Abstract
Objective:
Cuscuta campestris or common dodder is a holoparasitic plant that has been valorized for treatment of liver injury and cancer prevention in traditional medicine. Recently, extract of C. campestris had shown moderate antimicrobial properties and cytotoxic effects. In this study, we examined the level of cellular oxidants, cytotoxicity, apoptosis and differentiation induced by hydroalcoholic extract of C. campestris (CCE) (12.5-200 µg/ml), as well as arsenic trioxide (As2O3, 50 µM), in human leukemic (HL60 and NB4) and normal polymorph nuclear cells after 72 hr treatment.
Materials and Methods:
Resazurin assay was used to determine cell viability following treatment with C. campestris. Intracellular reactive oxygen species (ROS) and apoptotic cells were measured by fluorimetry using carboxy 2′, 7′-dichlorofluorescein diacetate and propidium iodide (PI), as staining reagents, respectively. The differentiation of leukemic cells was evaluated by Giemsa staining and nitro blue tetrazolium (NBT) reduction.
Results:
C. campestris inhibited cell viability with IC50 values of 23.9 µg/ml for HL60 and 60.3 µg/ml for NB4 cells after 72 hr treatment. ROS formation was also concentration-dependently increased following treatment with C. campestris. In addition, the number of apoptotic cells significantly increased to 88.4% and 62.3% in CCE (200 µg/ml)-treated HL60 and NB4 cells, respectively, which was higher than that of As2O3 (50 µM)-treated leukemic cells (p<0.001). Nonetheless, C. campestris did not induce differentiation of leukemic cells towards granulocytic pattern.
Conclusion:
The present study demonstrated that C. campestris induced apoptosis through ROS production without having differential effect on leukemic cells, in concentration- and time-dependent manners. Understanding of precise signaling pathway by which C. campestris induce apoptosis, needs further research.
Key Words: Cuscuta campestris, Leukemia, Apoptosis, Differentiation, ROS
Introduction
As hematologic malignancy, acute promyelocytic leukemia (APL) exhibits myeloid differentiation failure (Rodak et al., 2013 ▶). Arsenic trioxide (As2O3) is used clinically for treatment of APL; its anti-cancer mechanism includes induction of apoptosis, inhibition of growth, and promotion of differentiation. High doses of As2O3 cause various side effects and toxicity; however, substitution or combination of this drug with other anti-cancer compounds can reduce the required dose and side effects of As2O3 (Rodak et al., 2013 ▶). Therefore, search for novel anticancer agents is currently receiving great attention. Researchers believe that dietary phytochemicals may influence chemotherapy treatment and help to cure cancer (Alinejad et al., 2013 ▶). Different natural compounds can improve the efficiency of chemotherapeutic agents, decrease the resistance to these drugs, and alleviate their adverse effects (Sak, 2012 ▶). As a result, researchers attempt to employ different medicinal herbs and their effective phytochemicals in in-vitro and in-vivo models of cancer.
Cuscuta spp., also known as dodder is one of the medicinal herbs that belongs to the Convolvulaceae family and there are over 150 species of dodders in the world (McNeal et al., 2007 ▶). C. campestris or common dodder is a holoparasitic plant that been valorized for treatment of liver injury and cancer prevention in traditional medicine. C. campestris has mild laxative and diuretic properties and can be used for treatment of sciatica, scurvy and scrofula derma (Holm, 1997 ▶). Pharmacological properties of this herb include anti-seizure, anti-microbial and cytotoxic effects against cancer cell lines. The active compounds of Cuscuta species include flavonoids, lignans, quinic acid and polysaccharides (Mehrabani et al., 2007 ▶, Biswas et al., 2012 ▶).
In the present study, we investigated the cytotoxic and differentiation effects of C. campestris on human leukemic (HL60 and NB4) cells, compared to As2O3 (as positive control).
Materials and Methods
Preparation of the hydroalcoholic extract of C. campestris (CCE)
The aerial parts of C. campestris were prepared in the early autumn 2015 from Razavi Khorasan province (the Northeast of Iran) and authenticated by the herbarium of Ferdowsi University of Mashhad (No. 38195 FUMH). The aerial parts of the plant were cleaned and grounded to fine powder using a blender. Then, macerated extract was prepared as previously described (Shafiee-Nick et al., 2012 ▶). Briefly, 150 g of the powder was suspended in 500 ml of 56% ethanol and incubated for 72 hr at 37°C. The hydro-alcoholic extract was then dried on a water bath and the residue was dissolved in distilled water (The yield of extract was 14%).
