Abstract
Rods and cones mediate visual perception over 9 log units of light intensities, with both photoreceptor types contributing to a middle 3-log unit range that comprises most night-time conditions. Rod function in this mesopic range has been difficult to isolate and study in vivo because of the paucity of mutants that abolish cone signaling without causing photoreceptor degeneration. Here we describe a novel Gnat2 knockout mouse line (Gnat2−/−) ideal for dissecting rod and cone function. In this line, loss of Gnat2 expression abolished cone phototransduction, yet there was no loss of cones, disruption of the photoreceptor mosaic, nor change in general retinal morphology up to at least 9 months of age. Retinal microglia and Müller glia, which are highly sensitive to neuronal pathophysiology, were distributed normally with morphologies indistinguishable between Gnat2−/− and wildtype adult mice. ERG recordings demonstrated complete loss of cone-driven a-waves in Gnat2−/− mice; comparison to WT controls revealed that rods of both strains continue to function at light intensities exceeding 104 photoisomerizations rod−1 s−1. We conclude that the Gnat2−/− mouse is a preferred model for functional studies of rod pathways in the retina when degeneration could be an experimental confound.
Keywords: G-protein, Photoreceptor, Retina, Phototransduction
1. Introduction
The retinal circuits that relay rod and cone photoreceptor signals to retinal ganglion cells overlap extensively in the mammalian retina. Cones form chemical synapses with cone bipolar cells, which transmit visual signals to retinal ganglion cells (Strettoi et al., 2010). Rods transmit visual information via multiple pathways, all of which involve cone bipolar cells. The primary rod pathway employs chemical synapses between rods and rod bipolar cells, which pass information to A2 amacrine cells, which in turn modulate On- and Off-cone bipolar cells. The secondary rod pathway transmits rod responses via gap junctions directly to cones, which then relay the signals synaptically to cone bipolar cells (Deans et al., 2002). In the less common tertiary rod pathway, rods form chemical synapses directly with cone bipolar cells (Pang et al., 2010). Although convergent pathways from both photoreceptor classes are advantageous for signaling across a wide range of light intensities, such convergence also makes dissecting the functional contributions of rods and cones to the retinal circuitry challenging.
Performing experiments under scotopic or photopic conditions is a useful way to functionally isolate rod and cone signals, but only under those extreme conditions. At intermediate light levels, both rods and cones generate light responses, but the functional consequences of their individual contributions to a combined output are difficult to assess (e.g. Naarendorp et al., 2010). An ideal way to separate the contributions of rod and cone signaling would be to specifically render all the rods or all the cones insensitive to light without disrupting their synaptic connections and partners. Pharmacological manipulations such as superfusion with the metabotropic glutamate receptor agonist, 2-amino-4-phosphonobutyric acid (APB), are commonly used to overwhelm light-driven changes in synaptic release, but such manipulations affect all ON-bipolar cells or all Off-bipolar cells rather than all rod inputs or all cone inputs to the circuitry. Moreover, pharmacologically silencing the cone pathway downstream of cone photoreceptors necessarily eliminates some of the rod input to vision by silencing the secondary rod pathway.
Several mutant mouse lines have been created to genetically silence rod or cone light responses. For example, mice lacking the rod-specific G-protein transducin alpha subunit (Gnat1−/−) lack phototransduction signaling downstream of rhodopsin and thus have no rod electrical responses to light, with no detectable concurrent photoreceptor degeneration (Calvert et al., 2000). However, mutants with defects in the cone phototransduction cascade often exhibit morphological abnormalities. One mouse line commonly used to isolate rod function is the Gnat2cpfl3 mutant, which contains a single-base pair missense mutation in the gene encoding the cone-specific G-protein transducin alpha subunit, Gnat2 (Chang et al., 2006). In these mice, neither Gnat2 expression nor cone function is abolished initially, but both decline through adulthood and are finally undetectable by 9 months of age (Chang et al., 2006). Importantly, rod-driven responses in Gnat2cpfl3 mice also decline with age, with clear evidence of photoreceptor degeneration before 27 weeks of age, or approximately 6 months (Chang et al., 2006). More recently, the Gnat2c.518A > G mouse line was found to lack cone function due to an amino acid substitution that results in incorrect protein folding and ultimately a loss of Gnat2 function (Jobling et al., 2013). While the cones of Gnat2c.518A > G mice do not completely degenerate, they exhibit some structural abnormalities as early as 3 months of age, which become more pronounced and lead to Müller cell gliosis as the mice further age. These abnormalities include M-opsin mislocalization, displaced photoreceptor terminals, altered horizontal cell morphology, and altered bipolar cell morphology, suggesting significant disruption to both rod and cone pathways within the circuitry.
Here we describe a novel Gnat2 knock-out mouse line (Gnat2−/−) that exhibits a complete loss of cone function without retinal degeneration or other noticeable morphological abnormalities. Importantly, rod function in these animals appears to be completely normal throughout adulthood, revealing the upper limit of the mesopic range of rod function, and refining previous estimates for cone thresholds and the magnitude of cone signaling in the mouse.
