138–44. doi: 10.1002/uog.1938. [DOI] [PubMed] [Google Scholar]
- 28.Fait G, Har-Toov J, Gull I, Lessing JB, Jaffa A, Wolman I. Cervical length, multifetal pregnancy reduction, and prediction of preterm birth. J Clin Ultrasound. 2005;33:329–32. doi: 10.1002/jcu.20159. [DOI] [PubMed] [Google Scholar]
- 29.Arabin B, Roos C, Kollen B, Van Eyck J. Comparison of transvaginal sonography in recumbent and standing maternal positions to predict spontaneous preterm birth in singleton and twin pregnancies. Ultrasound Obstet Gynecol. 2006;27:377–86. doi: 10.1002/uog.2694. [DOI] [PubMed] [Google Scholar]
- 30.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–7. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 31.To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194:1360–5. doi: 10.1016/j.ajog.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–87. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 33.Klein K, Gregor H, Hirtenlehner-Ferber K, et al. Prediction of spontaneous preterm delivery in twin pregnancies by cervical length at mid-gestation. Twin Res Hum Genet. 2008;11:552–7. doi: 10.1375/twin.11.5.552. [DOI] [PubMed] [Google Scholar]
- 34.Fox NS, Saltzman DH, Klauser CK, Peress D, Gutierrez CV, Rebarber A. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies with the use of combined fetal fibronectin and cervical length. Am J Obstet Gynecol. 2009;201:313.e1–5. doi: 10.1016/j.ajog.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Honest H, Forbes CA, Duree KH, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2009;13:1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 36.Domin CM, Smith EJ, Terplan M. Transvaginal ultrasonographic measurement of cervical length as a predictor of preterm birth: a systematic review with meta-analysis. Ultrasound Q. 2010;26:241–8. doi: 10.1097/RUQ.0b013e3181fe0e05. [DOI] [PubMed] [Google Scholar]
- 37.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128.e1–12. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim AC, Hegeman MA, Huis In TVMA, Opmeer BC, Bruinse HW, Mol BW. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta-analysis. Ultrasound Obstet Gynecol. 2011;38:10–7. doi: 10.1002/uog.9013. [DOI] [PubMed] [Google Scholar]
- 39.Barros-Silva J, Pedrosa AC, Matias A. Sonographic measurement of cervical length as a predictor of preterm delivery: a systematic review. J Perinat Med. 2014;42:281–93. doi: 10.1515/jpm-2013-0115. [DOI] [PubMed] [Google Scholar]
- 40.Conde-Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am J Obstet Gynecol. 2014;211:583–95. doi: 10.1016/j.ajog.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Reeves M, Owen J, Keith LG. Precocious cervical ripening as a screening target to predict spontaneous preterm delivery among asymptomatic singleton pregnancies: a systematic review. Am J Obstet Gynecol. 2015;212:145–56. doi: 10.1016/j.ajog.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conde-Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2015;213:789–801. doi: 10.1016/j.ajog.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindinger LM, Poon LC, Cacciatore S, et al. The effect of gestational age and cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta-analysis. Bjog. 2016;123:877–84. doi: 10.1111/1471-0528.13575. [DOI] [PubMed] [Google Scholar]
- 44.Melamed N, Pittini A, Hiersch L, et al. Serial cervical length determination in twin pregnancies reveals 4 distinct patterns with prognostic significance for preterm birth. Am J Obstet Gynecol. 2016;215:476.e1–76.e11. doi: 10.1016/j.ajog.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandermolen BI, Hezelgrave NL, Smout EM, Abbott DS, Seed PT, Shennan AH. Quantitative fetal fibronectin and cervical length to predict preterm birth in asymptomatic women with previous cervical surgery. Am J Obstet Gynecol. 2016;215:480.e1–80.e10. doi: 10.1016/j.ajog.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Melamed N, Pittini A, Hiersch L, et al. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? Am J Obstet Gynecol. 2016;215:616.e1–16.e14. doi: 10.1016/j.ajog.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moroz LA, Brock CO, Govindappagari S, Johnson DL, Leopold BH, Gyamfi-Bannerman C. Association between change in cervical length and spontaneous preterm birth in twin pregnancies. Am J Obstet Gynecol. 