Abstract
Background
Evidence suggests a role of intestinal microbiota-host interactions in the pathophysiology and symptoms of irritable bowel syndrome (IBS).
Objective
The objective of this article is to assess the effects of Lactobacillus paracasei CNCM I-1572 on clinical and gut microbiota-related factors in IBS.
Methods
We conducted a multicenter, randomized, double-blind, cross-over, 18-week, placebo-controlled, pilot trial assessing the effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota composition, fecal short chain fatty acid (SCFA), immunoglobulin A, and cytokines in IBS. The intestinal microbial ecosystem was characterized by 16S rRNA gene profiling.
Results
Forty IBS patients were enrolled from five Italian centers. Lactobacillus paracasei CNCM I-1572 did not significantly improve IBS symptoms, including primary efficacy variables worst abdominal pain/discomfort and IBS degree of relief. Interestingly, Lactobacillus paracasei CNCM I-1572 induced a significant reduction in genus Ruminococcus, dominated by taxa related to Ruminococcus bromii and Ruminococcus callidus, a significant increase in the SCFAs acetate and butyrate, and a significant reduction in the pro-inflammatory cytokine interleukin-15.
Conclusions
This pilot study shows that Lactobacillus paracasei CNCM I-1572 is able to modulate gut microbiota structure/function and reduce immune activation in IBS. As no statistically significant effect on IBS-symptoms was found, further studies are necessary to determine the role of this probiotic in IBS. The study was registered at ClinicalTrials.gov registry under identifier NCT02371499.
Keywords: Irritable bowel syndrome, dietary compounds, probiotics, microbiota
Key summary
Although probiotics, as a class, have a small but significant therapeutic effect on irritable bowel syndrome (IBS) symptoms, the optimal probiotic strategy in IBS and the mechanism of action by which these compounds exert their beneficial actions in humans are virtually unknown.
Lactobacillus paracasei CNCM I-1572 induces a significant reduction in genus Ruminococcus, a significant increase in the fecal short chain fatty acids acetate and butyrate, and a significant reduction in the pro-inflammatory cytokine interleukin-15 in patients with IBS.
We identify plausible biological mechanisms by which this probiotic may exert its effects in patients with IBS.
Introduction
Irritable bowel syndrome (IBS) is characterized by abdominal pain and changes in bowel habits. IBS is one of the most common gastrointestinal disorders, affecting 11.2% of the population in the United States and Europe.1 Recently, advanced microscopic and molecular techniques have revealed alterations in the luminal factors, the epithelial barrier, and the immune, endocrine, and nervous systems in a large proportion of patients with IBS.2
Several lines of evidence suggest a pathogenetic contribution of the intestinal microbiota in IBS. Prospective studies have shown that 3% to 36% of enteric infections disrupting the intestinal ecosystem lead to de novo onset of so-called post-infection IBS.2,3 A number of studies have reported changes in the composition and stability of the intestinal microbiota in patients with IBS over time.4–6 Although these data do not allow us to determine if the abnormal microbiota is the cause or effect of IBS, the improvement of symptoms described in studies using probiotics7,8 or non-absorbable antibiotics9 implicates intestinal bacteria-host interactions in the pathophysiology and symptoms of this common disorder. However, current data are inconsistent because of the lack of control of diet, concomitant use of antibiotics, different bowel habit subtypes and gut transit.
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”10 Systematic reviews of the literature and meta-analyses indicate that probiotics, as a class, have a small but significant therapeutic effect on IBS symptoms.7,8 However, the quality of probiotic trials in IBS and their sample sizes remain suboptimal. The great variety of species, strains, and doses of probiotics tested in clinical trials makes it difficult to provide generalizable advice about the optimal probiotic strategy in IBS.11 For all these reasons, it is questionable if meta-analyses are really applicable to trials of probiotics. Understanding of the mechanism of action by which probiotics exert their beneficial actions in humans is limited because these aspects were evaluated mainly in pre-clinical studies or a small number of clinical trials.10,11 In one clinical study,12 probiotics were shown to have potent anti-inflammatory properties. In particular, Bifidobacterium longum subsp. infantis 35624 was capable of normalizing the interleukin (IL) 10/IL12 ratio, indicative, although not validated, of a pro-inflammatory T helper (Th)-1 type immune response, in patients with IBS.12 In a recent study of healthy volunteers,13 the intake of Lactobacillus paracasei CNCM I-1572 significantly modulated fecal Clostridiales bacteria and butyrate levels, potentially conferring a health benefit to the host. In addition, Lactobacillus paracasei CNCM I-1572 was able to modulate colonic microbiota in intestinal chronic inflammation, partly modifying Toll-like receptor expression when rectally administered.14,15
In this context, we designed a randomized, double-blind, placebo-controlled, cross-over pilot study to assess the efficacy, safety, and mechanism of action of Lactobacillus paracasei CNCM I-1572 in patients with IBS.