2. Materials and methods
2.1. Animals
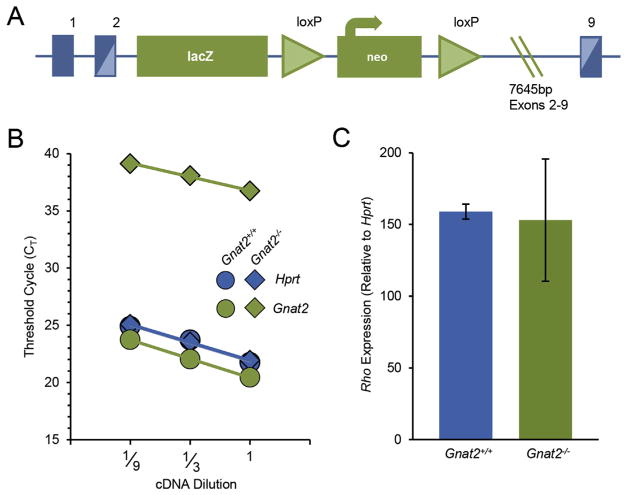
Mice were cared for and handled in accordance with NIH and UC Davis IACUC guidelines. C57BL/6J mice were obtained from the Jackson Laboratory (Sacramento CA). To disrupt Gnat2 expression, a 7654 base pair deletion spanning exons 2–9 of the Gnat2 gene on chromosome 3 was achieved by inserting a lacZ/Neo cassette using homologous recombination (Fig. 1A). ES cell clones were generated by the UC Davis Mouse Biology Program to yield Gnat2tm1(KOMP)Vlcg during targeting project VG17376, here referred to as Gnat2−/−. Vector clones and embryonic stem cells are commercially available from the UC Davis KOMP Repository (Davis, CA). Gnat2−/− ES cells were implanted into 129Sv/J host dams, and subsequently maintained on a C57Bl6/J background for more than 10 generations, with at least 3 rounds of out-and in-breeding Gnat2 heterozygotes with particular attention to genotyping for and selecting against the rd8 mutation. All mice used in this study lacked the rd8 mutation. For specific experiments, heterozygous Gnat2 mice were bred to generate both wild-type and knock-out littermates for direct comparisons; because wild-type littermates were phenotypically indistinguishable from C57BL/6J, the two data sets were ultimately pooled for comparisons. In each case, mice of both sexes were used.
Fig. 1.
Targeted deletion of Gnat2 decreases Gnat2 mRNA expression while Rho expression remains unchanged. (A) Targeted deletion strategy used to generate the Gnat2−/−mouse line (adapted from KOMP). (B) Representative qRT-PCR results from wild-type (filled circles) and Gnat2−/−(filled diamonds) littermates 3 months of age. Threshold cycle (CT) values are shown as a function of cDNA dilutions used for Gnat2 (green filled) and Hprt (blue filled). Across 4 independent experiments, the CT values for Gnat2 in the knockout were dramatically increased, indicating that there was at least a 60,000-fold reduction in expression in the knockout (p-value < 0.001). (C) Relative rhodopsin expression was unchanged between the knockout and control littermate (p-value = 0.75; n = 4–7). Bar graph shows mean 2−ΔΔCT ± SD.
2.2. Quantitative real time PCR
Whole retinas were isolated from 3–4-month-old Gnat2−/− and Gnat2+/+ littermate control mice. RNA was isolated from whole retinas using the RNeasy Plus Mini Kit (Qiagen) and cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (ThermoScientific). RNA and cDNA concentrations and quality were determined using a NanoDrop (ThermoScientific). TaqMan Gene Expression Assays (ThermoFisher Scientific) were used to quantify mRNA expression of Gnat2, Hprt, and Rho (Mm00492394_m1, Mm03024075_m1, and Mm01184405_m1 respectively) (UC Davis Real-Time PCR Research and Diagnostics Core Facility). Relative expression of Gnat2 and Rhodopsin were calculated using the comparative CT method (also known as the 2−ΔΔCT method; Schmittgen and Livak, 2008). This calculation assumes that the PCR efficiency for all genes is similar, which was confirmed for these probes and conditions using multiple dilutions (not shown). Some Gnat2−/− samples did not reach CT threshold for detection within the range of the apparatus although the wells were replicating; in such instances, the samples were assigned the maximum hypothetical CT value of 40 (based on the cycling conditions) for relative gene expression calculations. Statistical significance was calculated after combining the comparative CT calculations across independent experiments at a matching dilution using a two-tailed Student’s t-Test.
