Abstract
Background
A systematic review suggests that 25% of oesophageal adenocarcinomas (OAC) are ‘missed’ at index endoscopy for Barrett’s oesophagus (BO); however, this included few population-based studies and may be an overestimate.
Objective
The objective of this article is to quantify the ‘missed’ rates of high-grade dysplasia (HGD) and OAC at index BO endoscopy.
Methods
Patients from the Northern Ireland BO register diagnosed between 1993 and 2010 (n = 13,159) were linked to the Northern Ireland Cancer Registry to identify patients who developed OAC or HGD. Logistic regression analysis compared characteristics of ‘missed’ vs ‘incident’ HGD/OAC, defined as diagnoses within 3–12 months vs >1 year after incident BO, respectively.
Results
A total of 267 patients were diagnosed with HGD/OAC ≥3 months after BO diagnosis, of whom 34 (12.7%) were potentially ‘missed’. The proportion of ‘missed’ HGD/OAC was 25% among BO patients with low-grade dysplasia (LGD) and 9% among non-dysplastic BO patients. Older age and BO-LGD carried a higher risk of ‘missed’ HGD/OAC. Non-dysplastic BO patients were more often diagnosed with a ‘missed’ OAC (rather than HGD; 89%), compared with BO-LGD patients (40%).
Conclusions
Approximately one in 10 HGD/OAC cases are ‘missed’ at incident BO diagnosis, which is significant but lower than previous reports. However, ‘missed’ HGD/OAC cases represent only 0.26% of all BO patients.
Keywords: Barrett’s oesophagus, oesophageal cancer, endoscopy, surveillance, population-based
Key summary
- Established knowledge on this subject
- A systematic review suggests that 25% of oesophageal adenocarcinomas (OAC) are ‘missed’ at index endoscopy for Barrett’s oesophagus (BO); however, this review was severely lacking inclusion of robust, population-based data and included diagnoses within three months after the index BO endoscopy in their definition of a ‘missed’ cancer. Both of these considerations are likely to have resulted in an overestimate of the magnitude of ‘missed’ cancers.
- By performing one of the largest population-based studies to date, we aimed to quantify the ‘missed’ rates of high-grade dysplasia (HGD) and OAC at index BO endoscopy.
- Significant findings of this study
- We defined a ‘missed’ case as being diagnosed with HGD/OAC within 3–12 months after index BO diagnosis.
- Results showed a ‘missed’ HGD/OAC rate of 13%, approximately 1 in 10, at incident BO diagnosis, which is not negligible, but is substantially lower than rates suggested by a recent systematic review of this area.
- Increased awareness, adequate biopsy sampling and identifying biomarkers may reduce the number of BO patients with a ‘missed’ oesophageal malignant or premalignant lesion.
- However, such efforts must be balanced in the context of ‘missed’ cases representing a small minority of the overall BO patient population.
Introduction
Barrett’s oesophagus (BO) is currently the only known precursor for oesophageal adenocarcinoma (OAC), which has a poor prognosis with five-year survival rates between 15% and 20%.1 Although the incidence of BO and OAC are increasing in the Western world, only approximately 0.4% of BO patients will progress to OAC each year.2–5 This raises issues for how to manage the increasing number of patients with BO and how to identify high-risk patients, without overburdening services.
Endoscopic surveillance is recommended in BO patients to reduce morbidity and mortality through early detection of dysplasia and cancer.6,7 The British Society of Gastroenterology (BSG) guidelines recommend repeated endoscopy at three- to five-year intervals among BO patients with a Barrett’s length of under 3 cm, and repeated endoscopy at two- to three-year intervals is recommended for patients with longer Barrett’s segments or specialised intestinal metaplasia (SIM).6 Patients with low-grade dysplasia (LGD) should receive surveillance endoscopy at six-monthly intervals. However, as of 2015, endoscopic ablation, preferably with radiofrequency ablation (RFA), has been recommended for high-grade dysplasia (HGD) or LGD diagnosed on two occasions in addition to repeat surveillance endoscopy at six months for patients with LGD.6 In spite of relatively intensive surveillance, the impact of these programs on preventing deaths from OAC is equivocal.8–10 A contributing problem for the optimal management of BO surveillance is the occurrence of ‘interval’ and ‘missed’ cancers.11,12
‘Missed’ cancers can be defined as cancers that were already present at the index BO endoscopy, but were not detected, whereas it is hypothesised that truly incident cancers develop after the index BO endoscopy.13,14 A recent systematic review found that amongst BO patients, 25% of patients who later developed OAC, were diagnosed within one year after index BO endoscopy, and could therefore be considered as ‘missed’ cancers.14 However, this review included only a few population-based studies and included diagnoses within three months after the index BO endoscopy in their definition of a ‘missed’ cancer. Both of these considerations are likely to have resulted in an overestimate of the magnitude of ‘missed’ cancers. Therefore, this study aimed to quantify the ‘missed’ rates of HGD and OAC at index endoscopy among patients with a BO diagnosis utilising one of the largest population-based registers of BO worldwide. We further sought to identify risk factors which may contribute to these missed cases.
