Abstract
Objective
We sought to determine whether cumulative evidence of perinatal inflammation was associated with increased risk in a ‘multi-hit’ model of neonatal white matter injury.
Methods
This retrospective cohort study included very preterm (gestational ages at delivery <32 weeks) liveborn singleton neonates delivered at Hutzel Women’s Hospital, Detroit, MI, from 2006–2011. Four pathologists blinded to clinical diagnoses and outcomes performed histological examinations according to standardized protocols. Neurosonography was obtained per routine clinical care. The primary indicator of WMI was ventriculomegaly (VE). Neonatal inflammation-initiating illnesses included bacteremia, surgical necrotizing enterocolitis, other infections, and those requiring mechanical ventilation.
Results
A total of 425 liveborn singleton neonates delivered before the 32nd week of gestation were included. Newborns delivered of pregnancies affected by chronic chorioamnionitis who had histologic evidence of an acute fetal inflammatory response were at increased risk of VE, unlike those without funisitis, relative to referent newborns without either condition, adjusting for gestational age [OR 4.7; 95%CI 1.4–15.8 vs. OR 1.3; 95%CI 0.7–2.6]. Similarly, newborns with funisitis who developed neonatal inflammation initiating illness were at increased risk of VE, unlike those who did not develop such illness, compared to the referent group without either condition [OR 3.6; 95%CI 1.5–8.3 vs. OR 1.7; 95%CI 0.5–5.5]. The greater the number of these three types of inflammation documented, the higher the risk of VE (p<0.0001).
Conclusion
Chronic placental inflammation, acute fetal inflammation and neonatal inflammation-initiating illness seem to interact in contributing risk information and/or directly damaging the developing brain of newborns delivered very preterm.
Keywords: infection, FIRS, ventricular enlargement, ventriculomegaly
Introduction
Observational studies(1–5) and experimental animal models(3, 6–13) provide extensive evidence that neuroinflammation prompted by a fetal inflammatory response syndrome (FIRS) can damage the fetal brain(14–16). Extremely preterm newborns with funisitis (umbilical cord inflammation), a histopathological landmark of the fetal inflammatory response, are also significantly more likely to have elevated blood concentrations of inflammation-related proteins on postnatal day 7(17). The fetus accordingly might be able to initiate an inflammatory response that persists or influences subsequent immune response after birth.
Amassing evidence indicates that extremely low gestational age newborns who develop intermittent or sustained systemic neonatal inflammation(18) are at significantly elevated risk of perinatal brain damage (19–23). Systemic neonatal inflammation can also develop de novo in response to postnatal infection or other exposures unrelated to infection, such as mechanical ventilation(24, 25). A study of extremely preterm newborns reported that the association between persistent or recurrent systemic neonatal inflammation and neurodevelopmental impairment is independent of postnatal inflammation-initiating illnesses(19). Additionally, a combination of postnatal inflammation-initiating illnesses (bacteremia or necrotizing enterocolitis combined with those that require mechanical ventilation) was significantly associated with cognitive impairment when adjusting for markers of neonatal systemic inflammation.
In addition to acute inflammation, chronic inflammatory placental lesions have also been associated with indicators of perinatal brain damage, including intra-ventricular hemorrhage(26) and periventricular leukomalacia(27). Yet, it remains unclear if such risk varies according to the occurrence or absence of subsequent fetal and/or neonatal inflammation. In a multi-hit model of brain injury, it is possible that chronic inflammation may sensitize or predispose fetuses to be more vulnerable to subsequent inflammatory insults. It is also possible that the fetal inflammatory response promotes processes leading to systemic neonatal inflammation(17), and that this sensitizes the brain to be more vulnerable to subsequent inflammatory insults after birth(24). Evidence from experimental studies supports this view. In rats and mice, neonatal lipopolysaccharide pre-treatment can sensitize the brain to be more vulnerable to subsequent hypoxic-ischemic injury(28–30) and excitotoxicity(31). Evidence from an observational study also supports the potential for a two-hit model of brain injury to operate in extremely preterm newborns(32).
In this study, we examine i) to what extent chronic chorioamnionitis contributes information about risk of neonatal white matter injury depending on the occurrence of histopathologic landmarks of the fetal inflammatory response (i.e., inflammation of the umbilical cord or placental surface vessels(33)), ii) whether risk information provided by evidence of a fetal inflammatory response differs according to the occurrence or absence of postnatal inflammation-initiating illnesses (i.e., bacteremia, surgical necrotizing enterocolitis/ isolated bowel perforation, and those requiring mechanical ventilation), and iii) if there is evidence of higher risk of WMI with greater cumulative evidence of perinatal inflammation.
Methods
Study Design & Participants
This retrospective cohort study includes very preterm (gestational ages at delivery <32 weeks) singleton neonates cared for at Hutzel Women’s Hospital, Detroit, MI, who underwent routine neurosonographic examination from 2006–2011. Each of these newborns was delivered of a mother who participated in research conducted by the Perinatology Research Branch (PRB) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). All patients provided written informed consent and the use of clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
Histopathologic Examination of the Placenta
Microscopic analysis was performed by four pathologists who were blinded to clinical diagnoses and outcomes per standard protocols. This involved up to nine sections of the placenta, including at least two full thickness sections of the placental disc, two cord sections, and one membrane roll from the extraplacental membranes. At least one full thickness section was randomly taken from the center of the placenta,(34) others may have been taken from the placental margin.
Chronic placental inflammatory lesions include chronic chorioamnionitis, deciduaitis and villitis of unknown etiology (27, 35, 36). Chronic chorioamnionitis was diagnosed when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue is observed (37). Chronic deciduitis was defined by either a diffuse lymphocytic infiltration of the deciduas or any infiltrate accompanied by plasma cells(27). Villitis of unknown etiology (VUE) was defined as the presence of lymphohistiocytic infiltrate in varying proportion of the villous tree of placenta (35, 38).
Placental lesions consistent with maternal or fetal responses to amniotic fluid infection were diagnosed according to criteria established by the Perinatology Section of the Society for Pediatric Pathology(39). Lesions consistent with a maternal response include acute chorioamnionitis, whereas funinsitis (i.e., histologic inflammation of the umbilical cord) and inflammation of the extraplacental membranes are consistent with a fetal inflammatory response (i.e., umbilical phlebitis /chorionic vasculitis; umbilical arteritis; concentric umbilical perivasculitis or intense chorionic vasculitis with recent non-occlusive chorionic vessel thrombi).
