Abstract
Background
The sand fly Phlebotomus argentipes is the vector for visceral leishmaniasis (VL) in the Indian sub-continent. In Bangladesh since 2012, indoor residual spraying (IRS) was applied in VL endemic areas using deltamethrin. In Nepal, IRS was initiated in 1992 for VL vector control using lambda-cyhalothrin. Irrational use of insecticides may lead to vector resistance but very little information on this subject is available in both countries. The objective of this study was to generate information on the susceptibility of the vector sand fly, P. argentipes to insecticide, in support of the VL elimination initiative on the Indian sub-continent.
Methods
Susceptibility tests were performed using WHO test kits following the standard procedures regarding alpha cypermethrin (0.05%), deltamethrin (0.05%), lambda-cyhalothrin (0.05%), permethrin (0.75%), malathion (5%) and bendiocarb (0.1%) in six upazilas (sub-districts) in Bangladesh. In Nepal, the tests were performed for two insecticides: alpha cypermethrin (0.05%) and deltamethrin (0.05%). Adult P. argentipes sand flies were collected in Bangladesh from six VL endemic upazilas (sub-districts) and in Nepal from three endemic districts using manual aspirators.
Results
The results show that VL vectors were highly susceptible to all insecticides at 60 minutes of exposure in both countries. In Bangladesh, corrected mortality was 100% at 15 minutes as well as 30 minutes of exposure. The study sites in Nepal, however, showed some diverse results, with a mortality rate less than 90% for 15 minutes of exposure with alpha cypermethrin and deltamethrin in two districts but was above 95% after 30 minutes of exposure.
Conclusions
These results suggest that the insecticides tested can still be used in the national programmes of Bangladesh and Nepal. However, insecticide rotation should be performed to mitigate the possible development of insecticide resistance. Periodic susceptibility tests should be performed by the countries to get timely alerts regarding insecticide resistance.
Keywords: Susceptibility, Visceral leishmaniasis, Phlebotomus argentipes, Vector control, Alpha cypermethrin, Deltamethrin
Background
Visceral leishmanisis (VL) known as kala-azar in the Indian sub-continent is a deadly parasitic disease (if untreated) caused by Leishmania donovani Laveran & Mesnil (Kinetoplastida: Trypanosomatidae) which is transmitted by the bite of a female sand fly Phlebotomus argentipes Annandale & Brunneti (Diptera: Psychodidae). Phlebotomus argentipes is the only incriminated vector in the Southeast Asia Region. The disease is highly prevalent in the region and is considered to be anthroponotic, and the route of transmission from human to human. VL was virtually eliminated from this part of world during the malaria eradication era through massive DDT spraying for malaria vector control [1]. It has, however, reemerged since the 1970s after the relaxation of DDT spraying [1], indicating that effective vector management is crucial to control the disease. The use of DDT was banned in Bangladesh and Nepal in 1998 and 1995, respectively, due to its environmental hazard [2, 3].
VL is endemic in 45 [4] and 18 [5] districts in Bangladesh and Nepal, respectively. A memorandum of understanding (MoU) was signed by the honorable Health Ministers from Bangladesh, India and Nepal in 2005 to eliminate VL (less than one case per 10,000 people) by 2015 [6] which was extended to 2017 [7]. Five regional strategies, i.e. (i) early diagnosis and complete case management, (ii) integrated vector management and vector surveillance, (iii) effective disease surveillance through passive and active case detection; (iv) social mobilization and building partnerships, and (v) implementation and operational research, were set up to achieve the elimination target [6].
Since the signing of the MoU, substantial progress has been made in the last few years in Bangladesh and Nepal in terms of reducing VL cases by properly utilizing its regional strategies [8]. Nepal achieved the elimination target (less than one VL case per 10,000 people in all endemic districts) and has maintained it for the last two years. Bangladesh also achieved a similar target with the exception of two upazilas (sub-districts) (Fulbaria and Trishal) [8]. Integrated vector management (IVM) is one of the major components of the regional elimination strategy. The elimination initiative has so far been successful in Nepal and in most VL endemic parts of Bangladesh. However, it is important to maintain the efforts and avoid the above described re-emergence of vectors after the malaria eradication campaign. Both countries only rely on indoor residual spraying (IRS). IRS has been used in Nepal since 1992 using lambda-cyhalothrin and alpha cypermethrin alternatively every few years for kala-azar control [9] while in Bangladesh deltamethrin has been used since 2012 [4]. The first susceptibility test of VL vectors was conducted in Nepal in 1998 for different insecticides including DDT (4%), permethrin (0.25%), deltamethrin (0.025%), lambda-cyhalothrin (0.1%), malathion (5%) and bendiocarb (0.1%) showing vector mortality rates of 98, 99, 100, 100, 100 and 100%, respectively, at 60 minutes of exposure [9]. The susceptibility test for deltamethrin was done again in Nepal in 2009 showing 96–100% mortality [3]. In Bangladesh, in 2007, 100% sand fly mortality was observed using deltamethrin [4]. However, current information on the status of insecticide resistance in the sand fly population is limited. The objective of this study was to generate evidence on the current level of insecticide susceptibility in support of the VL vector control programmes in Bangladesh and Nepal.