Cell lines and reagents
HL60 and NB4 cells were obtained from the cell bank of Pasteur Institute (Tehran, Iran). Resazurin reagent [300 μM resazurin, 78 μM methylene blue, 1 mM potassium hexacyanoferrate III and 1 mM potassium hexacyanoferrate II], 2,7-dichlorofluorescin diacetate (DCFH-DA), propidium iodide (PI), nitroblue tetrazolium (NBT), phorbol-myristate-acetate (PMA) and arsenic trioxide (stock concentration 1 mM) were purchased from Sigma (St Louis, MO). High-glucose Roswell Park Memorial Institute medium (RPMI1640), penicillin-streptomycin and fetal bovine serum (FBS) were obtained from Gibco BRL Life Technologies (Grand Island, NY). Giemsa stain was purchased from MERK (Darmstadt, Germany).
Human normal cells isolation and culture
Polymorphonuclear cells (PMNs) were obtained from healthy volunteers under sterile conditions by two consecutive Ficoll-Hypaque (Pharmacia, Country) density gradient centrifugations (Cassatella et al., 1988 ▶). PMNs were purified to more than 97%, as judged by morphological examination of Wright-stained smears. After washing with sterile phosphate-buffered saline (PBS), the cells were resuspended in RPMI medium. HL60, NB4 and PMN cells were maintained in RPMI medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin at 37◦ C in a humidified atmosphere (90%) containing 5% CO2. Next, the cells were incubated with various concentrations of C. campestris extract (12.5-200 µg/ml) and As2O3 (50 µM) for 24, 48 and 72 hr. For each concentration and time course, there was a control sample which remained untreated and received an equal volume of medium. All different treatments were carried out in triplicate.
Cell viability
The cell viability was determined by resazurin reagent. For this purpose, 100 µl of the NB4, HL60 and PMN cells containing 1×105 cells were added to each well in 96-well tissue culture plates, then cells were treated with C. campestris extract (12.5-200 µg/ml) and As2O3 (50 µM) for 24, 48 and 72 hr. Then, 20 µl of resazurin reagent was added to each well and the plates were incubated for 4 hr. The fluorescence intensity of the product resorufin which is proportional to the number of viable cells per well, was measured by a fluorescence Victor X5 2030 Multilabel Plate Reader (PerkinElmer, Shelton, Connecticut) with excitation at 530 nm and emission at 590 nm (Mashkani et al., 2016 ▶).
Measurement of reactive oxygen species
In brief, HL60 and NB4 cells (105/well) were incubated with DCF-DA reagent (20 µM) for 30 min in the dark and then treated with C. campestris extract (12.5-200 µg/ml) or As2O3 (50 µM) for 2 hr. After that, the cells were transferred to a Falcon polystyrene tube. After washing twice with PBS, The DCF fluorescence intensity was detected using a fluorescence Victor X5 2030 Multilabel Plate Reader (PerkinElmer, Shelton, Connecticut) with excitation wavelength set at 485 nm and emission wavelength set at 530 nm (Hosseini and Rajabian, 2016 ▶).
Apoptosis
Apoptotic cells were detected using PI staining of the treated cells followed by flow cytometry to detect the so-called sub-G1 peak. Briefly, HL60 and NB4 cells were treated with C. campestris extract (50-200 µg/ml) or As2O3 (50 µM) for 48 hr. The cells were then incubated at 4 °C overnight in the dark with 500 µl of a PI hypotonic buffer (50 µg/ml). Samples were then analyzed by flow cytometry. A total of 10,000 events per sample were obtained and the data was analyzed using WinMDI software (Sadeghnia et al., 2014 ▶).