Postnatal Inflammation-Initiating Illnesses
Postnatal inflammation-initiating illnesses diagnosed in the first week of postnatal life included: 1) early-onset neonatal infection, 2) surgical necrotizing enterocolitis, 3) meningitis, 4) invasive fungal infection, and 5) those that required invasive mechanical ventilation in the first week of life. These conditions were selected based on the knowledge that neonatal sepsis (24, 40–47), necrotizing enterocolitis or bowel injury(34, 48–53), meningitis(54–57) as well as mechanical ventilation and its correlates(24, 58–60) are each associated with systemic inflammation.
Early-onset neonatal infection was diagnosed based on a positive blood culture in the first 72 hours of life, or the presence of clinical symptoms without a positive blood culture requiring at least 5 days of intravenous broad-spectrum antibiotics in the first postnatal week(61). Surgical necrotizing enterocolitis was defined as having a peritoneal drain or exploratory laparotomy performed as clinically indicated(62). Neonates with suspected spontaneous intestinal perforation (SIP) were excluded if they did not have any other inflammation-initiating illness as described above. Meningitis was diagnosed if the cerebrospinal fluid indicated a positive culture(61), leukocytosis, increased protein concentration, or decreased glucose concentration(63, 64).
White Matter Injury
Neurosonography was clinically obtained as part of routine care in the first 7–10 days of life, at 21–35 days and at discharge or 36 weeks postmenstrual age whichever came sooner. In addition, neonates with intracranial pathology were scanned weekly until the lesion remained stable or resolved. The following neurosonographic lesions were considered as evidence of WMI: 1) ventricular dilatation, or ventriculomegaly (VE); 2) periventricular echolucent lesion (PVE); and 3) periventricular leukomalacia(65) (PVL).
The primary indicator of WMI was VE; PVE and PVL were examined as secondary outcomes. This distinction was based on the knowledge that VE is a more reproducible neurosonographic diagnosis than PVE(66, 67) and also the small number of children who carried the diagnosis of PVL in this study. Ventriculomegaly (VE) was defined based on ventricular size on sagittal scan at the mid-body of the lateral ventricles(68). Periventricular echogenicity included focal or diffuse areas of increased echogenicity surrounding the lateral ventricles. PVL included cystic lesions in the brain parenchyma(68).
Statistical analysis
Binomial proportions with 95% confidence intervals (CI) and medians with interquartile ranges were calculated for categorical and arithmetic variables, respectively. Differences in distributions of arithmetic and categorical variables were respectively examined using the Mann-Whitney U-test and the Chi-square or Fisher’s exact test, where appropriate. Logistic regression models were fit to examine magnitudes of association. Covariables considered as potential confounders included gestational age at delivery, as well as maternal age, race, nulliparity, pre-pregnancy body mass index, smoking status, preeclampsia, birth weight in the bottom decile of a US reference population(69) (i.e., small for gestational age [SGA]), and male sex. Multivariable model reduction was performed based on change in main effect parameter estimates and statistical association between independent and dependent variables examined with respect to time-order.
To account for potential model over-fitting in light of small study group sizes when exploring a 3-hit model, a priori analyses used bootstrap estimated linear shrinkage factors to calculate conservative regression estimates less likely to be affected by over-fitting(70). Firth’s penalized maximum likelihood estimation was also performed to resolve separation issues(71). Statistical significance was defined by 95% confidence intervals (CI) that did not include the null estimate. Statistical analyses were performed using SAS version 9.3 (Carry, N.C.).
Results
Descriptive Characteristics
A total of 425 live born singleton newborns were delivered before the 32nd week of gestation during the study period, each underwent neurosonographic examination as part of routine care. Table 1 shows the descriptive characteristics of the study population. The majority (85%) of participants was identified as African American, 56% were born via cesarean delivery, 13% were born SGA, and 13% expired before discharge.
Table 1.
Descriptive Characteristics of the Study Population
| Characteristic | N or Median |
% or Inter-quartile range |
|---|---|---|
| Maternal Age* | 25 | 21–31 |
|
| ||
| Multiparous | 242 | 57 |
| Nulliparous | 182 | 43 |
|
| ||
| +African-American | 359 | 85 |
| − African-American | 64 | 15 |
|
| ||
| + Preeclampsia | 112 | 26 |
| − Preeclampsia | 313 | 74 |
|
| ||
| +Any Chronic Inflammatory lesion | 189 | 46 |
| − Any Chronic Inflammatory lesion | 226 | 54 |
|
| ||
| + Acute Chorioamnionitis | 201 | 48 |
| − Acute Chorioamnionitis | 214 | 52 |
|
| ||
| +Funisitis | 145 | 35 |
| − Funisitis | 269 | 65 |
|
| ||
| + pPROM ≥1 day | 123 | 29 |
| + pPROM < 1 day | 26 | 6 |
| − pPROM | 276 | 65 |
|
| ||
| + Antenatal Steroid | 386 | 93 |
| − Antenatal Steroid | 27 | 7 |
|
| ||
| + Magnesium Sulfate | 174 | 46 |
| − Magnesium Sulfate | 206 | 54 |
|
| ||
| + Cesarean section | 237 | 56 |
| − Cesarean section | 188 | 44 |
|
| ||
| Male | 222 | 53 |
| Female | 200 | 47 |
|
| ||
| Gestational Age at Delivery* | 29 | 26–30 |
|
| ||
| + Small for Gestational Age | 55 | 13 |
| − Small for Gestational Age | 370 | 87 |
|
| ||
| + Antibiotics continued to 7 days | 133 | 32 |
| − Antibiotics continued to 7 days | 283 | 68 |
|
| ||
| + Postnatal Steroid in first 7 days | 24 | 6 |
| − Postnatal Steroid in first 7 days | 398 | 94 |
|
| ||
| + Inflammation-Initiating Illness | 265 | 62 |
| − Inflammation-Initiating Illness | 160 | 38 |
Note: += presence, −= absence. Values are expressed as number (percentage) or
median (interquartile range). Missing values are as follows: Maternal Age, n=1; Gender, n=3; Parity, n=1; Acute Chorioamnionitis, n=10; Funisitis, n=11; Antenatal Steroid, n=12; Magnesium Sulfate, n=45; Antibiotics continued to 7 days, n=9; Postnatal Steroid in first 7 days, n=3.
