Abstract
The aim of this study was to evaluate the clinical usefulness of the chromogranin A (CgA) determination in patients with neuroendocrine neoplasms (NENs) of the digestive system and to analyse the association between concentration of the marker and progression-free survival (PFS) and overall survival (OS). Serum concentrations of CgA were determined before the treatment in 131 patients with NENs, including patients with tumours located in the pancreas, the small intestine, caecum, appendix and in the colon. No significant associations were identified in CgA concentrations between the control group and patients with NENs in appendix and colon. In patients with NENs of the pancreas and NENs of the small intestine and caecum, increased CgA levels were associated with lymph node involvement, distant metastases and a baseline liver involvement. Analyses revealed significantly higher CgA concentrations in patients with active disease compared to those without symptoms of NEN. In patients with NENs of the pancreas, CgA concentration was correlated with tumour grade and Ki67. Significantly higher CgA levels were also found in patients who died compared to those who lived. Analyses of PFS and OS revealed that CgA concentration was not a prognostic factor in patients with NENs of the pancreas. In patients with NENs of the small intestine and caecum, increased CgA concentrations are independent, poor prognostic factors for both PFS and OS. In conclusion, in patients with NENs in pancreas, CgA levels are associated with disease progression, while in patients with NENs in small intestine and caecum, its concentration is a predictive indicator for PFS and OS.
Keywords: chromogranin A, neuroendocrine neoplasms, prognostic factor, pancreas, small intestine
Introduction
Neuroendocrine neoplasms (NENs) constitute a heterogeneous group of increasingly diagnosed and detected tumours derived from neuroendocrine cells, scattered throughout the human body and forming a diffuse endocrine system. Neuroendocrine cells respond to various signals by releasing peptides, biogenic amines and other hormonally active substances. A characteristic feature of most well- and moderately differentiated NENs is the overexpression of somatostatin receptors (SSTs). These receptors are used in the search for a primary site when it cannot be identified by structural examinations and to assess the stage of the disease. In addition, evaluation of the degree of receptor expression based on in vivo imaging determines further treatment using synthetic SST receptor analogues. NENs differ in terms of the substances secreted by the cells, the presence or absence of hormonal activity, clinical symptoms, histopathological features and prognosis (1). Diagnostics and treatment monitoring use the ability of NEN cells to release non-specific markers into the bloodstream. The determination of non-specific marker – chromogranin A (CgA) – is applied in clinical practice (2, 3).
This substance belongs to acid glycoproteins contained in the granules of secretory neuroendocrine cells, both normal and those undergoing neoplastic transformations. The CgA concentration is significantly elevated in most locally advanced and disseminated NENs especially those in the midgut origin, although in very small lesions, its level may be within the normal range (4). CgA in gastroenteropancreatic neuroendocrine neoplasms (GEP-NEN) can also serve as an independent prognostic factor for the evaluation of survival of patients. Previous studies indicate some relationships between the degree of differentiation of NENs, the location of a primary lesion, the tumour mass and CgA levels (4, 5, 6, 7). Recent research confirms that CgA plays an important role in the regulation of angiogenesis, as well as in the modulation of the endothelial function as important processes in the tumour growth (8, 9, 10).
Previous studies have shown that intact CgA molecule has an anti-neoplastic effect and thus inhibitory properties in relation to the tumour growth by suppression of angiogenesis (9, 11). The modification of the molecule structure, however, leads to the formation of fragments of different biological activity. The clinical usefulness of the study results may depend on the sensitivity of methods used to detect various forms of CgA in the blood serum. In recent years, the presence of not only the native CgA molecule, but also of its various fragments (CgA1–78, CgA1–439, CgA1–373) has been demonstrated in the blood. The quantitative composition of these forms may differ depending on the diagnosis. Therefore, the variable profile of CgA derivatives, which are found in the patients’ blood serum, may create favourable conditions for the development of the disease (11, 12). The most common problems associated with the CgA determination include differences in the results depending on the reagent kit used (13, 14) as well as the impact of many other factors, such as the type of NEN, the location of a primary site, a degree of NEN cell differentiation, the presence of co-morbidities and the type of drugs used in the treatment.
The aim of this study was to evaluate the clinical usefulness of the CgA determination in patients with NENs of the digestive system by identifying the relationship with the clinical-pathological parameters and the analysis of the association between concentration of the marker and progression free survival (PFS) and overall survival (OS).
