Abstract
Background
We investigated whether metabolic syndrome exacerbated the risk of liver fibrosis among chronic hepatitis B patients and risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patients with components of metabolic syndrome.
Methods
This study included 1236 chronic hepatitis B patients with at least one component of metabolic syndrome. The controlled attenuation parameter and liver stiffness, patient information and relevant laboratory data were recorded.
Results
Controlled attenuation parameter was increased progressively with the number of metabolic syndrome components (p < 0.001). Multivariate analysis indicated younger age, high gamma-glutamyltransferase level, high waist-hip ratio, and high body mass index were independent risk factors associated with nonalcoholic fatty liver disease among chronic hepatitis B patients with metabolic syndrome. In the fibrosis and non-fibrosis groups, most of blood lipid was relatively lower in fibrosis group. An increased proportion of chronic hepatitis B patients with liver fibrosis was found concomitant with an increasing number of components of metabolic syndrome. Male gender, older age, smoking, aspartate aminotransferase levels, high body mass index, and low platelet level were identified as independent risk factors associated with liver fibrosis.
Conclusions
For chronic hepatitis B patients with coexisting components of metabolic syndrome, stratification by independent risk factors for nonalcoholic fatty liver disease and fibrosis can help with management of their disease.
Keywords: Hepatitis B, liver fibrosis, transient elastography, liver steatosis, metabolic syndrome
Introduction
Approximately 240 million people worldwide are chronically infected with hepatitis B virus (HBV), placing them at an increased risk of developing end-stage liver disease, including cirrhosis and hepatocellular carcinoma (HCC).1,2 The duration of the antiviral therapy required is very long and may even extend to life-long treatment.3 Early identification of liver fibrosis is important and necessary. The rising incidence of metabolic syndrome (MetS) is another grim health burden.4 Patients suffering from MetS have higher risks of cardiovascular morbidity and mortality, type 2 diabetes mellitus (T2DM), and HCC.5 There are patients who have concurrent chronic HBV infection and MetS, which can cause a clinical dilemma. Nonalcoholic fatty liver disease (NAFLD) is considered to be a hepatic manifestation of MetS. A study by Jin and colleagues6 reported that, among chronic hepatitis B (CHB) patients, the presence of NAFLD was an independent risk factor for poor clinical outcomes with entecavir treatment. Another study by Chan et al.7 reported that concurrent fatty liver disease in HBV-infected patients increased the risk of developing HBV-associated HCC by 7.3-fold. Hence, screening for NAFLD in CHB patients with MetS is an important clinical issue. Moreover, patients with MetS are at increased risk for liver fibrosis.8 Whether CHB patients with coexisting components of MetS have increased risk of liver fibrosis, and if so, how to identify the high-risk population among them, is an unsolved clinical problem.
Liver biopsy, until now, has been regarded as the gold standard for evaluating liver steatosis and fibrosis.9 However, it has also been criticized because of its invasive nature. Recently, a non-invasive measurement of liver steatosis and fibrosis by transient elastography (TE) to evaluate the controlled attenuation parameter (CAP) and liver stiffness (LS) has received increasing attention.10 However, because of the high cost of the equipment, and the lack of skilled operators, LS and CAP measured by TE have not been widely implemented clinically, especially in lower-to-middle income countries.11 It is more practical to stratify the risk among patients and perform TE and/or liver biopsy in the high-risk population. However, the risk factors associated with liver steatosis and fibrosis in CHB patients with components of MetS (CHBcM) has not been extensively examined.
In this study, the relationships of CAP, LS, demographics, and clinical parameters in CHB patients with one or more component of MetS were evaluated. The purpose of this study was to find out whether the component of MetS exacerbates the risk of liver fibrosis in CHB patients.
Subjects and methods
Subjects
The inclusion criteria were the presence of CHB and at least one MetS component. CHB was defined as showing a sero-positive of hepatitis B virus surface antigen (HBsAg) for ≥6 months with persistent or repetitive alanine aminotransferse (ALT) elevation.10 The MetS was defined according to the International Diabetes Federation by the presence of at least three of the following metabolic abnormalities: waist circumference >94 cm for men and >80 cm for women (as a pre-requisite diagnostic criterion); blood pressure ≥130/85 mm Hg; T2DM previously diagnosed by a physician, or a fasting plasma glucose level ≥5.6 mmol/l; triglyceride levels >1.7 mmol/l; and/or HDL-cholesterol <1.04 mmol/l for men and <1.29 mmol/l for women. Patients were excluded if (a) the patient used medications that can induce hepatic steatosis (e.g. corticosteroids, estrogen, methotrexate, or amiodarone) within six months of study inclusion, (b) had right-sided heart failure, (c) evidence of co-infection with hepatitis C, hepatitis D or human immunodeficiency virus (HIV), (d) had an autoimmune liver disease, or (e) heavy alcoholic abuse, defined as >30 g/day consumption.12 This is summarized on the flow chart shown in Supplementary Material Figure 1.