2017;216:159.e1–59.e7. doi: 10.1016/j.ajog.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Esplin MS, Elovitz MA, Iams JD, et al. Predictive Accuracy of Serial Transvaginal Cervical Lengths and Quantitative Vaginal Fetal Fibronectin Levels for Spontaneous Preterm Birth Among Nulliparous Women. Jama. 2017;317:1047–56. doi: 10.1001/jama.2017.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–86. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964–73. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 52.Figo Working Group On Best Practice In Maternal-Fetal M. Best practice in maternal-fetal medicine. Int J Gynaecol Obstet. 2015;128:80–2. doi: 10.1016/j.ijgo.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 53.National Institute for Health and Care Excellence. Preterm labor and birth. [November 12, 2017];NICE guideline. 2015 https://www.nice.org.uk/guidance/ng25/evidence/resources/full-guideline-2176838029. [PubMed]
- 54.Norman JE, Marlow N, Messow CM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet. 2016;387:2106–16. doi: 10.1016/S0140-6736(16)00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surveillance report (exceptional review) 2017 - Preterm labor and birth (2015) NICE guidelines NG25. [July 3, 2017]; http://www.nice.org.uk/guidance/ng25/resources/surveillance-report-exceptional-%20review-2017-preterm-labour-and-birth-2015-nice-guideline-ng25-5642777402053. [PubMed]
- 56.The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11–b13. doi: 10.1016/j.ajog.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth </= 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48:308–17. doi: 10.1002/uog.15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. Jama. 2015;313:1657–65. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 59.Higgins J, Altman D, Sterne J. Chapter 8: assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011 [Google Scholar]
- 60.Tierney JF, Vale C, Riley R, et al. Individual Participant Data (IPD) Meta-analyses of Randomised Controlled Trials: Guidance on Their Use. PLoS Med. 2015;12:e1001855. doi: 10.1371/journal.pmed.1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altman DG. Confidence intervals for the number needed to treat. Bmj. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebanoff MA. Subgroup analysis in obstetrics clinical trials. Am J Obstet Gynecol. 2007;197:119–22. doi: 10.1016/j.ajog.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. Jama. 2014;311:405–11. doi: 10.1001/jama.2013.285063. [DOI] [PubMed] [Google Scholar]
- 65.Klebanoff MA. 17 Alpha-hydroxyprogesterone caproate for preterm prevention: issues in subgroup analysis. Am J Obstet Gynecol. 2016;214:306–7. doi: 10.1016/j.ajog.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Schünemann H, Brozek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation. The GRADE Working Group. 2013 [Google Scholar]
- 67.Gradepro G. GRADEpro Guideline Development Tool [Software] McMaster University. 2015 [Google Scholar]
- 68.Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 69.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 70.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–96. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 71.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–9. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 72.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aboulghar MM, Aboulghar MA, Amin YM, AL-Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online. 2012;25:133–8. doi: 10.1016/j.rbmo.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Van Os MA, Van Der Ven AJ, Kleinrouweler CE, et al. Preventing Preterm Birth with Progesterone in Women with a Short Cervical Length from a Low-Risk Population: A Multicenter Double-Blind Placebo-Controlled Randomized Trial. Am J Perinatol. 2015;32:993–1000. doi: 10.1055/s-0035-1547327. [DOI] [PubMed] [Google Scholar]
- 75.Azargoon A, Ghorbani R, Aslebahar F. Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: A randomized placebo-controlled double-blind study. Int J Reprod Biomed (Yazd) 2016;14:309–16. [PMC free article] [PubMed] [Google Scholar]
