Abstract
Introduction:
Sepsis is a systemic inflammatory response to suspected or confirmed infection. Clinical evaluations are essential for its early detection and treatment. Blood cultures may take as long as 2 days to yield a result and are not always reliable. However, recent studies have suggested that neutrophil CD64 expression may be a sensitive and specific alternative for the diagnosis of systemic infection.
Objective:
The objective of the study was to analyze the difference in CD64 values between subjects with systemic inflammatory response syndrome (SIRS), suspected or confirmed sepsis, who meet diagnostic criteria for SIRS upon arriving at an emergency department.
Materials and Methods:
This was a prospective observational cohort study, an accuracy study of CD64 prospectively evaluated. The sample consisted of 109 patients aged 18 years with criteria for SIRS on arrival to emergency department. CD64 expression was measured within 6 h of hospital admission and once again after 48 h.
Results:
ROC curve analysis suggested that a cutoff of 1.45 for CD64 expression could diagnose sepsis with a sensitivity of 0.85, a specificity of 0.75, an accuracy of 82.08%, a positive predictive value of 0.96, a negative predictive value of 0.38 and a positive likelihood ratio of 3.33. The area under the curve was 0.83.
Conclusion:
CD64 seems to be a useful, sensitive, and specific biomarker in discriminating between SIRS and sepsis.
Keywords: CD64 index, sepsis, systemic inflammatory response syndrome
INTRODUCTION
Sepsis, severe sepsis, and septic shock are among the most common conditions in emergency departments and Intensive Care Units (ICUs) and are associated with high mortality rates, despite antibiotic treatment and respiratory and cardiovascular support. The incidence of sepsis, severe sepsis, and septic shock has been increasing steadily. Although the decrease in sepsis-associated mortality rates due to advances in early diagnosis and treatment, the absolute number of deaths from these conditions continues to increase.[1,2,3]
Some studies have focused on the search for diagnostic biomarkers for sepsis. Recent investigations have found neutrophil CD64 expression to be a sensitive and specific marker of systemic infection and sepsis. The CD64 monoclonal antibody is a high-affinity immunoglobulin receptor found in normal monocytes as well as some resting neutrophils. CD64 expression in neutrophils is regulated in a graded fashion which parallels the extent of the inflammatory response to infection or tissue damage. CD64 expression in neutrophils has proved to be a highly sensitive (>95%) and specific marker for systemic infection and sepsis in adults, children, and neonates.[4,5,6,7,8]
The quantitative expression of CD64 in neutrophils has been found to discriminate between sepsis and nonseptic systemic inflammatory response syndrome (SIRS). Some studies have also found that CD64 expression during the first 24 h of suspected clinical infection can allow clinicians to discontinue unnecessary antimicrobial treatments with no need to wait for confirmation by microbiological testing.[9,10,11,12,13,14]
The objective of the present study was to analyze the diagnostic accuracy of the CD64 test for sepsis.
MATERIALS AND METHODS
Study design
This was a prospective observational cohort study.
The project of this study was approved by the Committee of Ethics of Clinical Hospital of Porto Alegre (HCPA), number 130438.
Patients and samples
All patients with SIRS criteria with 6 h of admission in the HCPA Emergency Room were included in the study. A free informed consent for all patients who agreed to participate was applied, signed by the patient or their families, if the patient was not able to sign. Blood samples were obtained from all patients with SIRS seen at the emergency department of the HCPA in the first 6 h from admission in the emergency room for CD64 value measurement. An additional sample was collected after 48 h of hospitalization. The criteria we used for defining SIRS, according to the American College of Chest Physicians, were the presence of at least two of the following: body temperature >38°C (fever) or <36°C (hypothermia); respiratory rate >20 breaths/min (tachypnea) or partial arterial CO2 pressure <32 mmHg; heart rate >90 bpm; significantly increased or decreased peripheral leukocyte counts (>12,000 or <4,000 cells/mm3); or presence of more than 10% (>500) immature neutrophils (bands). Sepsis was diagnosed based on the presence of confirmed or suspected infection plus SIRS.
Exclusion criteria were under 18 years old, refusal to participate, and discharge or death before 48 h of admission.