LS and CAP measured by transient elastography
LS and CAP values were assessed by TE according to the manufacturer’s handbook (Echosens, Paris, France) by a professionally trained technician.13 LS analyses were expressed in kilopascals (kPa) and CAP decibels per meter (dB/m). The ratio of the interquartile range (IQR) of LS to the median (IQR/M) was calculated as an indicator of variability. Only procedures with at least 10 valid measurements, a success rate of at least 60%, and an IQR/M ratio of the LSM value <0.3 were considered reliable and then used for analysis. CAP was measured on the same signals with LS, ensuring that a liver ultrasonic attenuation was obtained simultaneously and in the same volume of liver parenchyma as the LS. The IQR of CAP in the study is less than 40 dB/m.14 The final CAP value was the median of individual measurements.
According to the METAVIR scoring system, a score of F1–F4 indicated fibrosis and a score of F2–F4 was defined as advanced fibrosis.15 Therefore, a LS >7.4 Kpa was defined as liver fibrosis whereas LS >9.8 kPa was the threshold for advanced fibrosis.16 CHB patients were diagnosed with hepatic steatosis at CAP values >310 dB/m, according to previous recommendations.17
Blood pressure was measured by a mercury sphygmomanometer twice at rest in a sitting position, spaced 1–2 min apart, and the average value of two blood pressure measurements was used in analysis. Hypertension was defined, according the 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension, as an office-sitting systolic blood pressure (SBP) of ≥140 mm Hg and/or office diastolic blood pressure (DBP) ≥90 mm Hg.18 According to current guidelines, for the diagnosis of hypertension, patients were divided into the following four groups. The normal group: SBP <139 mm Hg and/or DBP <89 mm Hg; Grade 1 hypertension group: SBP 140–159 mm Hg and/or DBP 90–99 mm Hg; Grade 2 hypertension group: SBP 160–179 mm Hg and/or DBP 100–109 mm Hg; Grade 3 hypertension group: SBP ≥180 mm Hg and/or DBP ≥110 mm Hg.
Demographical and laboratory data
When TE was performed, relevant patient information on lifestyle, including alcohol consumption and smoking, was also collected and analyzed. Laboratory tests, including platelet (PLT) levels, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), total cholesterol, high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol, triglycerides, and blood uric acid were assessed, according to standard procedures. These laboratory results were obtained by standard automated techniques within 14 days of the TE.
The medical history of T2DM, and physical examination data including age, height, weight, waistline, and hipline were measured and recorded. Waist-hip ratio (WHR) indicated ratio of waistline to hipline. Body mass index (BMI) was calculated using the formula: weight/height2 (kg/m2).
Compliance with ethical requirements
The Institutional Review Board of First Affiliated Hospital of Xiamen hospital approved the study (1 March 2013). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
Statistical analysis
The measurement units are expressed as mean±standard deviation (SD) for continuous data. Categorical data was expressed in percentages. The significance of differences was tested using either the Student's t-test (for continuous variables) or the chi-square test (for categorical variables). Significance of trends in the association between CAP values and the number of MetS components were assessed by one-way analysis of variance. Univariable and stepwise multivariable regression analyses were performed using logistic regression analysis and the results were expressed as odds ratio (OR) and 95% confidence interval (CI). All analyses were performed using SPSS (version 13.0) with an alpha level of 0.05.
Results
Non-NAFLD and NAFLD group characteristics
A total of 1236 CHB subjects were included in the current study. Demographics and clinical characteristics are shown in Supplementary Material Table 1. The CAP value of all participants is 236.16 ± 56.84. Among them, a total of 149 subjects with fatty liver of CAP>310 dB/m were defined as the NAFLD group, with all other subjects assigned to the non-NAFLD group. The difference in their characteristics is shown in Table 1. Surprisingly, the LS values were no different in the non-NAFLD group than in the NAFLD group.
Table 1.