Materials and methods
Study design
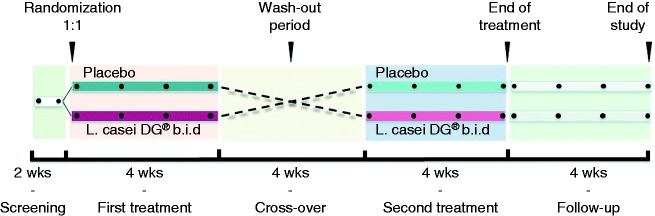
This was a multicenter, randomized, double-blind, cross-over, placebo-controlled, pilot trial designed to study the effect of Lactobacillus paracasei CNCM I-1572 (L. casei DG®, (LCDG), Enterolactis® plus, Sofar S.p.A., Trezzano Rosa, Milan, Italy, deposited at Institute Pasteur of Paris with number I1572) on the symptoms, fecal microbiota composition, and short chain fatty acid (SCFA), immunoglobulin (Ig) A, and cytokine levels in patients with IBS. The probiotic preparation consisted of a gelatin capsule containing at least 24 billion viable cells of the bacterial strain LCDG. Placebo and probiotic capsules, identical in color, texture, and taste, were delivered in aluminum boxes sealed with a plastic cap containing desiccant salts. Eligible patients entered a two-week run-in phase and were randomly assigned to either LCDG twice daily for four weeks or the equivalent product without bacteria (placebo), followed by a washout period of four weeks before crossing over to the alternate treatment (twice daily for four weeks). After 14 weeks, patients entered a four-week follow-up phase (Figure 1). Study visits occurred every four weeks during the treatment period and follow-up. The randomization schedule was determined by a computer-generated random code system. Intervention sequence assignments were not revealed until the study was completed. Patients, study investigators, and sponsor staff were blinded to the randomization codes. All participants underwent a formal clinical assessment and were further phenotyped using validated questionnaires as described below. In all cases, fecal samples were obtained at the start and end of the first (visits 2 and 3) and the second (visits 4 and 5) treatment period, and at the end of the follow-up.
Figure 1.
Study design. After a two-week run-in phase, patients were randomly (1:1) assigned to either Lactobacillus paracasei CNCM I-1572 twice daily for four weeks or placebo. This was followed by a washout period of four weeks before crossing over to the alternate treatment (twice daily for four weeks). After 14 weeks, patients entered a four-week follow-up phase. The total duration of the study was 18 weeks. Fecal samples were obtained at visits 2 and 3 (first period), visits 4 and 5 (second period), and at the end of follow-up.
The protocol was designed by the coordinating center. Data were collected by investigators and monitored by the sponsor with the supervision of OPIS, a contract research organization. OPIS personnel, in collaboration with the coordinating center, analyzed the trial data. A statistical analysis plan (SAP) was released and approved by the sponsor prior to the database lock and unblinding of the treatment sequence. The protocol was approved by an independent ethics committee at each center (in particular, it was approved by the Ethics Committee of St. Orsola-Malpighi Hospital of Bologna on October 7, 2014, approval identification no: 145/2014/O/Sper) and carried out according to the Declaration of Helsinki and the principles of good clinical practice. All patients provided written informed consent. All authors have access to the study data and reviewed and approved the final manuscript. The trial was registered in a public registry (ClinicalTrial.gov No. NCT02371499).
Patients
Eligible patients with symptoms meeting Rome III criteria for IBS,16 irrespective of bowel habit, were recruited from five Italian centers (for inclusion/exclusion criteria, see online supplementary material).
Study assessment
Data collection was carried out using an electronic clinical case report form (eCRF). Patients recorded all symptoms daily in a paper patient diary. The patients’ lifestyle and eating habits were controlled during the study and were the same throughout all the study periods. Compliance with the suggested lifestyle and eating habits was checked weekly and noted in the patient diary. Use of concomitant medication and adverse events were recorded at each visit.
