Abstract
Background/Objectives
Studies have suggested that smokers may have a lower risk of primary sclerosing cholangitis (PSC) although the results have been inconsistent. This systematic review and meta-analysis was conducted to summarize all available data to better characterize this association.
Methods
A comprehensive literature review was conducted using Medline and Embase databases through January 2018 to identify all studies that compared the risk of PSC among current/former smokers versus nonsmokers. Effect estimates from each study were extracted and combined using the random-effect, generic inverse variance method of DerSimonian and Laird.
Results
Seven case-control studies with 2,307,393 participants met the eligibility criteria and were included in the meta-analysis. The risk of PSC among current smokers and former smokers was significantly lower than nonsmokers with the pooled odds ratio of 0.31 (95% CI, 0.18–0.53) and 0.52 (95% CI, 0.44–0.61), respectively. The risk remained significantly lower among current smokers and former smokers compared with nonsmokers even when only patients with PSC without inflammatory bowel disease were included.
Conclusions
A significantly decreased risk of PSC among current and former smokers was demonstrated in this study.
Keywords: Primary sclerosing cholangitis, cholangitis, smoking, cigarettes, meta-analysis
Key points
Studies have suggested that smokers may have a lower risk of primary sclerosing cholangitis (PSC) although the results have been inconsistent.
This meta-analysis summarized all available data and demonstrated a statistically significant decreased risk of PSC among current and former smokers.
The risk remained significantly lower among current smokers and former smokers compared with nonsmokers even when only patients with PSC without inflammatory bowel disease were included.
Smoking has a wide range of effects on immunological function that may lower the chance of cell-mediated autoimmunity and decrease the risk of PSC.
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by progressive inflammation, destruction, and stricturing of the intrahepatic and extrahepatic bile ducts.1 The 2011 reported incidence rate of PSC was approximately 0.8 per 100,000 person-years in North America and Europe.2 The reported prevalence of PSC in the year 2000 from Olmsted County, Minnesota, USA, was 21 per 100,000 men and six per 100,000 women.3 About 50% to 80% of patients with PSC also have concomitant ulcerative colitis (UC).3,4 The exact etiology of PSC is not known but several factors, including genetic predisposition, autoimmunity, and chronic entry of bacteria into the portal circulation, have been linked to its pathogenesis.1,5
Cigarette smoking is one of the leading preventable causes of mortality worldwide as smokers have a higher risk of developing cardiovascular diseases, lung cancer, and chronic obstructive pulmonary disease.6,7 However, interestingly, smoking is associated with a lower risk of UC8–11 and smoking cessation in patients with UC is associated with increased disease activity and hospitalization.12 Smoking may also be associated with a lower risk of PSC as suggested by several epidemiologic studies, although the results from those studies were inconsistent.13–19 This systematic review and meta-analysis was conducted to summarize all available evidence with the aim of better characterizing the relationship between smoking and PSC.
Methods
Information sources and search strategy
A systematic literature search was conducted using the Embase and Medline databases from inception to January 2018 to identify all original studies that reported the association between smoking and PSC. The systematic literature review was independently conducted by three investigators (KW, PP, and PU) using the search strategy that included the terms for “primary sclerosing cholangitis,” “smoking,” and “cigarettes” as described in online Supplementary Data 1. A manual search for additional potentially relevant studies using references from the included studies and selected review articles was also performed. No language limitation was applied. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement, which is provided as online Supplementary Data 2. EndNote X7 (Clarivate Analytics, PA, USA) was used for study retrieval.
Selection criteria
Eligible studies must be case-control, cross-sectional or cohort studies that investigated the risk of PSC among current smokers and former smokers versus nonsmokers. They must provide the effect estimates (odds ratios (OR), relative risks, hazard ratios or standardized incidence ratio) with 95% confidence intervals (CI) or sufficient raw data to calculate the effect estimates. Inclusion was not restricted by study size. When more than one study using the same database/cohort was available, the study with the most comprehensive data/analyses was included.
Retrieved articles were independently reviewed for their eligibility by the same three investigators. Discrepancy was resolved by conference with all investigators. The Newcastle-Ottawa quality assessment scale was used to appraise the quality of study in three areas including the recruitment of cases and controls, the comparability between the two groups, and the ascertainment of the outcome of interest for cohort studies and the exposure of interest for case-control studies.20 The modified Newcastle-Ottawa scale as described by Herzog et al. was used for cross-sectional studies.21 Kappa statistics were used for evaluation of inter-rater agreement of the Newcastle-Ottawa scale.