Materials and methods
We analysed retrospectively the data of patients with NENs of the digestive system treated consecutively from 2014 to 2017 at The Maria Sklodowska-Curie Institute-Oncology Center in Warsaw. Serum concentrations of CgA were determined before the treatment, in 131 patients with NENs of the digestive tract, including 59 with tumours located in the pancreas and 72 with lesions in the small intestine, caecum and appendix (midgut – the tumours originating from the central part of the archenteron) and in the colon (hindgut – tumours of the posterior part of the archenteron). The study group included 80 women and 51 men; the median age was 58 years (range from 18 to 81 years). First group consisted of patients with diagnosis of pancreatic NEN, in accordance with the current WHO classification created in 2017 (Table 1): thirty patients with well-differentiated malignancy: NETG1, Ki-67 = 3%, 24 with moderately differentiated neoplasm: NETG2, Ki-67 3–20% and five with NETG3 or NEC (neuroendocrine carcinoma): Ki-67 >20%. In the second group, 48 patients had the primary lesion located in the small intestine and caecum, 13 in the appendix and 11 in the colon (2: sigmoid, 9: rectum). This group included (Table 1) 46 patients with NETG1, Ki-67 = 3%, 22 subjects with NETG2, Ki-67 3–20% and four with NEC (neuroendocrine carcinoma): Ki-67 >20%. Tumour size (pT), the involvement of the lymph nodes and presence of distant metastases (M) were determined in the study groups according to the TNM classification (Table 2).
Table 1.
Characteristics of patients according to WHO 2017 classification.
| Primary lesion | NETG1 (n) | NETG2 (n) | NETG3/NEC (n) |
|---|---|---|---|
| Pancreas | 30/59 | 24/59 | 5/59 |
| Small intestine | 24/39 | 14/39 | 1/39 |
| Caecum | 6/9 | 3/9 | 0 |
| Appendix | 10/13 | 2/13 | 1/13 |
| Colon | 5/11 | 4/11 | 2/11 |
| Sigmoid | 1/2 | 1/2 | 0 |
| Rectum | 4/9 | 3/9 | 2/9 |
NEC, neuroendocrine carcinoma; WHO, World Health Oraganization.
Table 2.
Clinicopathological characteristics of patients.
| Parameters | NENs of the pancreas | NENs of the small intestine and caecum |
|---|---|---|
| Number of patients (%) | Number of patients (%) | |
| Gender | ||
| Women | 31/59 (53) | 49/72 (68) |
| Men | 28/59 (47) | 23/72 (32) |
| Tumor size (T) | ||
| T1 | 16/59 (27) | 3/48 (6) |
| T2 | 15/59 (25) | 7/48 (15) |
| T3 | 15/59 (25) | 22/48 (46) |
| T4 | 6/59 (11) | 12/48 (25) |
| Tx | 7/59 (12) | 4/48 (8) |
| Lymph node status (N) | ||
| N0 | 32/59 (54) | 4/48 (8) |
| N1 | 27/59 (46) | 44/48 (92) |
| Distance metastasis (M) | ||
| M0 | 37/59 (63) | 18/48 (36) |
| M1 | 22/59 (37) | 30/48 (64) |
| Histological grade (G) | ||
| G1 | 30/59 (50) | 30/48 (63) |
| G2 | 24/59 (41) | 17/48 (35) |
| G3 | 5/59 (9) | 1/48 (2) |
| Indeks proliferation (Ki67) | ||
| =3 | 33/59 (56) | 32/48 (67) |
| 3–20 | 21/59 (35) | 15/48 (31) |
| >20 | 5/59 (8) | 1/48 (2) |
| Baseline liver involvement | ||
| Yes | 21/59 (35) | 22/48 (46) |
| No | 38/59 (65) | 26/48 (54) |
| Chromogranin A (ng/mL) | ||
| ≤84.7 | 30/59 (51) | 23/48 (48) |
| >84.7 | 29/59 (49) | 25/48 (52) |
NEN, neuroendocrine neoplasm.
Patients with bowel primary received SST analogues therapy. Patients with primary pancreas received SST analogues therapy or additional biological therapy (everolimus) after progression on SST or after radical surgery (R) – just ‘wait and watch’. Patients with bowel primary and with pancreatic NET after relapse on SST analogues therapy had peptide receptor radionuclide therapy.
All patients were informed of the study aims and procedures and signed an informed consent. The study was authorized by the Local Ethics Committee of University of Warmia and Masuria.
The control group consisted of 20 healthy subjects, including 11 women and 9 men, aged 24–75 years; the median was 45 years.
CgA concentrations were measured on the Kryptor system, B.R.A.H.M.S GmbH kits, Thermo Fisher. This assay is immunometric, using an anti-CgA mouse monoclonal antibody, with the time resolved amplified cryptate emission. The cut-off point for CgA = 84.7 ng/mL was adopted in accordance with the manufacturer’s instructions. The proton pump inhibitor was discontinued at least 1 week before blood sampling. Non-parametric tests, such as the Mann–Whitney U test, Kruskal–Wallis test and Spearman’s rank correlation coefficient, were used for statistical calculations. The PFS and OS analyses were performed on the basis of the Kaplan–Meier curves using a one-factor analysis, the log-rank test and multivariate Cox regression analysis. PFS was defined using standard oncological approach utilizing CT or MRI as initial imaging methods and as the follow-up imaging methods. The significance level was adopted at P < 0.05.