Blood cultures are not routinely collected in the emergency department of the HCPA.
Patients were divided into the categories SIRS and sepsis. Patients who met criteria for SIRS but not for suspected or confirmed sepsis were placed in the SIRS group. The sepsis group was divided into suspected versus documented sepsis. Documented sepsis group included patients with positive cultures. The suspected sepsis group included patients with no cultures performed or who produced contaminated samples, who met criteria for SIRS and were clinically suspected of infection due to fever and X-ray evidence of pneumonia, or leukocyte-and nitrite-positive urine.[15]
Sample size calculation
To detect a 50% difference in CD64 expression between all groups (SIRS no sepsis, sepsis, severe sepsis/septic shock) with a statistical power of 80% and a significance of <0.05, a total sample of 109 patients would be required. Spearman/pearson correlation coefficients were used to investigate associations between CD64 levels and outcomes such as the length of hospitalization, the duration of antibiotic therapy and mortality rates.
Instruments and data collection procedures
To evaluate the role of CD64 in monitoring sepsis, serial measurements of this biomarker were required. The first of these (T0) was obtained within 6 h of hospital admission, while the second (T1) was taken after 48 h of hospitalization.
Blood samples for CD64 assessment were drawn into tubes containing EDTA anticoagulant by adequately trained research assistants. All samples were immediately sent to the HCPA laboratory.
Neutrophil CD64 expression was analysed by flow cytometric immunophenotyping using the Leuko64 assay (Trillium Diagnostics, LLC, USA). Samples were analysed in a FACSCanto II cytometer (BD Biosciences) within 24 h (when kept at room temperature) or 48 h (2°C–8°C) of collection. As per manufacturer's instructions, 50uL of reagent A (mixture of murine monoclonal antibodies) were pipetted into a test tube, to which 50 uL of the patient's blood was added (if the number of leukocytes/uL <25,000). The contents were immediately vortexed and incubated for 10 min in the dark. Then, 1 mL of reagent B (red blood cell lysing solution) was added to each test tube, which was once again incubated for 15 min in the dark. Lastly, 5 uL of Leuko64 beads (reagent C) were added to the solution, and the contents of each tube were analyzed by cytometry using the software provided by the kit manufacturer. To ensure the quality of data analysis and quantification, CD64 expression in lymphocytes was used as a negative control (lymphocytes do not express CD64), while CD163 expression was used to exclude monocytes and also as a positive control (monocytes express both CD163 and CD64). A reference value of <1.00 ng/ml was used in this study. Neutrophils CD64 index above 1.50 ng/ml were classified as evidence of infection.
Secondary data were obtained from medical records. Patients were followed until hospital discharge or death, if occurring during hospitalization.
Statistical analysis
Continuous and normally distributed variables were described by mean and standard deviation (SD). Variables which did not show a normal distribution were described using medians and interquartile ranges. The comparison between mean values at baseline and after 48 h was performed using Wilcoxon paired-sample tests. Comparisons between all three patient groups were performed using the Kruskal-Wallis H-tests, followed by Bonferroni-corrected Mann–Whitney U-tests as a post hoc analysis. Results were considered significant when P < 0.05. Dichotomous variables were compared using Chi-square or Fischer's exact tests. Cut-offs to distinguish between SIRS and sepsis were determined using ROC curves.
RESULTS
From June to November 2014, 109 patients met inclusion criteria for the present study. Twelve (11%) had symptoms of SIRS, 45 (41.3%) had documented sepsis, and 52 (47.7%) were suspected of sepsis. The sample had a mean age of 60.5 years, with a SD of 16.27 years. Median age was 61 (interquartile range: 51–72 years). Male participants made up 52.5% (n = 57) of the sample. Over the course of the study, 18 patients (16.5%) died and 15 (13.8%) required mechanical ventilation.
The mean length of hospitalization was 14.64 (SD = 21.72) days. The median duration was 9 days, with an interquartile range of 5–16. Patients in the SIRS group (n = 12) were hospitalized for a mean of 23.83 days (SD = 54). The median length of hospitalization was 5.5 days, and the interquartile range, 3–17. The confirmed sepsis group (n = 45) was hospitalized for 14.13 days on average (SD = 12.75 days). The median duration of hospitalization was 11 days, and the interquartile range, 5–17.5. Lastly, patients with suspected sepsis (n = 45) were in the hospital for a mean of 12.96 days (SD = 13.83), and a median of 8 days (interquartile range: 5–15 days). The patient groups did not differ in terms of their clinical and anthropometric characteristics, or their risk factors for infection.