Methods
Study area and period
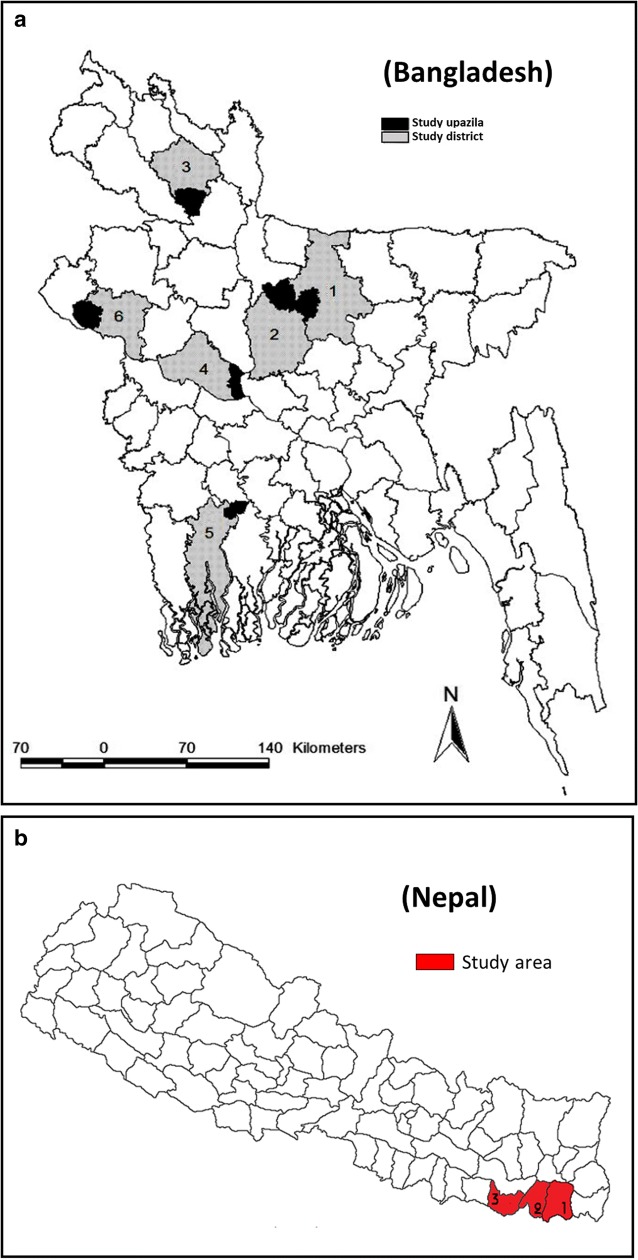
The study in Bangladesh was carried out in six VL endemic upazilas (sub-districts): Fulbaria, Terokhada, Bera, Pirganj, Godagari and Madhupur under Mymensingh, Khulna, Pabna, Rangpur, Rajshahi and Tangail districts, respectively (Fig. 1a) and Bhathigachha, Tanmuna and West Pipara village development committees under Morang, Sunsari and Saptari districts, respectively, (Fig. 1b) in Nepal from October 2015 to October 2016. The study areas were selected purposively in different locations of VL endemic areas of the country. The average incidence of VL was 17.82, 2.45, 1.46, 1.14 in Fulbaria, Terokhada, Godagari and Madhupur upazilas, respectively, from 2008 to 2013. Bera, Pirganj upazilas are also endemic but the incidence is less than one case in Bangladesh [4]. Morang district had reported the highest case numbers of VL in the year 2016, in Nepal. Hence, Morang and its two neighboring districts were selected for the study. The incidence of VL was 0.46, 0.79, 0.48 and 0.51 in Morang District; 0.14, 0.17, 0.24 and 0.09 in Sunsari District and 0.75, 0.35, 0.24 and 0.15 in Saptari District, for the years 2013, 2014, 2015 and 2016, respectively [10].
Fig. 1.
a Study area map of Bangladesh (Key to upazilas: 1, Fulbaria; 2, Madhupur; 3, Pirganj; 4, Bera; 5, Terokhada; 6, Godagari). b Study area map of Nepal (Key to districts: 1, Morang; 2, Sunssari; 3, Saptari)
Insecticides and control paper
The tests were performed with the following insecticides in Bangladesh: alpha cypermethrin (0.05%), deltamethrin (0.05%), lambda-cyhalothrin (0.05%), permethrin (0.75%), malathion (5%) and bendiocarb (0.1%). In Nepal, the tests were performed with two insecticides: alpha cypermethrin (0.05%) and deltamethrin (0.05%). Two types of oil treated control papers were used: in the case of pyrethroids, the control papers were treated with silicone oil and in the case of organophosphate/carbamate insecticides, the control papers were treated with olive oil.
Collection of sand flies from the field
Wild sand flies were collected from human dwellings in the above-mentioned areas in the evening from 6:30 to 20:30 h by manual aspirator and using torch light. Collected sand flies were transferred to a cage in the field laboratory where tests were performed after one hour or longer to settle down from transportation stress. Only P. argentipes sand flies were used for the testing after separating them from other species. A manual aspirator was used to transfer P. argentipes sand flies to the holding tube which was marked with a green dot. The exposure tubes were marked with a red dot and lined with insecticide-impregnated paper.
Test procedure
The tests were performed as per the World Health Organization (WHO) recommended procedure [11, 12] with the above-mentioned insecticides. WHO test kits, insecticide-impregnated and oil treated control papers were obtained from the Universiti Sains Malaysia (WHO collaborating Center). Tests were performed at a temperature and relative humidity of 28 ± 2 °C and 70–80%, respectively. For each district, we had an independent set of bioassays with an independent control group. In Bangladesh, six replicates containing 20–25 female P. argentipes sand flies in each replicate were used for each insecticide and control group. In Nepal, 5 replicates were used for each insecticide together with a control group. The other procedures remained the same. After exposure, knockdown (KD) was recorded after the test period for each insecticide and control. The holding tubes were kept for recovery in dark and cool places immediately after exposure for the requisite period. Fresh sugar solution on cotton was given as supplementary food during the recovery period for 24 h. The percent mortality was calculated by counting the dead and alive P. argentipes sand flies after 24 h of recovery; sand fly mortality was corrected by using Abbott’s formula , where P is the corrected mortality, Pi is the percent observed mortality in immediate exposed sand flies, and C is the percent mortality in control] where control mortality should be within 5–20%. Tests were invalid in the case of control mortality exceeding 20% and those tests were repeated. The cut-off percentage was < 20% [11]. The insecticide-impregnated papers were used 4 times and after use they were stored in their respective packets. A small line was drawn in one corner of the paper to mark the number of uses for each paper.