Placental Lesions
The presence or absence of histopathological placental lesions was recorded for 415 (98%) study participants. Nearly half of the examined placentas had one or more chronic inflammatory lesions (n=189, 46%), chronic chorioamnionitis was the most common of these lesions (n=132, 32%), and VUE was the least common (n=8, 2%). Pregnancies affected by chronic inflammatory lesions were significantly less likely to also be affected by acute chorioamnionitis (odds ratio 0.6; 95% confidence interval (CI) 0.4–0.9).
Acute chorioamnionitis was diagnosed in 49% of the studied pregnancies; among them, 71% delivered fetuses with funisitis. Four additional cases of funisitis were diagnosed among pregnancies not also affected by acute chorioamnionitis. About half of the newborns with lesions consistent with a fetal inflammatory response had stage 2 inflammation of the umbilical arteries and/or umbilical vein (n=76, 52%), whereas stage 1 acute chorionic vasculitis/umbilical phlebitis and stage 3 necrotizing funisitis were less common (n=50, 35% and n= 19, 13%, respectively).
Neonatal Inflammation-Initiating Illness
One or more neonatal inflammation-initiating illnesses was diagnosed in the first week of postnatal life in 62% of the study population (n=265). The prevalence of early-onset sepsis, surgical necrotizing enterocolitis, and conditions that required invasive mechanical ventilation in the first postnatal week was 32%, 2% and 37%, respectively. Meningitis was diagnosed in 7% (n=28) of the study population, respectively.
White Matter Injury
Neurosonographic evidence of VE, PVE and PVL was identified in 20% (n=83), 21% (n=88) and 4% (n=15) of the study population, respectively. The median duration of hospitalization (days) among these neonates diagnosed with WMI was 66 days (interquartile range 38–96 days).
Magnitudes of association
Table 2 shows the magnitudes of association among each indicator of WMI with placental lesions and, separately, neonatal inflammation-initiating illness.
Table 2.
Magnitudes of Association among Chronic Inflammatory Lesions of the Placenta, Acute Chorioamnionitis, Funisitis/Chorionic Vasculitis, Neonatal Inflammation-initiating illness, and each Indicator of White Matter Injury
| Type of inflammation |
N (%) | Ventricular Dilatation (n=83) |
Echodense Lesion (n=88) |
Periventricular Leukomalacia (n=14) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | aOR | 95%CI | OR | 95%CI | aOR | 95%CI | OR | 95%CI | aOR | 95%CI | |||||||||
| Chronic Chorioamnionitis | + | 132(32) | 1.1 | 0.7 | 1.9 | 1.3 | 0.8 | 2.3 | 1.0 | 0.6 | 1.7 | 1.1 | 0.6 | 1.9 | 1.2 | 0.4 | 3.6 | 1.3 | 0.4 | 4.2 |
| − | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | ||||||||
|
| ||||||||||||||||||||
| Chronic Deciduitis | + | 107(26) | 2.1 | 1.2 | 3.5 | 1.5 | 0.9 | 2.7 | 1.0 | 0.6 | 1.7 | 0.7 | 0.4 | 1.2 | 3.0 | 1.03 | 8.8 | 2.2 | 0.7 | 6.6 |
| − | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | ||||||||
|
| ||||||||||||||||||||
| Acute Chorioamnionitis | + | 203(49) | 2.5 | 1.5 | 4.1 | 1.9 | 1.1 | 3.3 | 1.3 | 0.8 | 2.1 | 0.9 | 0.5 | 1.5 | 1.4 | 0.5 | 4.2 | 1.0 | 0.3 | 3.1 |
| − | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | ||||||||
|
| ||||||||||||||||||||
| Funisitis/Chorionic Vasculitis | + | 145(35) | 1.8 | 1.1 | 3.0 | 1.5 | 0.9 | 2.7 | 1.3 | 0.8 | 2.1 | 1.1 | 0.7 | 1.9 | 1.2 | 0.4 | 3.7 | 0.8 | 0.3 | 2.5 |
| − | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | ||||||||
|
| ||||||||||||||||||||
| Any neonatal Inflammation-initiating illness | + | 265(62) | 2.7 | 1.5 | 4.7 | 2.6 | 1.4 | 4.7 | 1.1 | 0.7 | 1.8 | 0.9 | 0.6 | 1.6 | 2.3 | 0.6 | 8.5 | 2.1 | 0.6 | 7.8 |
| − | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | 1 | reference | ||||||||
Note: adjusted models included gestational age at delivery as an independent variable. OR= odds ratio; aOR=adjusted odds ratio, CI= confidence interval. Less than ten placentas had evidence of chronic villitis, results specific to this lesion are not reported.
Chronic chorioamnionitis was not associated with WMI. Newborns delivered of pregnancies affected by chronic deciduitis, however, were twofold more likely to develop VE, and, separately, threefold more likely to develop PVL, each compared to newborns delivered in the absence of chronic deciduitis [odds ratio (OR) 2.1; 95% confidence interval (CI) 1.2–3.5; OR 3.0; 95%CI 1.03–8.8, respectively]. However, multivariable adjustment for gestational age at delivery rendered these associations non-significant. Chronic vilitis was associated with PVL only after adjustment for gestational age, although this association should be considered in light of the small number of newborns with this lesion in this sample.
Acute chorioamnionitis, funisitis and neonatal inflammation-initiating illness were each significantly associated with increased risk of VE. However, adjustment for gestational age rendered the association between funisitis and VE non-significant. In contrast, acute chorioamnionitis and neonatal inflammation-initiating illness were each significantly associated with about twofold greater risk of VE compared to newborns without these conditions, respectively, with and without adjustment for gestational age.
Informed by the associations shown in Table 2, subsequent analyses focus on VE and not PVE or PVL.