Morphological and differentiation assay
Morphological characterization and differentiation assay were done by standard techniques using Giemsa-stained smears and was performed NBT reduction tests, respectively. Cells (1 × 106 cells/ml in 6-well plates) were treated with C. campestris extract (12.5 and 25 µg/ml) or As2O3 (0.5 µM) (Yan et al., 2005 ▶) for 3 days and then, washed twice with PBS and the pellets were resuspended in equal volumes of 0.2% NBT dissolved in Dulbecco’s phosphate-buffered saline containing 200 ng/ml of freshly diluted phorbol myristate acetate (PMA). After 25 min incubation at 37°C in the dark, slides were prepared and stained with Giemsa and 300 cells were scored for the presence of blue-black formazan granules (Gupta et al., 2016 ▶).
Statistical analysis
Statistical analysis were done by Graph Pad PRISM software (version 6, Graph Pad Software, Inc., San Diego, CA) using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison post-tests to evaluate differences among several treatment groups. p-values less than 0.05 were considered as statistically significant. The values were presented as the mean ± SEM of three independent experiments.
Results
C. campestris decreased cell viability in a concentration-dependent manner
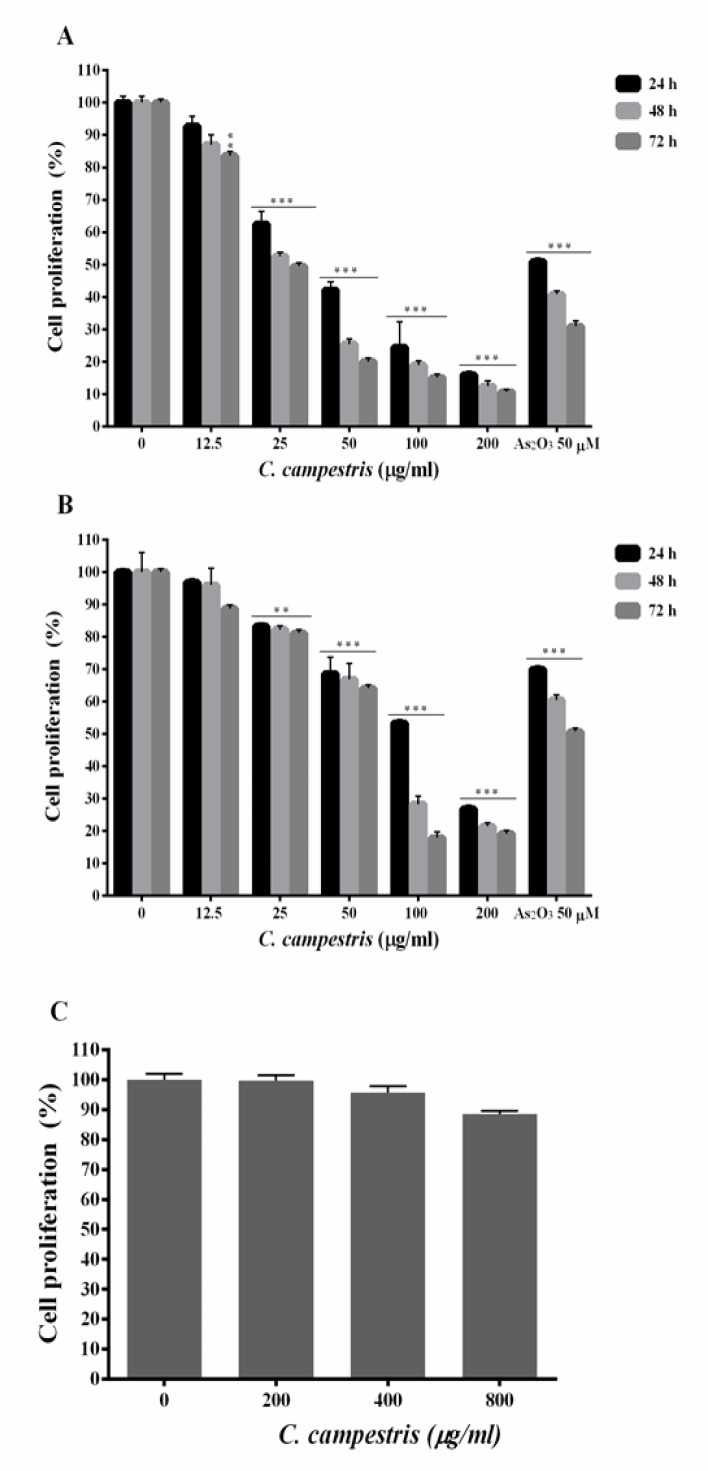
To evaluate the toxic effect of C. campestris, HL60 and NB4 cells were incubated with increasing concentrations of C. campestris (12.5-200 µg/ml) and As2O3 (50 µM), as a positive control, for up to 72 hr and the cell viability was measured by resazurin reagent (Figure 1). The normal PMN cells were incubated with C. campestris (200-800 µg/ml) for 24 hr. As compared to PMN cells, C. campestris-induced toxicity was significantly higher in the HL60 and NB4 cells. The results are graphically shown in Figure 1 and summarized in Table 1. Treatment of HL60 cells with relatively high concentration (>50 µg/ml) of C. campestris for 24, 48 and 72 hr significantly reduced cell viability to 42.1 ± 2.5, 25.4 ± 1.6 and 20.0 ± 1.0%, at 100 µg/ml, 24.4 ± 7.9, 18.9 ± 1.2 and 15.1 ± 1.0%, at 200 µg/ml, and 16.0 ± 1.0, 12.4 ± 1.6 and 10.7 ± 0.6% against 50.9 ± 1.0, 