Abstract
The morphological and physiological characteristics of Arabidopsis accessions differing in their phosphate acquisition efficiencies (PAEs) when grown on a sparingly soluble phosphate source (hydroxylapatite) were analyzed. A set of 36 accessions was subjected to an initial PAE evaluation following cultivation on synthetic, agarose-solidified media containing potassium phosphate (soluble) or hydroxylapatite (sparingly soluble). From the five most divergent accessions identified in this way, C24, Co, and Cal exhibited high PAEs, whereas Col-0 and Te exhibited low PAEs. These five accessions were analyzed in detail. Significant differences were found in root morphology, phosphate uptake kinetics, organic acid release, rhizosphere acidification, and the ability of roots to penetrate substrates. Long root hairs at high densities, high uptake per unit root length, and high substrate penetration ability in the efficient accessions C24 and Co mediate their high PAEs. The third accession with high PAE, Cal, exhibits a high shoot-to-root ratio, long roots with long root hairs, and rhizosphere acidification. These results are consistent with previous observations and highlight the suitability of using Arabidopsis accessions to identify and isolate genes determining the PAE in plants.
Phosphorus is a major mineral nutrient required by plants, but is one of the most immobile, inaccessible, and unavailable nutrients present in soils (Holford, 1997). In most soils, phosphorus is available primarily as cation precipitates or poorly soluble organic compounds or is bound to soil particles. The main inorganic phosphates in the soil are iron, aluminum, and calcium phosphates. Iron and aluminum phosphates are the major phosphate compounds in acidic soils, whereas calcium phosphates dominate in neutral to alkaline soil. At higher pH, calcium phosphate is present in the soil as hydroxylapatite [Ca5(PO4)3OH] or substituted apatite. Because these inorganic forms of phosphorus are sparingly soluble and organic phosphate must be enzymatically cleaved before uptake, phosphorus is often not readily accessible to plants. Its availability is a major growth-limiting factor for plants in many soils (Barber et al., 1963). The amelioration of phosphate deficiency by the application of costly and environmentally hazardous phosphate fertilizers is not an ideal solution and has raised serious concerns about the continued viability of contemporary agriculture practice. This has led to a search for more environmentally friendly and economically feasible strategies to improve crop production in low phosphorus soils. In an ideal manner, such strategies should enable the efficient use of phosphorus already present in the soil. A prerequisite for strategy development is the identification of plant traits that limit or enhance the uptake and utilization of phosphorus.
Plant species differ in the efficiency with which they acquire and utilize phosphorus. Phosphate acquisition efficiency (PAE) relates to the different extents to which plants are able to mobilize phosphorus from poorly soluble sources or to take up the soluble phosphorus available in the soil solution. Higher plants show several morphological and physiological adaptations that enable them to acquire phosphorus from sparingly soluble phosphorus soil fractions. These adaptations include root system enlargement (Anghinoni and Barber, 1980; Lynch, 1995), arbuscular mycorrhiza establishment (Smith et al., 1993), increased organic acid exudation (Johnson et al., 1996), rhizosphere acidification (Moorby et al., 1988), increased production of phosphatases (Duff et al., 1989; Goldstein, 1992; Loffler et al., 1992; Green, 1994; Bariola et al., 1994), and enhanced phosphate uptake rate (Schachtman et al., 1998). The genetic characterization of these mechanisms in crop plants is difficult because of the high cost and the large amount of time required for analysis. Furthermore, the analysis of these mechanisms is complicated by the existence of several potential sites of control and by the presence of adaptive responses. Screening programs directed toward the identification of adaptations in efficient genotypes should target quantitative processes and take into account the environmental factors that modify them (Smith et al., 1993).
The genetic model plant Arabidopsis, with its worldwide distribution, range of observed morphological variation, and its ability to adapt to different habitats and photoperiods (Karlsson et al., 1993) provides a good system with which to analyze phosphate efficiency. With the exception of mycorrhizal infections, all of the major characteristic responses of crop plants to phosphate starvation (such as changes in root-to-shoot ratio, anthocyanin accumulation in shoots, and increased phosphatase activity) also occur in Arabidopsis (Trull et al., 1997). In addition, Arabidopsis, due to its small size, can be grown at a high density under defined nutrient conditions, which facilitates the analysis of PAE. An approximate 400 diverse Arabidopsis accessions collected from different geographical locations are available through standardized sources including the following Arabidopsis stock centers: Arabidopsis Biological Resource Center, SENDAI Arabidopsis Seed Stock Center, and Nottingham Arabidopsis Stock Centre. The natural variations in PAE among these accessions provide a valuable resource for analyzing phosphate efficiency. One goal of this study was to characterize the variation in phosphorus acquisition from hydroxylapatite (HA), one of the major forms of phosphorus in alkaline soils, among Arabidopsis accessions. The other goal was to reveal potential morphological and physiological mechanisms involved in achieving high PAEs.
RESULTS
HA-PAE of Five Divergent Arabidopsis Accessions
A set of 36 different Arabidopsis accessions was subjected to a pre-screen for differences in phosphorus accumulation on HA medium. The five most divergent accessions selected for this study were C24, Co, and Cal with the highest and Col-0 and Te with the lowest abilities of HA-PAE. These accessions were studied in detail for PAE-related characteristics.
The five selected Arabidopsis accessions were evaluated in detail for their PAEs. To this end, shoot dry masses (Table I) and shoot phosphate contents (Table II) were measured for plants grown in modified MS medium (0.15% or 0.4% [w/v] agarose) containing HA (0.5 g L−1) or soluble phosphate (1 mm KH2PO4), respectively. The shoot dry mass values for accessions C24, Co, and Cal grown in HA medium solidified with 0.4% (w/v) agarose were higher than those of Col-0 and Te. When calculated as a percentage of those accumulated on KH2PO4 medium, the shoot dry masses of C24, Co, and Cal accessions ranged between 30% and 31%, whereas those of Col-0 and Te were significantly lower and ranged between 21% and 25%. In accordance with this, the latter two accessions were classified as inefficient.