Sand fly identification
Female P. argentipes sand flies were sorted by their morphological characteristics prior to conducting the tests. All evaluated sand flies (live and dead) were identified using the standard taxonomic key described by Lewis [13, 14] and Kalra & Bang [15].
Results
The results in Bangladesh of the susceptibility tests performed on wild populations of female P. argentipes sand flies with different insecticide-impregnated papers are shown in Tables 1, 2, 3, 4, 5, 6, 7, 8 and 9. The KD rate for 15, 30 and 60 minutes exposure to different insecticides varied between 19.17–40.98%, 40.48–74.19% and 81.15–96.77%, respectively. Tables 7 and 8 show that no KD was observed for any insecticides in any study sites in the control group and almost 100% of sand flies were alive 24 h post-exposure. The VL vector, P. argentipes, was found susceptible to all insecticides tested in the study as 100% corrected mortality was observed 24 h post-exposure (Table 9).
Table 1.
Mortality of P. argentipes to alpha-cypermethrin (0.05%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 120 [35 (29.16)] | 122 [65 (53.28)] | 125 [111 (88.80)] | 120 (100) | 122 (100) | 125 (100) |
| Madhupur | Tangail | 121 [32 (26.45)] | 125 [59 (47.20)] | 128 [115 (89.84)] | 121 (100) | 125 (100) | 128 (100) |
| Pirgonj | Rangpur | 122 [37 (30.33)] | 120 [69 (57.50)] | 122 [109 (89.34)] | 122 (100) | 120 (100) | 122 (100) |
| Bera | Pabna | 124 [39 (31.45)] | 121 [62 (51.24)] | 123 [111 (90.24)] | 124 (100) | 121 (100) | 123 (100) |
| Terokhada | Khulna | 123 [41 (33.33)] | 124 [71 (57.26)] | 126 [112 (88.89)] | 123 (100) | 124 (100) | 126 (100) |
| Godagari | Rajshahi | 126 [43 (34.13)] | 124 [63 (50.81)] | 122 [110 (90.16)] | 126 (100) | 124 (100) | 122 (100) |
Abbreviation: KD knockdown rate
Table 2.
Mortality status of P. argentipes to deltamethrin (0.05%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 120 [28 (23.33)] | 121 [53 (43.80) ] | 120 [105 (87.5)] | 120 (100) | 121 (100) | 120 (100) |
| Madhupur | Tangail | 124 [32 (25.81)] | 121 [55 (45.45)] | 121 [107 (88.43)] | 124 (100) | 121 (100) | 121 (100) |
| Pirgonj | Rangpur | 120 [35 (29.17)] | 126 [63 (50.00)] | 120 [109 (90.83)] | 120 (100) | 126 (100) | 120 (100) |
| Bera | Pabna | 126 [35 (27.78)] | 122 [59 (48.36)] | 120 [105 (87.5)] | 126 (100) | 122 (100) | 120 (100) |
| Terokhada | Khulna | 121 [42 (34.71)] | 122 [66 (54.10)] | 126 [112 (88.89)] | 121 (100) | 122 (100) | 126 (100) |
| Godagari | Rajshahi | 124 [39 (31.45)] | 123 [57 (46.34)] | 127 [114 (89.76)] | 124 (100) | 123 (100) | 127 (100) |
Abbreviation: KD knockdown rate
Table 3.
Mortality status of P. argentipes to lambda-cyhalothrin (0.05%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand fly exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 120 [23 (19.17)] | 122 [51 (41.80)] | 120 [108 (90.00)] | 120 (100) | 122 (100) | 120 (100) |
| Madhupur | Tangail | 126 [28 (22.22)] | 123 [52 (42.28)] | 122 [112 (91.80)] | 126 (100) | 123 (100) | 122 (100) |
| Pirgonj | Rangpur | 126 [35 (27.78)] | 121 [55 (45.45)] | 124 [112 (90.32)] | 126 (100) | 121 (100) | 124 (100) |
| Bera | Pabna | 122 [31 (25.41)] | 124 [59 (47.58)] | 120 [110 (91.67)] | 122 (100) | 124 (100) | 120 (100) |
| Terokhada | Khulna | 121 [29 (24.97)] | 120 [51 (42.50)] | 122 [108 (88.52)] | 121 (100) | 120 (100) | 122 (100) |
| Godagari | Rajshahi | 123 [24 (19.51)] | 126 [51 (40.48)] | 126 [110 (87.30)] | 123 (100) | 126 (100) | 126 (100) |
Abbreviation: KD knockdown rate
Table 4.
Mortality status of P. argentipes to permethrin (0.75%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 121 [26 (21.49)] | 123 [64 (52.03)] | 122 [108 (88.52)] | 121 (100) | 123 (100) | 122 (100) |
| Madhupur | Tangail | 126 [28 (22.22)] | 125 [65 (52.00)] | 120 [103 (85.83)] | 126 (100) | 125 (100) | 120 (100) |
| Pirgonj | Rangpur | 125 [30 (24.00)] | 122 [62 (50.82)] | 120 [105 (87.5)] | 125 (100) | 122 (100) | 120 (100) |
| Bera | Pabna | 121 [31 (25.61)] | 124 [57 (45.97)] | 122 [108 (88.52)] | 121 (100) | 124 (100) | 122 (100) |
| Terokhada | Khulna | 122 [28 (22.95)] | 121 [58 (47.93)] | 124 [105 (84.68)] | 122 (100) | 121 (100) | 124 (100) |
| Godagari | Rajshahi | 123 [30 (24.39)] | 120 [63 (52.50)] | 120 [106 (88.33)] | 123 (100) | 120 (100) | 120 (100) |
Abbreviation: KD knockdown rate
Table 5.