Methods
BO patients
The Northern Ireland Barrett’s register (NIBR) includes 13,294 patients with BO aged ≥ 16 years diagnosed between 1993 and 2010 in Northern Ireland (NI) (population of 1.8 million). Descriptions of the NIBR have been previously reported.4 Strict criteria for BO were used, which was defined as columnar-lined epithelium of the oesophagus. Trained staff extracted information on BO length, the presence of SIM and visible BO at endoscopy, using standardised guidelines, from all pathology reports relating to oesophageal biopsies carried out in NI over this time period. The date of the earliest (index) biopsy showing BO was taken as the date of entry into the register.
Outcomes
The NIBR was matched to the Northern Ireland Cancer Registry (NICR),15 which was used to identify BO patients who progressed to oesophageal or gastric cardia adenocarcinoma (hereafter referred to as OAC) between January 1993 and 2013 in NI. Gastric cardia adenocarcinoma was also included as an outcome because it is likely that these tumours in BO patients are oesophageal in origin. This process has been described previously.3 Histologically unspecified cancers were reviewed by a gastrointestinal pathologist. Oesophageal squamous cell carcinomas were excluded. Deaths were identified through matching to the NI Registrar General’s Office. Matching of BO patients diagnosed after 2005 with the NICR was performed by using the unique Health and Social Care Number, which is available for over 90% of patients. The remaining patients and patients diagnosed before 2005 were matched using patients’ forename, surname and date of birth.
BO patients who developed HGD were identified by examining all oesophageal pathology reports from NI for the period 1993–2013. Patients were considered to have HGD if diagnosed twice within one year or in two subsequent biopsies, even if the duration between them was more than one year, or if HGD was present in a single biopsy and the duration of available follow-up after the development of HGD was less than one year. HGD which occurred in squamous epithelium was not included as an outcome. According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee.
Statistical analysis
The primary outcome was ‘missed’ OAC and HGD after a BO diagnosis. Patients with HGD/OAC were divided into two categories: ‘missed’ and incident cases. In line with previous studies, ‘missed’ HGD/OAC was defined as diagnoses within 3–12 months after the index BO biopsy. An outcome less than three months after index BO could be part of the diagnostic work-up instead of ‘missed’ and therefore these patients were excluded from the analysis (n = 187).13,16 Incident HGD/OAC was defined as being diagnosed at least one year after index BO biopsy. Follow-up was defined from the first BO diagnosis until first HGD or OAC diagnosis and was available until 31 December 2013.
Data were analysed for the combined outcome of HGD and OAC, and for OAC only. Chi-squared tests and analysis of variance (ANOVA) were used to compare categorical and continuous variables, respectively, between patients diagnosed 3–12 months, one to three years and more than three years following BO diagnosis. Univariable and multivariable logistic regression were used to examine factors associated with being diagnosed within 3–12 months after a BO diagnosis vs being diagnosed later than one year after BO diagnosis.
Two analyses were performed among a selected group of BO patients. First, restriction was applied to the analysis to examine differences in the proportion of ‘missed’ HGD/OAC cases in the periods 1993–2001 and 2002–2010. Patients who progressed more than three years after BO diagnosis were excluded from this particular analysis as the maximum time of follow-up was three years for patients diagnosed with BO in 2010. Second, restriction was applied to the analysis to investigate tumour stage according to time between BO diagnosis and HGD/OAC diagnosis. As tumour stage was less accurately registered for BO patients who progressed to OAC before 2002, only patients diagnosed with BO as of 2002 were included. A secondary analysis compared median survival time between all ‘missed’ and incident OAC patients for whom survival time was defined from OAC diagnosis until death or until 9 December 2016, whichever occurred earlier. Statistical analyses were conducted using Intercooled STATA V11.0.