Table I.
Shoot dry masses of five Arabidopsis accessions after growth in media containing HA or KH2PO4, respectively.
| Accession | Shoot Dry Mass on MS Medium Solidified
with 0.15% (w/v) Agarose
|
Shoot Dry Mass on MS Medium
Solidified with 0.4% (w/v) Agarose
|
Shoot Dry Mass on HA
as a Percentage of Those on KH2PO4
|
|||
|---|---|---|---|---|---|---|
| HA (0.5 g L−1) | KH2PO4 (1 mm) | HA (0.5 g L−1) | KH2PO4 (1 mm) | 0.15% Agarose | 0.4% Agarose | |
| mg shoot−1 | ||||||
| C24 | 1.43 c | 5.52 b | 1.64 a | 5.23 b | 26 d | 31 a |
| Co | 1.48 c | 5.58 b | 1.71 a | 5.57 b | 27 d | 31 a |
| Cal | 1.87 b | 6.2 ab | 1.75 a | 5.83 ab | 30 c | 30 a |
| Col-0 | 2.64 a | 7.1 a | 1.28 b | 6.00 a | 37 b | 21 b |
| Te | 1.6 c | 3.9 c | 0.97 c | 3.87 c | 40 a | 25 b |
For each measurement, accession seedlings were grown for 24 d in modified Murashige and Skoog (MS) media (pH 7.0) containing sparingly soluble phosphate (0.5 g L−1 HA) or soluble phosphate (1 mM KH2RO4) solidified with 0.15% (w/v) agarose or 0.4% (w/v) agarose. Data are means of three replicate measurements of 10 pooled shoots each. Significant differences within columns (P ≤ 0.05) are indicated by different characters.
Table II.
Phosphate acquisition and PAE of five Arabidopsis accessions
| Accession | Plants Grown in Modified MS Medium
Solidified with 0.15% (w/v) Agarose (P contents in
shoot)
|
Plants Grown in Modified MS Medium Solidified
with 0.4% (w/v) Agarose (P contents in shoot)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 g L−1 HA | 1 mm KH2PO4 | PAE | 0.5 g L−1 HA | 1 mm KH2PO4 | PAE | |||||
| nmol mg−1 | Dry wt shoot nmol shoot−1 | nmol mg−1 | Dry wt shoot nmol shoot−1 | % | nmol mg−1 | Dry wt shoot nmol shoot−1 | nmol mg−1 | Dry wt shoot nmol shoot−1 | % | |
| C24 | 90 a | 130 cd | 213 b | 1,173 b | 11.0 ab | 61 a | 100.5 a | 236 b | 1,242 d | 8.1 a |
| Co | 98 a | 136 c | 217 b | 1,206 b | 11.8 a | 60 a | 102.5 a | 253 b | 1,404 c | 7.3 a |
| Cal | 85 a | 159 b | 326 a | 2,025 a | 7.9 c | 61 a | 106.0 a | 406 a | 2,370 a | 4.5 b |
| Col-0 | 77 a | 203 a | 304 a | 2,170 a | 9.3 bc | 57 a | 73.0 b | 358 a | 2,150 b | 3.4 c |
| Te | 82 a | 115 d | 292 a | 1,154 b | 11.4 ab | 47 b | 45.5 c | 366 a | 1,410 c | 3.2 c |
For each measurement, seedlings were grown for 24 d in modified MS media (pH 7.0) containing sparingly soluble phosphate (0.5 g L−1 HA) or soluble phosphate (1 mm KH2PO4) solidified with 0.4% or 0.15% (w/v) agarose. Data are means of three replicate measurements of 10 pooled shoots each. Significant differences between accessions (P ≤ 0.05) are indicated by different characters. Mean PAE (%) = mean (phosphate contents in shoot [nmol shoot−1] under phosphate-deficient conditions [0.5 g L−1 HA]/phosphate contents [nmol shoot−1] under normal phosphate supply [1 mm KH2PO4]) × 100.
This classification was confirmed by the analysis of the shoot phosphate contents accumulated when the plants were grown under phosphate-limiting conditions (in hydroxylapatite medium solidified with 0.4% [w/v] agarose). C24, Co, and Cal accumulated significantly more phosphate than Col-0 and Te (Table II). Although C24 and Co acquired more phosphate when supplied with HA, they showed relatively low phosphate contents after growth in soluble phosphate medium (phosphate-sufficient conditions). In comparison to the other accessions, Cal attained relatively high phosphate contents and Te relatively low phosphate contents regardless of the phosphate sources provided. C24 and Co showed the highest efficiencies in phosphorus acquisition from HA (expressed as a percentage of phosphorus accumulated from HA/phosphorus accumulated from KH2PO4), which were approximately 2.5 times greater than those of the less efficient accessions Col-0 and Te (Table II). The PAE of the accession Cal was intermediate between the two groups.
To study the effect of growth medium agar density on phosphate efficiency, the five Arabidopsis accessions were grown on low-density (0.15% [w/v] agarose) modified MS medium containing HA (0.5 g L−1) or soluble phosphate (1 mm KH2PO4). The shoot dry masses of efficient accessions C24 and Co grown in HA calculated as a percentage of those in KH2PO4 medium under these conditions were, however, lower than those of Cal, Col-0, and Te (Table I).