- 76.Crowther CA, Ashwood P, Mcphee AJ, et al. Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial. PLoS Med. 2017;14:e1002390. doi: 10.1371/journal.pmed.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre-term birth: a randomised trial in India. J Obstet Gynaecol. 2009;29:493–8. doi: 10.1080/01443610902980878. [DOI] [PubMed] [Google Scholar]
- 78.Akbari S, Birjandi M, Mohtasham N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications. Scientific Journal of Kurdistan University of Medical Sciences. 2009;14:11–19. [Google Scholar]
- 79.Jo B. Statistical power in randomized intervention studies with noncompliance. Psychol Methods. 2002;7:178–93. doi: 10.1037/1082-989x.7.2.178. [DOI] [PubMed] [Google Scholar]
- 80.Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. 2012;17:120–25. doi: 10.1016/j.siny.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raju TN, Langenberg P, Sen A, Aldana O. How much ’better’ is good enough? The magnitude of treatment effect in clinical trials. Am J Dis Child. 1992;146:407–11. doi: 10.1001/archpedi.1992.02160160027007. [DOI] [PubMed] [Google Scholar]
- 82.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42.e1–42.e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yusuf S, Wittes J. Interpreting Geographic Variations in Results of Randomized, Controlled Trials. N Engl J Med. 2016;375:2263–71. doi: 10.1056/NEJMra1510065. [DOI] [PubMed] [Google Scholar]
- 84.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374.e1–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 85.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433.e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarca AL, Fitzgerald W, Chaemsaithong P, et al. The cytokine network in women with an asymptomatic short cervix and the risk of preterm delivery. Am J Reprod Immunol. 2017;78 doi: 10.1111/aji.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Brien JM, Steichen JJ, Phillips JA, Creasy GW. 490: Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 2012;206:S223. [Google Scholar]
- 89.Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–40. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 90.Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38:272–80. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 91.McNamara HC, Wood R, Chalmers J, et al. STOPPIT Baby Follow-up Study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. PLoS One. 2015;10:e0122341. doi: 10.1371/journal.pone.0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vedel C, Larsen H, Holmskov A, et al. Long-term effects of prenatal progesterone exposure: neurophysiological development and hospital admissions in twins up to 8 years of age. Ultrasound Obstet Gynecol. 2016;48:382–9. doi: 10.1002/uog.15948. [DOI] [PubMed] [Google Scholar]
- 93.Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol. 2010;202:548.e1–8. doi: 10.1016/j.ajog.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Werner EF, Han CS, Pettker CM, et al. Universal cervical-length screening to prevent preterm birth: a cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38:32–7. doi: 10.1002/uog.8911. [DOI] [PubMed] [Google Scholar]
- 95.Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213:554.e1–6. doi: 10.1016/j.ajog.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 96.Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215:100.e1–7. doi: 10.1016/j.ajog.2016.01.192. [DOI] [PubMed] [Google Scholar]
- 97.Crosby DA, Miletin J, Semberova J, Daly S. Is routine transvaginal cervical length measurement cost-effective in a population where the risk of spontaneous preterm birth is low? Acta Obstet Gynecol Scand. 2016;95:1391–95. doi: 10.1111/aogs.13021. [DOI] [PubMed] [Google Scholar]
- 98.Pizzi LT, Seligman NS, Baxter JK, Jutkowitz E, Berghella V. Cost and cost effectiveness of vaginal progesterone gel in reducing preterm birth: an economic analysis of the PREGNANT trial. Pharmacoeconomics. 2014;32:467–78. doi: 10.1007/s40273-014-0133-2. [DOI] [PubMed] [Google Scholar]
- 99.Jain S, Kilgore M, Edwards RK, Owen J. Revisiting the cost-effectiveness of universal cervical length screening: importance of progesterone efficacy. Am J Obstet Gynecol. 2016;215:101.e1–7. doi: 10.1016/j.ajog.2016.01.165. [DOI] [PubMed] [Google Scholar]