Data abstraction
A structured data collection form was used to extract the following data from each study: title of the study, publication year, name of the first author, calendar year(s) when the study was conducted, country or countries where the study was conducted, number of participants, demographic data of participants, methods used to identify and verify diagnosis of PSC, as well as cigarette smoking status, adjusted effect estimates with 95% CI, and covariates that were adjusted in the multivariable analysis.
To ensure the accuracy, this data extraction process was independently performed by two investigators (KW and PP). Case record forms were cross-checked by the senior investigator (PU). Any data discrepancy was resolved by referring back to the original articles.
Statistical analysis
Data analysis was performed using the Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates for the association between smoking status and PSC from each study were extracted and combined using the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study in the pooled analysis inversely to its variance.22 Subgroup analysis on the association between smoking status and PSC without inflammatory bowel disease (IBD) was also performed using the same statistical technique.
In light of the high likelihood of between-study variance because of different study designs and ethnic backgrounds of the studied populations, the random-effect model was used. Cochran’s Q test and I2 statistic were used to determine the between-study heterogeneity. This I2 statistic quantifies the proportion of total variation across studies that is due to true heterogeneity rather than chance. A value of I2 of 0–25% represents insignificant heterogeneity, 26% to 50% represents low heterogeneity, 51% to 75% represents moderate heterogeneity, and more than 75% represents high heterogeneity.23 Visualization by funnel plot was used to assess the presence of publication bias.
Results
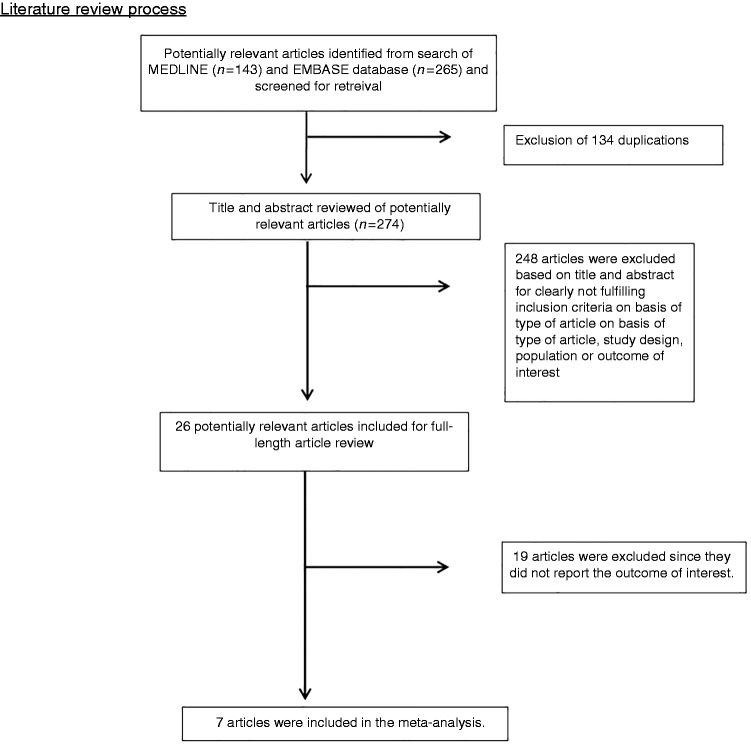
A total of 408 potentially eligible articles were identified using our search strategy (143 articles from Medline and 265 articles from Embase). After the exclusion of 134 duplicated articles, 274 articles underwent title and abstract review. A total of 248 articles were excluded at this stage since they clearly did not fulfill the eligibility criteria based on type of article (they were case reports, correspondences, review articles, in vitro studies, animal studies or interventional studies), leaving 26 articles for full-text review. Nineteen of them were excluded after the full-length review as they did not report the outcome of interest. Finally, seven case-control studies13–19 with 2,307,393 participants were included in the meta-analysis. The literature review and selection process are demonstrated in Figure 1. The characteristics and quality assessment of the studies are presented in Table 1. It should be noted that the inter-rater agreement for the quality assessment using the Newcastle-Ottawa scale was high with the kappa statistic of 0.68.