Two-Hit Model #1: Combined or isolated occurrence of chronic chorioamnionitis and funisitis/chorionic vasculitis
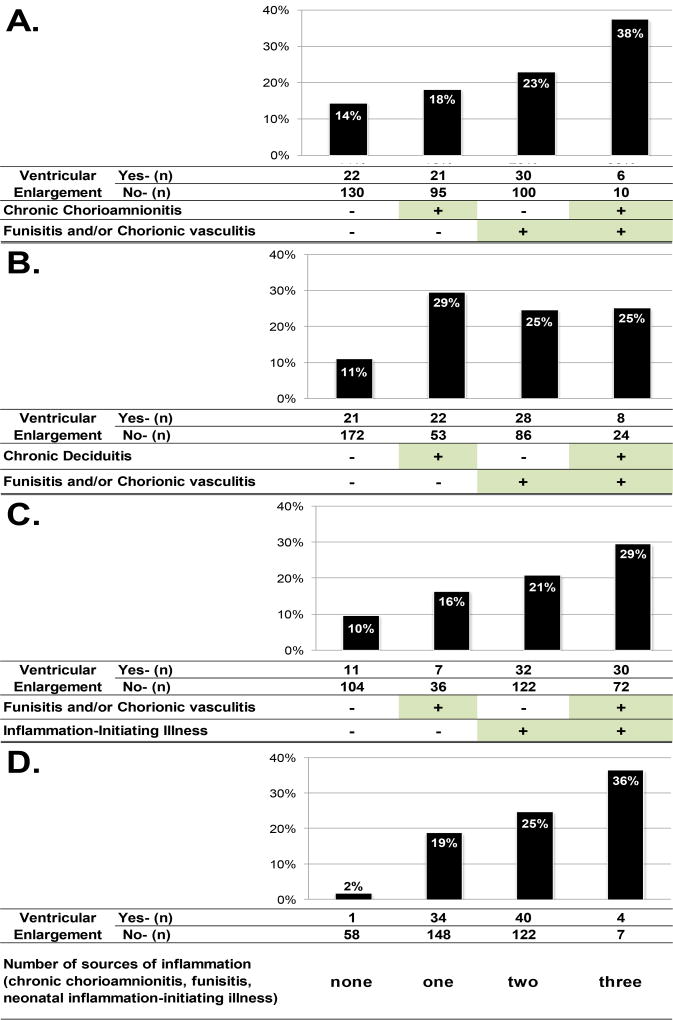
The first two-hit model examined whether risk information provided by the presence of chronic chorioamnionitis differs depending on the occurrence of funisitis, a histopathological landmark of the fetal inflammatory response. Specifically, the risk of VE was examined among four study groups formed based on the combined or isolated occurrence of each condition: 1) neonates exposed to chronic inflammatory lesions of the placenta who also developed funisitis; 2) neonates exposed to chronic inflammatory lesions of the placenta who did not develop funisitis; 3) neonates unexposed to chronic inflammatory lesions that developed funisitis; and 4) neonates who neither were exposed to chronic inflammatory lesions nor developed funisitis. Figure 1A shows that the prevalence of VE was greatest among newborns with chronic chorioamnionitis who developed funisitis. Table 3 shows that these newborns were more than three times as likely to develop VE as neonates without either condition [OR 3.5; 95%CI 1.2–10.7], and the association strengthened while remaining statistically significant with multivariable adjustments for gestational age, and other potential confounders (maternal age, pre-pregnancy body mass index, smoking, nulliparity, presence and duration of pre-labor rupture of membranes, small for gestational age and male sex). On the other hand, neither chronic chorioamnionitis nor funisitis alone (i.e., unaccompanied by the other) was significantly associated with VE in this sample.
Figure 1.
Risk of ventricular enlargement by the presence or absence of chronic chorioamnionitis, funisitis, and/or chorionic vasculitis and inflammation-initiating illness
Table 3.
Two-hit models of the risk of Ventriculomegaly Depending on Presence or Co-Occurrence of Chronic Chorioamnionitis, funisitis/chorionic vasculitis and inflammation-initiating illness
| 2-hit Models | Model I | Model II | Model III | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chronic Chorioamnionitis |
Acute Fetal Inflammatory Response |
OR | 95%CI | OR | 95%CI | OR | 95%CI | |||
| + | + | 3.5 | 1.2 | 10.7 | 4.7 | 1.4 | 15.8 | 4.5 | 1.3 | 15.8 |
| + | − | 1.3 | 0.7 | 2.5 | 1.3 | 0.7 | 2.6 | 1.0 | 0.5 | 2.2 |
| − | + | 1.8 | 0.96 | 2.3 | 1.5 | 0.8 | 2.8 | 1.2 | 0.6 | 2.4 |
| − | − | 1 | reference | 1 | reference | 1 | reference | |||
|
| ||||||||||
| Chronic Deciduitis | Acute Fetal Inflammatory Response | OR | 95%CI | OR | 95%CI | OR | 95%CI | |||
|
| ||||||||||
| + | + | 2.7 | 1.1 | 6.9 | 1.8 | 0.7 | 4.7 | 1.3 | 0.5 | 3.7 |
| + | − | 3.2 | 1.7 | 6.4 | 2.4 | 1.2 | 4.9 | 1.9 | 0.9 | 4.1 |
| − | + | 2.7 | 1.4 | 5.0 | 2.2 | 1.2 | 4.2 | 2.0 | 1.0 | 4.0 |
| − | − | 1 | reference | 1 | reference | 1 | reference | |||
|
| ||||||||||
| Acute Fetal Inflammatory Response | Neonatal Inflammation-Initiating Illness | OR | 95%CI | OR | 95%CI | OR | 95%CI | |||
|
| ||||||||||
| + | + | 4.3 | 1.9 | 9.6 | 3.6 | 1.5 | 8.3 | 3.4 | 1.4 | 8.2 |
| + | − | 2.0 | 0.7 | 6 | 1.7 | 0.5 | 5.5 | 1.4 | 0.4 | 4.4 |
| − | + | 2.7 | 1.3 | 6 | 2.5 | 1.1 | 5.7 | 2.5 | 1.1 | 5.6 |
| − | − | 1 | reference | 1 | reference | 1 | reference | |||
Model I: unadjusted
Model II: adjusted gestational age at delivery
Model III: adjusted for gestational age at delivery, maternal age, pre-pregnancy body mass index, smoking, nulliparity, presence and duration of pre-labor rupture of membranes, small for gestational age, male sex
Two-Hit Model #2: Combined or isolated occurrence of funisitis/chorionic vasculitis and Chronic Deciduitis
The second two-hit model examined whether risk information provided by chronic deciduitis varied according to the subsequent presence or absence of an acute fetal inflammatory response (funisitis or chorionic vasculitis). In contrast with the two-hit model involving chronic chorioamnionitis, Figure 1B shows that there was little difference in risk among study groups who had either chronic deciduitis or funisitis, or both. Table 3 also shows that newborns delivered of pregnancies affected by chronic deciduitis who developed funisitis were not at elevated risk of VE, unlike those who had either of these conditions alone, relative to the referent group with none of these conditions.