Results
First, the relationship between the CgA concentrations, measured before treatment in groups of patients with NENs of the digestive system, divided based on the location of a primary lesion and the concentrations in the healthy control group were analysed. Significantly higher CgA levels were found in patients with NENs of the pancreas (P = 0.019) and NENs of the small intestine and caecum (P = 0.0001) compared to healthy subjects. No significant association was observed in patients with tumour located both in the colon and appendix (Table 3).
Table 3.
Serum concentration of CgA in control group and in patients with GEN/NET.
| Chromogranin A (ng/mL) | %* | Control vs patients | ||
|---|---|---|---|---|
| Median | Range | |||
| Control group | 48.5 | 18.1–72.7 | 0 | |
| NENs of pancreas | 87.9 | 21.7–9803 | 49 | P = 0.019 |
| NENs of intestine | ||||
| Small intestine with caecum | 105.0 | 27.9–3776 | 52 | P = 0.001 |
| Appendix | 44.0 | 11.5–215.6 | 8 | NS |
| Colon | 60.0 | 9.3–397.0 | 27 | NS |
*The percentage of patients with elevated levels of CgA.
CgA, chromogranin A; NEN, neuroendocrine neoplasm; NS, not statistically significant.
In patients with NENs of the pancreas, the serum CgA concentrations were elevated in 49% of patients and the median concentration was 87.9 ng/mL (range: 21.7–9803 ng/mL); in patients with a primary lesion located in the small intestine and the median concentration was 105.0 ng/mL (range: 27.9–3776 ng/mL) and elevated concentrations were found in 52% of the subjects.
In the group of patients with tumour located in the colon and appendix, the median concentrations were below the cut-off point (60.0 ng/mL and 44.0 ng/mL, respectively), and the marker concentrations were increased in a small percentage of patients. Comparison of CgA levels in the study groups revealed that they were significantly higher in the group with a primary tumour located in the small intestine compared to those with the tumour identified in the colon (P = 0.012) and appendix (P = 0.001). There were no significant differences between CgA concentrations in patients with NENs of the pancreas and NENs of the small intestine (Table 3).
Because of the low sensitivity and the lack of differences between the CgA concentrations in patients with the location of the lesion in the colon and appendix and the control group, these patients were not included in further analyses.
Correlation with CgA serum levels and clinical-pathological features
The relationship between biomarker concentration and the following clinical-pathological features: the clinical stage (pTNM), a degree of tumour cell differentiation (G), mitotic activity (Ki-67 index – MIB1 antibody), gender and age, separately in two groups of patients: NENs of the pancreas and NENs of the small intestine were analysed. The characteristics of these patients are presented in Table 2. The comparison of the CgA concentrations in the patients’ blood serum depending on the size of the tumour (pT) showed no correlation between both groups. However, it was observed that the CgA levels increased with the tumour size in patients with NENs of the small intestine; however, this relationship was a trend (P = 0.07).
The Mann–Whitney U test revealed significant differences in the CgA concentrations depending on the lymph node involvement in both groups of patients. In subjects with NENs of the pancreas (P = 0.027) and NENs of the small intestine (P = 0.005) with the lymph node involvement, the CgA levels were significantly higher than those without neoplastic cells in the lymph nodes. Also, in both groups, significantly higher marker concentrations were found in patients with distant metastases (M1) (NENs of the pancreas P = 0.006, NENs of the small intestine P = 0.001) than in the subjects without distant metastases (M0). In patients with the baseline liver involvement, significantly higher CgA concentrations (P = 0.001) were confirmed both for NENs of the pancreas and NENs of the small intestine than in those without metastatic lesions in the liver.
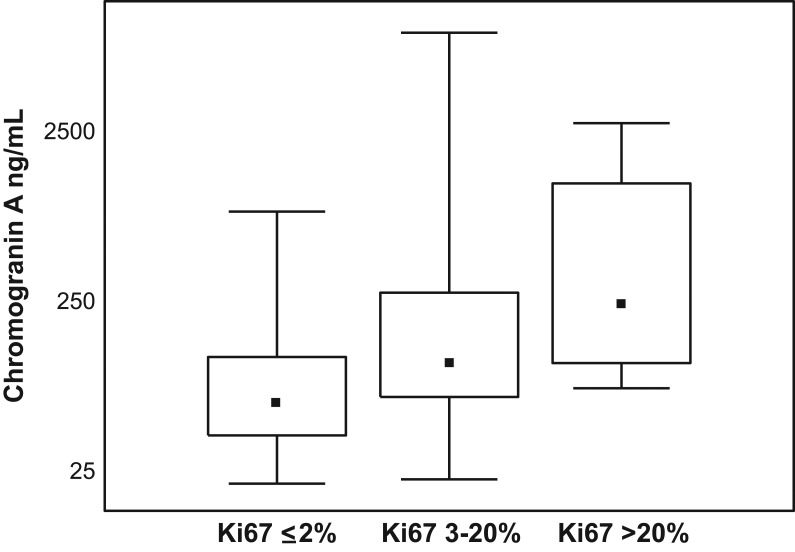
In the group with NENs of the pancreas, a positive correlation was found between the CgA concentrations, both G1 vs G2 tumour differentiation (P = 0.032, R = 0.33) and the Ki-67 proliferation index (P = 0.019, R = 0.43) (Fig. 1). The patients with G3 and Ki-67 >20% were not analysed due to their small number. There were no such associations found in the group with tumour located in the small intestine (Table 4). In the study groups with GEP-NEN (NENs of the pancreas, NENs of the small intestine), no relationship was demonstrated between the CgA concentration, gender and age.