40.8 ± 1.0 and 30.9 ± 1.6% in As2O3-treated cells (p<0.001), respectively. While in NB4 cells, C. campestris at concentrations >100 µg/ml resulted in more significant reductions in cell viability compared to As2O3, at 100 µg/ml 53.3±1.0, 28.3±2.4 and 17.9±1.7%, at 200 µg/ml 26.8±1.1, 21.4±1.2 and 19.2±1.1% against 70.0±1.0, 60.5±1.6 and 50.7±1.0% in As2O3 group (p<0.001). In contrast, the extract did not exhibit any cytotoxic effects, at concentrations up to 800 µg/ml, in PMN cells after 24 hr treatment (Figure. 1).
Figure 1.
Anti-proliferative effect of C. campestris on leukemic cells. (A) HL60 and (B) NB4 cells were treated with different concentrations of C. campestris (12.5-200 µg/ml) or As2O3 (50 µM) for 24-72 hr. (C) Normal polymorphnuclear cells treated with C. campestris (200-800 µg/ml) for 24 hr. The percentage of cell viability (quantitated by resazurin assay) was normalized against the negative controls for each cell type. Data are expressed as the mean ± SEM of three separate experiments. **p<0.01 and ***p<0.001 as compared to control value
Table 1.
IC50 (concentration required for 50% inhibition) values of different treatments of C. campestris against NB4 and HL60 cells at 24-72 hr
| Cell lines |
HL60 cells
|
NB4 cells
|
||||
|---|---|---|---|---|---|---|
| Treatment | 24 hr | 48 hr | 72 hr | 24 hr | 48 hr | 72 hr |
| C. campestris (µg/ml) | 43.09±0.05 | 29.10±0.06 | 24.85±0.04 | 105.7±0.03 | 73.63±0.06 | 60.36±05 |
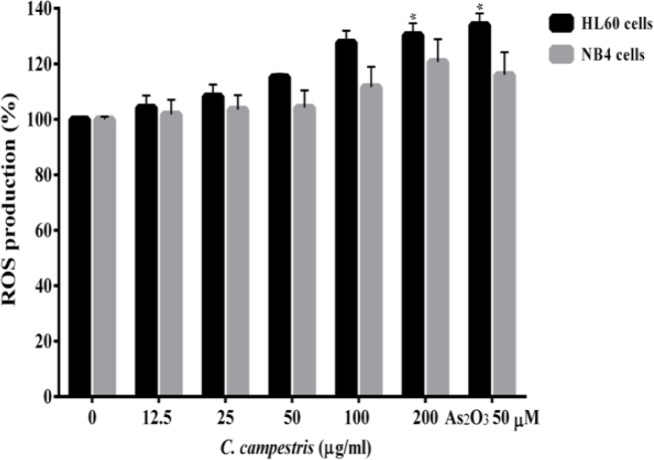
C. campestris concentration-dependently increased ROS formation
Our findings showed a regular and statistically significant time and concentration-dependent increase in ROS generation in HL60 and NB4 cells, 2 hr after treatment with C. campestris compared to control group (Figure 2). As compared with control HL60 cells, 200 µg/ml C. campestris significantly increased (p<0.001) ROS formation (130 ± 4.1%, comparable to that of As2O3 group; 134 ± 3.5%). There was also a significant increase (p<0.001) in ROS level in NB4 cells 2 hr after treatment with 200 µg/ml of C. campestris compared to control group (120 ± 8.1% comparable to that of As2O3 group; 116 ± 7.1%).