Primary efficacy variables were: (1) worst abdominal pain/discomfort in the last 24 hours (responders were defined as patients with ≥ 30% reduction in the weekly mean worst abdominal pain and/or discomfort score, versus mean value of the run-in period, in at least two of the four weeks of the treatment period) using a daily 11-point numeric rating scale (NRS); (2) IBS degree of relief in the past seven days compared to before the trial started (responders were defined as patients reporting being “completely relieved” or “considerably relieved” in at least two of the four weeks of the treatment period) using a weekly seven-point balanced ordinal scale; (3) daily stool frequency and consistency as assessed by the Bristol Stool Scale Form (BSSF); (4) gut microbiota composition, fecal SCFAs, immunoglobulin A (IgA), and cytokines assessed every four weeks during the treatment periods and at the end of follow-up.
Secondary efficacy variables included: (1) overall satisfaction with treatment at the end of both the treatment periods as assessed by a 10-point visual analog scale (VAS); (2) Hospital Anxiety and Depression Scale (HADS);17 (3) quality of life assessment using the validated Short-Form 12 Items Health Survey (SF-12)18 and (4) consumption of rescue medications.
Analysis of the bacterial composition of fecal samples
The bacterial community structure of the fecal microbiota was analyzed as described elsewhere13,19,20 (see online supplementary material).
Quantification of fecal SCFAs
SCFAs were quantified in the fecal samples as previously described19 (see online supplementary material).
Fecal IgA and cytokine analysis
Fecal IgA and cytokines (including interleukin (IL)6, IL8, IL10, IL12, IL15, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β) were detected by an enzyme-linked immunosorbent assay (ELISA) test as previously described21 (see online supplementary material).
Statistical analysis
This was a pilot study; thus, no sample size was calculated. Forty patients were included in the study based on feasibility criteria and previously published studies.22 Nevertheless, when the sample size in each sequence group is 20 (a total sample size of 40) a 2 × 2 cross-over design has 80% power to detect a difference between treatments, assuming a medium effect size, using a two group t test (cross-over analysis of variance (ANOVA)) with a 0.05 two-sided significance level.23
Continuous data were summarized by mean, standard deviation (SD), median, first and third quartile, minimum, and maximum. Categorical data were presented by absolute and relative frequencies or contingency tables. Patients were included in each analysis based on available assessments. The prevalence approach was applied unless otherwise indicated; therefore, missing data were not replaced.
The full analysis set (FAS) included all randomized patients. The safety set included all randomized patients who received at least one dose of the study treatment and had at least the post-baseline safety assessment. The intent-to-treat (ITT) set included all randomized patients who received at least one dose of the study treatment and had at least one efficacy assessment in each cross-over period. The per protocol (PP) set included all randomized patients who completed the study without any significant protocol violation. Primary efficacy analyses were performed on the ITT set and PP set provided supportive data.
For the binary efficacy variables, Prescott’s test for a direct treatment effect was applied after verifying the absence of a treatment-by-period interaction using the test proposed by Armitage and Hills.24 When a treatment-by-period interaction was evident, the analysis was based on the data from the first period only, using chi-square or Fisher’s exact test to determine the treatment effect. In addition, for primary variables, a generalized estimating equations model for repeated measures (i.e. subject within sequence) was applied considering sequence, period, and treatment as fixed effects. For the continuous efficacy variables, a mixed-effects model with repeated measures was applied after verifying the absence of a carryover effect.
All statistical tables, figures, listings, and analyses were produced using SAS® for Windows release 9.4 (64-bit) (SAS Institute Inc, Cary, NC, USA). Unless otherwise specified, each statistical test used a two-tailed α-level of 0.05 (see online supplementary material for the statistical analyses of data concerning the intestinal microbial ecosystem).