2.3. Immunohistochemistry
Mice 6 and 9 months of age were sacrificed by carbon dioxide euthanasia before enucleation. Eyes were fixed in 4% paraformaldehyde for 25–30 min, and the cornea and lens were removed during fixation. To examine histology and protein localization, fixed eyecups were embedded in agarose and sliced into 150 μm thick sections on a vibratome (Leica). Sections were blocked in normal goat serum at room temperature, followed by an overnight incubation with primary antibodies at 4 °C, and then incubation with secondary antibodies for 1.5–2 h at room temperature. To examine the number of cone photoreceptors and microglia morphology, retinal flatmounts were prepared rather than retinal slices. In brief, whole retinas were removed from the fixed posterior pole and cut to relax the curvature. Isolated retinas were incubated in 1% Triton X-100 in PBS overnight at 4 °C followed by blocking with normal goat serum for 2 h in a 37 °C water bath. Primary antibody incubation proceeded overnight at 4 °C, followed by incubation in secondary antibodies for 1.5–2 h in a 37 °C water bath. Both retinal sections and flat mounts were mounted on glass slides with Diamond Antifade Mountant (Life Technologies) before imaging. Primary antibodies and nuclei stains used are listed in Table 1, and secondary antibodies were Alexa Fluor-conjugated (Life Technologies) and used at 1:200 or 1:300 dilution. All sections and flat mounts were imaged using a Nikon A1 confocal microscope.
Table 1.
Antibodies and stains used for immunohistochemical staining of retinal sections and flat-mounts.
| Target | Host Species or Name | Catalog Number | Manufacturer | RRID | Dilution |
|---|---|---|---|---|---|
| Cones | Lectin PNA, Alexa Fluor 568 conjugated | L32458 | Life Technologies | NA | 1:100 |
| GFAP | Rabbit | Z0334 | Dako | AB_10013382 | 1:1000 |
| Gnat2 | Rabbit | LS-C321680 | LifeSpan Biosciences | none | 1:100 |
| Iba1 | Rabbit | 019–19741 | Wako Pure Chem Industries, Ltd. | AB_2665520 | 1:1000 |
| M-opsin | Rabbit | None | From Cheryl Craft | AB_2314755 | 1:100 |
| Nuclei | NucBlue® Fixed Cell Stain | R37606 | Life Technologies | NA | 1 drop/mL |
| S-opsin | Rabbit | None | From Cheryl Craft | none | 1:100 |
Cone photoreceptor density was assessed in 5 separate areas of each flat-mounted retina: superior, inferior, nasal, temporal, and central. Two squares of 150 μm × 150 μm were randomly chosen within each area of each retina, and cones were manually selected and quantified using a counting function (Nikon NES-Elements). Statistical significance was calculated using a two-tailed Student’s t-Test.
2.4. Optical coherence tomography
Mice 5 and 9 months of age were anesthetized with isoflurane and the pupils dilated with tropicamide and phenylephrine. High acquisition-speed Fd-OCT (132 nm @ 855 nm broadband light source [Superlum] and CMOS camera [Basler] operating at 100,000 A-scans/s) were used to obtain in vivo mouse retinal volumetric data sets as described in Zhang et al. (2015). Images were acquired over 1.9 × 1.9 mm retinal area (51 deg FOV; see Zhang et al., 2016). OCT volumes were flattened using a strip-registration algorithm to align each A-scan in the data set. To measure the thickness of retinal layers, all A-scan intensity profiles were averaged from the flattened OCT volume, and evaluated using the choroid, INL, and GCL as landmarks. The distance from choroid to INL defined the outer retinal thickness and the distance from INL to GCL was defined as inner retinal thickness.
2.5. Corneal electroretinography
After dark adaptation overnight, mice were anesthetized with isoflurane and the pupils dilated with tropicamide and phenylephrine. Electroretinograms (ERGs) were recorded with a Maxwellian-view Ganzfeld ERG system (Phoenix Research Labs), equipped with two independent calibrated LED sources (365 and 510 nm). A reference electrode was inserted subcutaneously above the eye, and a ground electrode was inserted subcutaneously at the base of the tail. Combined rod and cone photoreceptor function was characterized using 1 ms flashes of 365 and 510 nm light. Assuming an end-on collecting area of 0.87 μm2 for rods (Lyubarsky et al., 2004) and a collecting area of 1 μm2 for cones (Daniele et al., 2005), a flash delivering 100 photons μm−2 suppresses an average of ~50% of the circulating current of rods (Peinado Allina et al., 2017), but less than 4% of the circulating current of cones (Nikonov et al., 2006). We thus used this flash strength to probe the scotopic ERG in Fig. 4. In order to isolate cone photoreceptor function, a saturating probe flash (1 ms of 365 nm, 1.22 × 106 photons/ μm2) was delivered on top of a rod-desensitizing background (5 s of 510 nm light, delivering at least 1 × 105 photons μm−2 sec−1).
Fig. 4.
Knockout retinas display normal rod-driven a- and b-wave amplitudes. (A) Comparison of the average response of Gnat2−/− (green) and wild-type (WT, blue) animals to a flash of 100 photons μm−2. (B) Average bright flash responses of Gnat2−/− and WT animals (2 × 106 photons μm−2. (C–D) Peak electroretinogram a-wave (C) and b-wave (D) amplitudes of WT (blue circles) and Gnat2−/−(green diamonds) mice in response to increasingly bright flashes of 510 nm light (32–1.22 × 106 photons μm−2).