Mortality status of P. argentipes to malathion (5%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 122 [53 (43.44)] | 124 [92 (74.19)] | 124 [120 (96.77)] | 122 (100) | 124 (100) | 124 (100) |
| Madhupur | Tangail | 122 [50 (40.98)] | 120 [87 (72.50)] | 120 [112 (93.33)] | 122 (100) | 120 (100) | 120 (100) |
| Pirgonj | Rangpur | 120 [53 (44.17)] | 120 [83 (69.17)] | 121 [114 (94.21)] | 120 (100) | 120 (100) | 121 (100) |
| Bera | Pabna | 126 [57 (45.24)] | 122 [86 (70.49)] | 123 [111 (90.24)] | 126 (100) | 122 (100) | 123 (100) |
| Terokhada | Khulna | 125 [55 (44.00)] | 126 [91 (72.22)] | 122 [115 (94.26)] | 125 (100) | 126 (100) | 122 (100) |
| Godagari | Rajshahi | 121 [54 (44.63)] | 126 [89 (70.63)] | 124 [116 (93.55)] | 121 (100) | 126 (100) | 124 (100) |
Abbreviation: KD knockdown rate
Table 6.
Mortality status of P. argentipes to bendiocarb (0.1%) in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 122 [42 (35.00)] | 125 [81 (66.39)] | 123 [102 (82.93)] | 122 (100) | 125 (100) | 123 (100) |
| Madhupur | Tangail | 120 [48 (39.34)] | 122 [82 (67.21)] | 122 [99 (81.15)] | 118 (98.33) | 121 (99.18) | 122 (100) |
| Pirgonj | Rangpur | 122 [47 (38.52)] | 122 [79 (64.75)] | 120 [98 (81.67)] | 121 (99.18) | 121 (99.18) | 120 (100) |
| Bera | Pabna | 126 [52 (41.27)] | 120 [82 (67.21)] | 123[104 (84.55)] | 125 (99.21) | 119 (99.17) | 123 (100) |
| Terokhada | Khulna | 124 [51 (41.13)] | 120 [83 (68.03)] | 123[101 (82.11)] | 122 (98.39) | 120 (100) | 123 (100) |
| Godagari | Rajshahi | 125 [44 (35.20)] | 126 [85 (69.67)] | 120 [101 (84.17)] | 123 (98.4) | 124 (98.41) | 120 (100) |
Abbreviation: KD knockdown rate
Table 7.
Control mortality in silicon oil: PY status of P. argentipes in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 120 [0 (0)] | 124 [0 (0)] | 126 [0 (0)] | 0 | 0 | 7 (5.56) |
| Madhupur | Tangail | 122 [0 (0)] | 120 [0 (0)] | 120 [0 (0)] | 0 | 8 (6.67) | 0 |
| Pirgonj | Rangpur | 125 [0 (0)] | 120 [0 (0)] | 120 [0 (0)] | 0 | 0 | 0 |
| Bera | Pabna | 121 [0 (0)] | 123 [0 (0)] | 120 [0 (0)] | 0 | 0 | 0 |
| Terokhada | Khulna | 123 [0 (0)] | 122 [0 (0)] | 123 [0 (0)] | 0 | 0 | 9 (7.32) |
| Godagari | Rajshahi | 124 [0 (0)] | 120 [0 (0)] | 123 [0 (0)] | 0 | 9 (7.5) | 0 |
Abbreviations: KD knockdown rate, PY pyrethroid
Table 8.
Control mortality in olive oil: OP/CR status of P. argentipes in the WHO tube test (six replicates for each observation) in Bangladesh
| Upazila | District | No. of sand flies exposed in 6 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Fulbaria | Mymensingh | 121 [0 (0)] | 124 [0 (0)] | 126 [0 (0)] | 0 | 8 (6.45) | 0 |
| Madhupur | Tangail | 125 [0 (0)] | 120 [0 (0)] | 120 [0 (0)] | 0 | 0 | 9 (7.5) |
| Pirgonj | Rangpur | 120 [0 (0)] | 122 [0 (0)] | 120 [0 (0)] | 0 | 0 | 8 (6.67) |
| Bera | Pabna | 120 [0 (0)] | 123 [0 (0)] | 120 [0 (0)] | 0 | 0 | 0 |
| Terokhada | Khulna | 124 [0 (0)] | 120 [0 (0)] | 122 [0 (0)] | 0 | 0 | 0 |
| Godagari | Rajshahi | 123 [0 (0)] | 120 [0 (0)] | 121 [0 (0)] | 0 | 0 | 0 |
Abbreviations: KD knockdown rate, OP organophosphates, CR carbamate
Table 9.