Results
Study patients
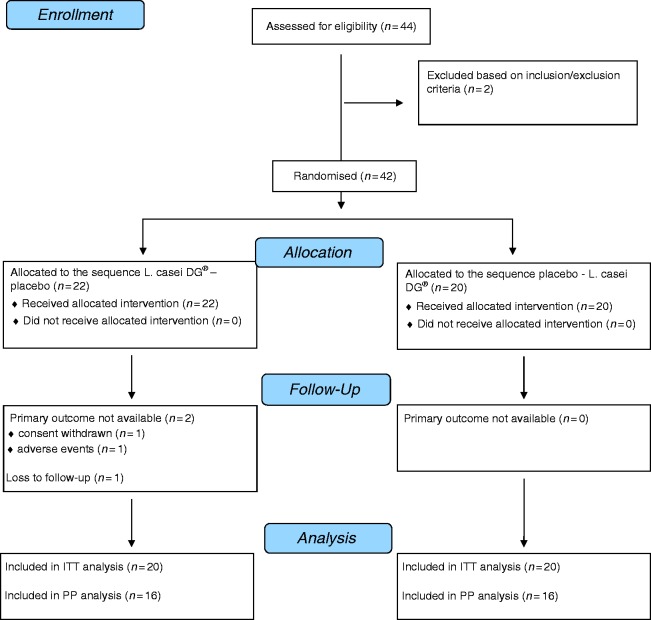
Study enrollment and randomization are shown in Figure 2. The study was conducted from January to November 2015. Forty-two patients (95.5%) were randomized (22 assigned to the LCDG-placebo sequence and 20 assigned to the placebo-LCDG sequence) and included in the FAS (all performed at visits 1 and 2). A total of 40 patients (90.9%) were seen at visits 3 and 4 and included in both the ITT set and safety set, whereas 39 patients remained for visit 5 and the follow-up phase. The primary reasons for study withdrawal were withdrawn consent, non-compliance, and adverse events. Almost all patients had a normal compliance (between 80% and 120%). The demographic and baseline characteristics of the subjects are reported in Table 1.
Figure 2.
DG® Flowchart of enrollment CNCM I-1572 and randomization of the study. L. casei: Lactobacillus paracasei; ITT: intent-to-treat; PP: per protocol.
Table 1.
Baseline characteristics of study participants.
| Characteristics | Placebo/Lactobacillus paracasei CNCM I-1572 (n = 20) | Lactobacillus paracasei CNCM I-1572/placebo (n = 20) |
|---|---|---|
| Age, years | 44.55 ± 12.98 | 37.35 ± 11.25 |
| Female gender | 15 (75%) | 11 (55%) |
| Ethnic origin | ||
| Caucasian | 20 (100%) | 20 (100%) |
| Other | 0 (%) | 0 (0%) |
| IBS subtype (4) | ||
| IBS-D | 6 (30%) | 8 (40%) |
| IBS-C | 7 (35%) | 5 (25%) |
| IBS-M | 1 (5%) | 2 (10%) |
| IBS-U | 6 (30%) | 5 (25%) |
| Abdominal pain scorea | 2.70 ± 1.24 | 3.28 ± 1.95 |
Data are presented as number of patients (%) or mean ± SD.
Mean value at run-in period.
IBS: irritable bowel syndrome; IBS-D: irritable bowel syndrome with diarrhea; IBS-C: irritable bowel syndrome with constipation; IBS-M: mixed irritable bowel syndrome; IBS-U: unsubtyped irritable bowel syndrome.
Effect of treatment on digestive symptoms
Abdominal pain/discomfort
Considering both treatment periods together, the proportion of responders was higher in patients who took LCDG (15/40, 37.5%) than placebo (12/40, 30%), but these differences were not significant in the model (p = 0.336). Analyzing the overall results by treatment in the PP set, the proportion of responders (overall) was the same in both groups of patients (11/32, 34.4%).
IBS degree of relief
Considering both treatment periods together, the proportion of responders was higher in patients who took LCDG (9/40, 22.5%) than placebo (6/39, 15.4%), but these differences were not significant in the model (p = 0.195). Similar results were obtained for the PP set.
Daily stool frequency and form
Stool frequency was collected daily and stool consistency was assessed using the BSSF. For both the features, no significant differences were found in either the ITT set or PP set. Although better results (i.e. bowel function normalization) were obtained in patients with IBS with diarrhea (IBS-D) and mixed IBS (IBS-M) treated with LCDG (see online supplementary material), there was no significant difference.
For all the investigated digestive symptoms, no carryover effect resulted statistically significant, indicating that values at the beginning of the second period are statistically equal to baseline values.
Effect of treatment on the gut microbiota
The within-sample biodiversity was analyzed in terms of bacterial richness and evenness (α-diversity) using the Chao1, Shannon, and InvSimpson indexes, while the inter-sample relationships (β-diversity) was measured by principal coordinate analysis (PCoA) based on weighted and unweighted UniFrac distances. The differences between LCDG and placebo in modulating α and β diversity were not significant (see online supplementary material Figures S1 and S2). Next, we assessed the effect of treatment on the modulation of specific bacterial taxa. We showed a significant increase in genus Lactobacillus (a plausible effect of the ingested probiotic cells) and Oscillospira, and reduction in genus Ruminococcus (Table 2(a)). In addition, only LCDG induced a significant change in the level of bacterial taxa; specifically, we observed an expansion of genera Parabacteroides, Lactobacillus, and an unidentified member of the family Barnesiellaceae (Table 2(b)).