Results
Proportion of ‘missed’ HGD/OAC cases
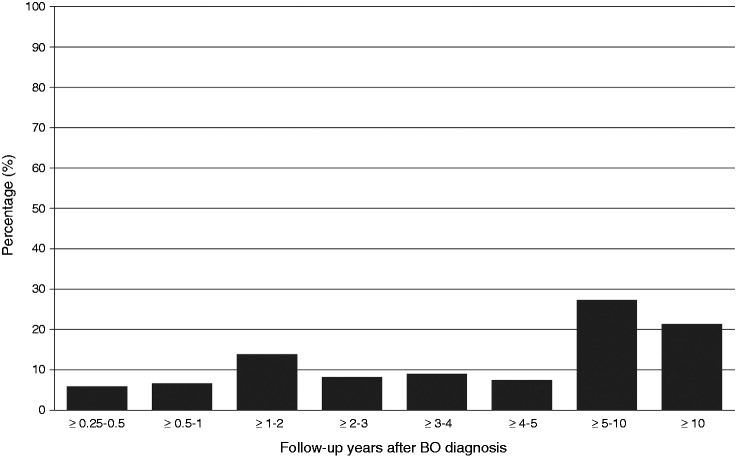
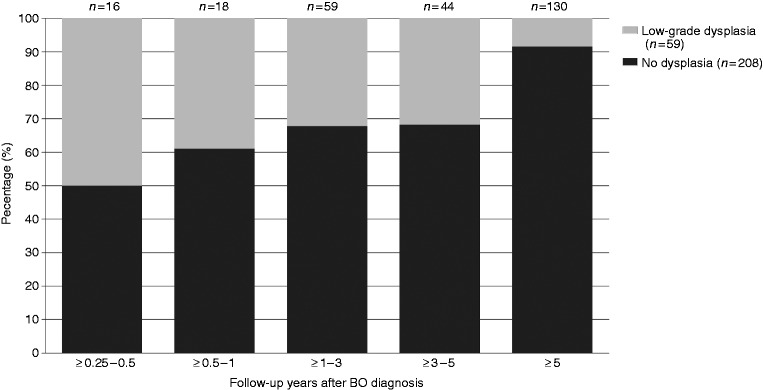
During the study period, n = 267 patients developed HGD/OAC after three months of follow-up, of whom n = 34 patients (12.7%) were diagnosed within 3–12 months after BO diagnosis (Table 1). The proportion of HGD/OAC classified as ‘missed’ was reduced in non-dysplastic BO (9%), whereas a higher proportion was observed in BO-LGD (25%). When restricting analysis to OAC progressors only, n = 210 patients developed OAC after three months of follow-up, of whom n = 26 patients (12%) were diagnosed within 3–12 months after BO diagnosis (Supplementary table 1). The distribution of HGD/OAC diagnoses over time is shown in Figures 1 and 2. Figure 1 shows that approximately half of HGD/OAC progressors were diagnosed more than five years after their first BO biopsy. Furthermore, the proportion of non-dysplastic BO patients increases, and the proportion of LGD-BO patients decreases with increasing follow-up years after first BO biopsy among patients who progressed in HGD/OAC (Figure 2).
Table 1.
Characteristics of patients with a Barrett’s oesophagus (BO) who progressed to HGD/OAC after three months after a Barrett’s diagnosis (n = 267).
| Features at index BO endoscopyb | HGD/OAC progressors ≥ 3–12 months N = 34 (13%) |
HGD/OAC progressors within ≥ 1–3 year N = 59 (22%) |
HGD/OAC progressors ≥ 3 years N = 174 (65%) |
||||
|---|---|---|---|---|---|---|---|
| N | %c | N | %c | N | %c | p value | |
| Sex | 0.601 | ||||||
| Female | 8 | 11.76 | 18 | 26.47 | 42 | 61.76 | |
| Male | 26 | 13.07 | 41 | 20.60 | 132 | 66.33 | |
| Median age (IQR) | 66.9 | 60.7–75.3 | 65.2 | 56.7–73.7 | 60.1 | 52.3–68.3 | <0.001 |
| Age group | 0.008 | ||||||
| <65 | 15 | 9.15 | 29 | 17.68 | 120 | 73.17 | |
| 65–74 | 10 | 15.38 | 20 | 30.77 | 35 | 53.85 | |
| ≥75 | 9 | 23.68 | 10 | 26.32 | 19 | 50.00 | |
| Socioeconomic statusa | 0.146 | ||||||
| Most deprived | 16 | 15.53 | 16 | 15.53 | 71 | 68.93 | |
| Middle deprived | 7 | 13.73 | 8 | 15.69 | 36 | 70.59 | |
| Least deprived | 9 | 9.68 | 29 | 31.18 | 55 | 59.14 | |
| Unknown | 2 | 10.00 | 6 | 30.00 | 12 | 60.00 | |
| Specialised intestinal metaplasia | 0.412 | ||||||
| Absent/unknown | 9 | 14.75 | 14 | 22.95 | 38 | 62.30 | |
| Present | 25 | 12.14 | 45 | 21.84 | 136 | 66.02 | |
| Visible segment seen at endoscopy | 0.843 | ||||||
| Unknown/no | 22 | 13.02 | 39 | 23.08 | 108 | 63.91 | |
| Yes | 12 | 12.24 | 20 | 20.41 | 66 | 67.17 | |
| Dysplasia | <0.001 | ||||||
| No dysplasia | 19 | 9.13 | 40 | 19.23 | 149 | 71.63 | |
| Low-grade dysplasia | 15 | 25.42 | 19 | 32.20 | 25 | 42.37 | |
Category ‘most deprived quintile’ and ‘quintile 2’ are merged into ‘most deprived’. Category ‘quintile 4’ and ‘Least deprived quintile’ were merged into ‘Least deprived’.