Susceptibility status of P. argentipes to different insecticides in the WHO tube test (six replicates for each observation) for 15, 30 and 60 minutes exposure in Bangladesh. Values without parentheses indicate observation at 15 minutes, those in parentheses - at 30 minutes and those in square brackets - at 60 minutes
| Insecticide | District | Mymensingh | Tangail | Rangpur | Pabna | Khulna | Rajshahi |
|---|---|---|---|---|---|---|---|
| Upazila | Fulbaria | Madhupur | Pirganj | Bera | Terokhada | Godagari | |
| Alpha-cypermethrin (0.05%) | No. of exposed | 120 (122) [125] | 121 (125) [128] | 122 (120) [122] | 124 (121) [123] | 123 (124) [126] | 126 (124) [122] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (0) [5.56] | 0 (6.67) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [7.32] | 0 (7.5) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S | |
| Deltamethrin (0.05%) | No. of exposed | 120 (121) [120] | 124 (121) [121] | 120 (126) [120] | 126 (122) [120] | 121 (122) [126] | 124 (123) [127] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (0) [5.56] | 0 (6.67) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [7.32] | 0 (7.5) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S | |
| Lambda-cyhalothrin (0.05%) | No. of exposed | 120 (122) [120] | 126 (123) [122] | 126 (121) [124] | 122 (124) [120] | 121 (120) [122] | 123 (126) [126] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (0) [5.56] | 0 (6.67) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [7.32] | 0 (7.5) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S | |
| Permethrin (0.75%) | No. of exposed | 121(123) [122] | 126 (125) [120] | 125 (122) [120] | 121 (124) [122] | 122 (121) [124] | 123 (120) [120] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (0) [5.56] | 0 (6.67) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [7.32] | 0 (7.5) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S | |
| Malathion (5%) | No. of exposed | 122 (124) [124] | 122 (120) [120] | 120 (120) [121] | 126 (122) [123] | 125 (126) [122] | 121 (126) [124] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (6.45) [0] | 0 (0) [7.5] | 0 (0) [6.67] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S | |
| Bendiocarb (0.1%) | No. of exposed | 120 (122) [123] | 122 (125) [122] | 122 (122) [120] | 126 (120) [123] | 124 (120) [123] | 125 (126) [120] |
| % mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| % control mortality | 0 (6.45) [0] | 0 (0) [7.5] | 0 (0) [6.67] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | |
| Corrected mortality | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | 100 (100) [100] | |
| Status | S | S | S | S | S | S |
Abbreviation: S susceptible
The study results in Nepal are shown in Tables 10, 11, 12, 13 and 14. They show that the KD rates of alpha cypermethrin and deltamethrin were 16.22–27.27%, 77.78–83.18% and 93.20–97.27% for 15, 30 and 60 minutes exposure, respectively. The mortality rate was 100 % for 60 minutes of exposure for both insecticides in all three study districts but different results were found for 15 and 30 minutes of exposure (Table 14). These results indicate the need for establishing the susceptibility status of vector sand flies for above tested (alpha-cypermethrin and deltamethrin) as well as other insecticides in the pipeline for IRS in Nepal.
Table 10.
Mortality status of P. argentipes to alpha-cypermethrin (0.05%) in the WHO tube test (five replicates for each observation) in Nepal
| District | Village Development Committee | No. of sand flies exposed in 5 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Morang | Bhathigachh | 111 [23 (20.72)] | 108 [84 (77.78)] | 109 [106 (97.25)] | 100 (89.89) | 108 (100) | 109 (100) |
| Sunsari | Tanmuna | 110 [30 (27.27)] | 107 [89 (83.18)] | 106 [100 (94.34)] | 105 (95.45) | 107 (100) | 106 (100) |
| Saptari | West Pipara | 111 [18 (16.22)] | 105 [82 (78.10)] | 104 [99 (95.19)] | 106 (95.10) | 105 (100) | 104 (100) |
Abbreviation: KD knockdown rate
Table 11.
Mortality status of P. argentipes to deltamethrin (0.05%) in the WHO tube test (five replicates for each observation) in Nepal
| District | Village Development Committee | No. of sand flies exposed in 5 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Morang | Bhathigachh | 114 [31 (27.19)] | 103 [75 (72.82)] | 110 [107 (97.27)] | 107 (93.86) | 99 (95.73) | 110 (100) |
| Sunsari | Tanmuna | 104 [21 (20.19)] | 108 [88 (81.48)] | 103 [96 (93.20)] | 93 (89.42) | 103 (95.37) | 103 (100) |
| Saptari | West Pipara | 114 [19 (16.67)] | 108 [79 (73.15)] | 109 [103 (94.50)] | 109 (95.48) | 108 (100) | 109 (100) |
Abbreviation: KD knockdown rate
Table 12.
Control mortality in silicon oil: PY status of P. argentipes in the WHO tube test (five replicates for each observation) for alpha-cypermethrin in Nepal
| District | Village Development Committee | No. of sand flies exposed in 5 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Exposure time (min) | Exposure time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Morang | Bhathigachh | 102 [0 (0)] | 104 [0 (0)] | 117 [0 (0)] | 2 (1.96) | 2 (1.92) | 6 (5.13) |
| Sunsari | Tanmuna | 104 [0 (0)] | 104 [0 (0)] | 107 [0 (0)] | 0 | 0 | 0 |
| Saptari | West Pipara | 112 [0 (0)] | 103 [0 (0)] | 115 [0 (0)] | 9 (8.04) | 2 (1.94) | 10 (8.70) |
Abbreviations: KD knockdown rate, PY pyrethroid
Table 13.
Control mortality in silicon oil: PY status of P. argentipes in the WHO tube test (five replicates for each observation) for deltamethrin in Nepal
| District | Village Development Committee | No. of sand flies exposed in 5 replicates [KD (%)] | No. of sand flies dead at 24 h (%) | ||||
|---|---|---|---|---|---|---|---|
| Observation time (min) | Observation time (min) | ||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| Morang | Bhathigachh | 102 [0 (0)] | 110 [0 (0)] | 120 [0 (0)] | 0 | 10 (9.09) | 0 |
| Sunsari | Tanmuna | 104 [0 (0)] | 100 [0 (0)] | 110 [0 (0)] | 0 | 0 | 0 |
| Saptari | West Pipara | 104 [0 (0)] | 104 [0 (0)] | 110 [0 (0)] | 3 (2.88) | 4 (3.85) | 5 (4.55) |
Abbreviations: KD knockdown rate, PY pyrethroid
Table 14.