All accessions accumulated more phosphate when grown in low-density (0.15%) agarose HA medium as compared with their phosphate contents on high-density agarose (0.4%) HA medium. The relative differences in phosphorus accumulation from the two media were more dramatic in the inefficient accessions, Col-0 and Te, than in the efficient accessions, C24 and Co. While the latter accumulated 37% and 54% more phosphate from the 0.15% (w/v) agarose medium, the increases in Col-0 and Te were 213% and 211%, respectively. The relative differences in phosphate concentration in the shoot tissue (nmol mg−1) of the plants grown on the two media were, however, unchanged (Table II). When grown in the low-density medium, the less efficient accession Col-0 acquired 203 nmol phosphate shoot −1 from sparingly soluble sources, which is approximately 50% more than that of efficient accessions C24 and Co. Furthermore, the differences in PAE between the accessions were less pronounced under these conditions and the ranking of the accessions was different.
Characterization of Morphological and Physiological Attributes Related to PAE among the Five Divergent Arabidopsis Accessions
Previous studies on plant responses to phosphate deficiency have shown that plant characteristics likely to be involved in the determination of the PAE include seed size (Sadeghian, 1991; Liao and Yan, 1999), root system size (Lynch and van Beem, 1993; Gahoonia et al., 1997), phosphate uptake rates (Schachtman et al., 1998), proton release (Moorby et al., 1988), and organic acid excretion (Van den Boogaard et al., 1992). In this study experiments were carried out to evaluate these attributes and to determine their relative contributions to the PAEs of the five divergent Arabidopsis accessions.
Seed Size
The comparison of seed masses (mg−1 1,000 seeds) of the five accessions shows that the efficient accession Cal has slightly higher seed mass. The other four accessions did not differ significantly in their seed masses (Table III). Therefore, variation in seed size could not account for the difference in dry masses observed for the accessions grown in hydroxylapatite medium.
Table III.
Seed sizes and shoot-to-root ratios of five Arabidopsis accessions
| Accessions | C24 | Co | Cal | Col-0 | Te |
|---|---|---|---|---|---|
| 1,000 Seeds dry mass (mg) | 17.3 ± 0.4 b | 17.0 ± 0.4 b | 18.2 ± 0.6 a | 16.5 ± 0.4 c | 16.7 ± 0.3 bc |
| Shoot/root at 0.5 g L−1 HA | 1.66 ± 0.21 b | 1.63 ± 0.16 b | 2.59 ± 0.10 a | 1.51 ± 0.11 c | 1.54 ± 0.13 c |
| Shoot/root at 1 mm KH2PO4 | 3.12 ± 0.30 bc | 3.44 ± 0.49 b | 4.05 ± 0.31 a | 2.70 ± 0.82 c | 2.07 ± 0.55 c |
One thousand dried seeds were sampled and weighed. Each value is the mean ± sd of three determinations. For each shoot-to-root ratio measurement, 25 seedlings were grown for 24 d in modified MS media (pH 7.0) containing sparingly soluble phosphate (0.5 g L−1 HA) or soluble phosphate (1 mm KH2PO4) solidified with 0.15% (w/v) agarose. Data are means ± sd of three replicate measurements of 10 pooled shoots and roots. Significant differences within Arabidopsis accessions (P ≤ 0.05) are indicated by different characters.
Shoot-to-Root Ratios
It has been generally observed that relative root growth appears to decrease with increasing phosphorus levels, whereas an adequate supply of inorganic phosphate significantly increases the formation of shoot dry matter. To determine whether the differences in PAE among the five Arabidopsis accessions are due to unequal growth partitioning, the shoot-to-root dry mass ratios of the accessions were compared. The ranking of shoot-to-root dry mass ratios of the five Arabidopsis accessions was found to be: Cal > C24 ≈ Co > Col-0 ≈ Te. The accession Cal had the highest shoot-to-root ratios in all measurements. When grown in HA medium all accessions showed a decreased shoot-to-root ratio in comparison to the situation in the soluble phosphate medium. The decrease in the efficient accessions (C24, Co, and Cal), however, was lower in comparison to that of the inefficient accessions (Col-0 and Te). In general, the efficient accessions appeared to have higher shoot-to-root ratios than the inefficient accessions (Table III).
Root Morphology
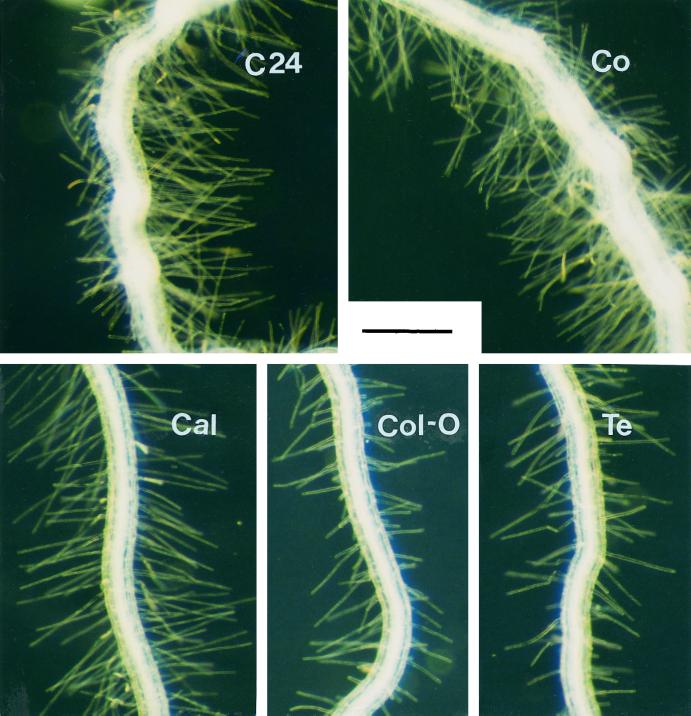
A number of root traits, including the size and structure of the root system, appear to affect PAE (Bates and Lynch, 1996). Therefore, it was desirable to determine the morphological differences in the root system among selected accessions to provide basic architectural information and to detect its potential correlations with the observed differences in PAE. The accessions differed significantly in root length, root hair length, and root hair density (Fig. 1; Tables IV and V).