- 100.Page J, Emerson J, Cahill A, et al. 126: The impact of cervical length on the costeffectiveness of vaginal progesterone as a preterm birth intervention. American Journal of Obstetrics & Gynecology. 2013;208:S66. [Google Scholar]
- 101.Brown S, Mozurkewich E. 397: Cost analysis of universal cervical length screening and progesterone therapy in remote populations. American Journal of Obstetrics & Gynecology. 2014;210:S201. [Google Scholar]
- 102.Fonseca EB, Nishikawa AM, Paladini L, Clark OA. Cervical Assessment With Progesterone in the Prevention of Preterm Birth: A Strategy Based On Cost-Effectiveness. Value Health. 2014;17:A510. doi: 10.1016/j.jval.2014.08.1565. [DOI] [PubMed] [Google Scholar]
- 103.Eke A, Buras A, Drnec S, Woo J. 756: Vaginal progesterone versus cervical cerclage for the prevention of preterm births in women with a sonographically short cervix–a cost effectiveness and decision analysis. American Journal of Obstetrics & Gynecology. 2015;212:S367–S68. [Google Scholar]
- 104.Green PM, Argyelan A, Mutual F, Nynas J, Williams J, Keeton K. Implementation of universal cervical length screening is associated with a reduction in the rate of spontaneous preterm delivery in a low-risk cohort. Am J Obstet Gynecol. 2017;216:S10. [Google Scholar]
- 105.Combs CA. Vaginal progesterone for asymptomatic cervical shortening and the case for universal screening of cervical length. Am J Obstet Gynecol. 2012;206:101–3. doi: 10.1016/j.ajog.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Khalifeh A, Berghella V. Universal cervical length screening in singleton gestations without a previous preterm birth: ten reasons why it should be implemented. Am J Obstet Gynecol. 2016;214:603.e1–5. doi: 10.1016/j.ajog.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 107.Wilson JMG, Jungner G, Organization WH. Principles and practice of screening for disease. 1968 [Google Scholar]
- 108.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med. 2014;19:15–26. doi: 10.1016/j.siny.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- 111.Iams JD. Clinical practice. Prevention of preterm parturition. N Engl J Med. 2014;370:254–61. doi: 10.1056/NEJMcp1103640. [DOI] [PubMed] [Google Scholar]
- 112.Kuon RJ, Abele H, Berger R, et al. Progesterone for Prevention of Preterm Birth--Evidencebased Indications. Z Geburtshilfe Neonatol. 2015;219:125–35. doi: 10.1055/s-0035-1545288. [DOI] [PubMed] [Google Scholar]
- 113.Conde-Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol. 2016;214:235–42. doi: 10.1016/j.ajog.2015.09.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goodnight W. Clinical Application of Progesterone for the Prevention of Preterm Birth, 2016. Am J Perinatol. 2016;33:253–7. doi: 10.1055/s-0035-1570378. [DOI] [PubMed] [Google Scholar]
- 115.O’Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17-alphahydroxyprogesterone caproate: a critical examination of efficacy and safety. Am J Obstet Gynecol. 2016;214:45–56. doi: 10.1016/j.ajog.2015.10.934. [DOI] [PubMed] [Google Scholar]
- 116.Vintzileos AM, Visser GH. Interventions for women with mid-trimester short cervix: which ones work? Ultrasound Obstet Gynecol. 2017;49:295–300. doi: 10.1002/uog.17357. [DOI] [PubMed] [Google Scholar]
- 117.Pedretti MK, Kazemier BM, Dickinson JE, Mol BW. Implementing universal cervical length screening in asymptomatic women with singleton pregnancies: challenges and opportunities. Aust N Z J Obstet Gynaecol. 2017;57:221–27. doi: 10.1111/ajo.12586. [DOI] [PubMed] [Google Scholar]
- 118.Newnham JP, Kemp MW, White SW, Arrese CA, Hart RJ, Keelan JA. Applying Precision Public Health to Prevent Preterm Birth. Front Public Health. 2017;5:66. doi: 10.3389/fpubh.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214:365.e1–5. doi: 10.1016/j.ajog.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 120.Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214:523.e1–8. doi: 10.1016/j.ajog.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orzechowski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124:520–5. doi: 10.1097/AOG.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 122.Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the United States preterm birth rate is declining. Am J Obstet Gynecol. 2015;213:175–80. doi: 10.1016/j.ajog.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 123.Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol. 2017;216:434–42. doi: 10.1016/j.ajog.2016.11.1037. [DOI] [PubMed] [Google Scholar]