Susceptibility status of P. argentipes to different insecticides in the WHO tube test (six replicates for each observation) for 15, 30 and 60 minutes exposure in Nepal. Values without parentheses indicate observation at 15 minutes, those in parentheses - at 30 minutes and those in square brackets - at 60 minutes
| Insecticide | District | Morang | Sunsari | Saptari |
|---|---|---|---|---|
| Village development committee | Bhathigachh | Tanmuna | West Pipara | |
| Alpha-cypermethrin (0.05%) | No. exposed | 111 (108) [109] | 110 (107) [106] | 111 (105) [104] |
| % mortality | 90.09 (100) [100] | 95.45 (100) [100] | 95.50 (100) [100] | |
| % control mortality | 1.96 (1.92) [5.13] | 0 (0) [0] | 8.04 (1.94) [8.70] | |
| Corrected mortality | 89.89 (100) [100] | 95.45 (100) [100] | 95.10 (100) [100] | |
| Status | S? | S | S | |
| Deltamethrin (0.05%) | No. exposed | 114 (103) [110] | 104 (108) [103] | 114 (108) [109] |
| % mortality | 93.86 (96.12) [100] | 89.42 (95.37) [100] | 95.61 (100) [100] | |
| % control mortality | 0 (9.09) [0] | 0 (0) [0] | 2.88 (3.85) [4.55] | |
| Corrected mortality | 93.86 (95.73) [100] | 89.42 (95.37) [100] | 95.48 (100) [100] | |
| Status | S | S? | S |
Abbreviations: S susceptible
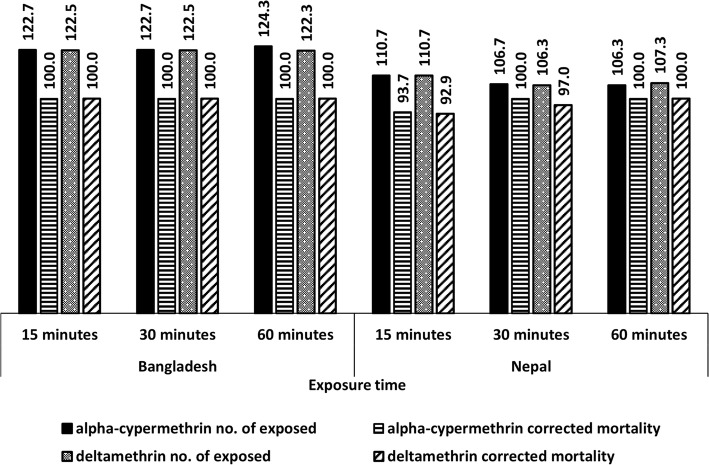
In this study, two insecticides, alpha-cypermethrin and deltamethrin, were used in both Bangladesh and Nepal; the tests performed, and the comparative findings are shown in Fig. 2. In Bangladesh, the mortality was 100% for both insecticides for 15, 30 and 60 minutes exposure, whilst this was the case only for 30 minutes exposure for alpha-cypermethrin and 60 minutes exposure for both insecticides in Nepal. In Nepal, the mortality dropped to 92.9 and 93.7 from 100% for 15 minutes exposure for both insecticides and to 97.0 from 100% at 30 minutes exposure for deltamethrin.
Fig. 2.
Average number of Phlebotomus argentipes exposed and their corrected mortality for two common insecticides, alpha-cypermethrin and deltamethrin tested in each study sites in Bangladesh (six study sites and six replicates in each site) and Nepal (three study sites and five replicates in each site)
Discussion
Insect-borne diseases remain a major public health concern in resource constrained situations. About 30 different species of phlebotomine sand flies are responsible for transmitting leishmaniasis worldwide [16]. Vector control is an important element to minimize the vector-borne disease burden in the countries and in fact, it heavily relies upon different insecticides. The inappropriate application (overuse and misuse) of insecticides have led to the emergence of resistance, which undermines the potency of vector control. Usually there are four possible types of insecticides resistance observed, i.e. (i) increased metabolism to non-toxic products, (ii) decreased target site sensitivity, (iii) decreased rates of insecticide penetration and (iv) increased rates of insecticide excretion, for four major classes of insecticides (organochlorines, organophosphates, carbamates and pyrethroids) [17]. Development of resistance is an evolutionary phenomenon caused by either behavioural avoidance or physiological or biochemical factors of any targeted insects. This can be tackled judiciously by implementing appropriate and comprehensive resistance monitoring and management strategies within the framework of integrated vector management. Insecticide resistance occurs when the insecticide no longer binds to its target or through detoxification enzyme-based resistance, which occurs when enhanced levels or modified activities of esterases, oxidases, or glutathione S-transferases prevent the insecticide from reaching its site of action [18].
In the present study, we did not perform assays of the susceptibility of sand flies because the lethal doses of all classes of insecticides are generally similar to those for mosquitoes [19] and determined the doses for the purpose of discriminating concentrations in routine insecticide resistance monitoring. This is now well established and widely adopted for the purposes of testing and monitoring insecticide resistance in mosquitoes and other disease vectors [20]. Wild-caught P. argentipes female sand flies were used in the present study as none of the study countries have a sand fly colony.