Figure 2.
Effect of C. campestris on intracellular reactive oxygen species (ROS) in leukemic cells. NB4 and HL60 cells were treated with different concentrations of C. campestris (12.5-200 µg/ml) or As2O3 (50 µM) for 2 hr. Data are expressed as the mean ± SEM of three separate experiments. *p<0.05 as compared to control value
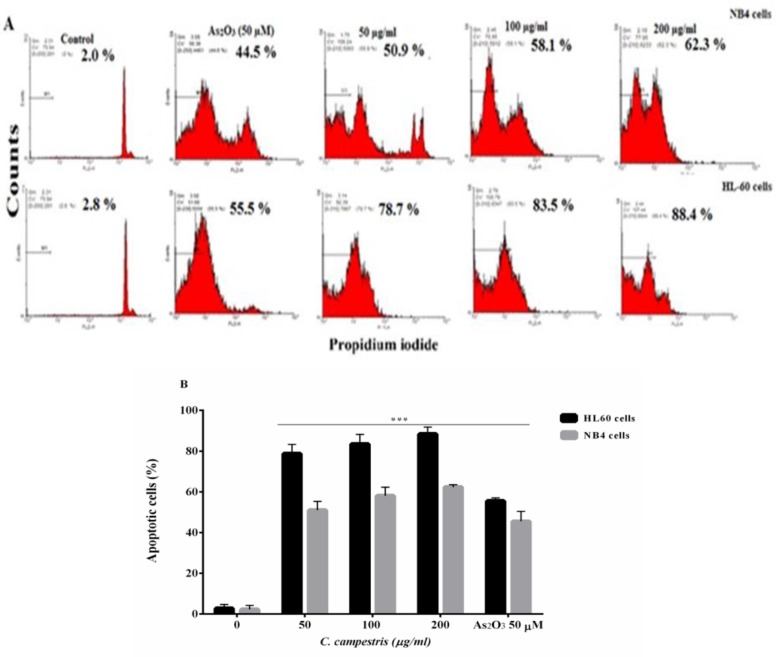
C. campestris concentration-dependently induced apoptotic cell death
Figure 3 shows that C. campestris at high concentration induces apoptosis in NB4 and HL60 cells, significantly. After 48 hr of treatment, C. campestris-treated HL60 cells showed 83.5 ± 4.7% (100 μg/ml) and 88.4 ± 3.4% (200 μg/ml) apoptosis rate, comparable to As2O3 (50 µM)-treated cells (55.5±1.5%) (p<0.001). Also, 62.3 ± 1.1% of C. campestris-treated NB4 cells undergone apoptosis at 200 μg/ml as compared to 45.5 ± 4.9% in As2O3 group (p<0.001). These results showed that the number of apoptotic cells increased with increasing concentrations, as compared to control cells (p<0.001, Figure 3).
Figure 3.
Apoptotic cell death induced by C. campestris in leukemic cells. (A) NB4 and HL60 cells were incubated with different concentrations of C. campestris (12.5-200 µg/ml) or As2O3 (50 µM) for 48 hr. Apoptosis was assayed by PI staining and analyzed by flow cytometry. (B) Apoptosis rate shown as bars. The data shown are the means ± SEM of three independent experiments. ***p<0.001 as compared to control value
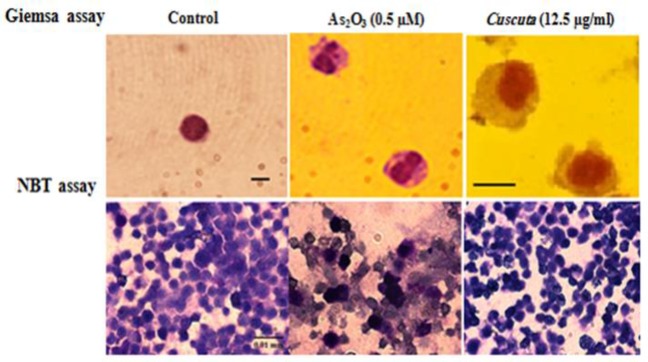
C. campestris did not induce differentiation of leukemic cells into granulocyte pattern
The Giemsa and NBT assays showed that C. campestris did not influence the maturation of leukemic cells toward neutrophils. In morphological Giemsa assay, cells showed promyelocytes characteristics with cytoplasmic granules, similar to control cells. In functional NBT assay, cells had no intracellular blue-black formazan deposits (Figure. 4).