Chronic hepatitis B (CHB) patient characteristics for the non-nonalcoholic fatty liver disease (NAFLD) and NAFLD groups.
| Characteristic | CHB with component of MetS |
||
|---|---|---|---|
| Non-NAFLD group | NAFLD group | p Value | |
| Sample size, n | 1087 | 149 | – |
| Sex (male), n (%) | 773 (71.1) | 116 (78.4) | 0.065 |
| Age (years) | 43.04 ± 12.18 | 40.49 ± 12.29 | 0.018 |
| Smoking, n (%) | 286 (26.3) | 42 (28.4) | 0.593 |
| Alcohol consumption, n (%) | 234 (21.5) | 50 (33.8) | 0.001 |
| T2DM, n (%) | 145 (13.3) | 21 (14.2) | 0.776 |
| Metabolic syndrome, n (%) | 115 (10.6) | 30 (20.3) | 0.001 |
| Weight, kg | 63.47 ± 11.15 | 80.21 ± 17.25 | < 0.001 |
| Height, cm | 165.35 ± 8.54 | 168.14 ± 7.32 | <0.001 |
| SBP, mm Hg | 126.92 ± 13.29 | 132.24 ± 13.28 | <0.001 |
| DBP, mm Hg | 81.21 ± 9.22 | 85.89 ± 9.88 | <0.001 |
| Waist circumference, cm | 80.06 ± 10.26 | 92.29 ± 8.75 | <0.001 |
| Hip circumference, cm | 91.10 ± 6.86 | 97.91 ± 7.23 | <0.001 |
| Liver stiffness, Kpa | 8.77 ± 9.51 | 7.75 ± 5.83 | 0.206 |
| PLT, 109/l | 242.72 ± 58.92 | 245.72 ± 62.47 | 0.567 |
| ALT, U/l | 91.12 ± 51.35 | 87.86 ± 42.42 | 0.473 |
| AST, U/l | 59.79 ± 35.67 | 61.03 ± 20.76 | 0.685 |
| GGT, U/l | 62.21 ± 34.07 | 72.81 ± 36.01 | 0.001 |
| Total cholesterol, mmol/l | 5.08 ± 0.75 | 5.22 ± 0.72 | 0.039 |
| HDL-cholesterol, mmol/l | 1.32 ± 0.31 | 1.31 ± 0.30 | 0.994 |
| LDL-cholesterol, mmol/l | 2.99 ± 0.69 | 3.19 ± 0.66 | 0.001 |
| Triglycerides, mmol/l | 1.38 ± 0.49 | 1.56 ± 0.52 | <0.001 |
| Uric acid, mmol/l | 413.36 ± 96.47 | 459.06 ± 123.11 | < 0.001 |
| HBV DNA, log10 IU/ml | 0.98 ± 1.72 | 0.97 ± 1.71 | 0.987 |
| HBeAg ( + ), n (%) | 630 (58.0) | 90 (60.4) | 0.570 |
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; HDL: high density lipoprotein; LDL: low density lipoprotein; MetS: metabolic syndrome; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus.
The CAP value increased progressively with the number of MetS components among the CHB patients (p < 0.001, Figure 1(a)). The CAP value was also significantly higher in patients with alcohol consumption (p < 0.001), hyperuricemia (p = 0.024), hypertension (p < 0.001), and hyperlipidemia (p = 0.007), as shown in Supplementary Material Figure 2. Additionally, the CAP value could also effectively distinguish between the different grades of hypertension (Supplementary Material Figure 3).
Figure 1.
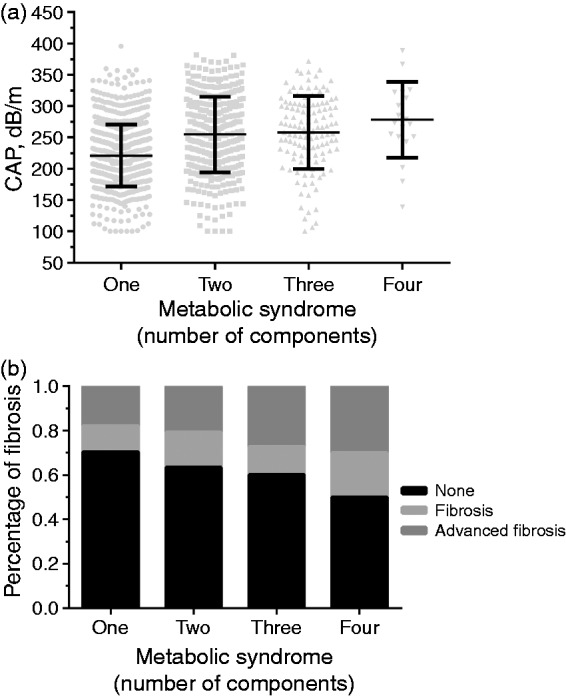
(a) Controlled attenuation parameter (CAP) value was increased progressively with the number of metabolic syndrome (MetS) components among the chronic hepatitis B (CHB) patients included (221.19 ± 49.43 vs 254.86 ± 60.28 vs 258.06 ± 58.32 vs 278.55 ± 60.57 dB/m, p < 0.001). (b) Increased proportion of CHB patients with liver fibrosis and advanced fibrosis with the number of components of MetS. A total of 11.7% and 17.9% of CHB patients had liver fibrosis and advanced fibrosis if they had one component of MetS, whereas 15.7% and 20.7% had fibrotic complications if they had two components, and 12.5% and 27.3% had the complications if they had three components, and 20.0% and 30.0% had complications if they had four components (p = 0.032).
