Abstract
Background
Data on surveillance for pancreatic ductal adenocarcinoma (PDAC) in high-risk individuals (HRIs) with “familial pancreatic cancer” (FPC) and specific syndromes are limited and heterogeneous.
Objective
We conducted a systematic review and meta-analysis of PDAC surveillance studies in HRIs.
Methods
Prevalence of solid/cystic pancreatic lesions and of lesions considered a successful target of surveillance (proven resectable PDAC and high-grade precursors) was pooled across studies. The rate of lesions diagnosed by endoscopic ultrasonography (EUS)/magnetic resonance imaging (MRI) and across different HRI groups was calculated.
Results
Sixteen studies incorporating 1588 HRIs were included. The pooled prevalence of pancreatic solid and cystic lesions was 5.8% and 20.2%, respectively. The pooled prevalence of patients with lesions considered a successful target of surveillance was 3.3%, being similar to EUS or MRI and varying across subgroups, being 3% in FPC, 4% in hereditary pancreatitis, 5% in familial melanoma, 6.3% in hereditary breast/ovarian cancer, and 12.2% in Peutz-Jeghers syndrome. The pooled estimated rate of lesions considered a successful target of surveillance during follow-up was 5/1000 person-years.
Conclusion
Surveillance programs identify successful target lesions in 3.3% of HRIs with a similar yield of EUS and MRI and an annual risk of 0.5%. A higher rate of target lesions was reported in HRIs with specific DNA mutations.
Keywords: Pancreatic cancer, meta-analysis, family history, screening
Key summary
- Summarize the established knowledge on this subject.
- Surveillance of pancreatic cancer is advised in individuals with “familial pancreatic cancer” (FPC) and specific genetic syndromes.
- No evidence-based consensus is available on the imaging test preferred between magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS).
- Whether surveillance protocols should be different in different high-risk individual (HRI) subgroups is unknown.
- What are the significant and/or new findings of this study?
- The rate of resected lesions considered a successful target of surveillance during pancreatic cancer surveillance programs in HRIs is 3.3% or 0.5% per year.
- There are no differences between EUS and MRI in diagnosing a “successful” target of screening.
- The rate of successful target lesions in FPC is lower compared to specific genetic syndromes, thus surveillance programs might need to be individualized accordingly.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an increasing cause of cancer-related death, partially because of delayed diagnosis.1,2 While precursor lesions, such as intraductal papillary mucinous neoplasms (IPMNs), can be detected at early stages, whether this is possible for pancreatic intraepithelial neoplasia (PanINs)3 is a matter of debate. At any rate, general population screening is not advised as the overall lifetime PDAC risk is relatively low.
However, since a hereditary component accounts for 5% to 10% of cases,3 surveillance is advised for high-risk individuals (HRIs). The International Cancer of the Pancreas Screening (CAPS) Consortium4 defined individuals with “familial pancreatic cancer” (FPC) or with hereditary syndromes of which PDAC is one phenotypic manifestation as HRIs. The FPC definition is not fully established, but an individual can be considered at high risk if ≥two blood relatives are affected by PDAC, of whom at least one is a first-degree relative (FDR). Regarding defined genetic syndromes, surveillance is indicated for all Peutz-Jeghers syndrome (PJS) patients regardless of family history. Furthermore, p16 (familial atypical multiple-mole melanoma syndrome, FAMMM), breast cancer type 2 susceptibility gene (BRCA2), partner and localizer of BRCA2 (PALB), and mismatch repair gene (hereditary nonpolyposis colorectal cancer, HNPCC) mutation carriers with one FDR or two other family members with PDAC should undergo surveillance.5
The ultimate goal of surveillance is to detect and surgically treat noninvasive precursor lesions, such as advanced PanINs or IPMNs with high-grade dysplasia, or early-stage PDAC, that are considered successful targets of surveillance according to the CAPS Consortium.4 Data on the efficacy of such surveillance programs in HRIs in terms of identification of the above-mentioned lesions are limited and heterogeneous, thus HRI surveillance is generally performed in the setting of research protocols.
Both magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS) are employed as first-line modalities for HRI surveillance, but no imaging test has gained evidence-based consensus.6,7 Furthermore, the results of screening might differ in terms of detected lesions in each HRI subgroup. As an example, patients with FAMMM were reported to develop more solid lesions while FPC individuals more cystic ones.8,9
This systematic review and meta-analysis is therefore aimed to assess in HRIs (a) the prevalence of solid and cystic lesions and of lesions considered a successful target of surveillance, (b) the prevalence of lesions diagnosed by EUS and/or MRI, and (c) the prevalence of lesions considered a successful target of the surveillance in different HRI subgroups.
Materials and methods
Search strategy
A PubMed and Scopus databases search (see Appendix 1) was run until June 2017. Duplicates were removed. The methodology was developed from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10
The titles of all identified articles were assessed for their relevance, and abstracts and/or full texts of potentially relevant papers screened and evaluated. A manual search of all relevant articles and references was conducted to identify further relevant studies.