Numbers for short, long and unknown Barrett’s segment are not presented due to small cell counts (<3) and to avoid disclosure of potentially identifiable information.
Percentages were calculated across the rows to emphasise the proportions of all missed or incident cancers over time, rather than calculating the percentages within the columns.
HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma; IQR: interquartile range.
Figure 1.
Distribution of time to HGD/OAC diagnosis among 267 detected cases of HGD/OAC.
BO: Barrett’s oesophagus; HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma.
Figure 2.
Dysplasia status at Barrett’s oesophagus (BO) diagnosis by time to HGD/OAC diagnosis among 267 detected cases of HGD/OAC. HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma.
Clinical factors associated with risk of ‘missed’ vs incident HGD/OAC
Patients with a ‘missed’ HGD/OAC were significantly older compared to patients diagnosed after three years with HGD/OAC (median age of 66.9 vs 60.1 years; Table 1). Approximately a quarter of the patients who were 75 years or older and progressed to HGD/OAC progressed within 3–12 months after a BO diagnosis, whereas only 9% of progressors younger than 65 years did so (p = 0.008; Table 1). In multivariable analysis, patients aged ≥75 v. <65 years still had higher odds of a ‘missed’ compared with incident HGD/OAC (odds ratio (OR) = 2.78 95% confidence interval (CI) 1.02–7.61). Overall, sex, SIM, length of Barrett’s segment, visible segment seen at index endoscopy and socioeconomic status were not associated with risk of a ‘missed’ compared with incident HGD/OAC (Table 2). Similar findings were observed when restricted to OAC progressors only (data not shown).
Table 2.
Univariable and multivariable logistic regression analysis to examine the likelihood of being diagnosed with HGD/OAC after 3–12 months compared to ≥ 1 year after a Barrett’s oesophagus (BO) diagnosis (n = 267).
| Features at index BO endoscopy | 3–12 months |
≥1 year |
Univariable |
Multivariableb |
||
|---|---|---|---|---|---|---|
| N = 34 | N = 233 | OR | 95% CI | OR | 95% CI | |
| Sex | ||||||
| Female | 8 | 60 | Ref | Ref | ||
| Male | 26 | 173 | 1.13 | 0.48–2.62 | 1.31 | 0.51–3.33 |
| Age group | ||||||
| <65 | 15 | 149 | Ref | Ref | ||
| 65–74 | 10 | 55 | 1.81 | 0.77–4.26 | 1.90 | 0.77–4.67 |
| ≥75 | 9 | 29 | 3.08 | 1.23–7.71 | 2.78 | 1.02–7.61 |
| Socioeconomic statusa | ||||||
| Most deprived | 16 | 87 | Ref | Ref | ||
| Middle deprived | 7 | 44 | 0.87 | 0.33–2.26 | 1.10 | 0.39–3.06 |
| Least deprived | 9 | 84 | 0.58 | 0.24–1.39 | 0.62 | 0.25–1.54 |
| Unknown | 2 | 18 | 0.60 | 0.13–2.86 | 0.75 | 0.15–3.79 |
| Specialised intestinal metaplasia | ||||||
| Absent/unknown | 9 | 52 | Ref | Ref | ||
| Present | 25 | 181 | 0.80 | 0.35–1.82 | 0.76 | 0.31–1.83 |
| Visible segment seen at endoscopy | ||||||
| No/unknown | 22 | 147 | Ref | Ref | ||
| Yes | 12 | 86 | 0.93 | 0.44–1.98 | 0.97 | 0.42–2.27 |
| Length of Barrett’s segmentc | ||||||
| Long ≥ 3 cm | NR | NR | 0.54 | 0.09–3.03 | 0.53 | 0.08–3.29 |
| Short < 3 cm | NR | NR | Ref | Ref | ||
| Unknown | 27 | 148 | 1.37 | 0.30–6.33 | 1.44 | 0.27–7.77 |
| Dysplasia at index biopsy | ||||||
| No dysplasia | 19 | 189 | Ref | Ref | ||
| Low-grade dysplasia | 15 | 44 | 3.39 | 1.60–7.20 | 3.48 | 1.56–7.76 |
Category ‘most deprived quintile’ and ‘quintile 2’ are merged into ‘most deprived’. Category ‘quintile 4’ and ‘Least deprived quintile’ were merged into ‘Least deprived’.