Dark intervals of 20–60 s were interposed between consecutive flashes to ensure complete recovery. These intervals also served to minimize oscillatory potentials that commonly develop over the course of a recording session. To measure a- and b-wave amplitudes, oscillatory potentials were removed prior to analysis by filtering with a 5 ms boxcar filter. A-wave amplitudes were measured from the baseline to the peak of the negative deflection of the unfiltered traces, and b-wave amplitudes were measured from the peak of the unfiltered a-wave to the peak of the filtered, positive-going deflection. All traces were analyzed using IgorPro 7.06 (WaveMetrics). Statistical significance of comparisons between wild-type and Gnat2−/− mice was calculated using a two-tailed Student’s t-Test. Mice used for ERGs were 3–5 months of age.
3. Results
3.1. Loss of Gnat2 expression does not produce cone dysmorphogenesis or retinal degeneration
Gnat2 expression was targeted for disruption by inserting a 7654 base pair deletion spanning exons 2–9 of the Gnat2 gene through homologous recombination (Fig. 1A; see Methods section 2.1). Successful disruption of gene expression was first confirmed by examining Gnat2 mRNA expression levels using quantitative real-time PCR (qRT-PCR). Gene expression was quantified using the comparative CT method, using Hprt as a reference gene. Gnat2 expression was undetectable; based on our detection limits, mRNA levels were decreased at least 6 × 104-fold in the knock-out retinas (p-value = 0.00055, Fig. 1B). Using the same analysis methods and different primers (see Methods), rhodopsin (Rho) expression was likewise quantified and found to be unchanged between Gnat2−/− and WT control littermates (p-value = 0.75, Fig. 1C), consistent with normal rod photoreceptor number and outer segment rhodopsin densities in the mutant retina.
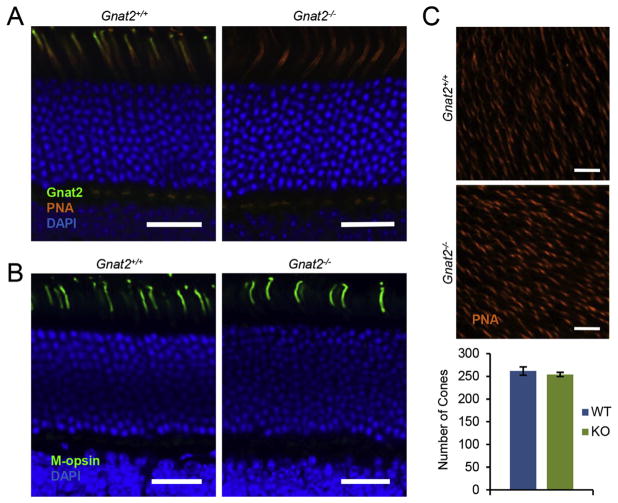
To examine Gnat2 protein expression as well as cone photoreceptor morphology, we used immunohistochemistry and peanut agglutinin (PNA) staining of cones in retinas of both knockout and wild-type littermates (Fig. 2). Staining with PNA revealed comparable outer segment and synaptic morphologies of cones from both genotypes (Fig. 2A). Cone synapses were never observed to be displaced to the inner or outer nuclear layers, and there were no morphological abnormalities observed in the outer plexiform layer. While there was clear Gnat2 immunoreactivity in the cone outer segments of wild-type retinas, there was no detectable staining in the Gnat2−/− retinas, consistent with complete loss of protein expression (Fig. 2A). DAPI staining revealed normal nuclear layers in Gnat2−/− retinas. Indeed, the outer nuclear layers of knock-out mice and wild-type litter mate controls were indistinguishable at 6 and 9 months (ONL thickness measurements, in μm: 6-month-old WT and Gnat2−/−, 51.72 ± 5.84 and 52.31 ± 3.87 respectively; 9-month-old Gnat2−/− 53.79 ± 5.63; no statistically significant differences, all p > 0.05). Additionally, staining for both M-opsin (Fig. 2B) and S-opsin (data not shown) was comparable between control and knockout mice, with no evident opsin mislocalization. Finally, the numbers of cones per unit retinal area were counted in retinal flat mounts, with both Gnat2−/− and wild-type littermates showing comparable cell densities (Fig. 2C). Thus, there were no signs of degeneration of cones in the Gnat2−/− mice up to 9 months of age.
Fig. 2.
Normal cone photoreceptor morphology and number in Gnat2−/− retinas. (A) In retinal sections, PNA staining (orange) showed normal cone morphology and Gnat2 staining (green) confirmed loss of Gnat2 protein in the knockout. Shown here are representative wild-type and Gnat2−/− littermates, 5 months of age. (B) M-opsin staining (green) was the same in wild-type and knockout littermates. (C) Representative 150 μm × 150 μm areas of control and knockout littermate retinal flat mounts stained with PNA to visualize cones. Quantification revealed no difference in the number of cones per unit area (p-value = 0.4817; n = 5 measurements per flatmount, 4 flatmounts from each strain). All scale bars are 25 μm.