Figure 1.
Medians and CgA concentrations in dependence on index proliferation Ki67 in NENs of pancreas patients. CgA, chromogranin A; NEN, neuroendocrine neoplasm.
Table 4.
Relationship between CgA serum levels and clinicopathological features in two groups of patients with GET/NEN.
| Parameters | Chromogranin A (ng/mL) | |
|---|---|---|
| NENs of pancreas | NENs of small intestine and caecum | |
| Gender | NS* | NS* |
| Localizacion | NS* | |
| Tumor size (T) | NS* | NS* |
| Lymph node status (N) | P = 0.027 | P = 0.005 |
| Distance metastasis (M) | P = 0.006 | P = 0.001 |
| Histological grade (G) | P = 0.032; R = 0.33 | NS* |
| Indeks proliferation (Ki67) | P = 0.019; R = 0.43 | NS* |
| Baseline liver involvement | P = 0.001 | P = 0.001 |
| Progresion | P = 0.034 | P = 0.001 |
| Survival status | P = 0.028 | P = 0.015 |
*NS, not statistically significant.
CgA, chromogranin A; NEN, neuroendocrine neoplasm.
Relationship between the CgA concentration and PFS and OS in patients with NENs of pancreas
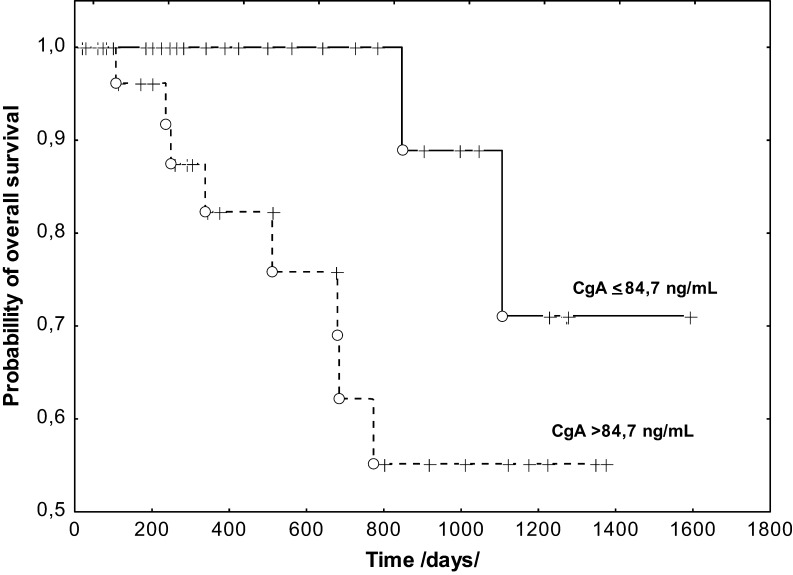
After about 3 years of follow-up (the median was 509 days), 46 patients showed no local recurrence or spread of the disease; NEN progression was observed in 13 (22%) patients, but 10 of them (17%) died. The subjects with progression had significantly higher CgA levels (P = 0.034) before the treatment than those without NEN progression (Table 4). Analysis of PFS revealed that the CgA concentration was not a prognostic factor. However, significantly higher CgA levels were found in patients with progression and subsequent death than in those who survived (P = 0.028) (Table 4). Apart from the clinical and pathological features: G, Ki-67 and M, the log-rank univariate analysis showed a prognostic value of CgA (P = 0.04) (Fig. 2). However, the Cox multivariate analysis did not confirm the significance of CgA as an independent prognostic factor for OS in patients with NENs of the pancreas.
Figure 2.
Kaplan–Meier curves estimates of overall survival of NENs of pancreas patients stratified by serum CgA levels. CgA, chromogranin A; NEN, neuroendocrine neoplasm.
Relationship between the CgA concentration and PFS and OS in patients with NENs of small intestine and caecum
During monitoring (the median was 882 days), 27 (35%) patients showed no active NEN disease; the progression was confirmed in 21 (44%) patients, and 10 subjects (21%) died during the clinical follow-up.
The Mann–Whitney U test revealed significantly higher CgA concentrations in patients with active disease (P = 0.001) compared to those without features of NEN. Significantly higher CgA levels were also found in patients who died compared to those who lived (P = 0.015).