Table 2.
Bacterial taxa that were significantly modified by probiotic (Lactobacillus paracasei CNCM I-1572) or placebo treatments. Median relative abundance before (baseline) and after treatment is shown.
| (a) | |||||
|---|---|---|---|---|---|
| Median relative abundance (%) |
|||||
|
L. paracasei CNCM I-1572 |
Placebo |
||||
| p value | Baseline | Post- treatment | Baseline | Post- treatment | |
| Family | |||||
| p_Firmicutes.c_Bacilli.o_Lactobacillales.f_Lactobacillaceae | 0.022 | 0.01 | 0.34 | 0.01 | 0.02 |
| Genus | |||||
| p_Firmicutes.c_Clostridia.o_Clostridiales.f_Ruminococcaceae.g_Ruminococcus | 0.042 | 4.44 | 3.94 | 5.25 | 5.62 |
| p_Firmicutes.c_Clostridia.o_Clostridiales.f_Ruminococcaceae.g_Oscillospira | 0.042 | 0.37 | 0.42 | 0.38 | 0.41 |
| p_Firmicutes.c_Bacilli.o_Lactobacillales.f_Lactobacillaceae.g_Lactobacillus | 0.011 | 0.01 | 0.34 | 0.01 | 0.02 |
Significant differences were determined according to repeated measure Friedman test (a) and Wilcoxon-Mann-Whitney test with Benjamini-Hochberg correction (b). Only taxa with a median relative abundance > 0.1 % were included in the analysis. The taxonomic lineage of each taxon is shown: k: kingdom; p: phylum; c: class; o: order; f: family; g: genus.
Because of the reported association between IBS and members of the genus Ruminococcus,25,26 we further investigated the data concerning this taxon. Using Basic Local Alignment Search Tool (BLASTn) and ClustalW global alignment algorithms, we assigned three of the most represented Ruminococcus-associated de novo sequences to the species R. bromii (67.7% of the Ruminococcus reads), R. bicirculans (7.7%), and R. callidus (4.3%) (Figure S3).
Effect of treatment on SCFAs
We demonstrated that SCFAs acetate and butyrate increased significantly with LCDG treatment, but no significant differences were found after placebo (Table 3; Figure S4). The median levels of acetate and, particularly, butyrate before the placebo were higher than before the probiotic treatment. Although this difference was not statistically significant, a carryover effect of the probiotic on SCFA levels (i.e. an insufficient washout period) cannot be excluded.
Table 3.
Fecal levels of short chain fatty acids (SCFAs) throughout treatment. Median values (±standard deviation) from before (baseline) and after treatment are given. Significant differences appear in bold and were determined by the Wilcoxon-Mann-Whitney test.
| L. paracasei CNCM I-1572 treatment | Median relative abundance (mmol/kg) |
||
|---|---|---|---|
| p value | Before | After | |
| Acetate | 0.021 | 36.63 (±22.62) | 47.83 (±26.14) |
| Propionate | 0.289 | 15.18 (±10.35) | 16.37 (±11.97) |
| Butyrate | 0.047 | 5.99 (±8.30) | 10.52 (±8.51) |
| Isobutyrate | 0.133 | 1.11 (±0.98) | 1.55 (±1.13) |
| Isovalerate | 0.428 | 1.14 (±0.81) | 1.04 (±1.03) |
| Valerate | 0.080 | 1.82 (±1.43) | 2.45 (±1.34) |
| Placebo treatment | |||
| Acetate | 0.388 | 43.06 (±26.65) | 33.08 (±26.70) |
| Propionate | 0.622 | 16.73 (±10.51) | 17.13 (±8.89) |
| Butyrate | 0.746 | 10.73 (±7.68) | 8.47 (±9.06) |
| Isobutyrate | 0.387 | 1.22 (±1.22) | 1.64 (±1.13) |
| Isovalerate | 0.36 | 0.95 (±1.12) | 1.28 (±1.22) |
| Valerate | 0.572 | 2.14 (±1.92) | 1.9 (±2.00) |
Effect of treatment on fecal IgA and cytokines
The mean fecal IgA level, expressed as ng/g, decreased during LCDG treatment (mean change −5.4), and increased during treatment with placebo (mean change 14.1), with a borderline difference (p = 0.068) (Table S1). The mean IL6 level, expressed as pg/g, decreased during LCDG treatment (mean change −0.2), and increased during treatment with placebo (mean change 0.7), with a borderline difference (p = 0.056) (Table S1). The mean IL15 level, expressed as pg/g, decreased during LCDG treatment (mean change −173.4), and increased during treatment with placebo (mean change 35.4), with a significant difference (p = 0.042) (Table S1). For the other fecal cytokines, no significant differences were found.