Baseline CD64 expression differed significantly between groups. The mean rank (MR) in the SIRS group was 22.79 ng/ml, while the corresponding values in the confirmed and suspected sepsis groups were 54.27 ng/ml and 63.07 ng/ml, respectively (P < 0.001). Post hoc analysis revealed a significant difference in CD64 expression between the confirmed sepsis group (MR = 32.63 ng/ml) and the SIRS group (MR = 15.38 ng/ml), (P = 0.001). These values also differed between the suspected sepsis group (MR = 36.79 ng/ml) and the SIRS group (MR = 13.92 ng/ml), (P < 0.001). However, no such differences were identified between patients with suspected (MR = 52.78 ng/ml) and confirmed sepsis (MR = 44.63 ng/ml), (P = 0.155).
Differences in CD64 expression remained significant after 48 h. The MR of the SIRS group was 30.83 ng/ml, while that of the confirmed sepsis group was 54.13 ng/ml, and that of the suspected sepsis group, 61.63 ng/ml (P < 0.01). The post hoc comparison between the MR of the SIRS group (MR = 19.83 ng/ml) and that of the confirmed sepsis group (MR = 31.44 ng/ml) yielded a P= 0.03, which meet the significance threshold of 0.05. However, the MR of the suspected sepsis group (35.96 ng/ml) did differ from that of the SIRS group (17.5 ng/ml) at P = 0.002. Patients with suspected (MR = 51.87 ng/ml) and confirmed sepsis (MR = 45.69 ng/ml) did not differ on this variable (P = 0.281). The baseline CD64 level in the first sample for SIRS, confirmed sepsis and suspected sepsis is shown in Figure 1. The baseline CD64 level in the second sample for SIRS, confirmed sepsis and suspected sepsis is shown in Figure 2.
Figure 1.
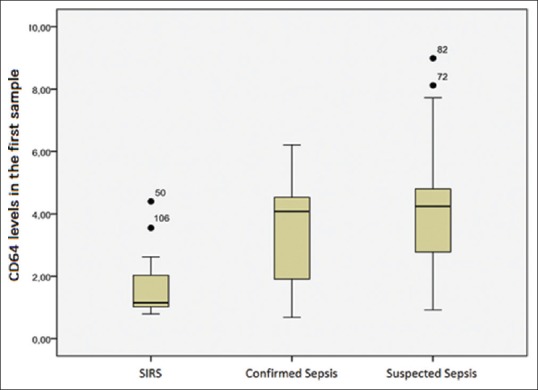
CD64 levels in the first sample for systemic inflammatory response syndrome, confirmed sepsis and suspected sepsis
Figure 2.
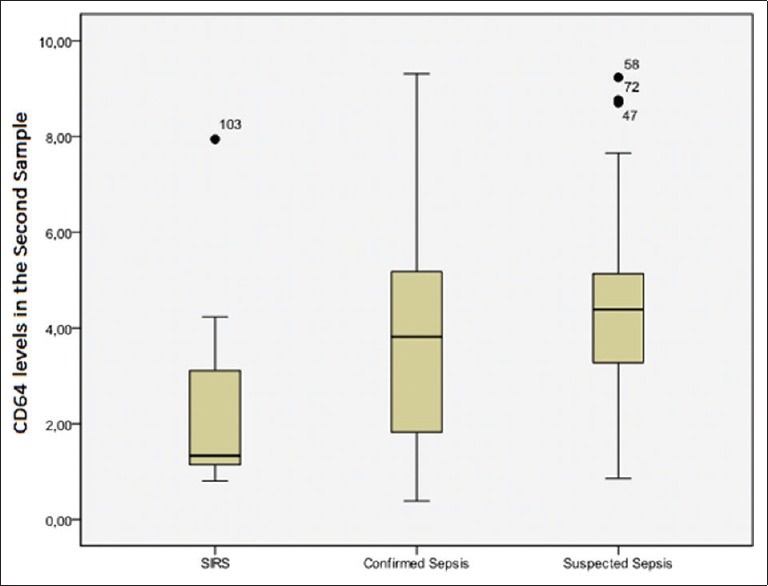
CD64 levels in the second sample for systemic inflammatory response syndrome, confirmed sepsis and suspected sepsis
Neutrophil CD64 expression also changed significantly from baseline to 48 h after hospital admission. Mean expression at baseline was 3.52 ng/mL with a SD of 1.88, a median value of 4.01 ng/mL and an interquartile range of 1.5–4.6. After 48 h, these values increased to 3.83 ng/mL (SD = 2.17) and 4.14 ng/mL (interquartile range: 1.83–5.05). The difference between these values was significant at P = 0.022.