Adjusted for all variables listed in Table 2.
Numbers for short and long Barrett’s segment are not presented due to small cell counts (<3) and to avoid disclosure of potentially identifiable information.
HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma; NR: not reported; OR: odds ratio; CI: confidence interval.
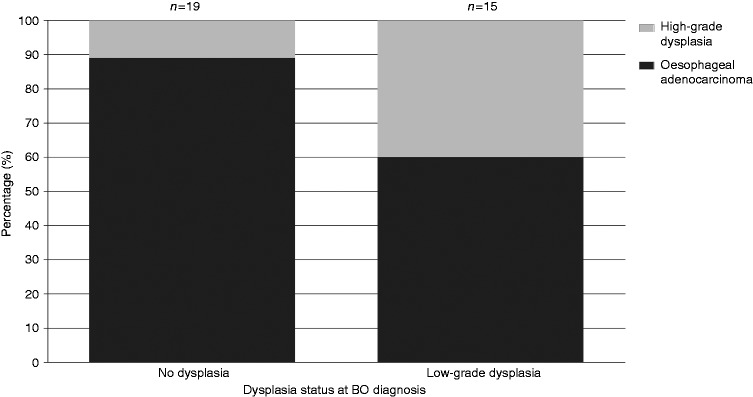
Patients with LGD had 3.5-fold higher odds of being diagnosed within 3–12 months rather than incident HGD/OAC compared to non-dysplastic BO patients (OR = 3.48 95% CI 1.56–7.76; Table 2). LGD or non-dysplastic status also influenced the severity of HGD/OAC detected within ‘missed’ cases. Among the BO-LGD patients, 40% developed HGD and 60% developed OAC. In contrast, within the non-dysplastic BO patients who developed a ‘missed’ HGD/OAC, only 11% had HGD detected and the majority (89%) had OAC detected (Figure 3).
Figure 3.
Progression in HGD/OAC according to dysplasia status among 34 ‘missed’ cases of HGD/OAC. BO: Barrett’s oesophagus; HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma.
Proportion of missed HGD/OAC by period of BO diagnosis
We then sought to evaluate if proportions of ‘missed’ HGD/OAC diagnoses had changed over time. Similar proportions of HGD/OAC cases diagnosed within 3–12 months after their BO diagnosis were observed in the earlier 1993–2001 time period (36%) and the more recent 2002–2013 period (38%) (Table 3). Results indicate a higher proportion of ‘missed’ cases compared to main results in Table 1 due to exclusion of patients diagnosed more than three years after a BO diagnosis.
Table 3.
Proportion of ‘missed’ HGD or OAC according to period of Barrett’s oesophagus (BO) diagnosis among patients who progressed in HGD or OAC within 3–36 months after their Barrett’s diagnosisa.
| Diagnosed 3–12 months after BO diagnosis N = 34 | Diagnosed ≥ 1–3 year after BO diagnosis N = 59 | p valueb | |
|---|---|---|---|
| Period of BO diagnosis | 0.835 | ||
| 1993–2001 | 20 (36%) | 36 (64%) | |
| 2002–2010 | 14 (38%) | 23 (62%) |
Patients diagnosed more than three years after a BO diagnosis were excluded from the analysis as the maximum follow-up is three years for BO patients diagnosed in 2010.
Based on a chi-squared test.
HGD: high-grade dysplasia; OAC: oesophageal adenocarcinoma.