Figure 1.
Literature review process.
Table 1.
Main characteristics of the studies included in this meta-analysis.
| Loftus et al.16 | van Erpecum et al.19 | Mitchell et al.18 | Andersen et al.13 | |
|---|---|---|---|---|
| Country | USA | The Netherlands | England | Norway |
| Study design | Case-control study | Case-control study | Case-control study | Case-control study |
| Year | 1996 | 1996 | 2002 | 2014 |
| Total number | 70 (35 cases with PSC and 35 controls without PSC) | 256 (59 cases with PSC and 197 controls without PSC) | 211 (41 cases with PSC and 170 controls without PSC) | 485 (240 cases with PSC and 245 controls without PSC) |
| Study sample | Cases: Cases were patients with PSC who were seen at Mayo Clinic, Minnesota, from January 1984 to December 1988 Controls: Controls were patients without PSC who were seen at Mayo Clinic, Minnesota, at the same period of time. Controls were sex-, age-, and geography-matched to cases. | Cases: Cases were patients with PSC who were seen at the University Hospitals Utrecht and Rotterdam from November 1994 to April 1995. Controls: Controls were patients without PSC who were seen at neurologic or orthopedic clinics of the University Hospital Utrecht from January 1995 to April 1995. | Cases: Cases were (a) patients with PSC who were seen at the liver and IBD clinics, (b) patients with PSC in the PSC database at the Oxford Radcliffe Hospital, and (c) patients with PSC from the National PSC Patient Group. Controls: Controls were patients without PSC who shared the same primary physicians with their corresponding cases. Controls were also sex- and age-matched to cases. | Cases: Cases were patients with PSC who were seen at Oslo University Hospital Rikshospitalet from 1992 to 2011. Controls: Controls without PSC were randomly chosen from the Norwegian Bone Marrow Donor Registry. Controls were sex- and age-matched to cases. |
| Determination of smoking status | Health questionnaires | Health questionnaires | Health questionnaires | Health questionnaires |
| Diagnosis of PSC | ERCP or MRCP and/or liver biopsy | ERCP or MRCP and/or liver biopsy | ERCP or MRCP and/or liver biopsy | ERCP or MRCP and/or liver biopsy |
| Confounder adjusted in multivariate analysis | Age | Sex, age, and history of previous appendectomy | None | Age, sex, and education level |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 2 Exposure: 2 | Selection: 3 Comparability: 1 Exposure: 3 | Selection: 4 Comparability: 2 Exposure: 3 | Selection: 4 Comparability: 2 Exposure: 3 |
PSC: primary sclerosing cholangitis; ERCP: endoscopic retrograde cholangiopancreatography; IBD: inflammatory bowel disease; GGT: gamma-glutamyltransferase; MRCP: magnetic resonance cholangiopancreatography; USA: United States of America.
Risk of PSC among current smokers
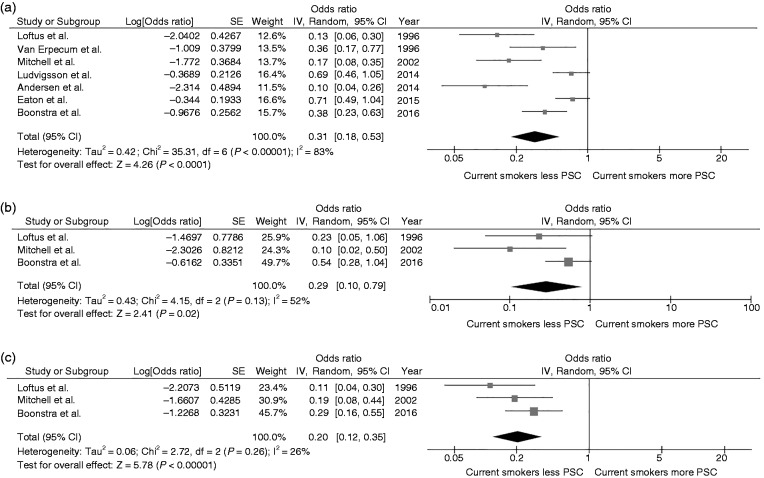
Overall, the pooled analysis demonstrated a significantly decreased risk of PSC among current smokers compared with nonsmokers with a pooled OR of 0.31 (95% CI, 0.18–0.53). Statistical heterogeneity was high with an I2 of 83% (Figure 2(a)). Three studies14,16,18 provided data on the association between subgroups of patients with PSC with and without IBD and smoking status. The pooled results of these three studies continued to show a significantly lower risk of PSC among current smokers compared with nonsmokers with a pooled OR of 0.29 (95% CI, 0.10–0.79; I2 52%) and pooled OR of 0.20 (95% CI, 0.12–0.35; I2 26%) for the PSC without IBD subgroup (Figure 2(b)) and PSC with IBD subgroup (Figure 2(c)), respectively.