Figure 1.
Root hairs in the primary roots of five Arabidopsis accessions. Seedlings were germinated and grown for 16 d in phosphate-free medium solidified with 0.15% (w/v) agarose. The regions of the roots where root hairs ceased to increase and had a uniform maximum size were observed under a binocular microscope and photographed. Bar represents 0.5 mm.
Table IV.
Root lengths, root diameters, and substrate penetration abilities of five Arabidopsis accessions
| Accessions | Root Length in MS
Medium
|
Root Diameter in MS Medium
|
Penetration Ability | ||
|---|---|---|---|---|---|
| Solidified with 0.4% (w/v) agarose | Solidified with 0.15% (w/v) agarose | Solidified with 0.4% (w/v) agarose | Solidified with 0.15% (w/v) agarose | ||
| cm | mm | % | |||
| C24 | 1.30 ± 0.19 c | 1.58 ± 0.30 c | 0.18 ± 0.01 | 0.18 ± 0.01 | 85.0 ± 18.2 a |
| Co | 1.18 ± 0.17 d | 1.50 ± 0.28 c | 0.18 ± 0.01 | 0.18 ± 0.01 | 79.6 ± 19.6 a |
| Cal | 1.85 ± 0.40 a | 2.80 ± 0.45 a | 0.17 ± 0.01 | 0.17 ± 0.01 | 74.0 ± 25.6 a |
| Col-0 | 1.55 ± 0.19 b | 3.02 ± 0.44 a | 0.16 ± 0.01 | 0.16 ± 0.01 | 53.4 ± 11.8 b |
| Te | 1.00 ± 0.23 e | 2.34 ± 0.60 b | 0.16 ± 0.01 | 0.16 ± 0.01 | 43.7 ± 12.7 b |
Root parameters were determined by microscopic observation of 16-d-old-seedlings grown in phosphate-free MS medium solidified with 0.4% (w/v) or 0.15% (w/v) agarose. Data are means ± sd of three replicate analyses of 20 seedling roots each. Significant differences within columns (P ≤ 0.05) are indicated by different characters. Mean penetration ability (%) = mean (root length at 0.4% [w/v] agarose MS medium/root length at 0.15% [w/v] agarose MS medium) × 100.
Table V.
Root hair lengths and densities of five Arabidopsis accessions
| Accessions | Root Hair Length in −P MS
Medium
|
Root Hair Density in −P MS Medium
|
||
|---|---|---|---|---|
| Solidified with 0.4% (w/v) agarose | Solidified with 0.15% (w/v) agarose | Solidified with 0.4% (w/v) agarose | Solidified with 0.15% (w/v) agarose | |
| mm | number mm−1 rootlength | |||
| C24 | 0.46 ± 0.09 a | 0.51 ± 0.08 a | 66 ± 9 a | 70 ± 9 a |
| Co | 0.47 ± 0.08 a | 0.50 ± 0.09 a | 64 ± 10 a | 72 ± 10 a |
| Cal | 0.35 ± 0.07 b | 0.58 ± 0.08 a | 42 ± 6 b | 39 ± 6 b |
| Col-0 | 0.30 ± 0.07 c | 0.35 ± 0.07 b | 38 ± 7 b | 40 ± 7 b |
| Te | 0.32 ± 0.13 c | 0.39 ± 0.12 b | 39 ± 6 b | 44 ± 6 b |
Root hair parameters were determined by microscopic observation and image analysis of 16-d-old-seedlings grown in phosphate-free modified MS medium solidified with 0.4% (w/v) agarose or 0.15% (w/v) agarose. Data are means ± sd of three replicate analyses of 20 seedling roots each. Significant differences within columns (P ≤ 0.05) are indicated by different characters.
When grown in 0.4% (w/v) agarose medium, the ranking of accessions according to their root length was Cal > Col-0 > C24 > Co > Te. The efficient accessions C24 and Co had relatively short roots, but their root hairs were the longest and had the highest density (Tables IV and V). Root hair length and density was intermediate in Cal and low for the less efficient accessions, Col-0 and Te. The high phosphate efficiency observed in C24 and Co thus coincided with the presence of long, dense root hairs.
When grown in 0.15% (w/v) agarose medium, plants of all accessions developed longer roots, but the relative differences in root length were accession specific (Table IV). Thus Te displayed the largest difference with 2.3-fold longer roots established in the 0.15% (w/v) agarose medium as compared with the roots grown in 0.4% (w/v) agarose medium. In the 0.15% (w/v) agarose medium Col-0 had the longest roots, approximately 2 times longer than those of C24 and Co. In accordance with this, the ranking of accessions with respect to their root lengths was Col-0 ≈ Cal > Te > C24 ≈ Co. When grown in 0.15% (w/v) agarose medium only Cal plants developed considerably longer root hairs as compared with the 0.4% (w/v) agarose medium (Table V). Only very small differences were observed in the root hair densities of plants grown in high and low strength medium within the accessions (Table V). The increased root lengths (and root hair length in Cal) in 0.15% (w/v) agarose medium coincided with strong increases in the PAEs of the accessions Cal, Col-0, and Te (Table II).
The observed differences in root lengths established upon growth in the 0.15% and 0.4% (w/v) agarose media were used to derive a measure for the substrate penetration ability of the roots of the different accessions. Determined as the ratios of the root lengths in 0.4% (w/v) agarose medium to the root lengths in 0.15% (w/v) agarose medium, substrate penetration abilities were higher for roots of the phosphate efficient accessions C24, Co, and Cal than for the inefficient accessions Col-0 and Te (Table IV).