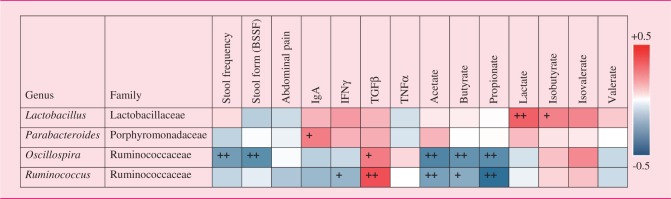
Correlations between microbiomic, clinical, and immunological features
The correlations between biological and clinical features are reported in Table 4 (see online supplementary material).
Table 4.
Correlation analyses performed using the relative abundances of the bacterial taxa modified by the Lactobacillus paracasei CNCM I-1572 treatment (predictors) and clinical parameters, immunological factors, and fecal SCFA levels (dependent variables).
BSSF: Bristol Stool Scale Form; IgA: immunoglobulin A; IFNγ: interferon gamma; TGFβ: transforming growth factor beta; TNFα: tumor necrosis factor alpha.
The colors of the spots in the table represent R values from Spearman’s Rank-Order correlation (blue: negative R values indicating inverse correlations; red: positive R values indicating positive correlations). +p < 0.01, ++p < 0.001 according to Kendall’s Rank Correlation.
Safety
Treatment-emergent adverse events during the study are reported in Table 5. Although no significant difference was found between the patients with at least one treatment-emergent adverse event in the two treatment groups (p = 0.742), one participant allocated to the sequence LCDG-placebo dropped out because of worsening of abdominal pain. No patient experienced a serious, severe, or related adverse event during the treatment period. All reported adverse events were unrelated to the experimental products.
Table 5.
Treatment-emergent adverse events during the study.
| Event | Placebo (n = 39) | L. paracasei CNCM I-1572 (n = 40) |
|---|---|---|
| Adverse events | ||
| Headache | 7 (17.9%) | 10 (25.0%) |
| Upper respiratory tract infection | 5 (12.8%) | 4 (10.0%) |
| Diarrhea | 3 (7.7%) | 3 (7.5%) |
| Abdominal pain | 2 (5.1%) | 3 (7.5%) |
| Asthenia | 1 (2.6%) | 3 (7.5%) |
| Nausea | 2 (5.1%) | 1 (2.5%) |
| Dyspepsia | 2 (5.1%) | 0 (0%) |
| Serious adverse events | ||
| 0 (0%) | 0 (0%) |
Adverse events are listed in descending order of frequency in the Lactobacillus paracasei CNCM I-1572 group. The adverse events listed were reported in ≥ 2% of the patients in either treatment group.
Discussion
LCDG significantly reduces the genus Ruminococcus, induces a significant increase in the fecal levels of SCFA butyrate, and significantly reduces the pro-inflammatory cytokine IL15. LCDG improves IBS symptoms, though the differences over placebo did not reach a statistical significance. Despite this, we identify plausible biological mechanisms by which this probiotic may exert its effects in patients with IBS.
Given the growing evidence of the role of dysbiosis in the pathophysiology of IBS,2,6 probiotics have been evaluated as a potential therapeutic option in these patients. Probiotics may reduce abdominal symptoms and benefit patients with IBS.7,8 A recent meta-analysis of 43 clinical trials of different products showed that probiotics improve global IBS symptoms, pain, bloating, and flatulence.8 Although probiotics may act through multiple mechanisms, whether they modify abdominal symptoms through direct modulation of the microbiota or indirect action via the gut immune system, or other ways, is unclear.10,11 In our study, LCDG was not statistically superior to placebo in any of the clinical efficacy variables evaluated. However, this was a pilot study not full powered for clinical endpoints aimed at investigating underlying mechanisms of action by which this probiotic induces its effect.