Figure 2.
(a) Forest plot of the meta-analysis evaluating the risk of all primary sclerosing cholangitis among current smokers compared with nonsmokers. (b) Forest plot of the meta-analysis evaluating the risk of primary sclerosing cholangitis without inflammatory bowel disease among current smokers compared with nonsmokers. (c) Forest plot of the meta-analysis evaluating the risk of primary sclerosing cholangitis with inflammatory bowel disease among current smokers compared with nonsmokers. CI: confidence interval; df: degree of freedom; IV: inverse variance; PSC: primary sclerosing cholangitis; SE: standard error.
Risk of PSC among former smokers
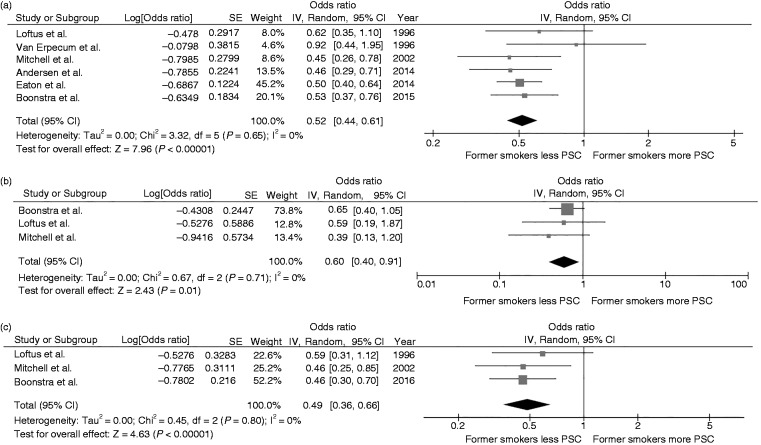
Overall, the pooled analysis demonstrated a decreased risk of PSC among former smokers compared with nonsmokers with a pooled OR of 0.52 (95% CI, 0.44–0.61). Statistical heterogeneity was not significant with an I2 of 0% (Figure 3(a)). Three studies14,16,18 provided data on the association between subgroup of patients with PSC with and without IBD and smoking status. The pooled results of these three studies continued to show a significantly lower risk of PSC among former smokers compared with nonsmokers with a pooled OR of 0.60 (95% CI, 0.40–0.91; I2 0%) and pooled OR of 0.49 (95% CI, 0.36–0.66; I2 0%) for the PSC without IBD subgroup (Figure 3(b)) and PSC with IBD subgroup (Figure 3(c)), respectively.
Figure 3.
(a) Forest plot of the meta-analysis evaluating the risk of all primary sclerosing cholangitis among former smokers compared with nonsmokers. (b) Forest plot of the meta-analysis evaluating the risk of primary sclerosing cholangitis without inflammatory bowel disease among former smokers compared with nonsmokers. (c) Forest plot of the meta-analysis evaluating the risk of primary sclerosing cholangitis with inflammatory bowel disease among former smokers compared with nonsmokers. CI: confidence interval; df: degree of freedom; IV: inverse variance; PSC: primary sclerosing cholangitis; SE: standard error.