Tumour stage and survival among ‘missed’ vs incident OAC patients
Patients diagnosed with a ‘missed’ OAC were diagnosed with an earlier or unknown tumour stage compared with OAC patients diagnosed after three years (p = 0·175). Among the patients with a ‘missed’ OAC, 33% had a stage I tumour, whereas 27% and 18% of the patients diagnosed within one to three years and after three years, respectively, had a stage I tumour (Supplementary figure 1). Better overall survival outcomes were also observed amongst ‘missed’ compared with incident OAC cases (median (interquartile range (IQR) survival 3.96 (0.90–9.46) and 1.94 (0.44–6.12) years, respectively).
Discussion
This is one of the largest population-based studies to date to investigate the magnitude of ‘missed’ HGD or OAC in patients with BO. We defined a ‘missed’ case as being diagnosed with HGD/OAC within 3–12 months after index BO diagnosis. Results showed ‘missed’ rates of 13% and 9% among all BO patients and all non-dysplastic BO patients, respectively, who were subsequently diagnosed with HGD/OAC. The proportion of ‘missed’ cases remained stable during the study period.
The ‘missed’ rate reported in the present study is significant but lower than previously reported estimates. A systematic review of 24 studies reported a ‘missed’ rate of 25%.14 Furthermore, three population-based studies, which were also included in the review, reported that 32%–66% of the patients who progressed in OAC were diagnosed within one year after BO diagnosis.2,3,17 In contrast with our study, these studies defined ‘missed’ as being diagnosed with HGD/OAC within one year after BO diagnosis. However, HGD/OAC patients diagnosed less than three months after BO may be part of the diagnostic work-up.16 Chadwick et al. also excluded patients diagnosed within three months after a BO diagnosis for the calculation of their ‘missed’ rate.13 They found that 7.8% of the patients with OAC underwent a previous endoscopy three to 36 months preceding diagnosis of OAC, which is similar to the ‘missed’ rate of 9% detected in non-dysplastic BO patients in the present study. Furthermore, Holmberg et al. also noted a high incidence of OAC within the first 100 days after BO diagnosis.16 Still, it is worth noting that all of the above reported ‘missed’ rates after an oesophagogastroduodenoscopy are unfavourable compared with reported rates of missed colorectal cancers after a colonoscopy, which ranges from 0.5% to 6%.18,19
There could be two overarching explanations for the ‘missed’ cancers. First, the missed cancers may be truly missed, which means that the cancer or premalignant lesions were already present at index endoscopy but not detected. A previous study has found that errors by the endoscopist account for the majority (73%) of ‘missed’ oesophageal or gastric cancers at endoscopy and the remaining 27% were related to errors by pathologists.20 It is possible that HGD or OAC was not detected due to features that make them less likely to be seen by the endoscopist such as oesophagitis, oesophageal stricture and ulceration.20 Methods to increase detection of HGD/OAC such as advanced endoscopic imaging techniques,6 greater time examining BO segments,21 greater number of targeted biopsies20 and dedicated time slots for examination22 may identify HGD or malignant lesions and decrease the burden of missed HGD/OAC through early detection of HGD/OAC, which could increase cure and survival rates.7,23
Cases may be truly missed if the second endoscopy was not part of routine surveillance. Based on a previous case note review (unpublished) among 60% of the HGD/OAC progressors, more than half of the ‘missed’ cases were not entered into routine surveillance and surveillance was probably performed due to new symptoms. These cases may be truly ‘missed’ cases. Moreover, taking into account the time interval between BO and OAC, one can suggest that the OAC cases were already present at index endoscopy. Nevertheless, the missed cases represents only 0.26% of all BO patients diagnosed in NI over this timeframe, and so the ever-important question of identifying the very small proportion of high-risk patients (‘missed’ or incident HGD/OAC) remains a considerable challenge.
Second, it is plausible that the missed cancers may be more aggressive cancers which have no visible evidence at index endoscopy but develop rapidly afterward. Therefore, biomarkers could assist in determining the risk of progression at BO diagnosis and guide the targeting of endoscopic surveillance.24 Previous studies indicate that there are two main pathways of progression among BO patients:25,26 a more indolent pathway which moves through to dysplasia to OAC, acquiring a variety of mutations and a more aggressive pathway dominated by genomic doubling with more frequent oncogenic amplification, and less frequent inactivation of tumour suppressors.25 Results from the present study provide some support for these two pathways, as non-dysplastic BO patients were more often diagnosed with ‘missed’ OAC than ‘missed’ HGD compared to LGD patients. However, the present study has found that patients diagnosed within 3–12 months after BO diagnosis had more often a stage I or stage II tumour and a longer median survival compared to patients diagnosed more than three years after BO diagnosis. Patients with a missed OAC had a better median survival probably because they had more often an earlier tumour stage which can effectively be treated with endoscopic techniques such as endoscopic resection and RFA.