- 124.Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol Rev. 1983;75:117–38. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 125.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–61. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 126.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–48. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- 127.Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial productinduced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med. 1998;7:222–6. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 128.Henson MC. Pregnancy maintenance and the regulation of placental progesterone biosynthesis in the baboon. Hum Reprod Update. 1998;4:389–405. doi: 10.1093/humupd/4.4.389. [DOI] [PubMed] [Google Scholar]
- 129.Bernal AL. Overview of current research in parturition. Exp Physiol. 2001;86:213–22. doi: 10.1113/eph8602178. [DOI] [PubMed] [Google Scholar]
- 130.Mesiano S. Roles of estrogen and progesterone in human parturition. Front Horm Res. 2001;27:86–104. doi: 10.1159/000061038. [DOI] [PubMed] [Google Scholar]
- 131.Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 132.Mendelson CR, Condon JC. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol. 2005;93:113–9. doi: 10.1016/j.jsbmb.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 133.Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–33. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 134.Blanks AM, Brosens JJ. Progesterone action in the myometrium and decidua in preterm birth. Facts Views Vis Obgyn. 2012;4:33–43. [PMC free article] [PubMed] [Google Scholar]
- 135.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–30. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012;26:1857–67. doi: 10.1210/me.2012-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Merlino A, Welsh T, Erdonmez T, et al. Nuclear progesterone receptor expression in the human fetal membranes and decidua at term before and after labor. Reprod Sci. 2009;16:357–63. doi: 10.1177/1933719108328616. [DOI] [PubMed] [Google Scholar]
- 138.Rajabi M, Solomon S, Poole AR. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology. 1991;128:863–71. doi: 10.1210/endo-128-2-863. [DOI] [PubMed] [Google Scholar]
- 139.Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol. 1999;180:614–20. doi: 10.1016/s0002-9378(99)70263-2. [DOI] [PubMed] [Google Scholar]
- 140.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314 e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 141.Nold C, Maubert M, Anton L, Yellon S, Elovitz MA. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol. 2013;208:223 e1–7. doi: 10.1016/j.ajog.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–92. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 143.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alphareductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–92. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 144.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 145.Kirby MA, Heuerman AC, Custer M, et al. Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition. Reprod Sci. 2016;23:1473–83. doi: 10.1177/1933719116650756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One. 2013;8:e81340. doi: 10.1371/journal.pone.0081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yellon SM. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod. 2017;96:13–23. doi: 10.1095/biolreprod.116.142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chwalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor. Role of progesterone and nitric oxide. Ann N Y Acad Sci. 1997;828:238–53. doi: 10.1111/j.1749-6632.1997.tb48545.x. [DOI] [PubMed] [Google Scholar]
- 149.Chwalisz K, Shao-Qing S, Garfield RE, Beier HM. Cervical ripening in guinea-pigs after a local application of nitric oxide. Hum Reprod. 1997;12:2093–101. doi: 10.1093/humrep/12.10.2093. [DOI] [PubMed] [Google Scholar]