Phosphate Uptake Kinetics
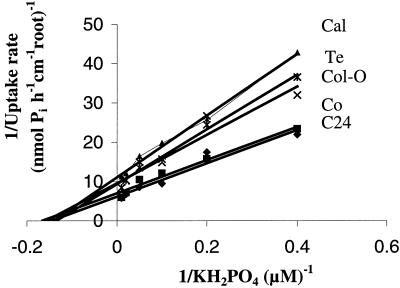
Accessions may vary in their effectiveness in acquiring phosphate through different levels of expression of transport proteins in roots (Imax), through differences in the affinity (Km) for phosphate, or through variation in Cmin (the minimum phosphate concentration in the growth medium at which no net uptake occurs into the roots) for phosphate. Phosphate uptake of the five Arabidopsis accessions was measured using seedlings grown in liquid culture for 7 to 8 d. The results of short-term uptake experiments performed with the plants grown in phosphate-sufficient (0.5 mm KH2PO4) or phosphate-deficient (2.5 μm KH2PO4) medium are shown in Table VI. Phosphate uptake over a concentration range of 0 to 300 μm can be described by Michaelis-Menten kinetics (Barber, 1995). Phosphate uptake was measured for concentrations ranging from 2.5 to 100 μm KH2PO4 (Fig. 2). Michaelis-Menten parameters describing the kinetics of phosphorus uptake were estimated separately for each accession by linear regression analysis.
Table VI.
Phosphate uptake kinetic parameters of five Arabidopsis accessions
| Accessions | Apparent
Km
|
Imax
|
Cmin | ||||
|---|---|---|---|---|---|---|---|
| 500 μm Phosphate | 2.5 μm Phosphate | 500 μm Phosphate | 2.5 μm Phosphate | 500 μm Phosphate | 2.5 μm Phosphate | ||
| μm | pmol h−1 cm−1 rootlength | nmol h−1g−1 fresh wt plant | nm | ||||
| C24 | 3.64 ± 0.72 | 6.84 ± 1.10 | 103 ± 19 a | 162 ± 35 a | 118 ± 22 b | 322 ± 70 b | 9 ± 3 ab |
| Co | 3.44 ± 0.65 | 6.00 ± 1.32 | 99 ± 22 a | 142 ± 25 a | 117 ± 26 b | 300 ± 53 b | 10 ± 3 a |
| Cal | 4.08 ± 1.26 | 7.07 ± 1.45 | 85 ± 18 ab | 90 ± 22 b | 181 ± 38 a | 447 ± 69 a | 11 ± 4 a |
| Col-0 | 3.92 ± 1.32 | 6.37 ± 1.61 | 74 ± 11 ab | 104 ± 10 b | 150 ± 22 a | 440 ± 42 a | 7 ± 1 b |
| Te | 3.91 ± 1.09 | 7.40 ± 2.1 | 62 ± 15 b | 107 ± 16 b | 149 ± 36 a | 494 ± 74 a | 11 ± 3 a |
The apparent kinetic parameters Km and Imax were determined by plotting phosphate uptake for 1 h as a function of phosphate concentrations. Uptake experiments were performed with 7- to 8-d-old-seedlings pre-grown in modified 0.5× Hoagland medium containing 500 or 2.5 μm KH2PO4, respectively. Cmins were determined through 7 h depletion of a solution initially containing 0.5 μm KH2PO4 by 10-d-old-seedlings pre-grown in modified 0.5× Hoagland medium lacking phosphate. Data are means ± sd of three replicate measurements of 300 seedlings each. Significant differences within columns (P ≤ 0.05) are indicated by different characters.
Figure 2.
Lineweaver-Burke plots of phosphate uptake into roots of five Arabidopsis accessions. For each measurement, 300 seedlings (on three nylon meshes) were grown in a modified 0.5× Hoagland medium containing 2.5 μm KH2PO4. Phosphate concentrations in the uptake solutions ranged from 2.5 to 100 μm. Plotted values are means of three replicate measurements. YC24 = 42.344XC24 + 6.1877, R2C24 = 0.9481; YCo = 42.162XCo + 7.0285, R2Co = 0.9723; YCal = 78.462XCal + 11.086, R2Cal = 0.993; YCol-0 = 61.345XCol-0 + 9.6375, R2Col-0 = 0.9262; YTe = 69.503XTe + 9.3792, R2 Te = 0.9829.
The apparent Km of the phosphate transporters of all five accessions were determined to be approximately 6.0 to 7.0 (±2.0) μm, which is characteristic for high affinity transporters. Although there were no significant differences among accessions for the estimated apparent Km value for phosphate-starved plants, the estimated Imax (pmol h−1 cm−1 root) values for C24 and Co were greater by a factor of about 1.5 than those of Cal, Co, and Col-0. Thus phosphate-starved C24 and Co plants show the highest phosphorus uptake rates per unit root length. On a plant biomass basis, however, the uptake rates of Cal, Col-0, and Te are higher (Table VI). This difference is due to the variation in root lengths, which, under the condition used, were greater for Cal, Col-0, and Te than for C24 and Co.
Another physiological parameter Cmin that can affect nutrient absorption was determined for the five accessions, but no obvious differences were observed. All accessions were able to deplete the 0.5-μm KH2PO4 solutions to between 7 and 11 nm (Table VI).
Organic Acid Exudations
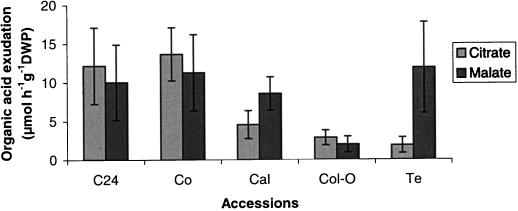
Organic acids released from the roots of phosphorus-efficient plants increase the availability of phosphorus by mobilizing sparingly soluble forms of phosphate such as calcium-phosphate (Dinkelaker et al., 1989). To determine if a similar mechanism operates in Arabidopsis, the accessions were examined for organic acid root exudation triggered by phosphate deficiency. Citric, malic, and succinic acids were measured in the root exudates of 8-d-old plants. The amount of organic acids released from roots of phosphorus-starved plants (grown at 10 μm KH2PO4) differed from those of plants grown in phosphorus-sufficient conditions (0.5 mm KH2PO4). When plants were supplied with adequate phosphorus, organic acids were released in very low amounts by all accessions. In contrast, these acids were present in higher concentrations in the bathing solutions when plants were pre-cultivated for 8 d under phosphate deficient conditions. The amounts of citric and malic acids released from the roots differed among accessions (Table VII; Fig. 3).