Two-Hit Model #3: Combined or isolated occurrence of funisitis/chorionic vasculitis and neonatal inflammation-initiating illness
The third two hit model examined whether risk information provided by funisitis/chorionic vasculitis differed according to the subsequent occurrence or absence of neonatal inflammation-initiating illness. Figure 1C shows that the highest prevalence of VE was observed among newborns with funisitis who subsequently developed neonatal inflammation-initiating illness. These newborns were four times as likely to have VE as those without either condition [OR 4.3; 95%CI 1.9–9.6]. In contrast, newborns with funisitis/chorionic vasculitis without subsequent inflammation-initiating illness were not at significantly greater risk of VE than neonates without these conditions [OR 2.0; 95%CI 0.7–6]. On the other hand, neonates without histologic evidence of an acute fetal inflammatory response who developed an inflammation-initiating illness were twofold as likely to have VE as newborns with neither of these two conditions [OR 2.7; 95%CI 1.3–6.0]. These magnitudes of association were reduced, yet were in the same direction and had consistent statistical significance with multivariable adjustment for gestational age at delivery, and further for additional potential confounders. [Table 3].
Three-Hit Models: Combined or isolated occurrence of chronic chorioamnionitis, funisitis/chorionic vasculitis and neonatal inflammation-initiating illness
The odds of having VE modeled according to study groups representing the presence or absence of three types of inflammatory conditions are shown relative to newborns without any evidence of inflammation, adjusted for gestational age, in Table 4. The first 3-hit model involved chronic chorioamnionitis, funisitis and neonatal inflammation initiating illness. Newborns delivered of pregnancies affected by chronic chorioamnionitis who had histologic evidence of an acute fetal inflammatory response and later developed neonatal inflammation-initiating illness were nine times as likely to have VE as those without such evidence of inflammation. Newborns exposed to two of these three conditions were also at 4 to 6 fold greater risk of VE. (Table 4) Alternatively, in this sample, funisitis occurring in the absence of chronic chorioamnionitis and subsequent neonatal inflammation-initiating illness was not significantly associated with VE.
Table 4.
Three-hit model of the risk of Ventriculomegaly Depending on Presence or Co-Occurrence of Chronic Chorioamnionitis or Chronic Deciduitis, funisitis/chorionic vasculitis and neonatal inflammation-initiating illness
| 3-hit Models | N | % | OR^ | 95%CI | |||
|---|---|---|---|---|---|---|---|
| Chronic Chorioamnionitis |
Acute Fetal Inflammatory Response |
Neonatal Inflammation- Initiating Illness |
|||||
| + | + | + | 11 | 2.7 | 9.7 | 2.1 | 45.2 |
|
| |||||||
| + | + | − | 5* | 1.2 | * | * | * |
|
| |||||||
| + | − | − | 53 | 12.8 | 4.7 | 1.3 | 17.3 |
| + | − | + | 63 | 15.2 | 4.8 | 1.3 | 17.1 |
| − | + | + | 94 | 22.7 | 6.1 | 1.8 | 21 |
| − | + | − | 36 | 8.7 | 2.6 | 0.6 | 10.8 |
| − | − | + | 93 | 22.5 | 5.6 | 1.6 | 19.4 |
| − | − | − | 59 | 14.3 | 1 | reference | |
|
| |||||||
| Chronic Deciduitis | Acute Fetal Inflammatory Response | Neonatal Inflammation-Initiating Illness | N | % | OR^ | 95%CI | |
|
| |||||||
| + | + | + | 21 | 5.1 | 3.0 | 1.02 | 9.0 |
|
| |||||||
| + | + | − | 11* | 2.7 | * | * | * |
|
| |||||||
| + | − | − | 32 | 7.7 | 1.8 | 0.6 | 5.3 |
| + | − | + | 43 | 10.4 | 4.4 | 1.8 | 10.8 |
| − | + | + | 84 | 20.3 | 3.4 | 1.5 | 7.8 |
| − | + | − | 30 | 7.3 | 1.7 | 0.5 | 5.3 |
| − | − | + | 113 | 27.3 | 2.0 | 0.8 | 4.6 |
| − | − | − | 80 | 19.3 | reference | ||
Note:
odds ratios (OR) are adjusted for gestational age, and were estimated using Firth’s penalized maximum likelihood estimation and a bootsrap-derived linear shrinkage factor estimated by 1,000 simulations was applied to estimate conservative regression coefficients.
data not reported due to small sample size.
The second 3-hit model differed from the first by incorporating chronic deciduitis instead of chronic chorioamnionitis, along with funisitis and neonatal inflammation-initiating illness. None of these three inflammatory conditions, when occurring in isolation, was associated with elevated risk of VE in this sample. (Table 4) In contrast, study groups with two or more types of inflammation were at three to four times greater risk of VE than newborns without these inflammatory conditions, adjusting for gestational age category.
Cumulative sources of inflammation
Figure 1D shows that the risk of VE increases with greater cumulative evidence of inflammation (chronic chorioamnionitis, funisitis and neonatal inflammation-initiating illness), irrespective of the timing of these conditions (Cochran-Armitage Trend Test, p < 0.0001).
Discussion
The principal findings of this study are
1) the contribution of chronic chorioamnionitis to elevated risk of ventriculomegaly depends on the occurrence or absence of an acute fetal inflammatory response, unlike chronic deciduitis; 2) the contribution of funisitis, a histological landmark of the fetal inflammatory response, to increased risk of ventriculomegaly similarly depends on whether or not subsequent neonatal inflammation initiating illness occurs; and 3) irrespective of time order, newborns with two or more of the studied perinatal inflammatory conditions more frequently had VE than those with one or none of these conditions. These findings support the view that chronic placental and acute fetal inflammation may each prime the developing brain to be more vulnerable to subsequent inflammation (e.g., that occurring as a consequence of neonatal illness). Thus, it seems that initiators of chronic maternal inflammation, acute fetal inflammation and neonatal inflammation-initiating illness might interact in contributing risk information and/or directly damaging the developing brain of newborns delivered very preterm.