Inclusion and exclusion criteria
Inclusion criteria were English language, patients belonging to FPC families and/or with other specific high-risk syndromes or germline mutation carriers, and surveillance carried out with MRI and/or EUS, with the prevalence and type of diagnosed pancreatic lesions (solid and/or cystic) being reported. In the case of duplicate publications, the most recent or most informative was included. Two independent reviewers (MS and GZ) carried out study identification and selection and discussed disagreements with a third reviewer (GC). Excluded studies and reasons for exclusion were recorded.
Data extraction and quality assessment
Two reviewers (MS and GZ) independently extracted data from each study into a Microsoft Excel spreadsheet (XP Professional Edition; Microsoft Corp, Redmond, WA, USA). Disagreements were resolved by consulting a third reviewer (GC). Study year, design and location, number of screened individuals, and type of high-risk subgroups, and of imaging, follow-up duration, number, and type of diagnosed lesions, and of patients with an indication for surgery and with an identified lesion considered to be a success of the surveillance or diagnosed with advanced/metastatic PDAC were recorded. A summary table of the relevant studies listing the population characteristics and outcomes was developed. The quality of the studies was evaluated independently by two reviewers (MS and GC) using the Newcastle-Ottawa Scale11 with a dedicated quality appraisal tool including seven items. Studies with a score ≥7 were considered of high quality.
Data analysis
We examined (a) the pooled prevalence rate of all solid or cystic lesions, and (b) the pooled prevalence of lesions being considered a successful target of surveillance as defined by gold-standard pathology after surgery. Lesions considered as successful targets of surveillance were: PanIN3 (or high-grade PanIN if not specified), IPMNs with high-grade dysplasia or main duct (MD)/mixed-type IPMN, and any resectable PDAC with R0 pathology. This definition is adapted from the CAPS one, as some of the papers did not provide enough information for detailed grouping; the pooled prevalence rate of advanced IPMNs and PanIN3 lesions was also calculated, considering them as “premalignant” target lesions; (c) the pooled prevalence rate of advanced/metastatic PDAC, not amenable to R0 resection; (d) the pooled prevalence of the above-mentioned lesions detected either by EUS or by MRI; and (e) the pooled prevalence of successful target lesions in each specific HRI group.
Data were combined to generate a pooled prevalence rate. To better reflect the incidence of detected lesions over time, we also calculated the incidence rates of lesions being a successful target of surveillance by dividing the total number of events by the total number of person-years (pyrs) of follow-up. If these latter data were not provided in a study, it was estimated by multiplying the number of patients who underwent surveillance by the reported mean follow-up time. The corresponding 95% confidence intervals (CIs) were calculated using exact methods and assuming a Poisson distribution. When the number of events was 0, a continuity correction of 0.5 was used for the purpose of calculation, as previously reported.12
A meta-analysis was performed using the software package Comprehensive Meta-Analysis (Biostat, Englewood, NJ, USA) by using a random-effects model.13 In addition to within-study variance, the random-effects model considers heterogeneity among studies and gives more conservative estimates. The quantity of heterogeneity was assessed by means of the I2 value.14 The I2 describes the percentage of total variation across studies that is caused by heterogeneity and not by chance. Publication bias was assessed using the Begg and Mazumdar test. A p value < 0.05 was accepted as statistically significant. We developed the following a priori hypotheses that would explain heterogeneity and planned sensitivity analyses for (a) area of origin (i.e., United States (US)/Canada or Europe) and (b) quality of the study (quality score >7 or ≤7).
Results
Search results and study selection and characteristics
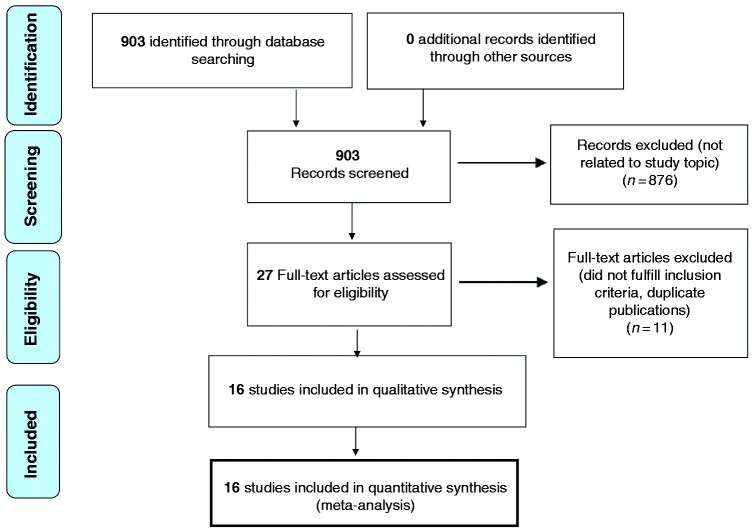
The study selection process is summarized in Figure 1. Sixteen studies met the eligibility criteria and were included for qualitative analysis and quantitative synthesis. One of them15 is a multicenter study whose findings were already reported in three previous single-center studies.9,16,17 As the population of this latter study was larger and results regarding the different HRIs subgroups more detailed, we used this manuscript for the analysis of pooled prevalence of overall lesions. However, as this more recent paper does not report the exact number of cystic/solid lesions diagnosed by either EUS or MRI, we used data from the older studies for the analyses on the role of MRI and EUS.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of assessment of studies identified in the preset systematic review.