Table VII.
Organic acid exudates from roots of five Arabidopsis accessions
| Accessions | Organic Acids
Exudation
|
Organic Acids Exudation
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0.5
mm Phosphate
|
10 μm
Phosphate
|
0.5 mm Phosphate
|
10
μm Phosphate
|
|||||
| Citrate | Malate | Citrate | Malate | Citrate | Malate | Citrate | Malate | |
| μmol h−1 g−1 dry wt plant | pmol h−1 cm−1 root | |||||||
| C24 | 2.6 ± 1.8 a | 2.2 ± 2.0 a | 12.2 ± 4.9 a | 10.0 ± 4.9 a | 172 ± 125 a | 145 ± 132 a | 428 ± 172 a | 391 ± 172 a |
| Co | 2.0 ± 1.5 ab | 2.0 ± 1.8 a | 13.6 ± 3.4 a | 11.6 ± 4.9 a | 120 ± 90 ab | 120 ± 108 a | 440 ± 125 a | 426 ± 180 a |
| Cal | 0.9 ± 0.8 b | 1.6 ± 1.0 ab | 4.5 ± 1.8 b | 8.5 ± 2.2 a | 33 ± 29 b | 59 ± 36 ab | 90 ± 36 b | 169 ± 43 b |
| Col-0 | 0.5 ± 0.5 b | 0.5 ± 0.2 b | 2.8 ± 1.0 c | 2.0 ± 1.0 b | 18 ± 18 b | 18 ± 8 c | 60 ± 21 bc | 42 ± 21 c |
| Te | 0.6 ± 0.5 b | 2.0 ± 1.0 a | 1.9 ± 1.0 c | 11.9 ± 5.9 a | 14 ± 12 b | 48 ± 24 b | 29 ± 16 c | 186 ± 92 b |
For each measurement, 400 seedlings (on four nylon meshes each with 100 seedlings) were grown for 8 d in a modified 0.5× Hoagland nutrient solution containing 10 μm KH2PO4 or 0.5 mm KH2PO4, respectively. Root exudates were collected in water for 1 h, concentrated, and analyzed using HPLC. Data are means ± sd of three replicate measurements with 400 seedlings each. Significant differences within columns (P ≤ 0.05) are indicated by different characters.
Figure 3.
Organic acid exudations (citrate and malate) by the roots of five Arabidopsis accessions. For each measurement, 400 seedlings (on four nylon meshes) were grown for 8 d in modified 0.5× Hoagland nutrient solution containing 10 μm KH2PO4. Root exudates were collected for 1 h, concentrated, and analyzed using HPLC. Each bar represents the total micromoles of organic acid released per gram seedling dry weight. Data are means ± sd of three replicate measurements.
In all accessions citrate and malate are the predominant organic acids released. The kinds and amounts of exuded organic acids appeared to be accession specific. In the accessions C24, Co, and Col-0, citrate exudation is slightly higher than is the exudation of malate, whereas in the accessions Cal and Te, malate is released in higher amounts than is citrate (in Te, malate exudation is six times higher than citrate).
In the efficient accessions C24 and Co the amounts of citrate and malate (μmol h−1 g−1 dry mass plants) in root exudates are higher than those of Cal, Col-0, and Te. When calculated on the basis of root lengths, these differences are even more dramatic. The accumulation of organic acids in the rhizosphere of C24 and Co plants is therefore expected to be drastically higher than that of Cal, Col-0, and Te plants (Table VII).
pH Changes and Its Effect on Phosphate Solubility
In alkaline soils, phosphorus mobilization occurs predominantly through rhizosphere acidification. To test whether organic anions are released in association with protons and whether rhizosphere acidification is correlated with organic anion secretion in Arabidopsis, pH changes in the growth media were measured.
As expected, since nitrogen was supplied exclusively in the NO3− form, cultivation of plants from all accessions resulted in pH increase of the medium. The observed pH changes in the growth medium, however, did not correlate to organic acid exudation. No significant differences in the pH of growth medium of high organic acid exuding, phosphate-efficient accessions (C24 and Co) and low organic acid exuding, phosphate-inefficient accessions (Col-0 and Te) were observed. Of all the accessions only Cal showed a slightly enhanced release of protons (Table VIII). Consistent with this observation, the accession Cal showed the highest PAE at higher buffer concentration (100 mm MOPS [3-(N-morpholino)-propanesulfonic acid], pH 7.0; data not shown).
Table VIII.
pH changes in growth media of five Arabidopsis accessions
| Accession Medium | C24 | Co | Cal | Col-0 | Te | Control Medium |
|---|---|---|---|---|---|---|
| pH | 5.65 ± 0.06 a | 5.64 ± 0.07 ab | 5.43 ± 0.06 d | 5.55 ± 0.08 c | 5.60 ± 0.10 b | 4.48 ± 0.05 |
For each measurement, 300 seedlings were grown aseptically for 10 d in a phosphate-free modified MS nutrient solution. pH of the growth medium was measured using a pH meter. Data are means ± sd of three replicate measurements. Significant differences (P ≤ 0.05) are indicated by different characters.