Differences in characteristics between the liver fibrosis group and the non-fibrosis group
Patients were divided into a liver fibrosis group (n = 415) and a non-fibrosis group (n = 821) as determined by a LS value for the former of >7.4 Kpa. As shown in Table 2. What was interesting was that lipid levels were relatively lower in the fibrosis group. The CAP values, unexpectedly, were not different in the two groups.
Table 2.
Chronic hepatitis B (CHB) patient characteristics for the non-fibrosis and fibrosis groups.
| Characteristic | CHB with component of MetS |
||
|---|---|---|---|
| Non-fibrosis group | Fibrosis group | p Value | |
| Sample size, n | 821 | 415 | |
| Sex (male), n (%) | 559 (68.1) | 331 (79.8) | <0.001 |
| Age (years) | 41.15 ± 11.49 | 45.91 ± 13.01 | <0.001 |
| Smoking, n (%) | 188 (22.9) | 141 (34.0) | < 0.001 |
| Alcohol consumption, n (%) | 160 (19.5) | 125 (30.1) | < 0.001 |
| T2DM, n (%) | 93 (11.3) | 73 (17.6) | 0.002 |
| Metabolic syndrome, n (%) | 84 (10.2) | 61 (14.7) | 0.021 |
| Weight, kg | 64.58 ± 12.76 | 67.29 ± 13.89 | 0.001 |
| Height, cm | 165.24 ± 8.96 | 166.58 ± 7.27 | 0.009 |
| SBP, mm Hg | 127.38 ± 13.19 | 127.91 ± 13.83 | 0.517 |
| DBP, mm Hg | 81.76 ± 9.27 | 81.79 ± 9.73 | 0.961 |
| Waist circumference, cm | 80.59 ± 10.32 | 83.37 ± 11.62 | < 0.001 |
| Hip circumference, cm | 91.70 ± 6.78 | 92.35 ± 8.07 | 0.145 |
| CAP, dB/m | 236.27 ± 55.29 | 235.35 ± 59.35 | 0.788 |
| PLT, 109/l | 257.09 ± 50.94 | 214.36 ± 64.61 | <0.001 |
| ALT, U/l | 90.97 ± 53.01 | 90.31 ± 44.89 | 0.832 |
| AST, U/l | 56.15 ± 34.11 | 67.32 ± 33.19 | <0.001 |
| GGT, U/l | 64.29 ± 35.64 | 61.86 ± 32.12 | 0.277 |
| Total cholesterol, mmol/l | 5.13 ± 0.70 | 5.03 ± 0.83 | 0.042 |
| HDL-cholesterol, mmol/l | 1.31 ± 0.30 | 1.32 ± 0.31 | 0.725 |
| LDL-cholesterol, mmol/l | 3.07 ± 0.66 | 2.87 ± 0.74 | < 0.001 |
| Triglycerides, mmol/l | 1.44 ± 0.52 | 1.35 ± 0.46 | 0.004 |
| Uric acid, mmol/l | 399.58 ± 76.73 | 458.62 ± 129.83 | <0.001 |
| HBV DNA, log10 IU/ml | 0.91 ± 1.81 | 1.12 ± 1.50 | 0.048 |
| HBeAg (+), n (%) | 471 (57.4) | 249 (60.0) | 0.376 |
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; CAP: controlled attenuation parameter; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; HDL: high density lipoprotein; LDL: low density lipoprotein; MetS: metabolic syndrome; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus.
There were increased proportions of CHB patients with fibrosis and advanced fibrosis as the number of components of MetS increased. The data are shown in Figure 1(b). However, the proportions with fibrosis and advanced fibrosis were similar in CHB patients with NAFLD or without NAFLD.
Table 3 shows the clinical and biochemical variables in the subgroup of CHB subjects with coexisting NAFLD, stratified by liver fibrosis. CHB with coexisting NAFLD had a higher weight and waist circumference. In contrast, LDL-cholesterol levels were significantly lower in the fibrosis group than in the non-fibrosis group.