A higher ‘missed’ rate of 25% among LGD-BO patients likely reflects appropriate clinical management and planned surveillance after BO diagnosis. Results of the present study support the effectiveness of BSG guidelines, which recommend more frequent surveillance endoscopy among LGD-BO patients, as these patients had a higher likelihood to have HGD/OAC diagnosed within 3–12 months, compared to non-dysplastic BO patients. This conclusion is supported by the proportion of ‘missed’ HGD cases among all ‘missed’ HGD/OAC cases being higher among patients with LGD-BO compared with non-dysplastic BO (60% vs 11%). Our study timelines pre-date the recent changes to BSG guidelines6 to allow endoscopic ablation, preferably with RFA, for LGD patients, instead of repeated endoscopy after six months of being treated with proton pump inhibitors (PPIs).6,27,28
We also explored if clinical or demographic features may differ between ‘missed’ or incident HGD/OAC cases. Having an older age was associated with a higher risk of a ‘missed’ HGD/OAC instead of an incident HGD/OAC. It is possible that simply the older you are the more likely you are to have cancer and therefore the more likely for it to be missed. However, higher rates of ‘missed’ cases among elderly patients may simply reflect shorter life expectancies and therefore a reduced likelihood of developing HGD/OAC three years after first BO biopsy. In addition, a previous study from Visrodia et al. found that the presence of a long-segment BO could place patients at greater risk of ‘missed’ HGD or OAC.29 In contrast, the length of Barrett’s segment was not associated with a higher risk of a ‘missed’ HGD or OAC in the present study. However, information on Barrett’s length was limited in our cohort.
This study has important strengths, in particular the completeness of identification of outcomes, large size and population-based analysis within a region with limited migration.15 However, this study also has some limitations. The exclusion of patients diagnosed within three months for the definition of ‘missed’ cases is somewhat arbitrary. However, a previous study also excluded these patients as a diagnosis within three months after BO diagnosis could be part of the diagnostic work-up.13 Furthermore, BO guidelines have been updated since conclusion of this study period. Within the updated BSG guidelines published in 2015, clinicians can now discharge patients from endoscopic surveillance who have a short Barrett’s segment and repeated confirmation that SIM is not present.6 Therefore, future research may need to reassess these estimates to evaluate any impact on potential ‘missed’ diagnoses; however, the perceived low cancer risk in these patients is likely to have minimal influence. In addition, information about PPI use was not available. Finally, we acknowledge that the term ‘missed’ is somewhat controversial in the capacity of this, and similar, studies. We retained the term in this report primarily to ensure comparability with previous publications. However, we call on researchers to adopt a more appropriate term, such as underdiagnosed or short-term interval cancers, for future manuscripts.
In conclusion, based upon a large population-based study, we observed a ‘missed’ HGD/OAC rate of 13%, which is not negligible, but is substantially lower than rates suggested by a recent systematic review of this area.14 Increased awareness, adequate biopsy sampling and identifying biomarkers may reduce the number of BO patients with a ‘missed’ oesophageal malignant or premalignant lesion. However, such efforts must be balanced in the context of ‘missed’ cases representing a small minority of the overall BO patient population.
Supplementary Material
Acknowledgements
This work was supported by the Sacha Swarttouw-Hijmans Foundation as they dedicated a travel grant to MP. Furthermore, we would like to thank the tumour verification officers in the Northern Ireland Cancer Registry and all staff in the Centre for Public Health of the Queens University who contributed to the development of the Northern Ireland Barrett’s register.
Declaration of conflicting interests
None declared.
Ethics approval
According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee.
Funding
The Northern Ireland Barrett’s register was funded by the UK Medical Research Council, Cancer Focus Northern Ireland (formerly the Ulster Cancer Foundation), Northern Ireland Health and Social Care Research and Development Office, and Cancer Research UK. The Northern Ireland Cancer Registry was funded by the Public Health Agency for Northern Ireland. The funding bodies had no role in the study design and all researchers involved in this study are independent of the funding bodies. HC and LM are co-investigators of the UKCRC Centre of Excellence for Public Health Northern Ireland.