Figure 4.
Effect of C. campestris on morphological and functional properties of granulocyte in leukemic cells. NB4 cells were treated with C. campestris (12.5 µM) or As2O3 (0.5 µM) for 72 hr. Morphologic and functional properties of granulocytes were determined by Giemsa and NBT assays, respectively. In Giemsa-stained slides, As2O3-treated cells (as positive control) showed polymorphonuclear morphology of granulocyte and C. campestris-treated cells showed promyelocytes characteristics similar to control. On NBT slides, As2O3-treated cells (as positive control) showed intracellular blue-black formazan deposits while C. campestris-treated cells showed no intracellular blue-black formazan deposits (bar represents 0.01 mm
Discussion
This study is the first to investigate the antineoplastic and differentiating effects of C. campestris in leukemic cells. We reported that C. campestris exhibits significant toxicity in the cancer cells, at tested concentrations. The cytotoxic effects of C. campestris were more pronounced against the neoplastic cells compared to human PMN cells. Our study also showed that C. campestris induces concentration-dependent apoptosis by increasing ROS generation. It is noteworthy to mention that these effects are comparable to standard anti-leukemic drug, As2O3. The results also showed that the rate of apoptosis induced by C. campestris in HL-60 (AML M2) cells was higher than that of NB4 (AML M3) cells. These may be related to inherent metabolic properties of the cancer cells. Previous studies indicated that NB4 cells show highly ‘‘glycolytic’’ properties (Suganuma et al., 2010 ▶, Estañ et al., 2014 ▶). Recent studies have also shown that some species of Cuscuta such as C. reflexa exhibit anti-tumor properties and are used for the treatment of prostate cancer (Suresh et al., 2011 ▶). It was shown that C. epithyum significantly reduced the viability of Hela, HT-29 and MDA-MB-468 cells (Jafarian et al., 2014 ▶). Previous studies also assessed the effects of different species of Cuscuta on various cell lines including lymphoblastic-like tumor cell line (HL60), epidermoid carcinoma cell line (MCF-7), human breast cancer cell line (T47D), human Caucasian acute lymphoblastic leukemia (CCRF-CEM) and Jurkat (JM) cell lines (Sepehr et al., 2011 ▶, Ghazanfari et al., 2013 ▶).
The phytochemical studies have shown that active compounds of Cuscuta species are flavonoids, lignans, quinic acid and polysaccharides. Flavonoids are considered effective antioxidants (Sepehr et al., 2011 ▶). Also, several studies have demonstrated that the pharmacological effects of C. chinensis and C. epithymum can be attributed to their main constituents including flavonoids, saccharids, alkaloids, lignans, saponins, and resin glycosides. Also, it has been shown that many flavonoids significantly decreased cell viability via enhancement of caspase activity (Sepehr et al., 2011 ▶). In the same way, several steroidal saponins possess marked cancer chemopreventive properties (Candra et al., 2002 ▶, Kajimoto et al., 2002 ▶, Yokosuka et al., 2002 ▶, Choi et al., 2003 ▶, Hu and Yao, 2003 ▶, Shen et al., 2003 ▶). The triterpene saponins, jenisseensosides A-D were found to increase the accumulation and cytotoxicity of the anticancer agent, cisplatin in human colon tumor cells (Gaidi et al., 2002 ▶).
In conclusion, the present study demonstrated that C. campestris inhibited the proliferation without affecting the differentiation of leukemic cells. The marked difference in cytotoxicity between cancer and normal cells suggests that C. campestris could be considered as a novel alternative to cancer therapeutics. The IC50 values reported in our study may become considerably lower if the isolated active compounds are used. C. campestris not only increased levels of ROS, but also induced concomitant increase of apoptosis. The precise signaling pathway via which C. campestris induce apoptosis needs further research.