Perinatal inflammation and brain damage
Microbial invasion of the amniotic cavity, intra-amniotic inflammation and both chronic and acute inflammatory placental lesions are frequently present in patients with spontaneous preterm labor who deliver preterm(72–82). Microorganisms in the amniotic cavity can attack the fetus, and this can initiate a systemic fetal inflammatory response syndrome(5, 14, 15), which can directly damage the brain (1–10, 15), and seems to promote tertiary damage mechanisms (83).
Intra-amniotic inflammation can also occur in the absence of a demonstrable microbial invasion of the amniotic cavity(84). This may be a consequence of organisms that escape detection using current cultivation or molecular microbiological techniques (e.g., certain viruses). Alternatively, it is well-recognized that immunologic “danger signals(85)” can elicit sterile inflammation. The immune system identifies these signals as “Danger Associated Molecular Patterns”, or DAMPs (i.e. IL-1α and HMGB-1). Recent evidence supports that DAMPs can occur in the setting of intra-amniotic inflammation, either without bacteria or when the inflammatory process induced by bacteria has generated tissue damage(86).
Postnatally, systemic neonatal inflammation prompted by infectious or non-infectious illnesses is also associated with perinatal brain damage (19, 87–89). Molecular mechanisms implicated in the pathogenesis of this damage include microglial activation and disturbances of apoptosis resulting in the arrest of oligodendrocyte maturation, and impaired neurogenesis, axonal growth and synaptogenesis(15, 87, 88, 90, 91).
Synthesis
Chronic and acute maternal, fetal and neonatal inflammation, each have been associated with elevated risk of brain injury, yet, many preterm newborns exposed to these conditions escape brain damage, why? We examined a multi-hit model to answer whether the risk of WMI differed according to the cumulative evidence of perinatal inflammation.
Our findings support the view that early inflammation may prime the developing brain to be more vulnerable to subsequent inflammation inducing events, and that the cumulative amount is indeed an important contributor of information about increased risk of perinatal brain injury. The basis for this hypothesis is the two-hit (or multi-hit) model of brain damage, in which an initial insult sensitizes or conditions the brain to be more vulnerable to a subsequent insult. In our case, chronic inflammation may be the first hit, an acute fetal inflammatory response the second, and neonatal inflammation initiating illness may constitute a third. This view is supported by experimental evidence indicating that perinatal inflammation can sensitize the brain to be more vulnerable to subsequent insults.(28–31)
Preclinical models have also shown that the timing of an inflammatory exposure may alter the course of subsequent brain injury (92–95). Subacute exposure to lipopolysaccharide (LPS) 24h prior to a second noxious exposure (e.g., hypoxia-ischemia or further LPS exposure) in a rodent model lessened brain injury as compared with exposure to the second insult alone. In contrast, very acute (6h prior) or remote exposures (72h prior) intensified injury following the second exposure, even when sub-threshold (not of enough severity to cause injury alone)(92). It is possible that chronic chorioamnionitis or funisitis/chorionic vasculitis identified on placental pathology at the time of birth may correspond to this subacute exposure which might have contributed to neonatal white matter injury.
Assessing postnatal inflammation-initiating illnesses
The term, ‘inflammation-initiating illness’, was coined by others(19) to emphasize the difference between inflammation occurring in the context of postnatal illness and that which appears to be initiated by the fetus or neonate in response to intrauterine infection/inflammation. In this study, we considered bacteremia as well as other infections, and conditions that required ventilation in the first week of postnatal life, as well as surgical necrotizing enterocolitis based on the knowledge that each is associated with systemic inflammation (24, 40–44)(48–50). None the less, direct measurement of postnatal systemic inflammatory markers would be desirable.
Strengths and limitations
The major strengths of this study include blinding of pathologists to obstetrical diagnoses and outcomes, the use of standardized protocols for placental examination, use of statistical techniques designed for small sample size estimation and definition of the study population by gestational age rather than birth weight(96). Limitations include the use of clinically obtained scans (without a central reader or assessment of inter-rater agreement), lack of information to classify severity of ventriculomegaly, and the possibility that some newborns may have developed WMI that was not detected. These findings may also be specific to like settings, given the high prevalence of VE in this study. Like all observational studies, time-ordered relationships warrant confirmation and should be interpreted appropriately with respect to sample size.
Conclusion
Chronic placental inflammation, acute fetal inflammation and neonatal inflammation-initiating illness interact in contributing risk information and/or directly damaging the developing brains of newborns delivered very preterm.
Acknowledgments
Financial support
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS, and the Wayne State University Perinatal Initiative.
Footnotes
Part of this work was presented in poster form at the 2014 Pediatric Academic Societies annual meeting.