Table 1 summarizes the characteristics of the 16 included studies. Two papers reported only the first surveillance round18,19 while two other studies did not report the exact follow-up period.20,21 The mean follow-up in studies reporting > one surveillance round2,6,7,15,22–26 was 32.4 months, and the total number of enrolled HRIs 1588. Considering the 1572 individuals for whom this information was available, the largest group of screened individuals was FPC (1043, 66.3% of total) followed by FAMMM (243, 15.4%) and hereditary breast and ovarian cancer (HBOC) syndrome individuals or BRCA1/2 mutation carriers (140, 8.9%). Some studies also enrolled individuals who did not meet the criteria to be designated as “HRI” according to the CAPS consortium.2,7,18–20 There were four patients with Li-Fraumeni syndrome,9,22 five with only one affected family member,25 nine with a family member with early-onset PDAC,24 and six with >1 relative with non-pancreatic cancers;2 all together these people accounted for 1.6% of the investigated individuals. Two studies enrolled patients with a very low risk of developing pancreatic cancer based on family history19,21 and those individuals therefore were not included in the analysis. Only four4,7,20,25 of the 16 studies were scored as of “high quality.”
Table 1.
Study demographics, population size, and characteristics.
| Study first author | Year | Country | Study design | Number screened | Mean age (range) | Types of high-risk group screened | Mean months of follow-up (intervals) | Type of imaging |
|---|---|---|---|---|---|---|---|---|
| Kimmey22 | 2002 | USA | Single center | 46 | NR | 46 FPC | 60 | EUS |
| Canto23 | 2006 | USA | Single center | 78 | 52 (32–77) | 72 FPC, 6 PJS | 12 (within one year) | EUS |
| Poley18 | 2009 | The Netherlands | Multicenter | 44 | NR (32–75) | 21 FPC, 3 BRCA1, 2 BRCA2, 2 PJS, 13 FAMMM, 2 HP, 1 LFS | Baseline only | EUS |
| Langer16 | 2009 | Germany | Multicenter | 76 | NR | FPC, FAMMMb | NR (annual) | EUS and MRI/MRCP with CE |
| Verna19 | 2010 | USA | Single center | 41 | 52 (29–77) | 30 FPC, 6 BRCA1/2, 5 OFMA | Baseline only | EUS and MRI (MRCP) |
| Ludwig20 | 2011 | USA | Single center | 109 | 54 (43–65) | 93 FPC ,7 BRCA, 9 EOPCF | NR | MRCP |
| Vasen15 | 2011 | The Netherlands | Single center | 79 | 56 (39–72) | 79 FAMMM | 48 (annual) | MRI/MRCP with CE |
| Canto (CAPS3)4 | 2013 | USA | Multicenter | 216 | 56 (28–79) | 195 FPC, 19 BRCA2, 2 PJS | 28.8 (one to three years) | EUS and CT and MRI/MRCP with CE and secretin |
| Al-Sukhni2 | 2012 | Canada | Single center | 226 | 54 (22–89) | 146 FPC, 51 BRCA2, 5 BRCA1, 10 FAMMM, 6 PJG, 2HP, 6 MCFDR | 50.4 (annual) | MRI/MRCP without CE |
| Sud24 | 2014 | USA | Single center | 16 | NR | FPC, BRCA1, BRCA2, PJS, FAMMM, HNPCCb | 12 (annual) | EUS |
| Harinck7 | 2016 | The Netherlands | Multicenter | 139 | 51 (20–73) | 68 FPC, 3 BRCA1, 20 BRCA2, 38 FAMMM, 7 PJS, 3 LFS | 12 (annual) | EUS and MRI/MRCP with CE |
| Del Chiaro25 | 2015 | Sweden | Single center | 40 | 50 (23–76) | 32 FPC, 3 BRCA2, 1 BRCA1, 4 FAMMM | 12.9 (annual) | MRI/MRCP with secretin |
| Mocci17 | 2015 | Spain | Multicenter | 41 | NR | 24 FPC, 12 HBOC, 5 EOPCF | 24 (3 to 12 months) | EUS and CT |
| Joergensen26 | 2016 | Denmark | Multicenter | 71 | 51 (27–72) | 40 FPC, 31 HP | 60 (annual) | EUS |
| Vasena15 | 2016 | The Netherlands, Germany, Spain | Multicenter | 411 | NR | 214 FPC, 178 FAMMM, 19 BRCA1/2 PALB2 | 43.2 (annual) | EUS and/or MRI |
| Chang25 | 2017 | Taiwan | Single center | 151 | NR | 1 BRCA2, 64 HP, 86 FPC | NR (annual) | MRI/MRCP with CE |
The exact number of high-risk individuals was not provided.