Table 3.
Characteristics of chronic hepatitis B (CHB) with nonalcoholic fatty liver disease (NAFLD) for the non-fibrosis and fibrosis groups.
| Characteristic | CHB with NAFLD |
||
|---|---|---|---|
| Non-fibrosis group | Fibrosis group | p Value | |
| Sample size, n | 92 | 57 | |
| Sex (male), n (%) | 68 (73.9) | 49 (86.0) | 0.082 |
| Age (years) | 40.00 ± 11.79 | 41.19 ± 13.06 | 0.566 |
| Smoking, n (%) | 25 (27.2) | 18 (31.6) | 0.564 |
| Alcohol consumption, n (%) | 30 (32.6) | 21 (36.8) | 0.597 |
| T2DM, n (%) | 10 (10.9) | 11 (19.3) | 0.151 |
| Metabolic syndrome, n (%) | 16 (17.4) | 14 (24.6) | 0.289 |
| Weight, kg | 77.44 ± 17.25 | 84.64 ± 16.27 | 0.013 |
| Height, cm | 167.69 ± 7.75 | 169.02 ± 6.59 | 0.286 |
| SBP, mm Hg | 131.59 ± 12.31 | 133.32 ± 14.66 | 0.443 |
| DBP, mm Hg | 85.49 ± 8.73 | 86.56 ± 11.45 | 0.523 |
| Waist circumference, cm | 90.08 ± 7.32 | 95.81 ± 9.68 | <0.001 |
| Hip circumference, cm | 96.64 ± 6.58 | 99.92 ± 7.78 | 0.007 |
| CAP, dB/m | 334.26 ± 16.66 | 336.40 ± 21.53 | 0.499 |
| PLT, 109/l | 253.51 ± 56.69 | 233.19 ± 69.49 | 0.056 |
| ALT, U/l | 87.96 ± 51.41 | 87.72 ± 24.41 | 0.973 |
| AST, U/l | 53.13 ± 16.53 | 73.23 ± 20.83 | <0.001 |
| GGT, U/l | 76.27 ± 39.66 | 67.42 ± 28.99 | 0.156 |
| Total cholesterol, mmol/l | 5.29 ± 0.58 | 5.08 ± 0.92 | 0.115 |
| HDL-cholesterol, mmol/l | 1.29 ± 0.30 | 1.34 ± 0.29 | 0.327 |
| LDL-cholesterol, mmol/l | 3.31 ± 0.54 | 2.98 ± 0.79 | 0.015 |
| Triglycerides, mmol/l | 1.61 ± 0.56 | 1.49 ± 0.45 | 0.180 |
| Uric acid, mmol/l | 405.11 ± 75.46 | 547.35 ± 135.06 | <0.001 |
| HBV DNA, log10 IU/ml | 0.95 ± 1.84 | 1.03 ± 1.51 | 0.769 |
| HBeAg ( + ), n (%) | 49 (53.3) | 41 (71.9) | 0.024 |
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; CAP: controlled attenuation parameter; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; HDL: high density lipoprotein; LDL: low density lipoprotein; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus.
Univariate and multivariate analysis of factors associated with NAFLD and fibrosis
Logistic regression was utilized to identify factors that were significantly associated with NAFLD in the CHBcM. It indicated in multivariate analysis that younger age, high GGT level, high WHR, and high BMI were independent risk factors associated with NAFLD among CHBcM (Table 4). However, when we removed WHR and BMI as covariates, the independent risk factors associated with NAFLD among CHBcM were high DBP (OR = 1.049, 95% CI: 1.026–1.072, p < 0.001), alcohol consumption (OR = 1.882, 95% CI: 1.215–2.916, p = 0.005), high GGT level (OR = 1.008, 95% CI: 1.003–1.014, p = 0.003), and younger age (OR = 0.975, 95% CI: 0.958–0.992, p = 0.005). The results indicated that relationship between fatty liver and low-level alcohol consumption in our study may due to the obesity caused by alcohol consumption, rather than to alcohol itself. As shown in Table 5, according to multivariate analysis, male gender, older age, smoking, high AST level, and BMI, and a low PLT level were independent risk factors. Among subjects with CHB and NAFLD, the multivariate analysis showed that, interestingly, only high AST level, high WHR and high BMI were independent risk factors associated with liver fibrosis among subjects with CHB and NAFLD, as shown in Table 6.
Table 4.
Univariate and multivariate analyses of factors associated with nonalcoholic fatty liver disease (NAFLD).