Next, we evaluated the thickness and integrity of the retina layers by in vivo optical coherence tomography (OCT; Fig. 3A). OCT uses interferometry to extract depth-dependent measures of back-scattered light, and the intensity and axial distribution of this signal are useful means to quantify subtle differences in retinal structure between mice or within an individual animal over time (e.g. Levine et al., 2014; Zhang et al., 2015). OCT B-scans showed the retinal layers in the Gnat2−/− mice to be indistinguishable from those of wild-type mice up to 9 months of age, with all retinas showing clearly delineated nuclear and plexiform layers, transparent (non-scattering) outer nuclear layers as well as an intact external limiting membrane, all signs of healthy photoreceptors (Fig. 3A; Levine et al., 2014). Measurements of outer (Fig. 3B) and inner retina thicknesses (data not shown) revealed no differences between wild-type and knockout strains at any age examined. Thus, there were no overt signs of photoreceptor or retinal degeneration in vivo.
Fig. 3.
Gnat2−/− retinas do not exhibit retinal degeneration. (A) OCT B-scans through the optic nerve head show normal layering of WT and Gnat2−/− retinas. Shown here are a representative 5-month-old WT retina (top) and a representative 9-month-old Gnat2−/− retina (bottom). The optic nerve head is located at the far right of each image. Scale bars indicates 100 μm. (B) The outer retinal thickness (choroid to INL) was on average the same in 5-month-old (5 mo) WT and Gnat2−/− mice, as well as 9 month old (9 mo) Gnat2−/− mice. Data displayed are mean ± range (n = 4–6 eyes for each). (C) Maximum intensity projections (through 5 μm in z) of the outer plexiform layer of Gnat2+/+ and Gnat2−/− littermate flat mounts stained for Iba1 (green, microglia) and PNA (orange, cones) reveal normal ramified microglia at the photoreceptor synapses. Scale bars indicate 50 μm. (D) Representative images of retinal sections from C57BL/6J and Gnat2−/− stained for CRALBP (orange) and GFAP (green) reveal no differences in reactive gliosis. Scale bars indicate 50 μm. IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigmented epithelium.
To look for more subtle changes in retinal health, we examined the morphology and localization of retinal microglia, the resident macrophages that respond to neuronal injury or stress by changing their morphology and migrating to the site of insult. Staining both retinal sections (data not shown) and flat mounts (Fig. 3C) with the microglial marker Iba1 revealed normal ramified morphologies and normal densities of microglial cells in the outer plexiform layer with no microglial invasion into the nuclear layers. We also examined retinal sections for signs of Müller cell gliosis by immunostaining for GFAP. No difference was detectable between control or knockout retinas (Fig. 3D). These results provide further evidence that degeneration does not occur in the Gnat2−/− retina by 9 months of age.
3.2. Absence of cone-driven retinal signaling in the absence of Gnat2
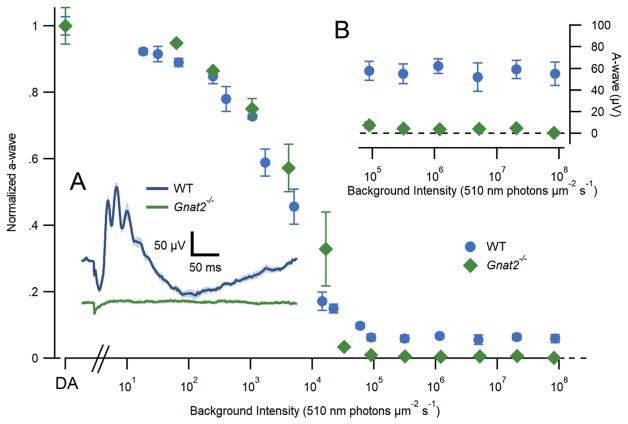
To assess retinal function in the absence of Gnat2, we used in vivo electroretinograms (ERGs). The ERG provides information about the electrical responses of both photoreceptors (the a-wave) and bipolar cells (the b-wave). We first probed rod signaling in dark-adapted animals by recording ERGs in response to a flash that strongly activated rods, but negligibly activated cones (100 photons μm−2; see Methods section 2.5). The ERGs elicited by such a flash were indistinguishable in both amplitude and time course between Gnat2−/− and wild-type animals (Fig. 4A), suggesting that rod function and rod-driven bipolar cell signaling were normal in Gnat2−/− mice. Strongly consistent with this hypothesis, intensity-response functions for the a-wave and b-wave amplitudes recorded from dark-adapted animals were indistinguishable between mouse strains (Fig. 4C and D). Moreover, the ERG elicited by a stimulus calculated to saturate both rod and cone responses (1 ms flash of 2 × 106 photons μm−2; Fig. 4B) had nearly identical saturating a-wave amplitudes (WT: 1035 ± 55 μV, mean ± SEM, n = 5 and Gnat2−/−: 985 ± 25 μV, n = 3; p-value = 0.462207), consistent with the scotopic a-wave being dominated by rods. The completely normal characteristics of scotopic ERGs of the Gnat2−/− mice reveal that rod function is unperturbed by the loss of Gnat2 expression in cones.