References
- 1.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. AMNIOTIC-FLUID INTERLEUKIN-6 - A SENSITIVE TEST FOR ANTENATAL DIAGNOSIS OF ACUTE INFLAMMATORY LESIONS OF PRETERM PLACENTA AND PREDICTION OF PERINATAL MORBIDITY. Am J Obstet Gynecol. 1995;172(3):960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 2.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174(5):1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1 beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177(1):19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 4.Heep A, Behrendt D, Nitsch P, Fimmers R, Bartmann P, Dembinski J. Increased serum levels of interleukin 6 are associated with severe intraventricular haemorrhage in extremely premature infants. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2003;88(6):F501–F4. doi: 10.1136/fn.88.6.F501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. Journal of perinatology : official journal of the California Perinatal Association. 2008;28(11):759–65. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean JM, Farrag D, Zahkouk SA, Yamany E, Zawahry E, Hagberg H, et al. Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep. Neuroscience. 2009;60(3):606–15. doi: 10.1016/j.neuroscience.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 7.Gavilanes AWD, Strackx E, Kramer BW, Gantert M, van den Hove D, Steinbusch H, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol. 2009;200(4) doi: 10.1016/j.ajog.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Wallace K, Veerisetty S, Paul I, May W, Miguel-Hidalgo JJ, Bennett W. Prenatal Infection Decreases Calbindin, Decreases Purkinje Cell Volume and Density and Produces Long-Term Motor Deficits in Sprague-Dawley Rats. Dev Neurosci. 2010;32(4):302–12. doi: 10.1159/000319506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodygensky GA, West T, Stump M, Holtzman DM, Inder TE, Neil JJ. In vivo MRI analysis of an inflammatory injury in the developing brain. Brain Behavior and Immunity. 2010;24(5):759–67. doi: 10.1016/j.bbi.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177(4):797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 11.Shen Y, Yu HM, Yuan TM, Gu WZ, Wu YD. Intrauterine infection induced oligodendrocyte injury and inducible nitric oxide synthase expression in the developing rat brain. J Perinat Med. 2007;35(3):203–9. doi: 10.1515/JPM.2007.058. [DOI] [PubMed] [Google Scholar]
- 12.Lyng K, Munkeby BH, Scheie D, Mallard C, Hagberg H, Stray-Pedersen B, et al. Fetal brain injury in experimental intrauterine asphyxia and inflammation in Gottingen minipigs. J Perinat Med. 2006;34(3):226–34. doi: 10.1515/JPM.2006.041. [DOI] [PubMed] [Google Scholar]
- 13.Yuan TM, Yu HM, Gu WZ, Li JP. White matter damage and chemokine induction in developing rat brain after intrauterine infection. J Perinat Med. 2005;33(5):415–22. doi: 10.1515/JPM.2005.074. [DOI] [PubMed] [Google Scholar]
- 14.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 15.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24(9):1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34(1):5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 17.Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90(2):235–43. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014 doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Shea TM, Allred EN, Kuban KCK, Dammann O, Paneth N, Fichorova R, et al. Elevated Concentrations of Inflammation-Related Proteins in Postnatal Blood Predict Severe Developmental Delay at 2 Years of Age in Extremely Preterm Infants. The Journal of Pediatrics. 2012;160(3):395–401.e4. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leviton A, Allred EN, Dammann O, Engelke S, Fichorova RN, Hirtz D, et al. Systemic Inflammation, Intraventricular Hemorrhage, and White Matter Injury. J Child Neurol. 2012 doi: 10.1177/0883073812463068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, et al. The Relationship between Early Concentrations of 25 Blood Proteins and Cerebral White Matter Injury in Preterm Newborns: The ELGAN Study. The Journal of Pediatrics. 2011;158(6):897–903.e5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70(4):550–65. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 24.Chang BA, Huang Q, Quan J, Chau V, Ladd M, Kwan E, et al. Early inflammation in the absence of overt infection in preterm neonates exposed to intensive care. Cytokine. 2011;56(3):621–6. doi: 10.1016/j.cyto.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohlen G, Antal EA, Castellheim A, Thaulow E, Kielland A, Saugstad OD. Hyperoxic resuscitation after hypoxia-ischemia induces cerebral inflammation that is attenuated by tempol in a reporter mouse model with very young mice. J Perinat Med. 2013;41(3):251–7. doi: 10.1515/jpm-2012-0135. [DOI] [PubMed] [Google Scholar]
- 26.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 27.Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31(3):292–5. doi: 10.1016/s0046-8177(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang XY, Hagberg H, Nie CX, Zhu CL, Ikeda T, Mallard C. Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxia-ischemia. J Neuropathol Exp Neurol. 2007;66(6):552–61. doi: 10.1097/01.jnen.0000263870.91811.6f. [DOI] [PubMed] [Google Scholar]
- 29.Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci. 2001;13(6):1101–6. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Sameshima H, Ikeda T, Ikenoue T. Lipopolysaccharide administration enhances hypoxic-ischemic brain damage in newborn rats. J Obstet Gynaecol Res. 2004;30(2):142–7. doi: 10.1111/j.1447-0756.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 31.Rousset CI, Kassem J, Olivier P, Chalon S, Gressens P, Saliba E. Antenatal bacterial endotoxin sensitizes the immature rat brain to postnatal excitotoxic injury. J Neuropathol Exp Neurol. 2008;67(10):994–1000. doi: 10.1097/NEN.0b013e31818894a1. [DOI] [PubMed] [Google Scholar]
- 32.Leviton A, Fichorova RN, O'Shea TM, Kuban K, Paneth N, Dammann O, et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res. 2012 doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185(2):496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 34.van Vliet EO, de Kieviet JF, Oosterlaan J, van Elburg RM. Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatr. 2013;167(7):662–8. doi: 10.1001/jamapediatrics.2013.1199. [DOI] [PubMed] [Google Scholar]
- 35.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. Journal of immunology (Baltimore, Md : 1950) 2009;182(6):3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PloS one. 2011;6(2):e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Modern Pathology. 2010;23(7):1000–11. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Human pathology. 2007;38(10):1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation - A workshop report. Placenta. 2005;26:S114–S7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Leviton A, O'Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O, et al. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr. 2012;101(4):355–9. doi: 10.1111/j.1651-2227.2011.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocabas E, Sarikcioglu A, Aksaray N, Seydaoglu G, Seyhun Y, Yaman A. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor-necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49(1):7–20. [PubMed] [Google Scholar]
- 42.Kurt ANC, Aygun AD, Godekmerdan A, Kurt A, Dogan Y, Yilmaz E. Serum IL-1 beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Mediators Inflamm. 2007 doi: 10.1155/2007/31397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnon S, Litmanovitz I. Diagnostic tests in neonatal sepsis. Current Opinion in Infectious Diseases. 2008;21(3):223–7. doi: 10.1097/QCO.0b013e3282fa15dd. [DOI] [PubMed] [Google Scholar]