Acknowledgment
This work was supported by a grant from the Vice Chancellor for Research and Technology of Mashhad University of Medical Sciences, Mashhad, Iran. The authors appreciate this financial support.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Alinejad B, Ghorbani A, Sadeghnia HR. Effects of combinations of curcumin, linalool, rutin, safranal, and thymoquinone on glucose/serum deprivation-induced cell death. Avicenna J Phytomed. 2013;3:321–328. [PMC free article] [PubMed] [Google Scholar]
- Benner SE, Hong WK. Clinical chemoprevention: developing a cancer prevention strategy. J Natl Cancer Inst. 1993;85:1446–1447. doi: 10.1093/jnci/85.18.1446. [DOI] [PubMed] [Google Scholar]
- Biswas S, Chowdhury A, Das J, Karamkar U, Raihan S, Das A. Phytochemical investigation and chromatographic evaluation with antimicrobial and cytotoxic potential of Cuscuta epithymum. Int J Pharm. 2012;8:422–427. [Google Scholar]
- Candra E, Matsunaga K, Fujiwara H, Mimaki Y, Kuroda M, Sashida Y, Ohizumi Y. Potent apoptotic effects of saponins from Liliaceae plants in L1210 cells. J Pharm Pharmacol. 2002;54:257–262. doi: 10.1211/0022357021778286. [DOI] [PubMed] [Google Scholar]
- Cassatella M, Cappelli R, Della Bianca V, Grzeskowiak M, Dusi S, Berton G. Interferon-gamma activates human neutrophil oxygen metabolism and exocytosis. Immunology. 1988;63:499. [PMC free article] [PubMed] [Google Scholar]
- Choi HH, Jong H-S, Park J-H, Choi S, Lee JW, Kim T-Y, Otsuki T, Namba M, Bang Y-J. A novel ginseng saponin metabolite induces apoptosis and down-regulates fibroblast growth factor receptor 3 in myeloma cells. Int J Oncol. 2003;23:1087–1094. [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Estañ MC, Calviño E, Calvo S, Guillén-Guío B, del Carmen Boyano-Adanez M, de Blas E, Rial E, Aller P. Apoptotic efficacy of etomoxir in human acute myeloid leukemia cells Cooperation with arsenic trioxide and glycolytic inhibitors, and regulation by oxidative stress and protein kinase activities. PLoS One. 2014;9:e115250. doi: 10.1371/journal.pone.0115250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidi G, Correia M, Chauffert B, Beltramo J-L, Wagner H, Lacaille-Dubois M-A. Saponins-mediated potentiation of cisplatin accumulation and cytotoxicity in human colon cancer cells. Planta Med. 2002;68:70–72. doi: 10.1055/s-2002-19873. [DOI] [PubMed] [Google Scholar]
- Ghazanfari T, Naseri M, Shams J, Rahmati B. Cytotoxic effects of Cuscuta extract on human cancer cell lines. Food Agric Immunol. 2013;24:87–94. [Google Scholar]
- Gupta K, Stefan T, Ignatz-Hoover J, Moreton S, Parizher G, Saunthararajah Y, Wald DN. GSK-3 inhibition sensitizes acute myeloid leukemia cells to 1, 25D-mediated differentiation. Cancer Res. 2016;76:2743–2753. doi: 10.1158/0008-5472.CAN-15-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L. John Wiley & Sons; 1997. [Google Scholar]
- Hosseini A, Rajabian A. Protective effect of Rheum turkestanikum root against doxorubicin-induced toxicity in H9c2 cells. Rev Bras Farmacogn. 2016;26:347–351. [Google Scholar]
- Hu K, Yao X. The cytotoxicity of methyl protoneogracillin (NSC‐698793) and gracillin (NSC‐698787) two steroidal saponins from the rhizomes of Dioscorea collettii var hypoglauca against human cancer cells in vitro. Phytother Res. 2003;17:620–626. doi: 10.1002/ptr.1211. [DOI] [PubMed] [Google Scholar]
- Jafarian A, Ghannadi A, Mohebi B. Cytotoxic effects of chloroform and hydroalcoholic extracts of aerial parts of Cuscuta chinensis and Cuscuta epithymum on Hela, HT29 and MDA-MB-468 tumor cells. Res Pharm Sci. 2014;9:115. [PMC free article] [PubMed] [Google Scholar]