The present study confirmed that P. argentipes sand flies in Bangladesh are susceptible to alpha-cypermethrin, deltamethrin, lambda-cyhalothrin, permethrin, malathion and bendiocarb as 100% mortality was observed at 60 minutes of exposure. In Nepal, incipient signs of resistance development were observed: the mortality rate was below 90% after 15 minutes and about 95% after 30 minutes of exposure to pyrethroid insecticides but 100% after 60 minutes of exposure for both insecticides which is slightly different (the mortality was between 96–99% for 60 minutes for deltamethrin 0.05%) from an earlier study conducted in Nepal and India [3]. Two insecticides (deltamethrin and alpha-cypermethrin) were tested in the both countries and the findings indicate that the P. argentipes populations are on the way to developing tolerance against both insecticides in Nepal whereas they are still 100% susceptible in Bangladesh. In Nepal, pyrethroid based (alpha-cypermethrin, lambda-cyhalothrin and deltamethrin) IRS has been in practice for a long time and various types of pyrethroids are in use in different years. Rotation of insecticide for IRS is still not foreseen in Nepal, hence it is essential to monitor the available insecticide for its efficacy to control vectors of VL. It is also worth mentioning that if the potential emergence of resistance is observed, we need to go for next step (molecular mechanism) of confirmation resistance test.
The VL vector control programmes in Bangladesh and Nepal are completely dependent on insecticide-based interventions, mainly IRS using synthetic pyrethroids (deltamethrin, alpha-cypermethrin and lambda-cyhalothrin) in human dwellings as well as in cattle sheds. Nepal reached the elimination target (less than one case per 10,000 people at district level) for the last few years; Bangladesh has yet to achieve it as a few upazilas (sub-districts) are still reporting incidence rates above the elimination target. Continuous vector control activities with rational use of quality insecticide are essential to sustain this achievement and eliminate the disease. As mentioned before, VL was virtually eliminated from Bangladesh and Nepal due to the massive use of DDT for IRS during the malaria eradication era [1, 2] but reappeared in the sub-continent due to a lack of effective vector control activities in the post-eradication phase. IRS is an expensive operation so it must be carried out using appropriate insecticides to achieve the optimum results against the targeted vector. Periodic monitoring insecticide efficacy through susceptibility tests (by WHOPES suggested method) is needed to have updated information on vector susceptibility to insecticides. IRS is an operationally challenging activity in terms of maintaining its optimum quality. Due to poor operation and relatively lower disease burden, the community acceptance of IRS is gradually decreasing in the Indian sub-continent (personal observation of RC). A study in India and Nepal identified that IRS is effective on vector reduction when it was carried out by the research team in a controlled situation but it was deficient when it was carried out by the National Programme [21]. Long-term irrational use of insecticide may develop tolerance or resistance on its targeted insects which we observed in Nepal. The WHOPES-recommended susceptibility test kits and insecticide-impregnated paper are only available at Universiti Sains Malaysia and to obtain them in time is difficult which affects the planned activity so WHO may come forward to find out the solution.
In the case of Bangladesh, the chance of cross-resistance is very unlikely as there were virtually no vector control activities for a long time (1997–2012) [4, 22]. Limited scale IRS was conducted in 2012 using deltamethrin 5WP in Bangladesh and since then it has continued, usually with two rounds of spraying. While IRS has been carried out in Nepal using synthetic pyrethroids for a long time as part of VL vector control activities, the current study did not evaluate the cross-resistance as a fully susceptible reference strain of P. argentipes was not available.
Conclusions
In conclusion, it is recommended that the national control programme in Bangladesh and Nepal can use alpha-cypermethrin, deltamethrin, lambda-cyhalothrin, permethrin, malathion and bendiocarb for VL vector control. In Nepal, however, deltamethrin or alpha-cypermethrin should be used judiciously. In both cases, insecticide rotation should be performed to mitigate the possible development of insecticide resistance. Periodic susceptibility tests should be performed by the countries to get timely alerts regarding insecticide resistance.
Acknowledgments
We gratefully acknowledge Nurul Alam, Muzahidul Islam, Beauti Akter and Shahidullah in Bangladesh and Shashi Majhi, Manish Karn, Satyanarayan Bhagat and Kailash Majhi in Nepal for their field assistance in conducting the study. The authors are also thankful to the UHFPOs and HI in-charge of Fulbaria, Madhupur, Pirgonj, Bera, Terokhada and Godagari upazilas and their staff in Bangladesh and District Public Health Officers and vector control officers of Morang, Sunsari and Saptari districts in Nepal, for providing the information on kala-azar and assistance in conducting the study. We highly acknowledge M Mamun Huda and Md. Sahidul Islam for their comments and critical review. The study was supported by the Special Programme for Tropical Diseases Research and Training (TDR) at the World Health Organization (WHO). In Bangladesh, susceptibility kits, insecticide-impregnated and control papers were provided by the NTD Department of WHO.
Funding
The study was funded by the Special Programme for Tropical Diseases Research and Training (TDR) at the World Health Organization (WHO), Geneva, Switzerland.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Abbreviations
- BMRC
Bangladesh Medical Research Council
- BPKIHS
BP Koirala Institute of Health Sciences
- DDT
Dichlorodiphenyltrichloroethane
- IRS
Indoor residual spraying
- IVM
Integrated vector management
- KD
Knockdown
- MoU
Memorandum of understanding
- OP/CR
Organophosphate/carbamate
- PY
Pyrethroid
- VL
Visceral leishmanisis
- WHO
World Health Organization
- WHOPES
World Health Organization Pesticide Evaluation Scheme
- WP
Wettable powder
Authors’ contributions
RC, MLD, DA and AK drafted the manuscript. RC, MLD, VC, NPM and AK conceived and designed the study. LR, SF, JP, SA and RKK collected the data. RC, MLD and VC monitored the study activities. VC performed overall data analysis. RC, MLD, NPM, DA and AK acquired funding for the study. All authors read and approved the final manuscript except NPM.