- 150.Chwalisz K, Garfield RE. Nitric oxide as the final metabolic mediator of cervical ripening. Hum Reprod. 1998;13:245–8. doi: 10.1093/humrep/13.2.245. [DOI] [PubMed] [Google Scholar]
- 151.Saito Y, Takahashi S, Maki M. Effects of some drugs on ripening of uterine cervix in nonpregnant castrated and pregnant rats. Tohoku J Exp Med. 1981;133:205–20. doi: 10.1620/tjem.133.205. [DOI] [PubMed] [Google Scholar]
- 152.Garfield RE, Puri CP, Csapo AI. Endocrine, structural, and functional changes in the uterus during premature labor. Am J Obstet Gynecol. 1982;142:21–7. doi: 10.1016/s0002-9378(16)32279-7. [DOI] [PubMed] [Google Scholar]
- 153.Zuidema LJ, Khan-Dawood F, Dawood MY, Work BA., Jr Hormones and cervical ripening: dehydroepiandrosterone sulfate, estradiol, estriol, and progesterone. Am J Obstet Gynecol. 1986;155:1252–4. doi: 10.1016/0002-9378(86)90154-7. [DOI] [PubMed] [Google Scholar]
- 154.Stiemer B, Elger W. Cervical ripening of the rat in dependence on endocrine milieu; effects of antigestagens. J Perinat Med. 1990;18:419–29. doi: 10.1515/jpme.1990.18.6.419. [DOI] [PubMed] [Google Scholar]
- 155.Ito A, Imada K, Sato T, Kubo T, Matsushima K, Mori Y. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301(Pt 1):183–6. doi: 10.1042/bj3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 157.Marx SG, Wentz MJ, Mackay LB, et al. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–39. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 158.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453.e1–4. doi: 10.1016/j.ajog.2006.09.009. discussion 21. [DOI] [PubMed] [Google Scholar]
- 159.Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4:369–77. doi: 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- 160.Wolf JP, Sinosich M, Anderson TL, Ulmann A, Baulieu EE, Hodgen GD. Progesterone antagonist (RU 486) for cervical dilation, labor induction, and delivery in monkeys: effectiveness in combination with oxytocin. Am J Obstet Gynecol. 1989;160:45–7. doi: 10.1016/0002-9378(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 161.Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978;130:414–8. doi: 10.1016/0002-9378(78)90282-x. [DOI] [PubMed] [Google Scholar]
- 162.Elovitz MA, Mrinalini C. The use of progestational agents for preterm birth: lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–10. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 163.Sato T, Ito A, Mori Y, Yamashita K, Hayakawa T, Nagase H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem J. 1991;275(Pt 3):645–50. doi: 10.1042/bj2750645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Imada K, Ito A, Sato T, Namiki M, Nagase H, Mori Y. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod. 1997;56:575–80. doi: 10.1095/biolreprod56.3.575. [DOI] [PubMed] [Google Scholar]
- 165.Choi SJ, Oh S, Kim JH, Roh CR. Changes of nuclear factor kappa B (NF-kappaB), cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9) in human myometrium before and during term labor. Eur J Obstet Gynecol Reprod Biol. 2007;132:182–8. doi: 10.1016/j.ejogrb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 166.Duggan SV, Lindstrom T, Iglesias T, Bennett PR, Mann GE, Bartlett SR. Role of atypical protein kinase C isozymes and NF-kappaB in IL-1beta-induced expression of cyclooxygenase-2 in human myometrial smooth muscle cells. J Cell Physiol. 2007;210:637– 43. doi: 10.1002/jcp.20901. [DOI] [PubMed] [Google Scholar]
- 167.Vidaeff AC, Ramin SM, Gilstrap LC, 3rd, Bishop KD, Alcorn JL. Impact of progesterone on cytokine-stimulated nuclear factor-kappaB signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23–8. doi: 10.1080/14767050601128019. [DOI] [PubMed] [Google Scholar]
- 168.Zaragoza DB, Wilson RR, Mitchell BF, Olson DM. The interleukin 1beta-induced expression of human prostaglandin F2alpha receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFkappaB. Biol Reprod. 2006;75:697–704. doi: 10.1095/biolreprod.106.053439. [DOI] [PubMed] [Google Scholar]