In contrast, cone-driven ERGs were undetectable in the absence of Gnat2 expression. In wild-type mice, a bright flash delivered in the presence of a rod-saturating background elicited a photopic ERG response with a cone-driven a-wave of approximately 60 μV in amplitude, while no such cone-driven a-wave was detectable in Gnat2−/−mice (Fig. 5A–B). Because Gnat2−/− mice show normal cone morphology and normal rod function but lack visual transduction in cones, this strain is ideal for dissecting the contributions of rods and cones to the ERG across a range of steady light intensities. To examine the conditions under which cone function is manifest in the ERGs of the wild-type mice, we measured the response to an intense ultraviolet flash (365 nm, 1 ms, 1.22 × 106 photons μm−2) in the presence of a 510 nm backgrounds with intensities ranging over 7 log10 units (Fig. 5). A UV flash was used because it strongly activates the predominant mouse cone opsin, S-opsin, which has a 360 nm λmax (Lyubarsky et al., 1999). In contrast, the 510 nm backgrounds negligibly activate the S-opsin, while strongly suppressing the rod circulating current. In the presence of scotopic and mesopic backgrounds up to 1 × 104 photons μm2 s−1 (~104 R*/rod/s), both wild-type and Gnat2−/− mice showed indistinguishable a-wave amplitudes, consistent with predominance of rod electrical responses in this intensity regime. However, as the background intensity increased above 5 × 104 photons μm−2 s−1, Gnat2−/−mice failed to generate detectable ERG responses, whereas wild-type mice continued to show cone-driven flash responses whose maximal amplitude changed negligibly over a 1000-fold range of backgrounds (Fig. 5, insets). These results establish the absence of phototransduction in Gnat2−/− cones, while also revealing the level of rod excitation (~104 R*/rod/s) at which cones become the predominant source underlying the a-wave.
Fig. 5.
Cone-driven ERG responses are absent in Gnat2−/−mice. Electroretinogram a-wave amplitudes of WT (blue circles) and Gnat2−/− (green diamonds) mice in response to saturating flashes (365 nm; 1.22 × 106 photons μm−2) delivered on increasingly intense 510 nm backgrounds. Insets: (A) Average electroretinogram of WT (blue) and Gnat2−/−(green) mice in response to a 1 ms saturating flash delivered on top of a rod-saturating background (510 nm; 1 × 105 photons μm−2 s−1). The small deflection in the Gnat2−/−trace is an electrical artifact. (B) Expanded view of WT and Gnat2−/− a-wave amplitudes at the brightest background intensities. Error bars (frequently smaller than the points themselves) represent SEM.
4. Discussion
Many studies that have attempted to dissect rod and cone contributions to retinal signaling have utilized Gnat1−/− mice, whose rods are rendered completely insensitive to light by the loss of the alpha subunit of the rod G protein, transducin (Calvert et al., 2000). In contrast, the use of the analogous disruption in cone phototransduction through genetic deletion of the cone G protein, Gnat2, has been hampered by concurrent rod pathway dysfunction or degeneration (Chang et al., 2006; Jobling et al., 2013), effects that can confound conclusions about rod function. Here we have presented evidence that cone photoreceptors can be rendered insensitive to light by abolishing Gnat2 expression without any loss of cone cell numbers or rod function. Thus, for the first time there is a genetic line of mice lacking cone function but having intact rod circuitry, and thus an opportunity to fully examine to the extent to which rods are capable of signaling under mesopic and photopic conditions. Here we summarize our findings in the context of previous efforts in this realm and then discuss the significance of rod signaling at high light intensities for future work.
4.1. Comparison of Gnat2−/− mice to other common cone-silent mice
The Gnat2−/− mouse line characterized here is the first example of complete loss of cone phototransduction without degeneration or other morphological abnormalities that have been observed in other mice with Gnat2 disruption. For example, in the Gnat2c.518A > G mouse, in which a missense mutation results in the misfolding of the protein, distinct retinal remodeling is apparent as early as 3 months of age, despite a normal rod ERG. This remodeling includes disruptions in the outer plexiform and nuclear layers, most notably displaced photoreceptor synapses, horizontal cell processes, and bipolar cell dendrites into the outer nuclear layer. These disruptions then become more pronounced with age, as does Müller cell gliosis, both of which are extensive by 12 months of age (Jobling et al., 2013). Such retinal remodeling, especially the displacement of rod bipolar cells into the outer nuclear layer, suggests that rod primary and secondary pathways in the retina are likely perturbed as early as 3 months of age. Presumably, the worsening of this remodeling contributes to the decrease in the rod ERG with age in Gnat2c.518A > G mice.
Another commonly used cone phototransduction-incompetent mouse line is Gnat2cpfl3, in which a missense mutation results in an early stop codon in the Gnat2 gene. In this line, loss of cone function is coincident with outer nuclear layer thinning and outer segment vacuolization by 6 months. This is accompanied by a mild but clearly detectable decrease in rod-mediated ERGs with age (Chang et al., 2006), and it has been shown that the secondary rod pathway is entirely abolished in these mice (Nusinowitz et al., 2007). Thus, while both Gnat2c.518A > G and Gnat2cpfl3 lines have allowed significant research into rod-mediated vision (e.g. Umino et al., 2008; Naarendorp et al., 2010; Pahlberg et al., 2017), their use has the potential to introduce significant confounds, particularly for adult or aging studies.