FPC: familial pancreatic cancer; BRCA: breast cancer susceptibility gene; PALB2: partner and localizer of BRCA2; CAPS: International Cancer of the Pancreas Screening; FAMMM: familial atypical multiple-mole melanoma syndrome; HNPCC: hereditary nonpolyposis colorectal cancer; HBOC: hereditary breast-ovarian cancer syndrome; PJS: Peutz-Jeghers syndrome; HP: hereditary pancreatitis; LFS: Li-Fraumeni syndrome; EOPCF: early-onset pancreatic cancer family; MCFDR: multicancers first-degree relatives; OFMA: one family member affected; EUS: endoscopic ultrasonography; MRI: magnetic resonance imaging; MRCP: magnetic resonance cholangiopancreatography; CE: contrast enhancement; CT: computed tomography; NR: not reported; USA: United States of America.
Prevalence of solid pancreatic lesions in HRIs
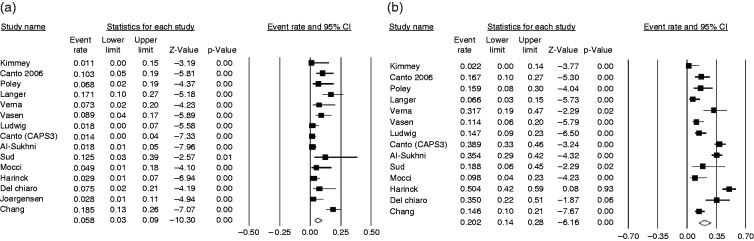
A total of 79 pancreatic solid lesions were detected, with a pooled prevalence of 5.8% (95% CI 3%–9%; I2 = 77.5%) (Figure 2). No publication bias was found (Begg and Mazudmar Kendall’s tau = –0.21; p = 0.27). When considering only the studies conducted in the USA or Canada, the pooled estimate prevalence was 3.8% (95% CI 2%–8%; I2 = 68.8%), compared to 6.8% with similar heterogeneity (95% CI 4%–12%; I2 = 61.2%) in the studies from Europe. The pooled prevalence of solid lesions in studies of high quality4,7,20,25 was 2.8% (95% CI 1%–6%) compared to 7.7% (95% CI 5%–12%) in the 11 studies of lower quality,2,9,16–19,21–24,26 with lower heterogeneity (I2 = 38.2% vs I2 = 72.3%) in high-quality studies.
Figure 2.
Forest plot showing the pooled prevalence of pancreatic solid lesions (panel (a), on the left) diagnosed in high-risk individuals in all the 14 included studies and of the pooled prevalence of cystic lesions (panel (b), on the right) diagnosed in high-risk individuals in the 13 studies reporting this information. Random-effects model demonstrating a pooled prevalence of 5.8% (95% confidence interval (CI) 3%–8%) with moderate heterogeneity (I2 = 77.5%) for solid lesions and a pooled prevalence of 20.2% (95% CI 14%–29%) with considerable heterogeneity (I2 = 88.9%) for cystic ones.
Prevalence of cystic pancreatic lesions
A total of 340 pancreatic cystic lesions were detected, with a pooled prevalence of 20.2% (95% CI 14%–28%; I2 = 88.9%) (Figure 2). Information on prevalence of pancreatic cystic lesions was not provided in one study.26 No publication bias was found (Begg and Mazudmar Kendall’s tau = –0.34; p = 0.09). The pooled prevalence of cystic lesions was 23.4% (95% CI 16%–34%; I2 = 84.1%) in the studies conducted in the USA and Canada, and 18.4% (95% CI 8%–37%; I2 = 92.1%) in the studies conducted in Europe. In studies with a high-quality score, the pooled prevalence of cystic lesions was 33.6% (95% CI 21%–49%; I2 = 90.3%), being higher than the 15.4% (95% CI 10%–24%) of studies with a low-quality score, yet with similar heterogeneity (I2 = 83.7%).
Prevalence of successful target lesions of surveillance
Of 1588 screened HRIs, 95 were considered to have an indication for surgery (pooled prevalence 6.8%; 95% CI 4%–11%; I2 = 81%). However, the pooled prevalence of individuals for whom surveillance identified a lesion considered a successful target of surveillance was 3.3% (95% CI 2%–5%; I2 = 40.5%) (Figure 3). In high-quality studies, this pooled prevalence was 2.9% (95% CI 1%–8%; I2 = 69.2%), being 3.4% (95% CI 2%–5%; I2 = 23.4%) in studies of lower quality. In the sensitivity analysis by country of origin, the pooled prevalence was 2.7% (95% CI 1%–5%; I2 = 43.3%) for studies conducted in the USA or Canada and 4.1% (95% CI 2%–8%; I2 = 52.2%) for studies conducted in Europe. No publication bias was found (Begg and Mazudmar Kendall’s tau = –0.16; p = 0.45). Furthermore, when we repeated this analysis excluding individuals who were not at high risk according to the guidelines,2,7,18–20 the pooled prevalence was 3.4% (95% CI 2%–5%; I2 = 44.7%). As the ideal target of the surveillance programs should be the diagnosis of “premalignant” lesions, the pooled prevalence rate of advanced IPMNs and PanIN3 lesions was also calculated, and resulted in 1.6% (95% CI 1%–2%; I2 = 0%) (see Supplementary Figure 1).