At baseline, the confirmed and suspected sepsis groups combined (n = 97) had a mean CD64 index of 3.74 (SD = 1.83) and a median of 4.17 ng/mL (interquartile range: 2.1–4.69). After 48 h, the median increased to 4.25 ng/mL (2.05–5.22), while the mean rose to 4.02 ng/mL (SD = 2.21). These values differed at P = 0.025.
In the SIRS group (n = 12), median CD64 expression at baseline was 1.5 ng/mL (interquartile range: 1.02–2.32), while that observed at T1 was 1.32 ng/mL (interquartile range: 1.10–3.46). The difference between these values was not significant (P = 0.239).
The Spearman correlation coefficient between CD64 expression at baseline and T1 was rho = 0.85, P < 0.001.
Based on the results of the ROC curve analysis, the best cutoff for CD64 expression for sepsis diagnosis was 1.45 ng/mL [Figure 3]. This value had a sensitivity of 0.85, a specificity of 0.75, an accuracy of 82.08%, a positive predictive value of 0.96, a negative predictive value of 0.38 and a positive likelihood ratio of 3.34. The area under the curve was 0.832.
Figure 3.
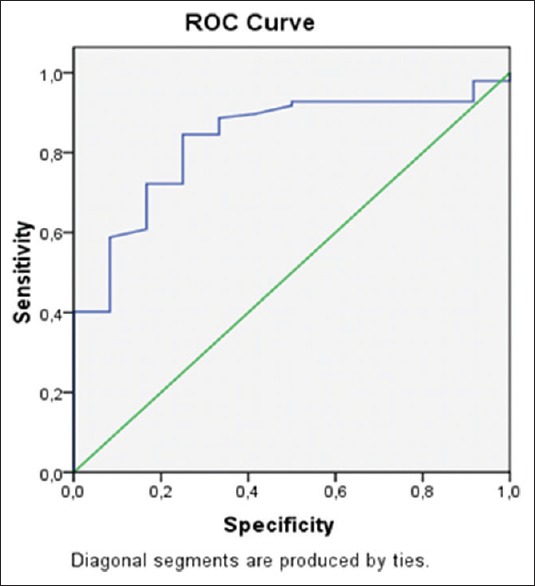
The receiver operating characteristic curve
DISCUSSION
CD64 expression was able to differentiate sepsis from SIRS with 82.1% accuracy early on admission to the emergency department.
Similar results were found by Ulla et al. in a study of sepsis biomarkers, with samples collected during the first assessment (T0), as well as 24 (T1) and 72 (T2) hours after admission. These authors found significantly higher values of biomarkers at T0 as compared to T1 and T2.[16] Fourteen other patients in the suspected sepsis group had no positive cultures and were highly suspected of infection. In these cases, antibiotic treatment was initiated before cultures were collected. Twelve of these patients had blood cultures, eight had urine cultures, one had a negative CSF culture, and another had a negative sputum smear. The contaminated samples included 17 sputum smears, six urine samples and only one blood culture. All patients in the severe sepsis and septic shock group had positive cultures.
According to Carvalho and Trotta Ede, despite all efforts to isolate microorganisms from blood cultures, these tend to be positive in 34% of patients, with estimates ranging from 9% to 64%.[17]
It is possible to observe the importance of the CD64 in the diagnosis of sepsis in different studies. Gerdes described blood cultures as the gold-standard for the diagnosis of sepsis. However, its positivity rates vary widely, and range from 30% to 87%.[18]
Therefore, to facilitate and possibly accelerate the diagnosis of sepsis, clinicians must complement their examination with additional diagnostic tests.