DISCUSSION
By analysis of Arabidopsis accessions, morphological and physiological traits that are likely to affect the PAE of higher plants were identified. At first the experiments were focused on identifying Arabidopsis accessions exhibiting PAE variations. Five divergent accessions selected after screening 36 accessions were analyzed in detail to characterize the adaptations responsible for PAE on HA. Different results may be obtained upon supply of the plants with other forms of sparingly soluble phosphate such as organic phosphate, iron phosphate, and aluminum phosphate. To be able to modify precisely and reproducibly growth conditions such as phosphorus source and supply, as well as the strength of the substrate, the experiments were performed in agarose-solidified modified MS medium. This experimental setup furthermore allowed in situ root morphology analysis. Similar conditions were used in previous studies on phosphate uptake in Arabidopsis accessions (Krannitz et al., 1991), on isolation and characterization of the Arabidopsis pho1 and pho2 mutants (Poirier et al., 1991; Delhaize and Randall, 1995), as well as mutants altered in organic acid exudation (Larsen et al., 1996). Potential effects of oxygen gradients in the media (Chung and Ferl, 1999) were restricted by the use of a maximum thickness of the media of 4 mm. A potential influence of oxygen gradients on root growth was furthermore excluded by the use of media with different agarose concentrations in which root growth rates were different and in which different relative PAEs were observed. The C24, Co, and Cal accessions showed relatively high shoot phosphate accumulation when grown in medium containing sparingly soluble phosphate and solidified with 0.4% (w/v) agarose, whereas accessions Col-0 and Te acquired less phosphate under identical conditions. However, accessions Cal, Col-0, and Te showed increased phosphate contents on low-density agarose medium, apparently due to dramatic increases in root length. Variations among these accessions in PAE-related traits such as root morphology (root length, root hair length, and root hair density), transport activity per unit length, organic acid exudation, and root penetration ability have been identified. Most probably, however, the predominant parameter determining the PAEs of the analyzed Arabidopsis accessions is the root morphology affected by the strength of the growth substrate.
Effect of Root Morphology and Penetration Ability on Phosphate Efficiency
Root hairs substantially extend the root surface area available for nutrient uptake from the soil. Theoretical calculations (Nye, 1966), autoradiographs (Lewis and Quirk, 1967), mechanistic models (Föhse et al., 1991), and experimental evidence (Gahoonia and Nielsen, 1998) suggest a relationship between root hair length and density and phosphate uptake from the soil. The Arabidopsis accessions tested for root morphology differed widely in their root hair parameters. The accessions C24, Co, and Cal have long, dense root hairs, whereas Col-0 and Te have shorter and fewer root hairs. The contribution of root hairs to phosphate acquisition depends upon the diffusion of nutrients (Barber and Silberbrush, 1984). Long root hairs may intercept the phosphate diffusing toward the root at some distance. If the root hairs are short and develop in pre-existing zones depleted by the absorption of phosphate by the rhizodermis, they contribute only a little to phosphate acquisition. The presence of long and dense root hairs in C24, Co, and Cal might well explain the superior PAE exhibited by these accessions when grown in HA medium. A wide variation in root hairs of cereal varieties and the correlation of long root hairs to high phosphate uptake has been reported (Gahoonia et al., 1997). Root hair growth appears to be controlled by multiple genetic loci and to be strongly influenced by environmental conditions (Schiefelbein and Somerville C, 1990).
Root parameters were measured under two different conditions since it was found that they changed with changes in growth medium density (Tables IV and V). Root elongation of the five accessions decreased when the growth media agarose concentration increased (0.15%–0.4%). The high acquisition of phosphate from sparingly soluble phosphate medium, as well as from full phosphate medium in Cal is probably due to its long roots and long root hairs (i.e. high general uptake efficiency). The comparison of the root lengths of the accessions furthermore showed that the efficient accessions C24 and Co have slightly shorter roots as compared with the less efficient accession Col-0. C24 and Co, however, display highest root hair densities and root hair lengths among all accessions tested when grown in 0.4% (w/v) agarose medium. The inefficient accessions Col-0 and Te have much shorter and fewer root hairs. However, when grown in low-density agarose medium (0.15% [w/v] agarose), Col-0 and Te showed strongly increased PAEs. In case of Col-0 this results in a PAE equivalent to those exhibited by the efficient accessions C24 and Co. The change in phosphate efficiency of accessions Col-0 and Te under low agarose density is obviously due to the dramatic increase in their root lengths, and consequently their root surface areas and, therefore, the volume of the medium, which they can exploit.
The development of a root system capable of anchoring the shoot and obtaining sufficient water and nutrients is essential for the survival of most terrestrial plants (Bengough et al., 1997). In the soil, roots experience a range of penetration resistances depending upon soil type. Since the selected accessions were sampled from different habitats, their adaptations potentially included differences in soil penetration abilities. The differential changes in the root growth of the different accessions with varying densities of the agarose medium reflect differences in their penetration abilities. The root penetrating abilities of C24 and Co were found to be approximately two times higher than those of Col-0 and Te. In accordance with this, the C24 and Co accessions showed higher PAEs when grown in high-density agarose medium (0.4%).
Mucilage exudation and sloughing of root cap cells may be involved in decreasing the frictional resistance in soil penetration by roots. Increases in the exudation of mucilage are found in roots growing in compressed soil when compared with those in loose soil (Boeuf-Tremblay et al., 1995). Whether similar mechanism influences the root penetration ability in Arabidopsis is presently unknown.
Diversity among Accessions in Phosphate Uptake
The results of uptake measurements show that the accessions differed in their phosphate uptake characteristics. Differences in the phosphate uptake have also been identified among crops plants (Barber, 1995; Horst et al., 1996; Römer and Schenk, 1998). In the uptake experiments described here all Arabidopsis accessions showed single-phase Michalis-Menten kinetics when supplied with phosphate in the range of 2.5 to 100 μm. The apparent Km values of phosphate uptake, determined for plants of the accessions C24, Co, Cal, Col-0, and Te grown under phosphate deficient conditions, are characteristic for high affinity transporters (Schachtman et al., 1998). Similar results for the high affinity system have been reported in Arabidopsis despite differences in experimental procedure and age of plants used (Dunlop et al., 1997; Dong et al., 1999). It is surprising that slightly higher affinities were observed for C24, Co, Cal, Col-0, and Te as determined by apparent Km values (which could be determined here with lower precision) under phosphate-sufficient conditions.