Figure 3.
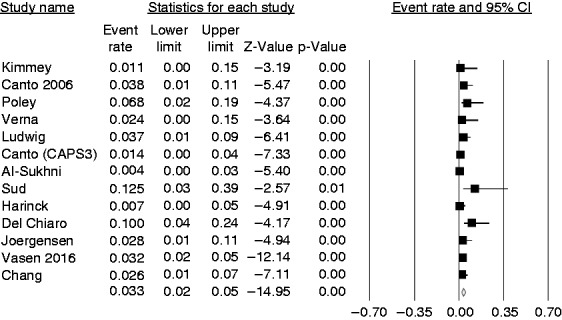
Forest plot showing the overall pooled prevalence of successful target lesions of the surveillance that is equal to 3.3% (95% confidence interval (CI) 2%–5%), with moderate heterogeneity (I2 = 40.5%).
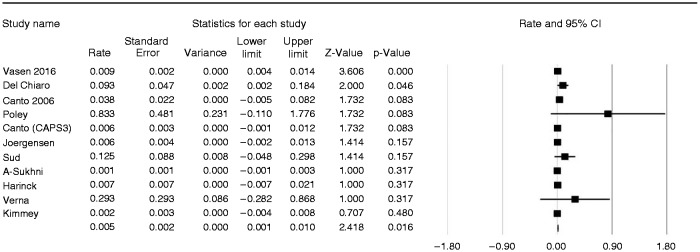
In detail, 26 (1.6%) patients were diagnosed with a resectable PDAC, 11 (0.7%) with branch duct (BD)-IPMNs with high-grade dysplasia or an MD-IPMN, and four (0.3%) with advanced PanINs. Six individuals were diagnosed with pancreatic neuroendocrine neoplasms (pNENs).2,6,15,24 Four of them were resected and all but one2 had a diameter <15 mm. Type and number of histologically confirmed lesions, including those successfully operated on, are summarized in Supplementary Table 1. The pooled estimate rate of lesions considered a successful target of surveillance was calculated for 11 studies in which follow-up length was reported, and resulted in 0.005/pyrs (95% CI 0.001%–0.005%; I2 = 56%), equal to 5/1000 pyrs (Figure 4).
Figure 4.
Forest plot showing the pooled estimate rate of successful target lesions of the surveillance in the 11 studies that reported the follow-up length. The pooled estimate rate resulted of 5/1000 person years with moderate heterogeneity (I2 = 56%).
Prevalence of advanced/metastatic pancreatic adenocarcinoma
During the surveillance programs, nine advanced/metastatic adenocarcinoma were diagnosed. Six metastatic PDAC were diagnosed and histologically confirmed by percutaneous or EUS-guided fine needle aspiration;2,9,19,23 the other three underwent surgical resection but histology showed a positive resection margin.9,15 The pooled prevalence of HRIs for which surveillance identified advanced PDAC was 1.0% (95% CI 1%–2%), without heterogeneity (I2 = 0%).
Prevalence of pancreatic lesions diagnosed either by EUS or by MRI
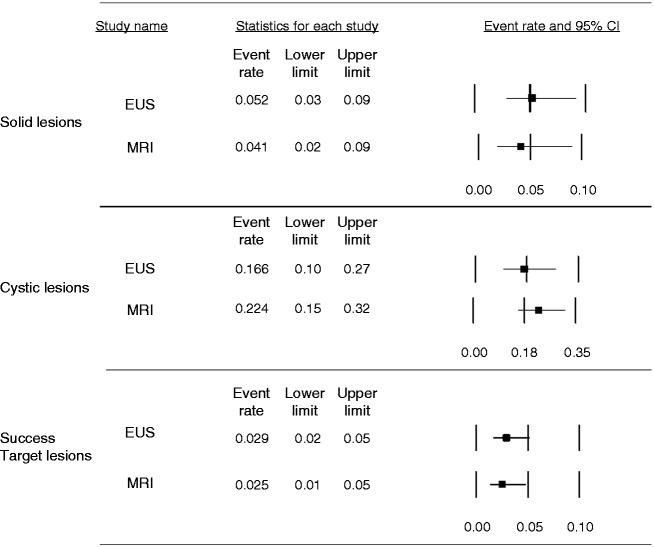
Ten studies employed EUS6,7,16–19,22–24,26 and nine MRI.2,6,7,9,16,19–21,25 The pooled prevalence of solid lesions was higher in studies employing EUS (5.2%, 95% CI 3%–9%; I2 = 60.6%) compared with those using MRI (4.1%, 95% CI 2%–9%; I2 = 83%) (Figure 5). The pooled prevalence of cystic lesions was instead 22.4% (95% CI 15%–32%; I2 = 89.3%) with MRI and 16.6% (95% CI 10%–27%; 85.7%) with EUS in the eight studies providing this information, which was lacking in two studies.17,26 The pooled prevalence of pancreatic lesions considered a successful target of surveillance was 2.9% with EUS (95% CI 2%–5%; I2 = 27.4%) and 2.5% with MRI (95% CI 1%–5%; I2 = 51.7%) (see Figure 5).