In contrast, at approximately the same ages, the Gnat2−/− mouse line showed no differences in retinal thickness between knockout and wild-type littermates both by OCT and standard histological measures of nuclear layer thickness, nor was there any detectable decrease in rod-mediated ERGs. Additionally, there were no differences between knockout and control retinas in Müller cell gliosis or microglial activation, both markers of retinal dysfunction and photoreceptor loss. Most importantly, loss of Gnat2 expression did not result in any detectable change in cone morphology, cell densities, opsin localizations, or rod-driven a- or b-waves. Taken together, these results suggest the Gnat2−/− line is a valuable tool for isolating rod function under mesopic conditions, such as is needed for work investigating rod secondary and tertiary retinal pathways and for behavioral studies. Future studies that rely on downstream retinal circuitry should also take care to examine more subtle features of cone synapses, such as their electrical coupling and horizontal and bipolar cell processes, to ensure there are not as yet undetected compensatory changes resulting from loss of Gnat2.
4.2. The contribution of rods to the normal murine ERG under mesopic intensities
Rod and cone function can be easily separated and examined at very dim and very bright light levels, respectively. However, the wide middle range of light intensities over which phototransduction drives changes in membrane currents concurrently in both rods and cones creates ambiguity both about the absolute sensitivity of cones, as well as the state of functional adaptation of rods. Some behavioral experiments using the Gnat2cpfl3 mouse line (Umino et al., 2008), have concluded that rod signaling becomes negligible at intensities greater than −2.0 log cd m−2 or roughly 1 photoisomerizations rod−1 s−1. In contrast, more recent work in mouse retinal slices has found the primary rod pathway can function at light levels similar to our results, although this work did not entirely rule out an indirect contribution of cones (Long et al., 2013). Behavioral experiments using the Gnat2cpfl3 mouse line have found sustained rod function in the presence of background illumination of 103 (Nathan et al., 2006) and even 104 photons μm−2 s−1 (Naarendorp et al., 2010), similar to the upper functional limit of rod a-waves found here (Fig. 5A). Various states of rod degeneration may explain some of the differences between these studies, and lead to underestimating rod contributions. Future studies utilizing the Gnat2−/− mouse should be useful in sorting out these discrepancies.
The Gnat2−/− mouse will likely also be useful for investigating the influence of rod signaling pathways on light adaptation mechanisms in the retina. For example, it has been shown that the cone-driven b-wave increases in amplitude during light adaptation, yet the cause of this increase remains unclear (Alexander et al., 2006; Tanikawa et al., 2004; Garon et al., 2010). Because the Gnat2−/− mouse line does not exhibit observable deficiencies in rod signaling, it could be used to dissect the unique contributions of the rod-pathway to post-photoreceptor light adaptation.
4.3. Gnat2−/− mouse as a model of achromatopsia
Finally, the Gnat2−/− line may also be of interest to the community as a mouse model of achromatopsia, a heterogeneous disorder in which the loss of cone function results in deficits in visual acuity, nystagmus, photophobia, and other clinical symptoms. Indeed, although the most common causative achromatopsia mutations are in the cone cyclic nucleotide-gated channel, some cases of achromatopsia have also been causally linked to mutations in Gnat2 (Kohl et al., 2004). While achromatopsia is defined as a lack of cone function, human achromatopsia patients also exhibit a wide range of rod function, ranging from essentially normal to dramatic deficits (Maguire et al., 2017). Both Gnat2cpfl3 and Gnat2c.518A > G have been used as mouse models of achromatopsia, generally as models for achromatopsia cases where secondary deficiencies arise (Chang et al., 2006; Jobling et al., 2013). In contrast, this Gnat2−/− mouse line may be useful in modeling the less severe achromatopsia cases, where rod function remains normal (Maguire et al., 2017; Hansen, 1990), or have normal scotopic ERGs yet display dramatic deficiencies in other measures of scotopic function, such as fast flicker responses (Hansen, 1990). Thus, the Gnat2−/− mouse should be a useful model for identifying where in the rod parallel pathways these specific deficiencies arise.
Acknowledgments
We thank Dr. Sarah Karlen for helpful discussions and technical assistance, and Dr. Mayank Goswami for preliminary OCT assistance. This work was supported by the National Institutes of Health [NEI R01-14047]; and the UC Davis Training Program in Molecular and Cellular Biology [GM T32-007377]. The authors declare no competing interests.
Footnotes
Author contributions
KER performed and analyzed qRT-PCR, performed and analyzed IHC, obtained some ERGs, wrote and edited the manuscript and created figures; GP performed and analyzed ERGs, assisted with qRT-PCR, helped with creation of Figs. 4 and 5; EBM performed OCT imaging, analysis, retinal thickness measurements, and drafted the OCT methods; RJZ provided OCT imaging expertise and edited the OCT methods; ENP guided ERG experiments and analysis, and extensively edited the manuscript; RH designed and engineered the Gnat2 knock out construct and edited the manuscript; MEB directed experimental design and oversaw data interpretation, performed initial genotyping, and wrote and edited the manuscript.
Declarations of interest
None.