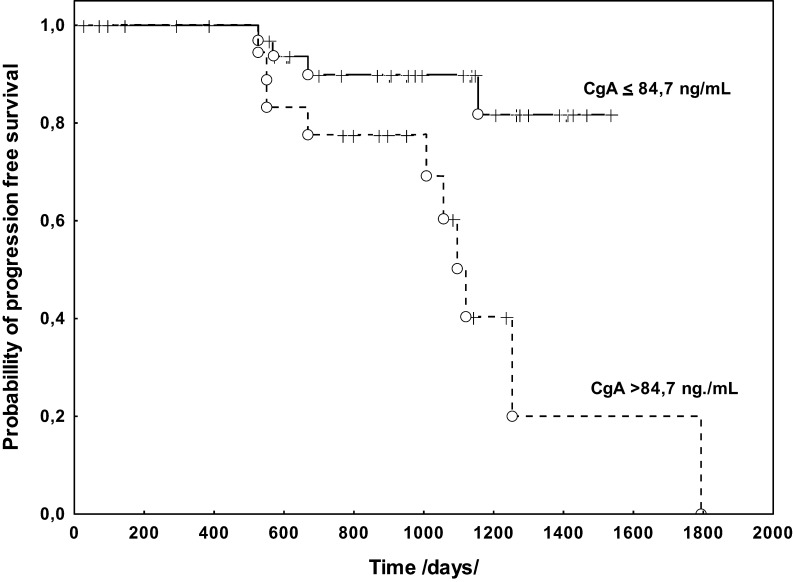
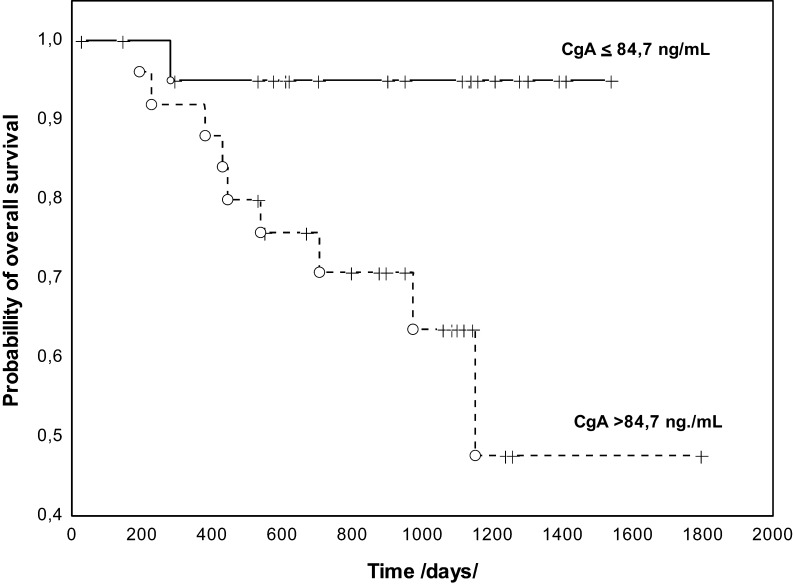
The assessment of the prognostic PFS value using univariate analysis showed that elevated CgA concentrations were associated with PFS (P = 0.001). Among the clinical-pathological features, the presence of distant metastases (P = 0.014) was associated with the shorter PFS. Cox analysis of PFS, which took into account the M features and CgA concentrations, revealed that only CgA concentrations were an independent prognostic factor (HR: 5.026, 95% CI: 3.672–6.380, P = 0.019) (Fig. 3). The analysis of OS and the log-rank test demonstrated a prognostic value only for CgA concentrations (P = 0.014), and this was further confirmed in the Cox analysis (HR: 8.73, 95% CI: 6.658–10.810, P = 0.041) (Fig. 4).
Figure 3.
Kaplan–Meier curves estimates of PFS of NENs of small intestine and caecum patients stratified by serum CgA levels. CgA, chromogranin A; NEN, neuroendocrine neoplasm; PFS, progression-free survival.
Figure 4.
Kaplan-Meier curves estimates of OS of NENs of small intestine and caecum patients stratified by serum CgA levels. CgA, chromogranin A; NEN, neuroendocrine neoplasm; OS, overall survival.
Discussion
The most common location of neuroendocrine tumours is the digestive system (GEP-NEN GEP). The diagnosis is usually made at the advanced stage of the disease when the surgical treatment is impossible. Majority of patients with GEP-NEN have atypical clinical symptoms, suggestive of other conditions, and despite the slow development of NENs, tumours are not correctly recognized. The diagnosis of this group of tumours is based on some common features of the neoplastic cells, such as the expression of SST used in imaging techniques to determine the location of a primary lesion and the assessment of the stage of progression as well as the qualification of patients for targeted treatment using SST receptor analogues. An additional feature is the release of non-specific markers, such as CgA (5, 15, 16).