We showed that LCDG significantly reduces Ruminococcus. Members of the intestinal microbiota ascribed to the genus Ruminococcus have been found to be increased in IBS patients.5,25–27 Therefore, the observed ability of LCDG to reduce the relative abundance of this taxon can be considered beneficial in IBS. In particular, we ascribed most of the Ruminococcus-associated reads (∼72%) to the species R. bromii and R. callidus, which were recently proposed as potential microbial biomarkers for diagnosing IBS (patent WO/2011/043654). Correlation analyses supported the proposed dominant involvement of bacteria from the genus Ruminococcus in IBS. We found that Ruminococcus negatively correlates with fecal levels of the main SCFAs in the human gut (i.e. acetate, butyrate, and propionate), which play important roles in maintaining intestinal homeostasis.28,29 Accordingly, an ecological link could exist between the significant reduction in Ruminococcus, which is a dominant genus of the microbiota (overall median relative abundance ∼5%), and the increase in butyrate and acetate observed over the course of the LCDG intervention. The data on intestinal microbial ecology presented in this study agree with the results of a previous intervention study that demonstrated the ability of LCDG to modulate SCFAs and Clostridiales bacteria in healthy adults.13 In addition, the inverse correlation between the Clostridiales genus Oscillospira, which was modulated by LCDG but not placebo, and stool frequency and form suggests that the active treatment may regulate gut physiology.
We assessed the fecal levels of IL6, IL8, IL12, TNF-α, and IFN-γ, which are typical Th-1 pro-inflammatory cytokines, and TGF-β and IL10, regulatory cytokines capable of suppressing inflammatory responses.30 In addition to its well-known pro-inflammatory role, IL6 also possesses anti-inflammatory properties exerted through its ability to stimulate IgA secretion.31,32 This evidence may explain why, in our study, the significant decrease in IL6 levels is also accompanied by a decrease in fecal IgA levels after treatment with LCDG, but not placebo.31,32 IL15 is produced by intestinal epithelial cells and able to stimulate intraepithelial lymphocytes and their interactions with enterocytes. IL15 plays a primary role in the development of several inflammatory diseases, including celiac disease and IBD, affecting the integrity of the mucosal barrier.33 The significant decrease in IL15 levels observed in our study after treatment with LCDG, but not placebo, suggests that this product may play an important role in the restoration of intestinal regulation and mucosal integrity.33,34 The role of IL15 in IBS should be clarified in ad hoc studies.
The strength of this study is that we used the same rigorous criteria, design, and endpoints as classical pharmacological efficacy studies. In addition, as suggested by recent guidelines,11 we previously demonstrated that the test organism was present in the stools of exposed individuals;13 here, we clarified the mechanisms by which it may benefit patients with IBS. However, we acknowledge the limitations of the present study. Clearly, we recognize the downsides of the cross-over design, particularly in studies of patients with functional bowel disorders; however, we opted for this design because it seemed most applicable in pathophysiological studies in which endpoints are measured objectively. Furthermore, because of the pilot and mechanistic nature of the study, the sample size was limited and clearly not powered for clinical endpoints. We did not show any significant differences between the active treatment and placebo, though better results were obtained with LCDG. Whether this absence of significant differences reflects a true treatment ineffectiveness or a type 2 error should be clarified in ad hoc studies. Finally, for all these reasons, the generalizability of our results requires caution and further confirmation.
In conclusion, although causality is not proven and only an association can be reported, we showed that LCDG improves IBS symptoms, though not in a statistically significant manner, through modulation of the gut microbiota, its metabolic pathways, and pro-inflammatory cytokines. As in this study no statistically significant effect on IBS symptoms was found, further studies are necessary to determine the role of LCGD in the management of IBS.
Supplementary Material
Acknowledgments
All authors have approved the final version of the article and the list of authors. The authors are grateful to Laura Patrucco, Gabriella Bartesaghi, and Ruggero Rossi from Sofar S.p.A. and to Aldo Poli from OPIS for their constant support in all phases of the study, as well as their assistance in preparing the manuscript. We are grateful to Dr Claudio Gardana for his technical assistance. We would like to thank Paul Kretchmer, PhD, Managing Director, San Francisco Edit, Mill Valley, CA, USA, for editing the manuscript. Costs of the editing were supported by Sofar S.p.A., Trezzano Rosa, Milan, Italy.