- Kajimoto S, Takanashi N, Kajimoto T, Xu M, Cao J, Masuda Y, Aiuchi T, Nakajo S, Ida Y, Nakaya K. Sophoranone, extracted from a traditional Chinese medicine Shan Dou Gen, induces apoptosis in human leukemia U937 cells via formation of reactive oxygen species and opening of mitochondrial permeability transition pores. Int J Cancer. 2002;99:879–890. doi: 10.1002/ijc.10414. [DOI] [PubMed] [Google Scholar]
- Mashkani B, Tanipour MH, Saadatmandzadeh M, Ashman LK, Griffith R. FMS-like tyrosine kinase 3 (FLT3) inhibitors: Molecular docking and experimental studies. Eur J Pharmacol. 2016;776:156–166. doi: 10.1016/j.ejphar.2016.02.048. [DOI] [PubMed] [Google Scholar]
- McNeal JR, Arumugunathan K, Kuehl JV, Boore JL. Systematics and plastid genome evolution of the cryptically photosynthetic parasitic plant genus Cuscuta (Convolvulaceae) BMC Biol. 2007;5:1. doi: 10.1186/1741-7007-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabani M, Modirian E, Ebrahimabadi A, Vafazadeh J, Shahnavaz S, Heidari MR. Study of the Effects of hydro-methanol extracts of lavandula vera DC and cuscuta epithymum murr on the seizure induced by pentylentetranzol in Mice. J Kerman Uni Med Sci. 2007;14:25–32. [Google Scholar]
- Rodak BF, Fritsma GA, Keohane E. Hematology: clinical principles and applications. Elsevier Health Sciences; 2013. pp. 340–373. [Google Scholar]
- Sadeghnia HR, Ghorbani Hesari T, Mortazavian SM, Mousavi SH, Tayarani-Najaran Z, Ghorbani A. Viola tricolor induces apoptosis in cancer cells and exhibits antiangiogenic activity on chicken chorioallantoic membrane. BioMed Res Inter. 2014;2014 doi: 10.1155/2014/625792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract. 2012;2012 doi: 10.1155/2012/282570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr MF, Jameie SB, Hajijafari B. The Cuscuta kotschyana effects on breast cancer cells line MCF7. J Med Plant Res. 2011;5:6344–6351. [Google Scholar]
- Shafiee-Nick R, Ghorbani A, Vafaee Bagheri F, Rakhshandeh H. Chronic administration of a combination of six herbs inhibits the progression of hyperglycemia and decreases serum lipids and aspartate amino transferase activity in diabetic rats. Adv Pharmacol Sci. 2012;2012 doi: 10.1155/2012/789796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Wang S-L, Liu X-K, Yang C-R, Cai B, Yao X-S. Steroidal Saponins from Rhizomes of Tupistra wattii HOOK. f. Chem Pharm Bull (Tokyo) 2003;51:305–308. doi: 10.1248/cpb.51.305. [DOI] [PubMed] [Google Scholar]
- Suganuma K, Miwa H, Imai N, Shikami M, Gotou M, Goto M, Mizuno S, Takahashi M, Yamamoto H, Hiramatsu A. Energy metabolism of leukemia cells: glycolysis versus oxidative phosphorylation. Leuk. Lymphoma. 2010;51:2112–2119. doi: 10.3109/10428194.2010.512966. [DOI] [PubMed] [Google Scholar]
- Suresh V, Sruthi V, Padmaja B, Asha V. In vitro anti-inflammatory and anti-cancer activities of Cuscuta reflexa Roxb. J Ethnopharmacol. 2011;134:872–877. doi: 10.1016/j.jep.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Yan H, Peng Z-G, Wu Y-L, Jiang Y, Yu Y, Huang Y, Zhu Y-S, Zhao Q, Chen G-Q. Hypoxia-simulating agents and selective stimulation of arsenic trioxide-induced growth arrest and cell differentiation in acute promyelocytic leukemic cells. Haematologica. 2005;90:1607–1616. [PubMed] [Google Scholar]
- Yokosuka A, Mimaki Y, Sashida Y. Spirostanol saponins from the rhizomes of Tacca chantrieri and their cytotoxic activity. Phytochemistry. 2002;61:73–78. doi: 10.1016/s0031-9422(02)00168-1. [DOI] [PubMed] [Google Scholar]