The CgA expression has been confirmed both in neuroendocrine tumours and other neoplasms, such as breast cancer, non-small-cell lung cancer and prostate cancer, which may undergo neuroendocrine differentiation (17, 18, 19). Results of our studies show the clinical evaluation of the usefulness of CgA determination in patients with GEP-NEN. Our research demonstrated significantly higher CgA levels in these subjects compared to healthy controls, which was consistent with previous reports (20). There were differences between the marker concentrations depending on the location of a primary lesion in the digestive system. Higher CgA levels were observed in patients with non-functional pancreatic tumours than in those with an intestinal location, although these differences were not statistically significant. Literature reports often emphasize the high diagnostic sensitivity of CgA in patients with NENs of the pancreas compared to other pancreatic diseases, including insulinoma (21, 22, 23, 24). Significant differences were demonstrated in the CgA concentrations depending on the location of a primary lesion in the intestines. Consistent with other studies, the elevated CgA concentrations were most frequently observed in patients with tumour located in the small intestine and caecum. These concentrations were significantly higher in comparison to those reported in patients with tumour located in the colon and appendix (25). Epidemiological data indicate increased incidence of neuroendocrine tumours located in the appendix, and this ranks third in terms of location, after the small intestine and rectum (26). In patients with primary lesion in the appendix who usually have small tumours, similar to other reports, we observed a significant percentage of subjects with the CgA levels below the cut-off point (27). As in other studies, there was no correlation between the marker concentrations and age and sex of the patients (28). CgA concentrations did not differ statistically depending on the size of the tumour, which was not consistent with the results presented by other researchers (21, 22, 24). Only in patients with the tumour located in the intestine, the marker concentrations increased with the size of tumour. Our studies showed, however, that elevated marker concentrations were associated with lymph node involvement. Similarly, higher marker levels were observed in patients with metastases to the liver or other distant organs. This relationship was also described in the works of other authors (21, 22, 28).
The value of the proliferation index, which is related to the degree of NEN cell differentiation, plays an important role in making therapeutic decisions. High values of the Ki-67 index are an unfavourable prognostic factor, which is associated with the shortening of PFS and OS in these patients. Our studies demonstrated an association between the CgA concentration and Ki-67 only for patients with the primary NEN located in the pancreas. These results are consistent with observations of Massironi et al., though other authors report no relationship between CgA concentrations and the degree of cell differentiation (6, 28).
The assessment of the usefulness of CgA determination in predicting OS and PFS, which is available in the literature, is also ambiguous. The unfavourable effect of the elevated CgA levels on OS has been confirmed (6, 28, 29). Our studies showed that CgA levels obtained before treatment were significantly higher in patients with progression or death during clinical follow-up, regardless of the tumour location (the pancreas or small intestine). The analysis of PFS conducted in patients with NENs of the pancreas revealed that the CgA values were an independent prognostic factor. Apart from the clinical and pathological features: G, Ki67 and M, the assessment of OS in the univariate analysis revealed a prognostic value of CgA. Cox multivariate analysis did not show the importance of CgA as an independent prognostic factor in patients with NENs of the pancreas. These results were consistent with the work of Sherman et al. who did not confirm the significance of CgA concentrations as an independent prognostic factor, although in the univariate analysis, the effect of the marker levels on both PFS and OS was demonstrated. Authors found an association between elevated CgA concentration, which were determined at the end of treatment and OS (30). However, our study showed that in the group of patients with the tumour located in the small intestine, the CgA levels were an independent prognostic factor for both PFS and OS.
Results of studies on the usefulness of CgA concentrations as a universal NEN marker are ambiguous, and research is often conducted in very few groups of patients. Only in the digestive system, we can obtain different test results depending on the location of a primary lesion. Our work showed that marker concentrations in patients with tumour located in the pancreas are more useful to determine the severity of the disease – a relationship with the parameters, such as the Ki-67 mitotic index and the degree of NEN cell differentiation – than in subjects with a small intestine location. In contrast, in patients with NENs of the small intestine, the CgA levels are undoubtedly an unfavourable prognostic factor for PFS and OS.
Despite numerous controversies, most authors point to the validity of the CgA determination in clinical practice in patients with GEP-NEN (5, 31, 32, 33). However, the heterogeneity of neuroendocrine tumours, their rare incidence in connection with the diversity of marker forms, makes it necessary to conduct further research in these patients. It will be particularly important to identify groups who will benefit the most from the determination of CgA concentration.
In conclusion, in patients with NENs in pancreas, CgA levels are associated with disease progression, while in patients with NENs in small intestine and caecum, its concentration is a predictive indicator for PFS and OS.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
The authors thank Dr Joanna Achinger-Kawecka from Epigenetics Research Laboratory, Genomics and Epigenetics Division, Garvan Institute of Medical Research and St. Vincent’s Clinical School, Faculty of Medicine, University of New South Wales, Darlinghurst, Australia for careful proofreading of the manuscript in English.