References
- Alexander KR, Raghuram A, Rajagopalan AS. Cone phototransduction and growth of the ERG b-wave during light adaptation. Vis Res. 2006;46(22):3941–3948. doi: 10.1016/j.visres.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicoló M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN, Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducing alpha -subunit. Proc Natl Acad Sci Unit States Am. 2000;97(25):13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca-Sonmez P, Nusinowitz S, Heckenlively JR. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. IOVS. 2006;47(11):5017–5021. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46(6):2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36(4):703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon ML, Rufiange M, Hamilton R, McCulloch DL, Lachapelle P. Asymmetrical growth of the photopic hill during the light adaptation effect. Doc Ophthalmol. 2010;121(3):177–187. doi: 10.1007/s10633-010-9243-0. [DOI] [PubMed] [Google Scholar]
- Hansen E. In: Clinical Aspects of Achromatopsia. Night Vision: Basic, Clinical and Applied Aspects. Hess RF, Sharpe LT, Nordby K, editors. Cambridge University Press; 1990. [Google Scholar]
- Jobling AI, Vessey KA, Waugh M, Mills SA, Fletcher EL. A naturally occurring mouse model of achromatopsia: characterization of the mutation in cone transducin and subsequent retinal phenotype. IOVS. 2013;54:3350–3359. doi: 10.1167/iovs.13-11831. [DOI] [PubMed] [Google Scholar]
- Kohl S, Jägle J, Wissinger B. Achromatopsia. In: Pagon RA, Adam MP, Ardinger HH, editors. GeneReviews® [Internet] University of Washington; Seattle, Seattle, WA: 2004. pp. 1993–2017. updated 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1418/ [PubMed] [Google Scholar]
- Levine ES, Zam A, Zhang P, Pechko A, Wang X, FitzGerald P, Pugh EN, Jr, Zawadzki RJ, Burns ME. Rapid light-induced activation of retinal microglia in mice lacking Arrestin-1. Vis Res. 2014;102:71–79. doi: 10.1016/j.visres.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JH, Arshavsky VY, Burns ME. Absence of synaptic regulation by phosducin in retinal slices. PLoS One. 2013;8(12):e83970. doi: 10.1371/journal.pone.0083970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19(1):442–455. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vis Res. 2004;44(28):3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Maguire J, Parry NRA, Kremers J, Murray IJ, McKeefry D. The morphology of human rod ERGs obtained by silent substitution stimulation. Doumenta Ophthalmologica. 2017;134(1):11–24. doi: 10.1007/s10633-017-9571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP, Pugh EN., Jr Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010;30(37):12495–12507. doi: 10.1523/JNEUROSCI.2186-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan J, Reh R, Ankoudinova I, Ankoudinova G, Chang B, Heckenlively J, Hurley JB. Scotopic and photopic visual thresholds and spatial and temporal discirimination evaluated by behavior of mice in a water maze. Photochem Photobiol. 2006;82(6):1489–1494. doi: 10.1562/2006-02-27-RA-818. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-come photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127(4):359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinowitz S, Ridder WH, 3rd, Ramirez J. Temporal response properties of the primary and secondary rod-signaling pathways in normal and Gnat2 mutant mice. Exp Eye Res. 2007;84(6):1104–1114. doi: 10.1016/j.exer.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pahlberg J, Frederiksen R, Pollock GE, Miyagishima KJ, Sampath AP, Cornwall MC. Voltage-sensitive conductances increase the sensitivity of rod photoresponses following pigment bleaching. J Physiol. 2017;595(11):3459–3469. doi: 10.1113/JP273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Gao F, Lem J, Bramblett DE, Paul DL, Wu SM. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci Unit States Am. 2010;107(1):395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado Allina G, Fortenbach C, Naarendorp F, Gross OP, Pugh EN, Jr, Burns ME. Bright flash response recovery of mammalian rods in vivo in rate limited by RGS9. J Gen Physiol. 2017;149(4):443–454. doi: 10.1085/jgp.201611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Novelli E, Mazzoni F, Barone I, Damiani D. Complexity of retinal cone bipolar cells. Prog Retin Eye Res. 2010;29(4):272–283. doi: 10.1016/j.preteyeres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa A, Bush RA, Takada Y, Mears AJ, Swaroop A, Sieving PA. Functional rods are required for photopic ERG amplitude increase during light adaptation: study of NRL−/− and RHO−/− mice. Invest Ophthalmol Vis Sci. 2004;45 ARVO E-Abstract 811. [Google Scholar]
- Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci. 2008;28(1):189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Goswami M, Zawadzki RJ, Pugh EN., Jr The photosensitivity of rhodopsin bleaching and light-induced increases of fundus reflectance in mice measured in vivo with scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2016;57(8):3650–3664. doi: 10.1167/iovs.16-19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zam A, Jian Y, Wang X, Li Y, Lam KS, Burns ME, Sarunic MV, Pugh EN, Zawadzki RJ. In vivo wide-field multispectral scanning laser ophthalmoscopy-optical coherence tomography mouse retina imager: longitudinal imaging of ganglion cells, microglia, and Müller glia, and mapping of the mouse retinal and choroidal vasculature. J Biomed Optic. 2015;20(12):126005. doi: 10.1117/1.JBO.20.12.126005. [DOI] [PMC free article] [PubMed] [Google Scholar]