Guarantor of article: Prof Giovanni Barbara
Author contributions: Giovanni Barbara, Vincenzo Stanghellini and Cesare Cremon planned the study, designed the protocol, contributed to the writing of the manuscript, and were involved in the screening and periodic visits of the patients. Simone Guglielmetti contributed to the writing of the text concerning the analysis of the intestinal microbial ecosystem (IME). Simone Guglielmetti, Giorgio Gargari, and Valentina Taverniti carried out the IME analyses, bioinformatic and statistical analysis of IME data, and the preparation of fecal waters. Anna Maria Castellazzi, Chiara Valsecchi, and Carlotta Tagliacarne contributed to the experimental design, to the writing of the manuscript, and carried out the analyses on IgA and cytokines. Walter Fiore contributed to the writing of the protocol and manuscript. Massimo Bellini, Lorenzo Bertani, Dario Gambaccini, Michele Cicala, Bastianello Germanà, Maurizio Vecchi, Isabella Pagano, Maria Raffaella Barbaro, and Lara Bellacosa contributed to the experimental design, and were involved in the screening and periodic visits of the patients.
Declaration of conflicting interests
None declared.
Ethics approval
The protocol was approved by an independent ethics committee at each center (in particular, it was approved by the Ethics Committee of St. Orsola-Malpighi Hospital of Bologna on October 7, 2014, approval identification no: 145/2014/O/Sper) and carried out according to the Declaration of Helsinki and the principles of good clinical practice.
Funding
This work was supported by Sofar S.p.A., Trezzano Rosa, Milan, Italy. The funding agency had no role in the study design, collection, analysis, data interpretation, or writing of the report.
Informed consent
All patients provided written informed consent.
References
- 1.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 2.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016; 150: 1305–1318. [DOI] [PubMed] [Google Scholar]
- 3.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009; 136: 1979–1988. [DOI] [PubMed] [Google Scholar]
- 4.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in faecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 5.Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014; 63: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 6.Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013; 62: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010; 59: 325–332. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–1561. [DOI] [PubMed] [Google Scholar]
- 9.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11: 506–514. [DOI] [PubMed] [Google Scholar]
- 11.Irvine EJ, Tack J, Crowell MD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2016; 150: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 12.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128: 541–551. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario C, Taverniti V, Milani C, et al. Modulation of faecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr 2014; 144: 1787–1796. [DOI] [PubMed] [Google Scholar]
- 14.Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: Mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease—a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 2013; 38: 741–751. [DOI] [PubMed] [Google Scholar]
- 15.D’Incà R, Barollo M, Scarpa M, et al. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci 2011; 56: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 16.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 19.Gargari G, Taverniti V, Balzaretti S, et al. Consumption of a Bifidobacterium bifidum strain for 4 weeks modulates dominant intestinal bacterial taxa and faecal butyrate in healthy adults. Appl Environ Microbiol 2016; 82: 5850–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duranti S, Gaiani F, Mancabelli L, et al. Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiol Ecol 2016; 92: pii: fiw191–pii: fiw191. [DOI] [PubMed] [Google Scholar]
- 21.Avanzini MA, Plebani A, Monafo V, et al. A comparison of secretory antibodies in breast-fed and formula-fed infants over the first six months of life. Acta Paediatr 1992; 81: 296–301. [DOI] [PubMed] [Google Scholar]
- 22.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014; 146: 67–75.e5. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988.
- 24.Armitage P, Hills M. The two-period crossover trial. Statistician 1982; 31: 119–131. [Google Scholar]
- 25.Taverniti V, Guglielmetti S. Methodological issues in the study of intestinal microbiota in irritable bowel syndrome. World J Gastroenterol 2014; 20: 8821–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajilić-Stojanović M, Jonkers DM, Salonen A, et al. Intestinal microbiota and diet in IBS: Causes, consequences, or epiphenomena? Am J Gastroenterol 2015; 110: 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigsbee L, Agans R, Shankar V, et al. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2012; 107: 1740–1751. [DOI] [PubMed] [Google Scholar]
- 28.Corrêa-Oliveira R, Fachi JL, Vieira A, et al. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology 2016; 5: e73–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ríos-Covián D, Ruas-Madiedo P, Margolles A, et al. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016; 7: 185–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagarasan S, Honjo T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat Rev Immunol 2003; 3: 63–72. [DOI] [PubMed] [Google Scholar]
- 32.Goodrich ME, McGee DW. Preferential enhancement of B cell IgA secretion by intestinal epithelial cell-derived cytokines and interleukin-2. Immunol Invest 1999; 28: 67–75. [DOI] [PubMed] [Google Scholar]
- 33.van Heel DA. Interleukin 15: Its role in intestinal inflammation. Gut 2006; 55: 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagliari D, Cianci R, Frosali S, et al. The role of IL15 in gastrointestinal diseases: A bridge between innate and adaptive immune response. Cytokine Growth Factor Rev 2013; 24: 455–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.