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 1.485 | 0.983–2.243 | 0.060 | |||
| Age | 0.982 | 0.967–0.997 | 0.016 | 0.973 | 0.953–0.993 | 0.008 |
| SBP | 1.028 | 1.016–1.041 | <0.001 | |||
| DBP | 1.052 | 1.033–1.071 | <0.001 | |||
| Hyperuricemia | 1.447 | 0.972–2.154 | 0.069 | |||
| Hyperlipidemia | 1.521 | 1.017–2.275 | 0.041 | |||
| T2DM | 1.066 | 0.651–1.746 | 0.800 | |||
| Alcohol consumption | 1.897 | 1.313–2.741 | 0.001 | |||
| Smoking | 1.136 | 0.778–1.660 | 0.509 | |||
| ALT | 0.999 | 0.995–1.002 | 0.473 | |||
| AST | 1.001 | 0.996–1.006 | 0.685 | |||
| GGT | 1.008 | 1.003–1.013 | 0.001 | 1.008 | 1.002–1.014 | 0.010 |
| PLT | 1.001 | 0.998–1.004 | 0.567 | |||
| WHR (<0.9 vs ≥0.9) | 4.669 | 3.154–6.910 | <0.001 | 3.148 | 1.918–5.164 | <0.001 |
| BMI (<28 vs ≥28) | 10.950 | 7.376–16.257 | <0.001 | 7.417 | 4.547–12.099 | <0.001 |
| HBV DNA | 0.999 | 0.904–1.104 | 0.987 | |||
| HBeAg status | 1.107 | 0.780–1.570 | 0.570 | |||
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; BMI: body mass index; CI: confidence interval; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; OR: odds ratio; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; WHR: waist-hip ratio.
Table 5.
Univariate and multivariate analyses of factors associated with liver fibrosis.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 1.847 | 1.395–2.446 | <0.001 | 1.713 | 1.169–2.511 | 0.006 |
| Age | 1.032 | 1.022–1.042 | <0.001 | 1.032 | 1.019–1.045 | <0.001 |
| SBP | 1.003 | 0.994–1.012 | 0.517 | |||
| DBP | 1.000 | 0.988–1.013 | 0.961 | |||
| Hyperuricemia | 1.039 | 0.773–1.395 | 0.801 | |||
| Hyperlipidemia | 1.017 | 0.751–1.376 | 0.915 | |||
| T2DM | 1.653 | 1.184–2.307 | 0.003 | |||
| Alcohol consumption | 1.773 | 1.350–2.327 | <0.001 | |||
| Smoking | 1.727 | 1.330–2.241 | <0.001 | 1.471 | 1.024–2.113 | 0.037 |
| ALT | 1.000 | 0.997–1.002 | 0.832 | |||
| AST | 1.010 | 1.006–1.013 | <0.001 | 1.010 | 1.006–1.015 | <0.001 |
| GGT | 0.998 | 0.994–1.002 | 0.277 | |||
| PLT | 0.987 | 0.984–0.989 | <0.001 | 0.988 | 0.985–0.990 | <0.001 |
| WHR (< 0.9 vs ≥ 0.9) | 1.673 | 1.316–2.128 | <0.001 | |||
| BMI (< 28 vs ≥ 28) | 2.194 | 1.552–3.102 | <0.001 | 2.678 | 1.703–4.212 | <0.001 |
| HBV DNA | 1.070 | 1.000–1.145 | 0.049 | |||
| HBeAg status | 1.115 | 0.877–1.417 | 0.376 | |||
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; BMI: body mass index; CI: confidence interval; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; OR: odds ratio; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; WHR: waist-hip ratio.
Table 6.
Univariate and multivariate analysis for fibrosis among chronic hepatitis B (CHB) coexist with nonalcoholic fatty liver disease (NAFLD).