- 169.Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3:e169. doi: 10.1371/journal.pmed.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lappas M, Yee K, Permezel M, Rice GE. Lipopolysaccharide and TNF-alpha activate the nuclear factor kappa B pathway in the human placental JEG-3 cells. Placenta. 2006;27:568– 75. doi: 10.1016/j.placenta.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 171.Schmitz T, Souil E, Herve R, et al. PDE4 inhibition prevents preterm delivery induced by an intrauterine inflammation. J Immunol. 2007;178:1115–21. doi: 10.4049/jimmunol.178.2.1115. [DOI] [PubMed] [Google Scholar]
- 172.Bennett P, Allport V, Loudon J, Elliott C. Prostaglandins, the fetal membranes and the cervix. Front Horm Res. 2001;27:147–64. doi: 10.1159/000061024. [DOI] [PubMed] [Google Scholar]
- 173.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 174.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–33. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 175.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–44. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 176.Rodriguez R, Carson MA, Weigel NL, O’Malley BW, Schrader WT. Hormone-induced changes in the in vitro DNA-binding activity of the chicken progesterone receptor. Mol Endocrinol. 1989;3:356–62. doi: 10.1210/mend-3-2-356. [DOI] [PubMed] [Google Scholar]
- 177.Denner LA, Weigel NL, Maxwell BL, Schrader WT, O’Malley BW. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–3. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 178.Allan GF, Tsai SY, Tsai MJ, O’Malley BW. Ligand-dependent conformational changes in the progesterone receptor are necessary for events that follow DNA binding. Proc Natl Acad Sci U S A. 1992;89:11750–4. doi: 10.1073/pnas.89.24.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Power RF, Conneely OM, O’Malley BW. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992;13:318–23. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 180.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 181.Henderson D, Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol. 2001;185:579–85. doi: 10.1067/mob.2001.116753. [DOI] [PubMed] [Google Scholar]
- 182.Brosens JJ, Tullet J, Varshochi R, Lam EW. Steroid receptor action. Best Pract Res Clin Obstet Gynaecol. 2004;18:265–83. doi: 10.1016/j.bpobgyn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 183.Meizel S, Turner KO. Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol. 1991;77:R1–5. doi: 10.1016/0303-7207(91)90080-c. [DOI] [PubMed] [Google Scholar]
- 184.Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–12. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 185.Luconi M, Bonaccorsi L, Maggi M, et al. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab. 1998;83:877–85. doi: 10.1210/jcem.83.3.4672. [DOI] [PubMed] [Google Scholar]
- 186.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 187.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 188.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19:763–72. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 189.Mesiano S. Myometrial progesterone responsiveness. Semin Reprod Med. 2007;25:5–13. doi: 10.1055/s-2006-956771. [DOI] [PubMed] [Google Scholar]
- 190.Romero R, Scoccia B, Mazor M, Wu YK, Benveniste R. Evidence for a local change in the progesterone/estrogen ratio in human parturition at term. Am J Obstet Gynecol. 1988;159:657–60. doi: 10.1016/s0002-9378(88)80029-2. [DOI] [PubMed] [Google Scholar]
- 191.Cicinelli E, De Ziegler D. Transvaginal progesterone: evidence for a new functional ’portal system’ flowing from the vagina to the uterus. Hum Reprod Update. 1999;5:365–72. doi: 10.1093/humupd/5.4.365. [DOI] [PubMed] [Google Scholar]
- 192.Amini P, Michniuk D, Kuo K, et al. Human Parturition Involves Phosphorylation of Progesterone Receptor-A at Serine-345 in Myometrial Cells. Endocrinology. 2016;157:4434–45. doi: 10.1210/en.2016-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. doi: 10.1038/ncomms11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peters GA, Yi L, Skomorovska-Prokvolit Y, et al. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology. 2017;158:158–69. doi: 10.1210/en.2016-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Furcron AE, Romero R, Plazyo O, et al. Vaginal progesterone, but not 17alphahydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol. 2015;213:846.e1–46.e19. doi: 10.1016/j.ajog.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