References
- 1.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Custer S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010. 39 707–712. ( 10.1097/MPA.0b013e3181ec124e) [DOI] [PubMed] [Google Scholar]
- 2.Kos-Kudla B, Blicharz-Dorniak J, Strzelczyk J, Baldys-Waligorska A, Bednarczuk T, Bolanowski M, Boratyn-Nowicka A, Borowska M, Cichocki A, Cwikła JB, et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Polish Endokrynology 2017. 68 79–110. ( 10.5603/EP.2017.0015) [DOI] [PubMed] [Google Scholar]
- 3.Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape UF, Perren A, Rindi G, Ruszniewski P, et al. Antibes Consensus Conference participants. ENETS Consensus Guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology 2017. 105 201–221. ( 10.1159/000472254) [DOI] [PubMed] [Google Scholar]
- 4.Landry CS, Cavaness K, Celinski S, Preskitt J. Biochemical prognostic indicators for pancreatic neuroendocrine tumors and small bowel neuroendocrine tumors. Gland Surgery 2014. 3 215–218. ( 10.3978/j.issn.2227-684X.2014.10.01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Law C. Chromogranin A: a sensitive biomarker for the detection and post-treatment monitoring of gastroenteropancreatic neuroendocrine tumors. Expert Review of Gastroenterology and Hepatology 2012. 6 313–334. ( 10.1586/egh.12.15) [DOI] [PubMed] [Google Scholar]
- 6.Massironi S, Rossi RE, Casazza G, Conte D, Ciafardini C, Galeazzi M, Peracchi M. Chromogranin A in diagnosing and monitoring patients with gastro-entero-pancreatic neuroendocrine neoplasms. A large series from a single institution. Neuroendocrinology 2014. 100 240–249. ( 10.1159/000369818) [DOI] [PubMed] [Google Scholar]
- 7.Rogowski W, Wachula E, Lewczuk A, Kolesinska-Cwikla A, Izycka-Swieszewska E, Sulzyc-Bielicka V, Cwikła JB. Baseline chromogranin A and its dynamics are prognostic markers in gastroenteropancreatic neuroendocrine tumors. Future Oncology 2017. 13 1069–1079. ( 10.2217/fon-2016-0455) [DOI] [PubMed] [Google Scholar]
- 8.Corti A, Ferrero E. Chromogranin A and the endothelial barrier function. Current Medicinal Chemistry 2012. 19 4051–4058. ( 10.2174/092986712802429975) [DOI] [PubMed] [Google Scholar]
- 9.Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, Loh YP, Curnis F, Corti A. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 2013. 121 392–402. ( 10.1182/blood-2012-05-430314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helle KB, Corti A. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cellular and Molecular Life Sciences 2015. 72 339–348. ( 10.1007/s00018-014-1750-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curnis F, Dallatomasina A, Bianco M, Gasparri A, Sacchi A, Colombo B, Fiocchi M, Perani L, Venturini M, Tacchetti C, et al. Regulation of tumor growth by circulating full-length chromogranin A. Oncotarget 2016. 7 72716–72732. ( 10.18632/oncotarget.12237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti A, Marcucci F, Bachetti T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflügers Archiv: European Journal of Physiology 2018. 470 199–210. ( 10.1007/s00424-017-2030-y) [DOI] [PubMed] [Google Scholar]
- 13.Brehm Hoej L, Parkner T, Soendersoe Knudsen C, Grønbaek H. A comparison of three chromogranin A assays in patients with neuroendocrine tumours. Journal of Gastrointestinal and Liver Diseases 2014. 23 419–424. [DOI] [PubMed] [Google Scholar]
- 14.van der Knaap RHP, Kwekkeboom DJ, Ramakers CRB, de Rijke YB. Evaluation of a new immunoassay for chromogranin A measurement on the Kryptor system. Practical Laboratory Medicine 2015. 1 5–11. ( 10.1016/j.plabm.2015.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh YP, Cheng Y, Mahata SK, Corti A, Tota B. Chromogranin A and derived peptides in health and disease. Journal of Molecular Neuroscience 2012. 48 347–356. ( 10.1007/s12031-012-9728-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanno Y, Toyama H, Matsumoto I, Otani K, Asari S, Goto T, Ajiki T, Zen Y, Fukumoto T, Ku Y. Baseline plasma chromogranin A levels in patients with well-differentiated neuroendocrine tumors of the pancreas: a potential predictor of postoperative recurrence. Pancreatology 2017. 17 291–294. ( 10.1016/j.pan.2016.12.012) [DOI] [PubMed] [Google Scholar]
- 17.Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. Granins and granin-related peptides in neuroendocrine tumours. Regulatory Peptides 2010. 165 12–20. ( 10.1016/j.regpep.2010.02.011) [DOI] [PubMed] [Google Scholar]