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 2.162 | 0.896–5.214 | 0.086 | |||
| Age | 1.008 | 0.981–1.036 | 0.564 | |||
| SBP | 1.010 | 0.985–1.035 | 0.441 | |||
| DBP | 1.011 | 0.978–1.046 | 0.521 | |||
| Hyperuricemia | 1.242 | 0.586–2.630 | 0.572 | |||
| Hyperlipidemia | 1.530 | 0.720–3.250 | 0.269 | |||
| T2DM | 1.961 | 0.774–4.967 | 0.156 | |||
| Alcohol consumption | 1.206 | 0.603–2.410 | 0.597 | |||
| Smoking | 1.237 | 0.600–2.549 | 0.564 | |||
| ALT | 1.000 | 0.992–1.008 | 0.973 | |||
| AST | 1.060 | 1.036–1.084 | <0.001 | 1.068 | 1.038–1.100 | <0.001 |
| GGT | 0.993 | 0.982–1.003 | 0.185 | |||
| PLT | 0.995 | 0.989–1.000 | 0.058 | |||
| WHR (< 0.9 vs ≥ 0.9) | 4.306 | 1.663–11.148 | 0.003 | 4.632 | 1.333–16.096 | 0.016 |
| BMI (< 28 vs ≥ 28) | 3.013 | 1.515–5.990 | 0.002 | 2.948 | 1.140–7.620 | 0.026 |
| HBV DNA | 1.029 | 0.850–1.247 | 0.767 | |||
| HBeAg status | 2.249 | 1.108–4.566 | 0.025 | |||
ALT: serum aspartate aminotransferase; AST: alanine aminotransferase; BMI: body mass index; CAP: controlled attenuation parameter; CI: confidence interval; DBP: diastolic blood pressure; GGT: gamma-glutamyltransferase; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; OR: odds ratio; PLT: platelet; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; WHR: waist-hip ratio.
Discussion
In this study, we found that both the CAP values and the proportion of fibrosis increased with an increase in the number of MetS components. And, unexpectedly, we did not find in this study that CHB with coexisting NAFLD is associated with severe liver fibrosis. An epidemiology study conducted by Jinjuvadia and Liangpunsakul19 found that the prevalence of MetS was 10.4% in HBV-infected patients and 25.6% in controls. The authors believed that there was an inverse correlation between MetS and HBV infection. In our study, the prevalence of MetS among CHB patients was 11.7%, significantly lower than the 25.6% value reported in healthy controls. This may simply reflect a lower prevalence of MetS in CHB patients.
The serum lipid profile in CHB patients has received some attention. It is known that serum cholesterol level correlates with liver function and prognosis in patients with advanced liver disease.20 Low total cholesterol was thought to be associated with incipient liver failure.21 The explanation could partially explain why lipids were significantly lower in the fibrosis group compared to the non-fibrosis group in the CHB patients in our study. A study reported by Hsieh et al.21 suggested that GGT was the most representative liver enzyme related to MetS in the non-hepatitis population. According to our study, a high GGT level could also be regarded as a marker for NAFLD, together with younger age, WHR, and BMI, and could assist stratification of the risk for CHB patients with a component of MetS.
Liver fibrosis would impair the synthetic and metabolic functions of the liver. It is well established that CHB, NAFLD, and MetS lead to the development of liver fibrosis, cirrhosis, and HCC.22–24 A prospective study from Taiwan indicated that a high BMI at baseline correlated with the incidence of liver cirrhosis and HCC.25 In our study, we found that CHB patients with MetS components had accelerated rates of liver fibrosis. The more components present in the CHB patient, the higher the potential for liver fibrosis. More importantly, smoking is an independent risk factor for liver fibrosis among CHBcM. The results indicated that, for CHBcM, smoking cessation is important and necessary, as recommended by recent guidelines.26 Behavioral therapy and motivational counseling is beneficial for all these patients, but is essential for those not yet ready to quit. For CHBcM, identifying smoking triggers, avoiding high-risk situations, and motivational counseling may all be useful.
A surprising result in our study was that the combination of CHB and NAFLD did not accelerate the development of fibrosis. The explanation for this is unknown and needs further investigation. Some studies have indicated that hepatitis B virus could influence hepatocyte metabolism, and therefore coined the term “metabolovirus.”27,28 On the one hand, HBV could regulate many key metabolic genes in infected hepatocytes.29 On the other hand, it is well known that HBV DNA load increases the risk of liver fibrosis and cirrhosis.30
The current study has limitations. It was a retrospective investigation, carried out in a single center, and performed by clinicians from that same center. The occurrence of NAFLD may differ according to regional dietary habits that may vary geographically in China, or around the world. The potential limitations of the present report could be overcome in future studies through the incorporation of multiple centers, in several regions and/or countries. Nevertheless, the current study provides useful data derived from a large number of CHB patients who also had components of MetS, which to our knowledge had not been reported previously.
Conclusion
In conclusion, in CHB patients, the CAP value and the proportion of fibrosis increased with an increasing number of MetS components. For such patients, stratification by independent risk factors for NAFLD and fibrosis can help with management of their disease.
Summarize the established knowledge on this subject
Clear evidence indicated that CHB, NAFLD, and MetS can lead to the development of liver fibrosis.
NAFLD, as the hepatic manifestation of MetS, increased risk of end-stage of liver disease in CHB patients.
What are the significant and/or new findings of this study?
Level of CAP and fibrosis increased with increasing numbers of MetS components among CHB patients.