Figure 5.
Summary of the pooled prevalence of pancreatic lesions (solid, cystic and successful target lesions of the surveillance) diagnosed either by endoscopic ultrasonography (EUS) or magnetic resonance imaging (MRI). CI: confidence interval.
Prevalence of lesions considered a successful target of surveillance in different HRI subgroups
The pooled prevalence of lesions considered a successful target of surveillance was 3% (95% CI 2%–5%; I2 = 22.2%) in FPC individuals. In people with a specific genetic syndrome it was 4% in HP (95% CI 1%–14%), 5% for FAMMM (95% CI 3%–9%), 6.3% in HBOC or BRCA1/2, PALB2 mutation carriers (95% CI 3%–14%), and 12.2% in PJS (95% CI 4%–32%), without heterogeneity (I2 = 0%) in all these subgroups except for people with HP (I2 = 12.2%) (Figure 6). We also analyzed the pooled prevalence rate of histologically confirmed solid lesions diagnosed at the baseline examination in each subgroup. These data were available for all studies but one.21 The pooled rate of solid lesions at baseline resulted respectively in: 1.6% (95% CI 1%–3%; I2 = 0%) in FPC, 5.8% (95% CI 2%–14%; I2 = 0.8%) in HBOC or BRCA1/2 mutation carriers, 4.6% (95% CI 2%–12%; I2 = 35%) in FAMM, 12% (95% CI 4%–32%; I2 = 0%) in PJS, and 7.2% (95% CI 1%–30%; I2 = 0.8%) in HP. The number of pancreatic cancer cases and the relative proportion of unresectable/metastatic cases were respectively 12 (25% metastatic) in FPC, 15 (20% metastatic) in FAMMM, four (25% metastatic) in HBOC, and one (0% metastatic) in HP. No PDAC cases were diagnosed in PJS patients.
Figure 6.
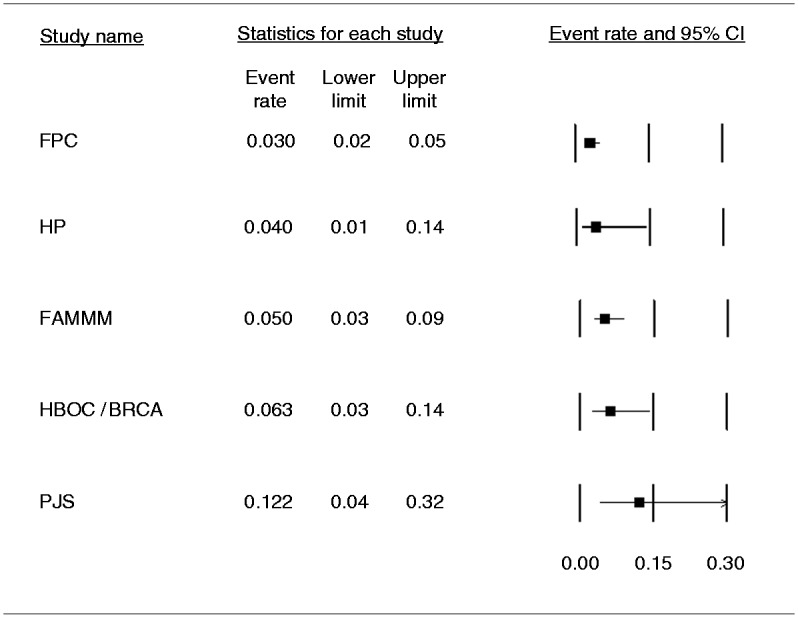
Pooled prevalence of lesions considered a successful target of surveillance in the different high-risk individual subgroups. The pooled prevalence of lesions considered a successful target of surveillance diagnosed in familial pancreatic cancer (FPC) was 3% (95% confidence interval (CI) 2%–5%) with moderate heterogeneity (I2 = 22.2%). The pooled prevalence in familial atypical multi-mole melanoma syndrome (FAMMM) was 5% (95% CI 3%–9%), in hereditary pancreatitis (HP) was 4% (CI 1%–14%), in hereditary breast-ovarian cancer syndrome (HBOC) or BRCA1/BRCA2 or PALB2 mutation carriers was 6.3% (95% CI 3%–14%), and in Peutz-Jeghers syndrome (PJS) it was 12.2% (95% CI 4%–32%). Notably, in all these genetic syndromes but HP, there was no heterogeneity (I2 = 0%).
Discussion
As data on the prevalence of lesions diagnosed during surveillance programs in individuals at high risk of PDAC are scanty and heterogeneous, we conducted a meta-analysis to estimate the pooled prevalence of solid and/or cystic lesions, and more important, whether detected lesions could be considered a successful target of surveillance. We also calculated the pooled estimated rate of detected lesions during the course of subsequent surveillance rounds, the prevalence of lesions diagnosed by either EUS or MRI, and the differential prevalence of lesions among the various HRI subgroups.