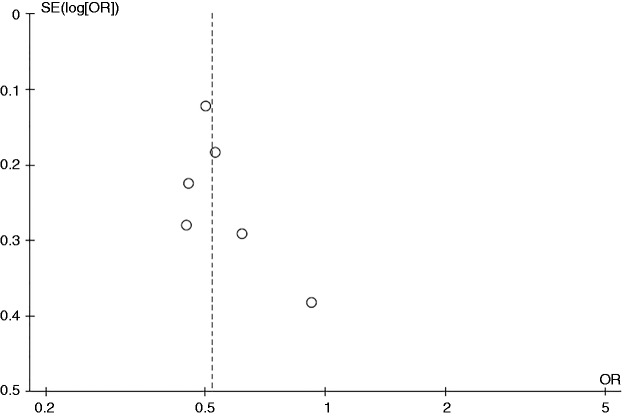
Evaluation for publication bias
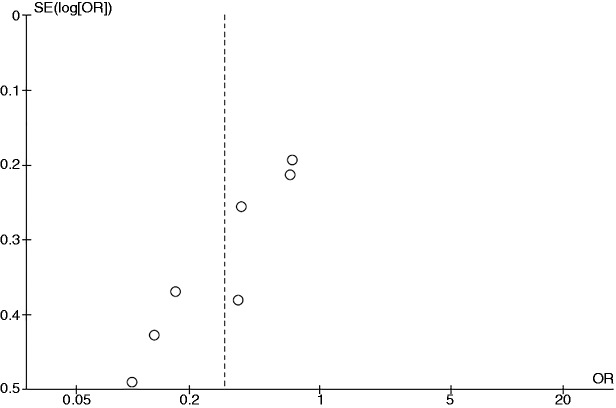
Figure 4 is the funnel plot used to assess the presence of publication bias in the meta-analysis of the risk of PSC among current smokers. The graph is asymmetric and suggests that publication bias in favor of protective results may have been present.
Figure 4.
Funnel plot of the studies evaluating the risk of all primary sclerosing cholangitis among current smokers compared with nonsmokers. OR: odds ratio; SE: standard error.
Figure 5 is the funnel plot used to assess the presence of publication bias for the meta-analysis of the risk of PSC among former smokers. The graph is fairly symmetric and is not suggestive of publication bias.
Figure 5.
Funnel plot of the studies evaluating the risk of all primary sclerosing cholangitis among former smokers compared with nonsmokers. OR: odds ratio; SE: standard error.
Discussion
The current study is the first systematic review and meta-analysis that summarizes all available data on the risk of PSC among current and former smokers. We found an approximately 70% and 50% decreased risk of PSC among current smokers and former smokers, respectively, compared with nonsmokers. The results may suggest the protective effect of cigarette smoking against development of PSC and the effect may attenuate after smoking cessation. However, the exact mechanisms behind this apparent protective effect are not known.
The association between smoking and a lower risk of UC is well known.8–11 Studies have suggested that cigarette smoking may decrease the risk of UC via alteration in intestinal permeability, immune response, colonic mucosal blood flow, and mucus production.24,25 Since UC is a strong predisposing factor for PSC, smokers may have a lower risk of PSC as they develop UC less often compared with nonsmokers. Nonetheless, UC is not the sole explanation for this negative association as subgroup analysis continued to show a significantly lower risk of PSC without IBD among current and former smokers compared with nonsmokers.
Although the pathogenesis of PSC is well understood, it is widely accepted that immunologically mediated bile duct injury is one of the key mechanisms26 and bile duct epithelial cells in PSC are often targeted by T cells for immune-mediated attack.27 Smoking has a wide range of effects on immunological function, including suppression of T cells and B-cell functions,28,29 which may lower the chance of cell-mediated autoimmunity and thus decrease the risk of PSC.
Although the literature review process of this study was robust and the included studies were of high quality, we acknowledge that this study had some limitations and the results should be interpreted with caution.
First, the statistical heterogeneity was moderate to high in the meta-analyses of risk of PSC among current smokers. Second, publication bias in favor of studies that showed a protective effect of smoking may have been present. Third, this is a meta-analysis of observation studies that can demonstrate only an association between the two conditions but could not establish causality. It is still possible the observed negative association is actually a function of confounders.
In summary, this systematic review and meta-analysis demonstrated a significantly lower risk of PSC among current and former smokers.
Supplemental Material
Supplemental material, Supplementary data1 for Association between smoking and risk of primary sclerosing cholangitis: A systematic review and meta-analysis by Karn Wijarnpreecha, Panadeekarn Panjawatanan, Omar Y Mousa, Wisit Cheungpasitporn, Surakit Pungpapong and Patompong Ungprasert in United European Gastroenterology Journal
Supplemental Material
Supplemental material, Supplementary data2 for Association between smoking and risk of primary sclerosing cholangitis: A systematic review and meta-analysis by Karn Wijarnpreecha, Panadeekarn Panjawatanan, Omar Y Mousa, Wisit Cheungpasitporn, Surakit Pungpapong and Patompong Ungprasert in United European Gastroenterology Journal
Acknowledgment
All authors had access to the data and a role in writing the manuscript.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable as this study does not directly involve human subjects.