The recent interest in using neutrophil CD64 expression as a biomarker for sepsis may be attributable to its high clinical applicability, and to the importance of early diagnosis and treatment for the prognosis of this condition. Our findings regarding the clinical utility of CD64 expression in sepsis patients corroborate those of Icardi et al., who collected blood cultures from 109 patients over the course of 2 months, and found that a CD64 index of 1.19 could predict a diagnosis of infection with a sensitivity of 94.6%, a specificity of 88.7%, a positive predictive value of 89.8%, and a negative predictive value of 94%. Based on these findings, the authors concluded that, in addition to being easily measured, CD64 levels may be a useful and inexpensive tool to improve the diagnosis and treatment of patients with bacterial infection.[8]
Elevated levels of CD64 expression in patients with sepsis as compared to those with SIRS have also been found by other studies in the literature. In a review of the literature on the use of neutrophil CD64 expression as a biomarker for sepsis, Hoffmann analysed eight studies with a combined sample of 907 patients. The author found that CD64 could detect infection in adult patients with a sensitivity of 88.3% (95% confidence interval: 78.1–94.1%) and a specificity of 87.6% (71.8–95.2%). All included studies reviewed produced similar findings to those of the present study.[11]
Lewis et al. evaluated serum CD64 expression in patients with sepsis, and found a marked increase in the percentage of CD64-bearing neutrophils in these individuals (69%) as opposed to healthy subjects (17%), (P < 0.001). Interestingly, this finding was specific to patients with sepsis, and was not observed in patients with community-acquired infections who did not develop the condition or in those with acute or chronic inflammation with no evidence of infection.[19]
Gerrits et al. compared the CD64 index of patients with sepsis and SIRS admitted to an ICU with that of a control group of outpatients. The authors determined the CD64 index in residual EDTA blood samples from 25 patients with sepsis, 19 subjects with SIRS, and 24 outpatient controls. Neutrophil and eosinophil granulocyte counts were also performed, while CRP and erythrocyte sedimentation rates were measured. The analysis revealed a higher CD64 index in patients with sepsis as compared to those with SIRS and outpatient controls (P < 0.0001). The CD64 index had higher sensitivity and specificity than other routine tests, such as CRP and white blood cell counts.[20]
Dimoula et al. measured neutrophil CD64 expression in patients with sepsis upon hospital admission and daily until discharge or death. CRP levels were also measured on a routine basis. The authors found that CD64 expression could be used to detect sepsis with a sensitivity of 89% (81%–94%) and a specificity of 87% (83%–90%). The combination of CRP and CD64 levels could detect sepsis with a positive predictive value of 92% and a negative predictive value of 99%. Additionally, the authors found that CD64 levels tended to decrease in patients receiving adequate antibiotic treatment, but remained persistently high in subjects receiving inadequate antibiotics. Based on these findings, the authors concluded that the combination of CRP and CD64 levels may contribute significantly to the diagnosis of sepsis.[21]
This study seems to have a good external validation. With the limitation of current diagnostic tests for sepsis, together with the clinical context severity of this disease, requiring early treatment for the best outcome for the patient, the great challenge studies with biomarkers in sepsis seems to be how to avoid selection bias in the definition of suspected sepsis. We observed that the allocation of patients in the suspected sepsis group was appropriate, since this group behaved similar to documented sepsis group and differently of SIRS group. This fact indicates the importance of considering that tests routinely used for diagnosis of sepsis may be negative and may overlook many patients with this clinical condition.
One limitation of the present study was the collection of patient data from medical records. In some cases, severe sepsis and septic shock were diagnosed based on blood pressure measurements after fluid administration, as registered on medical records. In a few records, this information was described in the section reserved for the doctor's diagnostic impression, which led us to combine patients with both of these conditions into a single “sepsis” group. Although the fact our sample had a larger amount of suspected lower airways infections than urinary tract infections, our study found more germs isolated in urine culture than in sputum culture. However, in our department, asking for sputum culture is less frequent than urine culture. This limitation may be due to the fact that in case of strong suspicion of airways infection by chest radiography, with strongly suggestive of infectious cause consolidations complementing the clinical setting of the patient, sputum culture is not done routinely, in order to prioritize an early treatment.