Data from 1588 enrolled HRIs were included. The pooled prevalence of solid and cystic lesions in these individuals was 5.8% and 20.2%, respectively (Figure 2). The pooled prevalence of lesions considered a successful target of surveillance according to the CAPS definition was 3.3% (Figure 3), while the actual pooled prevalence of “preneoplastic” target lesions (advanced IPMNs and PanIN3 lesions) was 1.6% (see Supplementary Figure 1). The pooled estimated rate of lesions considered a successful target of surveillance during follow-up amounted to five cases per 1000 pyrs, equal to an annual risk of 0.5% (Figure 4).
EUS seemed able to diagnose more solid lesions and MRI more cystic ones (Figure 5). Moreover, the rate of lesions considered a successful target of surveillance was much lower in FPC compared to HRI with specific syndromes (Figure 6). This is not surprising as in FPC the causal mutation is unknown despite a clear autosomal dominant inheritance pattern. Therefore, half of FPC individuals undergo surveillance without carrying the causal mutation.
Of the 1588 screened HRIs, 6.8% underwent surgery, with histologically confirmed lesions considered a successful target of surveillance in 3.3%. To date, there is little consensus about which lesions detected by surveillance represent an indication for surgery,4 considering the morbidity of pancreatic surgery.27 It is unknown whether for example in the case of BD-IPMNs the same criteria for resection apply in HRIs compared to sporadic cases.28 A recent study showed that cystic lesions diagnosed in HRIs with a known mutation are more prone to progress compared to those discovered in FPC individuals, although this latter group had a significantly higher prevalence of cystic lesions.29 There is also evidence of a high rate of lymph node involvement and poor prognosis in HRIs with PDAC even with very small lesions.9,18 This might justify a more aggressive attitude toward resecting precursor lesions in this setting.
A proportion of patients diagnosed with PDAC (n = 9, pooled prevalence 1%) were identified at an advanced/metastatic stage. Two of them were prevalent cases diagnosed at baseline. The other patients who underwent surgical resection with positive resection margins, or who were diagnosed with an unresectable interval cancer during subsequent follow-up, however, should be considered a failure of surveillance. The proportion of unresectable PDAC was similar in people with FPC, FAMMM, and HBOC. This raises concerns about the validity of currently performed surveillance programs.
In four cases the resected lesions were pNENs, only one2 with diameter >1.5 cm. The European Neuroendocrine Tumours Society guidelines30 would not recommend surgery for incidentally detected pNENs <2 cm.
Few studies compared the diagnostic yield of EUS and MRI/magnetic resonance cholangiopancreatography. A high concordance between the two methods was described by Canto et al.,6 while only a 55% agreement was shown by Harinck et al.7 for the detection of clinically relevant lesions. In the present study, the pooled prevalence of solid lesions detected by EUS was higher compared to MRI (5.2% vs 4.1%), while MRI had a higher yield for cystic lesions (22.4% vs 16.6%). The pooled prevalence of lesions considered a successful target of surveillance was similar for EUS and MRI. A limitation of this analysis is the high heterogeneity between studies in terms of MRI protocols, and the use of radial EUS in some studies, while linear EUS is able to detect more pancreatic lesions in HRIs.31 The two methods might be considered complementary rather than interchangeable in surveillance programs,7 and their use should be tailored considering local expertise.
The yield of surveillance programs in different HRI subgroups is another interesting subject. The pooled prevalence of lesions considered a successful target of surveillance in the present meta-analysis was 3% in FPC individuals, representing the majority of people screened, 4% in HP, 5% in FAMMM, 6.3% in HBOC, BRCA1/2, or PALB2 mutations carriers, and 12.2% in PJS. Notably, while the results obtained in FPC showed a certain heterogeneity, this was not the case in patients with genetic syndromes. It would be attractive to tailor surveillance in terms of age at which to start, modality, and follow-up intervals based on the frequency and growth characteristics of the lesions diagnosed in each HRI subgroup.
Vasen et al.15 recently reported that IPMNs with high-grade dysplasia and multifocal PanINs3 were more frequent in FPC compared to FAMMM patients, while the rate of diagnosed PDAC was higher in this latter group. Further studies into the differential risk and growth characteristics of the various subgroups of HRIs are needed.
This is the first study to systematically appraise the available literature evidence from surveillance studies in HRIs for developing PDAC. Although we developed a priori hypotheses for sensitivity analyses considering likely sources of heterogeneity, the observed heterogeneity between studies reflecting differences in surveillance tests, intervals, type of reported lesions, and kind of HRIs enrolled is a potential limitation. The lack of individual patient data limited the possibility of performing any analysis other than that of aggregate data, and the influence of factors such as the age of the individuals enrolled in the surveillance programs, and the relevance of risk factors such as smoking, could not be appropriately considered.
In conclusion, the pooled prevalence rate of resected lesions that can be considered a successful target of surveillance during PDAC surveillance programs in HRIs is 3.3% with an annual risk of 0.5%. The pooled prevalence rate of successful “premalignant” target lesions is, however, lower and equal to only 1.6%. A higher prevalence rate was observed in HRI carriers with a specific DNA mutation compared to HRIs with FPC in whom the mutation is unknown.