Younger age, high GGT levels, high WHR, and high BMI were independent risk factors for NAFLD in the CHB patients with MetS.
Male gender, older age, smoking, high AST level and BMI, and low PLT level were independent risk factors for liver fibrosis among CHB patients with MetS.
Supplementary Material
Acknowledgments
The authors wish to thank Caixia Zheng for his helpful assistance in the study.
Declaration of conflicting interests
The authors declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Ethics approval
The Institutional Review Board of First Affiliated Hospital of Xiamen University, had approved the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
Funding
This work was supported by Science and Technology Planning Project of Guangdong Province,China (Self-raised, No.2016ZC0068).
Informed consent
Each enrolled patient provided informed consent.
References
- 1.Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30: 2212–2219. [DOI] [PubMed] [Google Scholar]
- 2.Jarcuska P, Janicko M, Kruzliak P, et al. Hepatitis B virus infection in patients with metabolic syndrome: A complicated relationship. Results of a population based study. Eur J Intern Med 2014; 25: 286–291. [DOI] [PubMed] [Google Scholar]
- 3.Cai S, Yu T, Jiang Y, et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-Week result. Clin Exp Med 2016; 16: 429–436. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Schmeltz LR. Metabolic syndrome management. Expert Opin Pharmacother 2007; 8: 2059–2075. [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology 2008; 135: 111–121. [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Chen YP, Yang YD, et al. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One 2012; 7: e34198–e34198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AW, Wong GL, Chan HY, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol 2017; 32: 667–676. [DOI] [PubMed] [Google Scholar]
- 8.EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed]
- 9.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344: 495–500. [DOI] [PubMed] [Google Scholar]
- 10.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int 2016; 10: 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou H, Cai S, Liu Y, et al. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol 2017; 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EASL clinical practical guidelines: Management of alcoholic liver disease. J Hepatol 2012; 57: 399–420. [DOI] [PubMed]
- 13.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010; 51: 828–835. [DOI] [PubMed] [Google Scholar]
- 14.Wong VW, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol 2017; 67: 577–584. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, Bedossa P. Age and platelet count: A simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat 1997; 4: 199–208. [DOI] [PubMed] [Google Scholar]
- 16.Chen YP, Liang XE, Zhang Q, et al. Larger biopsies evaluation of transient elastography for detecting advanced fibrosis in patients with compensated chronic hepatitis B. J Gastroenterol Hepatol 2012; 27: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 17.de Ledinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2016; 31: 848–855. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014; 23: 3–16. [DOI] [PubMed] [Google Scholar]
- 19.Jinjuvadia R, Liangpunsakul S. Association between metabolic syndrome and its individual components with viral hepatitis B. Am J Med Sci 2014; 347: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarcuska P, Drazilova S, Fedacko J, et al. Association between hepatitis B and metabolic syndrome: Current state of the art. World J Gastroenterol 2016; 22: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh MH, Ho CK, Hou NJ, et al. Abnormal liver function test results are related to metabolic syndrome and BMI in Taiwanese adults without chronic hepatitis B or C. Int J Obes (Lond) 2009; 33: 1309–1317. [DOI] [PubMed] [Google Scholar]
- 22.Zeng J, Cai S, Liu J, et al. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med 2017; 36: 261–268. [DOI] [PubMed] [Google Scholar]
- 23.Cai S, Cao J, Yu T, et al. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017; 96: e7021–e7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue X, Cai S, Ou H, et al. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence 2017; 11: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu MW, Shih WL, Lin CL, et al. Body-mass index and progression of hepatitis B: A population-based cohort study in men. J Clin Oncol 2008; 26: 5576–5582. [DOI] [PubMed] [Google Scholar]
- 26.Shields PG, Herbst RS, Arenberg D, et al. Smoking cessation, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14: 1430–1468. [DOI] [PubMed] [Google Scholar]
- 27.Chiang CH, Huang KC. Association between metabolic factors and chronic hepatitis B virus infection. World J Gastroenterol 2014; 20: 7213–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geier A. Hepatitis B virus: The “metabolovirus” highjacks cholesterol and bile acid metabolism. Hepatology 2014; 60: 1458–1460. [DOI] [PubMed] [Google Scholar]
- 29.Shlomai A, Shaul Y. The “metabolovirus” model of hepatitis B virus suggests nutritional therapy as an effective anti-viral weapon. Med Hypotheses 2008; 71: 53–57. [DOI] [PubMed] [Google Scholar]
- 30.Cai SH, Lv FF, Zhang YH, et al. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis 2014; 14: 85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