Ethics approval
The need for ethics approval by institutional board review was waived as this study does not directly involve human subjects.
References
- 1.Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis—a comprehensive review. J Hepatol 2017; 67: 1298–1323. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: A systematic review and meta-analysis. Hepatology 2011; 53: 1590–1599. [DOI] [PubMed] [Google Scholar]
- 3.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003; 125: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 4.Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis 1991; 11: 31–39. [DOI] [PubMed] [Google Scholar]
- 5.Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med 1995; 332: 924–933. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep 2008; 57: 1226–1228. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58: 1227–1232. [PubMed] [Google Scholar]
- 8.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 1989; 34: 1841–1854. [DOI] [PubMed] [Google Scholar]
- 9.Fraga XF, Vergara M, Medina C, et al. Effects of smoking on the presentation and clinical course of inflammatory bowel disease. Eur J Gastroenterol Hepatol 1997; 9: 683–687. [DOI] [PubMed] [Google Scholar]
- 10.Merrett MN, Mortensen N, Kettlewell M, et al. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut 1996; 38: 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas GA, Rhodes J, Green JT. Inflammatory bowel disease and smoking—a review. Am J Gastroenterol 1998; 93: 144–149. [DOI] [PubMed] [Google Scholar]
- 12.Beaugerie L, Massot N, Carbonnel F, et al. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol 2001; 96: 2113–2116. [DOI] [PubMed] [Google Scholar]
- 13.Andersen IM, Tengesdal G, Lie BA, et al. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2014; 12: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 14.Boonstra K, de Vries EM, van Geloven N, et al. Risk factors for primary sclerosing cholangitis. Liver Int 2016; 36: 84–91. [DOI] [PubMed] [Google Scholar]
- 15.Eaton JE, Juran BD, Atkinson EJ, et al. A comprehensive assessment of environmental exposures among 1000 North American patients with primary sclerosing cholangitis, with and without inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41: 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus EV, Jr, Sandborn WJ, Tremaine WJ, et al. Primary sclerosing cholangitis is associated with nonsmoking: A case-control study. Gastroenterology 1996; 110: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Bergquist A, Ajne G, et al. A population-based cohort study of pregnancy outcomes among women with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2014; 12: 95–100. e101. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell SA, Thyssen M, Orchard TR, et al. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: A case control study. Gut 2002; 51: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Erpecum KJ, Smits SJ, van de Meeberg PC, et al. Risk of primary sclerosing cholangitis is associated with nonsmoking behavior. Gastroenterology 1996; 110: 1503–1506. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 21.Herzog R, Álvarez-Pasquin MJ, Díaz C, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13: 154–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pullan RD. Colonic mucus, smoking and ulcerative colitis. Ann R Coll Surg Engl 1996; 78: 85–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava ED, Russell MA, Feyerabend C, et al. Effect of ulcerative colitis and smoking on rectal blood flow. Gut 1990; 31: 1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollheimer MJ, Halilbasic E, Fickert P, et al. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol 2011; 25: 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf GM, Vierling JM. Disappearing intrahepatic bile ducts: The syndromes and their mechanisms. Semin Liver Dis 1993; 13: 261–275. [DOI] [PubMed] [Google Scholar]
- 28.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax 1987; 42: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002; 2: 372–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary data1 for Association between smoking and risk of primary sclerosing cholangitis: A systematic review and meta-analysis by Karn Wijarnpreecha, Panadeekarn Panjawatanan, Omar Y Mousa, Wisit Cheungpasitporn, Surakit Pungpapong and Patompong Ungprasert in United European Gastroenterology Journal
Supplemental material, Supplementary data2 for Association between smoking and risk of primary sclerosing cholangitis: A systematic review and meta-analysis by Karn Wijarnpreecha, Panadeekarn Panjawatanan, Omar Y Mousa, Wisit Cheungpasitporn, Surakit Pungpapong and Patompong Ungprasert in United European Gastroenterology Journal