Supplementary Material
Appendix 1
Search strategy
The following search strategy was employed: (Neoplasm, Pancreatic OR Pancreatic Neoplasm OR Neoplasms, Pancreas OR Pancreas Neoplasm OR Neoplasms, Pancreatic OR Cancer of Pancreas OR Pancreas Cancers OR Pancreas Cancer OR Cancer, Pancreas OR Cancers, Pancreas OR Pancreatic Cancer OR Cancer, Pancreatic OR Cancers, Pancreatic OR Pancreatic Cancers OR Cancer of the Pancreas) AND (Cancer Early Detection OR Cancer Screening OR Screening, Cancer OR Cancer Screening Tests OR Cancer Screening Test OR Screening Test, Cancer OR Screening Tests, Cancer OR Test, Cancer Screening OR Tests, Cancer Screening OR Early Diagnosis of Cancer OR Cancer Early Diagnosis) AND (High Risk OR High-Risk individuals OR High-Risk patients OR High-Risk cohort OR High-Risk population OR FPC OR familial pancreatic cancer OR inherited pancreatic cancer OR HBOC OR hereditary breast and ovarian cancer syndrome OR BRCA OR FAMMM OR familial atypical multiple mole melanoma OR PJS OR Peutz-Jeghers syndrome OR HNPCC OR hereditary nonpolyposis colorectal cancer OR PALB OR mismatch repair gene mutation OR Genetic Susceptibility OR Genetic Susceptibilities OR Susceptibilities, Genetic OR Susceptibility, Genetic OR Genetic Predisposition OR Genetic Predispositions OR Predispositions, Genetic OR Predisposition, Genetic).
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: An eight-year experience. J Gastrointest Surg 2012; 16: 771–783. [DOI] [PubMed] [Google Scholar]
- 3.Del Chiaro M, Zerbi A, Capurso G, et al. Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: A position paper from the Italian Registry. Dig Liver Dis 2010; 42: 597–605. [DOI] [PubMed] [Google Scholar]
- 4.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capurso G, Signoretti M, Valente R, et al. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc 2015; 7: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142: 796–804. quiz e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016; 65: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 8.Potjer TP, Schot I, Langer P, et al. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res 2013; 19: 442–449. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011; 140: 850–856. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Reprint—preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys Ther 2009; 89: 873–880. [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014, accessed 1 october 2017).
- 12.Sutton AJ. Methods for meta-analysis in medical research. Chichester, New York: J. Wiley, 2000, pp.xvii, 317.
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: Outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol 2016; 34: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 16.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009; 58: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 17.Mocci E, Guillen-Ponce C, Earl J, et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer 2015; 51: 1911–1917. [DOI] [PubMed] [Google Scholar]
- 18.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 19.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: A comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–5037. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011; 106: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang MC, Wu CH, Yang SH, et al. Pancreatic cancer screening in different risk individuals with family history of pancreatic cancer—a prospective cohort study in Taiwan. Am J Cancer Res 2017; 7: 357–369. [PMC free article] [PubMed] [Google Scholar]
- 22.Kimmey MB, Bronner MP, Byrd DR, et al. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc 2002; 56(4 Suppl): S82–S86. [DOI] [PubMed] [Google Scholar]
- 23.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–781. quiz 665. [DOI] [PubMed] [Google Scholar]
- 24.Sud A, Wham D, Catalano M, et al. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas 2014; 43: 458–461. [DOI] [PubMed] [Google Scholar]
- 25.Del Chiaro M, Verbeke CS, Kartalis N, et al. Short-term results of a magnetic resonance imaging-based Swedish screening program for individuals at risk for pancreatic cancer. JAMA Surg 2015; 150: 512–518. [DOI] [PubMed] [Google Scholar]
- 26.Joergensen MT, Gerdes AM, Sorensen J, et al. Is screening for pancreatic cancer in high-risk groups cost-effective?—Experience from a Danish national screening program. Pancreatology 2016; 16: 584–592. [DOI] [PubMed] [Google Scholar]
- 27.Balzano G, Zerbi A, Capretti G, et al. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg 2008; 95: 357–362. [DOI] [PubMed] [Google Scholar]
- 28.Mandai K, Uno K, Yasuda K. Does a family history of pancreatic ductal adenocarcinoma and cyst size influence the follow-up strategy for intraductal papillary mucinous neoplasms of the pancreas? Pancreas 2014; 43: 917–921. [DOI] [PubMed] [Google Scholar]
- 29.Konings IC, Harinck F, Poley JW, et al. Prevalence and progression of pancreatic cystic precursor lesions differ between groups at high risk of developing pancreatic cancer. Pancreas 2017; 46: 28–34. [DOI] [PubMed] [Google Scholar]
- 30.Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016; 103: 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin EJ, Topazian M, Goggins MG, et al. Linear-array EUS improves detection of pancreatic lesions in high-risk individuals: A randomized tandem study. Gastrointest Endosc 2015; 82: 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.