CONCLUSION
The present findings are in agreement with the existing literature, and suggest that CD64 may be a useful biomarker for distinguishing between SIRS and sepsis, whether confirmed or suspected, with adequate sensitivity and specificity. CD64 measurements are comparable in speed to a hemogram, and may therefore contribute significantly to the early diagnosis and treatment of sepsis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Martin G. Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–6. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 4.Venet F, Lepape A, Monneret G. Clinical review: Flow cytometry perspectives in the ICU-From diagnosis of infection to monitoring of injury-induced immune dysfunctions. Crit Care. 2011;15:231. doi: 10.1186/cc10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 6.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130:654–61. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: Neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 8.Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol. 2009;47:3914–9. doi: 10.1128/JCM.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsh M, Mahamid E, Bashenko Y, Hirsh I, Krausz MM. Overexpression of the high-affinity Fcgamma receptor (CD64) is associated with leukocyte dysfunction in sepsis. Shock. 2001;16:102–8. doi: 10.1097/00024382-200116020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Groselj-Grenc M, Ihan A, Derganc M. Neutrophil and monocyte CD64 and CD163 expression in critically ill neonates and children with sepsis: Comparison of fluorescence intensities and calculated indexes. Mediators Inflamm 2008. 2008:202646. doi: 10.1155/2008/202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann JJ. Neutrophil CD64: A diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47:903–16. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez MC, Alvarez Perez SJ, Castro M. Epidemiology, diagnosis, imunologic biomarkers and sepsis prognosis [thesis] Rio de Janeiro: Faculdade de Ciências Médicas da Universidade do Estado do Rio de Janeiro. 2009;Vol. xii:106 f. [Google Scholar]
- 13.Mokuda S, Doi O, Takasugi K. Simultaneous quantitative analysis of the expression of CD64 and CD35 on neutrophils as markers to differentiate between bacterial and viral infections in patients with rheumatoid arthritis. Mod Rheumatol. 2012;22:750–7. doi: 10.1007/s10165-011-0587-4. [DOI] [PubMed] [Google Scholar]
- 14.Rudensky B, Sirota G, Erlichman M, Yinnon AM, Schlesinger Y. Neutrophil CD64 expression as a diagnostic marker of bacterial infection in febrile children presenting to a hospital emergency department. Pediatr Emerg Care. 2008;24:745–8. doi: 10.1097/PEC.0b013e31818c2679. [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 16.Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study. Crit Care. 2013;17:R168. doi: 10.1186/cc12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho PR, Trotta Ede A. Advances in sepsis diagnosis and treatment. J Pediatr (Rio J) 2003;79(Suppl 2):S195–204. doi: 10.2223/jped.1096. [DOI] [PubMed] [Google Scholar]
- 18.Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am. 2004;51:939. doi: 10.1016/j.pcl.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SM, Treacher DF, Bergmeier L, Brain SD, Chambers DJ, Pearson JD, et al. Plasma from patients with sepsis up-regulates the expression of CD49d and CD64 on blood neutrophils. Am J Respir Cell Mol Biol. 2009;40:724–32. doi: 10.1165/rcmb.2008-0252OC. [DOI] [PubMed] [Google Scholar]
- 20.Gerrits JH, McLaughlin PM, Nienhuis BN, Smit JW, Loef B. Polymorphic mononuclear neutrophils CD64 index for diagnosis of sepsis in postoperative surgical patients and critically ill patients. Clin Chem Lab Med. 2013;51:897–905. doi: 10.1515/cclm-2012-0279. [DOI] [PubMed] [Google Scholar]
- 21.Dimoula A, Pradier O, Kassengera Z, Dalcomune D, Turkan H, Vincent JL. Serial determinations of neutrophil CD64 expression for the diagnosis and monitoring of sepsis in critically ill patients. Clin Infect Dis. 2014;58:820–9. doi: 10.1093/cid/cit936. [DOI] [PubMed] [Google Scholar]