- 18.Bogina G, Munari E, Brunelli M, Bortesi L, Marconi M, Sommaggio M, Lunardi G, Gori S, Massocco A, Pegoraro MC, et al. Neuroendocrine differentiation in breast carcinoma: clinicopathological features and outcome. Histopathology 2016. 68 422–432. ( 10.1111/his.12766) [DOI] [PubMed] [Google Scholar]
- 19.Heck MM, Thaler MA, Schmid SC, Seitz AK, Tauber R, Kübler H, Maurer T, Thalgott M, Hatzichristodoulou G, Höppner M, et al. Chromogranin A and neurone-specific enolase serum levels as predictors of treatment outcome in patients with metastatic castration-resistant prostate cancer undergoing abiraterone therapy. BJU International 2017. 119 30–37. ( 10.1111/bju.13493) [DOI] [PubMed] [Google Scholar]
- 20.Wang YH, Yang QC, Lin Y, Xue L, Chen MH, Chen J. Chromogranin a as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014. 93 e247 ( 10.1097/MD.0000000000000247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belli SH, Oneto A, Aranda C, O’Connor JM, Domenichini E, Roca E, Méndez G, Bestani MC, Parma P, Giacomi N, et al. Chromogranin A as a biochemical marker for the management of neuroendocrine tumors: a multicenter study developed in Argentina. Acta Gastroenterologica Latinoamericana 2009. 39 184–189. [PubMed] [Google Scholar]
- 22.Hijioka M, Ito T, Igarashi H, Fujimori N, Lee L, Nakamura T, Jensen RT, Takayanagi R. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer Sciences 2014. 105 1464–1471. ( 10.1111/cas.12533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao XW, Qiu L, Chen YJ, Meng CT, Sun Z, Bai CM, Zhao DC, Zhang TP, Zhao YP, Song YL, et al Chromogranin A is a reliable serum diagnostic biomarker for pancreatic neuroendocrine tumors but not for insulinomas. BMC Endocrine Disorders 2014. 14 64 ( 10.1186/1472-6823-14-64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun E, Kim SC, Song KB, Hwang DW, Lee JH, Shin SH, Hong SM, Park KM, Lee YJ. Diagnostic value of chromogranin A in pancreatic neuroendocrine tumors depends on tumor size: a prospective observational study from a single institute. Surgery 2017. 162 120–130. ( 10.1016/j.surg.2017.01.019) [DOI] [PubMed] [Google Scholar]
- 25.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-biological function and clinical utility in neuroendocrine tumor disease. Annals of Surgical Oncology 2010. 17 2427–2443. ( 10.1245/s10434-010-1006-3) [DOI] [PubMed] [Google Scholar]
- 26.Shaib W, Krishna K, Kim S, Goodman M, Rock J, Chen Z, Brutcher E, Staley CI, Maithel SK, Abdel-Missih S, et al. Appendiceal neuroendocrine, goblet and signet-ring cell tumors: a spectrum of diseases with different patterns of presentation and outcome. Cancer Research and Treatment 2016. 48 596–604. ( 10.4143/crt.2015.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandraki KI, Kaltsas GA, Grozinsky-Glasberg S, Chatzellis E, Grossman AB. Appendiceal neuroendocrine neoplasms: diagnosis and management. Endocrine-Related Cancer 2016. 23 27–41. ( 10.1530/ERC-15-0310) [DOI] [PubMed] [Google Scholar]
- 28.Tian T, Gao J, Li N, Li Y, Lu M, Li Z, Lu Z, Li J, Shen L. Circulating chromogranin A as a marker for monitoring clinical response in advanced gastroenteropancreatic neuroendocrine tumors. PLoS ONE 2016. 11 e0154679 ( 10.1371/journal.pone.0154679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ter-Minassian M, Chan JA, Hooshmand SM, Brais LK, Daskalova A, Heafield R, Buchanan L, Qian ZR, Fuchs CS, Lin X, et al Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocrine-Related Cancer 2013. 20 187–196. ( 10.1530/ERC-12-0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman SK, Maxwell JE, O’Dorisio MS, O’Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Annals of Surgical Oncology 2014. 21 2971–2980. ( 10.1245/s10434-014-3728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Yang Y, Li Z, Cheng C, Yang T, Wang C, Liu Ln, Liu S. Diagnostic value of circulating chromogranin A for neuroendocrine tumors: a systematic review and meta-analysis. PLoS ONE 2015. 10 e0124884 ( 10.1371/journal.pone.0124884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X, Zhang C, Tang M, Xu X, Liu L, Ji Y, Pan B, Lou W. The value of serum chromogranin A as predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. European Journal of Gastroenterology and Hepatology 2015. 27 227–235. ( 10.1097/MEG.0000000000000332) [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Sun Z, Bai C, Yan X, Qin R, Meng C, Ying H. Serum chromogranin A levels for the diagnosis and follow-up of well-differentiated non-functioning neuroendocrine tumors. Tumor Biology 2016. 37 2863–2869. ( 10.1007/s13277-015-4114-7) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a