- 44.Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198(4) doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121(1):129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 47.Sood BG, Shankaran S, Schelonka RL, Saha S, Benjamin DK, Jr, Sanchez PJ, et al. Cytokine profiles of preterm neonates with fungal and bacterial sepsis. Pediatr Res. 2012;72(2):212–20. doi: 10.1038/pr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103(4):766–71. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 49.Ng PC, Ang IL, Chiu RWK, Li KR, Lam HS, Wong RPO, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest. 2010;120(8):2989–3000. doi: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KCK, et al. Neurodevelopment of Extremely Preterm Infants who had Necrotizing Enterocolitis with or without Late Bacteremia. J Pediatr. 2011;157(5) doi: 10.1016/j.jpeds.2010.05.042. 751-U82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatia AM, Stoll BJ, Cismowski MJ, Hamrick SE. Cytokine Levels in the Preterm Infant with Neonatal Intestinal Injury. Am J Perinatol. 2013 doi: 10.1055/s-0033-1353437. [DOI] [PubMed] [Google Scholar]
- 52.Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PloS one. 2012;7(5):e36977. doi: 10.1371/journal.pone.0036977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azuma H, Tsuda N, Sasaki K, Okuno A. Clinical significance of cytokine measurement for detection of meningitis. The Journal of Pediatrics. 1997;131(3):463–5. doi: 10.1016/s0022-3476(97)80079-0. [DOI] [PubMed] [Google Scholar]
- 55.Moller K, Tofteng F, Qvist T, Sahl C, Sonderkaer S, Pedersen BK. Cerebral output of cytokines in patients with pneumococcal meningitis. Crit Care Med. 2005;33(5):979–83. doi: 10.1097/01.ccm.0000162494.84354.9d. [DOI] [PubMed] [Google Scholar]
- 56.Barichello T, Generoso JS, Silvestre C, Costa CS, Carrodore MM, Cipriano AL, et al. Circulating concentrations, cerebral output of the CINC-1 and blood-brain barrier disruption in Wistar rats after pneumococcal meningitis induction. Eur J Clin Microbiol Infect Dis. 2012;31(8):2005–9. doi: 10.1007/s10096-011-1533-2. [DOI] [PubMed] [Google Scholar]
- 57.Dulkerian SJ, Kilpatrick L, Costarino AT, Jr, McCawley L, Fein J, Corcoran L, et al. Cytokine elevations in infants with bacterial and aseptic meningitis. J Pediatr. 1995;126(6):872–6. doi: 10.1016/s0022-3476(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 58.Bose CL, Laughon MM, Allred EN, O'Shea TM, Van Marter LJ, Ehrenkranz RA, et al. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61(1):315–22. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gitto E, Reiter RJ, Cordaro SP, La Rosa M, Chiurazzi P, Trimarchi G, et al. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am J Perinatol. 2004;21(4):209–16. doi: 10.1055/s-2004-828610. [DOI] [PubMed] [Google Scholar]
- 60.Krediet TG, Kavelaars A, Vreman HJ, Heijnen CJ, van Bel F. Respiratory distress syndrome-associated inflammation is related to early but not late peri/intraventricular hemorrhage in preterm infants. J Pediatr. 2006;148(6):740–6. doi: 10.1016/j.jpeds.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 61.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama-Journal of the American Medical Association. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 62.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srinivasan L, Shah SS, Padula MA, Abbasi S, McGowan KL, Harris MC. Cerebrospinal fluid reference ranges in term and preterm infants in the neonatal intensive care unit. J Pediatr. 2012;161(4):729–34. doi: 10.1016/j.jpeds.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivasan L, Shah SS, Abbasi S, Padula MA, Harris MC. Traumatic lumbar punctures in infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J. 2013;32(10):1150–2. doi: 10.1097/INF.0b013e31829862b7. [DOI] [PubMed] [Google Scholar]
- 65.Banker BQ, Larroche J-C. Periventricular leukomalacia of infancy: a form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 66.O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KCK, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brouwer MJ, de Vries LS, Groenendaal F, Koopman C, Pistorius LR, Mulder EJ, et al. New reference values for the neonatal cerebral ventricles. Radiology. 2012;262(1):224–33. doi: 10.1148/radiol.11110334. [DOI] [PubMed] [Google Scholar]
- 68.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–38. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 69.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 70.Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 71.FIRTH D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 72.Romero R, Gómez R, Chaiworapongsa T, Conoscenti G, Cheol Kim J, Mee Kim Y. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 73.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11(1) doi: 10.1016/s0891-5520(05)70347-0. 135-&. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Gomez R, Ghezzi F, Yoon BY, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179(1):186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. INFECTION AND LABOR .5. PREVALENCE, MICROBIOLOGY, AND CLINICAL-SIGNIFICANCE OF INTRAAMNIOTIC INFECTION IN WOMEN WITH PRETERM LABOR AND INTACT MEMBRANES. Am J Obstet Gynecol. 1989;161(3):817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 76.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, et al. INFECTION AND LABOR .6. PREVALENCE, MICROBIOLOGY, AND CLINICAL-SIGNIFICANCE OF INTRAAMNIOTIC INFECTION IN TWIN GESTATIONS WITH PRETERM LABOR. Am J Obstet Gynecol. 1990;163(3):757–61. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 77.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179(5):1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 78.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 79.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183(5):1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 80.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92(1):77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 81.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38(3):261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J, Kim JS, Park JW, Park CW, Park JS, Jun JK, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34(8):681–9. doi: 10.1016/j.placenta.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurology. 2012;11(6):556–66. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Chaiworapongsa T, Savasan ZA, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. Journal of Maternal-Fetal & Neonatal Medicine. 2011;24(12):1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns - dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79(1):1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Dammann O, Leviton A. Perinatal Brain Damage Causation. Dev Neurosci. 2007;29(4–5):280–8. doi: 10.1159/000105469. [DOI] [PubMed] [Google Scholar]
- 89.Paneth N, Korzeniewski SJ, Hong T. The Role Of The Intrauterine And Perinatal Environment in Cerebral Palsy. NeoReviews. 2005;6:e133–e44. [Google Scholar]
- 90.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58(6):821–8. doi: 10.1002/ana.20662. [DOI] [PubMed] [Google Scholar]
- 91.Kaindl AN, Favrais G, Gressens P. Molecular Mechanisms Involved in Injury to the Preterm Brain. J Child Neurol. 2009;24(9):1112–8. doi: 10.1177/0883073809337920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatric research. 2005;58(1):112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 93.Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. Journal of neurosurgery. 2005;103(4):715–23. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- 94.Ikeda T, Yang L, Ikenoue T, Mallard C, Hagberg H. Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatric research. 2006;59(1):56–60. doi: 10.1203/01.pdr.0000191140.87314.ce. [DOI] [PubMed] [Google Scholar]
- 95.Bordet R, Deplanque D, Maboudou P, Puisieux F, Pu Q, Robin E, et al. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(8):1190–6. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. VERY-LOW-BIRTH-WEIGHT - A PROBLEMATIC COHORT FOR EPIDEMIOLOGIC STUDIES OF VERY SMALL OR IMMATURE NEONATES. Am J Epidemiol. 1991;134(6):604–13. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]