Ethics approval and consent to participate
The study proposal was approved by the Bangladesh Medical Research Council (BMRC) and in Nepal by the BP Koirala Institute of Health Sciences (BPKIHS) Institutional Review Committee and Nepal Health Research Council (NHRC). Prior to funding, the proposal was reviewed by the Special Programme for Tropical Diseases Research and Training (TDR) at the World Health Organization (WHO), Geneva, Switzerland. Prior permission was obtained from household head of residences for the collection of sand flies.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Rajib Chowdhury and Murari Lal Das contributed equally to this work.
Narayan Prosad Maheswary deceased
Contributor Information
Rajib Chowdhury, Email: rajib478@yahoo.com.
Murari Lal Das, Email: mldas_29@yahoo.com.
Vashkar Chowdhury, Email: prostatguru@gmail.com.
Lalita Roy, Email: roy_lalita@yahoo.com.
Shyla Faria, Email: shylafaria@yahoo.com.
Jyoti Priyanka, Email: jyopriyanka016@gmail.com.
Sakila Akter, Email: sakilaakter95@yahoo.com.
Narayan Prosad Maheswary, Email: rajib.chowdhuryk@gmail.com.
Rajaul Karim Khan, Email: md.rajaulkarim@yahoo.com.
Daniel Argaw, Email: danielargaw@gmail.com.
Axel Kroeger, Email: kroegera@who.int.
References
- 1.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh and prospects for improved control. Indian J Med Res. 2006;123:275–288. [PubMed] [Google Scholar]
- 2.Rahman M. Insecticide substitutes for DDT to control mosquitoes may be causes of several diseases. Environ Sci Pollut Res Int. 2013;20:2064–2069. doi: 10.1007/s11356-012-1145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4:e859. doi: 10.1371/journal.pntd.0000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury R, Mondal D, Chowdhury V, Faria S, Alvar J, Nabi SG, et al. How far are we from visceral leishmaniasis elimination in Bangladesh? An assessment of epidemiological surveillance data. PLoS Negl Trop Dis. 2014;8:e3020. [DOI] [PMC free article] [PubMed]
- 5.Annual Report of Department of Health Services 2072/73 (2015/2016) Department of Health Services, Ministry of Health, Government of Nepal. 2016. [Google Scholar]
- 6.World Health Organization . Regional strategic framework for elimination of kala-azar from South East Asia region (2005–2015) New Delhi: SEA-VBC-85-REV-1; 2005. [Google Scholar]
- 7.WHO Regional Committee for South-East Asia . Report of the sixty-seventh session, Dhaka, Bangladesh; 2014 September 9–12. New Delhi: World Health Organization, Regional Office for South-East Asia; 2014. pp. 1–149. [Google Scholar]
- 8.Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: a threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Negl Trop Dis. 2017;11:e0005877. doi: 10.1371/journal.pntd.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiology and Disease Control Division (EDCD). The annual internal assessment of Malaria and Kala-azar control activities 1999. Department of Health Services. Epidemiology and Disease Control Division and Vector Borne Disease Research and Training Centre. Ministry of Health, HMG/N, Nepal; 2000.
- 10.Lal BK. Meeting to update National Strategic Guideline on Kala-azar Elimination Program in Nepal, 8–9 January 2018. Kathmandu; 2018.
- 11.World Health Organization . Instructions for determining the susceptibility or resistance of adults blackflies, sandflies and biting midges to insecticides. Geneva: WHO; 1981. [Google Scholar]
- 12.World Health Organization . Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces, Report of the WHO Informal Consultation. Geneva: WHO; 1998. [Google Scholar]
- 13.Lewis DJ. The phlebotomine sand flies (Diptera: Psychodidae) of oriental region. Bull Br Mus (Nat Hist) Entomol. 1978;37:217–343. [Google Scholar]
- 14.Lewis DJ. A taxonomic review of the genus phlebotomus (Diptera: Psychodidae) Bull Br Mus (Nat Hist). 1982;45:121–209. [Google Scholar]
- 15.Kalra NL, Bang YH. Manual on entomology in visceral leishmaniasis. Document SEA/VBC/35. New Delhi: World Health Organization; 1998. [Google Scholar]
- 16.Bailey MS, Lockwood DN. Cutaneous leishmaniasis. Clin Dermatol. 2007;25:203–211. doi: 10.1016/j.clindermatol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Techniques to detect insecticide resistance mechanism (Field and laboratory manual); World Health Organization, Department of Disease Prevention & Control, WHO Communicable Diseases (CDS); WHO/CDS/CPCIMALl98.6; 1998. http://apps.who.int/iris/bitstream/10665/83780/1/WHO_CDS_CPC_MAL_98.6.pdf; Accessed 14 Aug 2017.
- 18.Brogdon WG, McAllister JC. Insecticide resistance and vector control. J Agromedicine. 2004;9:329–45. [PubMed]
- 19.World Health Organization. Pesticides and their application for the control of vectors and pests of public health importance (6th edition). 2006. Department of Control of Neglected Tropical Diseases, WHO Pesticide evaluation scheme (WHOPES). http://apps.who.int/iris/bitstream/10665/69795/1/WHO_CDS_NTD_WHOPES_GCDPP_2006.1_eng.pdf; Accessed 25 July 2017.
- 20.World Health Organization . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes (2nd edition). Global Malaria Programme. Geneva: World Health Organization; 2016. [Google Scholar]
- 21.Chowdhury R, Huda MM, Kumar V, Das P, Joshi AB, Banjara MR, et al. The Indian and Nepalese programmes of indoor residual spraying for the elimination of visceral leishmaniasis: performance and effectiveness. Ann Trop Med Parasitol. 2011;105:31–35. doi: 10.1179/136485911X12899838683124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondal D, Alam MS, Karim Z, Haque R, Boelaert M, Kroeger A. Present situation of vector control in Bangladesh: a wake up call. Health Policy. 2008;87:369–376. doi: 10.1016/j.healthpol.2008.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.