References
- 1.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013; 381: 400–412. [DOI] [PubMed] [Google Scholar]
- 2.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011; 365: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 3.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: Results from a large population-based study. J Natl Cancer Inst 2011; 103: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman HG, Bhat S, Murray LJ, et al. Increasing incidence of Barrett’s oesophagus: A population-based study. Eur J Epidemiol 2011; 26: 739–745. [DOI] [PubMed] [Google Scholar]
- 5.Masclee GM, Coloma PM, de Wilde M, et al. The incidence of Barrett’s oesophagus and oesophageal adenocarcinoma in the United Kingdom and The Netherlands is levelling off. Aliment Pharmacol Ther 2014; 39: 1321–1330. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]
- 7.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: A population-based study. Gut 2015; 64: 20–25. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett’s oesophagus: Results from a population based study. Gut 2003; 52: 1081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010; 8: 235–244. quiz e232. [DOI] [PubMed] [Google Scholar]
- 10.Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol 2004; 39: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 11.Qiao Y, Hyder A, Bae SJ, et al. Surveillance in patients with Barrett’s esophagus for early detection of esophageal adenocarcinoma: A systematic review and meta-analysis. Clin Transl Gastroenterol 2015; 6: e131–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramus JR, Gatenby PA, Caygill CP, et al. Surveillance of Barrett’s columnar-lined oesophagus in the UK: Endoscopic intervals and frequency of detection of dysplasia. Eur J Gastroenterol Hepatol 2009; 21: 636–641. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick G, Groene O, Hoare J, et al. A population-based, retrospective, cohort study of esophageal cancer missed at endoscopy. Endoscopy 2014; 46: 553–560. [DOI] [PubMed] [Google Scholar]
- 14.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology 2016; 150: 599–607.e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney TM, Donnelly C, Kelly JM, et al. Validation of the completeness and accuracy of the Northern Ireland Cancer Registry. Cancer Epidemiol 2015; 39: 401–404. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg D, Ness-Jensen E, Mattsson F, et al. Risk of oesophageal adenocarcinoma in individuals with Barrett’s oesophagus. Eur J Cancer 2017; 75: 41–46. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge PJ, van Blankenstein M, Looman CW, et al. Risk of malignant progression in patients with Barrett’s oesophagus: A Dutch nationwide cohort study. Gut 2010; 59: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 18.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: A population-based analysis. Gastroenterology 2007; 132: 96–102. [DOI] [PubMed] [Google Scholar]
- 19.Ee HC, Semmens JB, Hoffman NE. Complete colonoscopy rarely misses cancer. Gastrointest Endosc 2002; 55: 167–171. [DOI] [PubMed] [Google Scholar]
- 20.Yalamarthi S, Witherspoon P, McCole D, et al. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004; 36: 874–879. [DOI] [PubMed] [Google Scholar]
- 21.Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 2012; 76: 531–538. [DOI] [PubMed] [Google Scholar]
- 22.Ooi J, Wilson P, Walker G, et al. Dedicated Barrett’s surveillance sessions managed by trained endoscopists improve dysplasia detection rate. Endoscopy 2017; 49: C1–C1. [DOI] [PubMed] [Google Scholar]
- 23.Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. Epub ahead of print 18 August 2017. DOI: 10.1136/gutjnl-2017-314109. [DOI] [PMC free article] [PubMed]
- 24.Bird-Lieberman EL, Dunn JM, Coleman HG, et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology 2012; 143: 927–935.e923. [DOI] [PubMed] [Google Scholar]
- 25.Stachler MD, Taylor-Weiner A, Peng S, et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. 2015; 47: 1047-1055. [DOI] [PMC free article] [PubMed]
- 26.Martinez P, Timmer MR, Lau CT, et al. Dynamic clonal equilibrium and predetermined cancer risk in Barrett’s oesophagus. Nat Commun 2016; 7: 12158–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: A randomized clinical trial. JAMA 2014; 311: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 28.Haidry RJ, Lipman G, Banks MR, et al. Comparing outcome of radiofrequency ablation in Barrett’s with high grade dysplasia and intramucosal carcinoma: A prospective multicenter UK registry. Endoscopy 2015; 47: 980–987. [DOI] [PubMed] [Google Scholar]
- 29.Visrodia K, Iyer PG, Schleck CD, et al. Yield of repeat endoscopy in Barrett’s esophagus with no dysplasia and low-grade dysplasia: A population-based study. Dig Dis Sci 2016; 61: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.