Imax values, under phosphate-deficient conditions, were within the range of 90 to 152 pmol/h−1 cm−2 root, which is approximately 1.5-fold higher than the Imax values determined under sufficient phosphate supply. Increases in Imax have also been reported for various species under phosphate deficiency by several researchers (Lee 1982; Drew et al., 1984; Dunlop et al., 1997) and have been attributed to increased expression of Pi transporters in tobacco suspension cells (Shimogawara and Usuda, 1995).
Dunlop et al. (1997), however, suggested the existence of a low-affinity system (two-phase kinetics) in Arabidopsis at higher phosphate concentrations. Under phosphate-deficient conditions the efficient accessions C24 and Co have approximately 1.5 times higher Imax (pmol h−1 cm−1 root) than those of Col-0 and Te and, consequently, they can acquire more phosphate per centimeter of root than the latter (less efficient) accessions. Because no obvious differences were observed in the apparent Km values among the different accessions, the differences in the phosphorus uptake systems of the accessions are probably manifested in different frequencies of active phosphate transporters (difference in Imax) rather than in expression of different kinds of phosphate transporter genes. Variation in the numbers of functional ion transporters per unit length of root may be caused by differential rates of synthesis and breakdown of the transport proteins, a process likely to cause changes in the maximum transport capacity of the root (Lee, 1982). The tomato phosphate transporter genes LePT1 and LePT2 show predominant expression in the root epidermis and the root hairs (Daram et al., 1998; Liu et al., 1998). The phosphate transport activity may therefore also be influenced by the frequencies and sizes of the root hairs, which differ in the various accessions and are highest in C24 and Co. The lower phosphate transport activity per centimeter of root in Cal, Col-0, and Te is over-compensated by the elevated root lengths in these accessions when grown in low strength medium. This results in higher phosphate transport activity per gram of plant fresh weight in Cal, Col-0, and Te under these conditions in comparison to C24 and Co. The superior PAEs displayed by C24 and Co when grown in high-strength medium, therefore, are most probably conferred by a combination of high root penetration abilities, high root surface areas, and high phosphate transport activities per centimeter of root.
Another physiological parameter, Cmin, the concentration of phosphate in the growth medium at which no net uptake or release of ions occurs, is a measure of the ability of a plant to acquire phosphate at low concentrations and of the degree to which the soil solution can be depleted. This threshold may be set by a limited affinity of the carrier sites for the ions or it may be the point at which influx is balanced by the efflux (Marschner, 1988). This parameter of ion uptake is also strongly affected by the plant nutritional status (like Imax and Km). Measurement of phosphate-starved plants showed that all tested accessions (C24 and Co, Cal, Col-0, and Te) displayed Cmin between 7 and 11 nm. In accordance with this, this measure does not contribute substantially to the differences in phosphate efficiency in Arabidopsis as in other plant species (Jungk et al., 1990). The Cmin values determined in this study were four to six times lower than those measured by Krannitz et al. (1991) in 25 Arabidopsis genotypes. The reason for this discrepancy may reside in the different measurement conditions used.
Phosphate Mobilization by Root Exudates
In addition to root morphology and uptake activity, the release of organic anions from roots of can contribute to PAE through the dissolution of sparingly soluble phosphates (Dinkelaker et al., 1989). It is widely believed that the efficiency of uptake is of minor importance for phosphorus acquisition from soils because availability of Pi to the root surface rather than its uptake is the limiting factor (Barber, 1995). The efficient accessions C24 and Co exude four to five times more citrate than the less efficient accessions Col-0 and Te. The increased exudation of organic anions, particularly citrate and malate, in the accession C24 and Co may enable them to acquire phosphate from hydroxylapatite. Differences in the exudation of citrate and malate among the members of the Brassicaceae, which correlated with their PAEs, have been reported previously (Van den Boogaard et al., 1992). Consistent with these data, elevated root citrate and malate exudation has been observed in the efficient Arabidopsis accessions C24 and Co in this study.
Polar substances like organic acids can diffuse into the rhizosphere due to the high electrochemical potential gradient existing between the cytoplasm of root cells and the soil solution (Jones et al., 1994). This passive diffusion of citrate, however, is slow ranging from 14.4 to 93.6 pmol h−1 (cm root length)−1 for wheat and tomato and 100 to 200 pmol h−1 (cm root length)−1 from non-proteoids roots of white lupin as compared with the active citrate exudation from the proteoid roots of phosphorus-deficient white lupin, which is approximately 30- to 70-fold higher (Neumann and Römheld, 1999; Neumann et al., 1999). Citric acid exudation rates in white lupin depend upon the age of proteoids roots and reaches up to 6,700 pmol h−1 (cm root length)−1 in mature proteoid roots (Neumann et al., 1999). Exudation of organic acids at a high level in response to phosphorus stress has been documented also for other species such as oilseed rape (Hoffland et al., 1992) and alfalfa (Lipton et al., 1987). The carboxylates that are most effective in mobilizing phosphate from sparingly soluble sources are citrate > oxalate > malate (Bar-Yosef, 1996).
The root exudation of citrate and malate in the efficient accessions C24 and Co is lower as compared with white lupin proteoid roots, but it is comparable with oilseed rape (Hoffland et al., 1989). In contrast to white lupin and oilseed rape, however, the high citrate and malate exudation in the Arabidopsis accessions C24 and Co are not accompanied by strong proton extrusion. All accessions displayed a net alkalinization of the growth medium. To monitor potential spatial variation of root-induced pH changes, other experimental procedures need to be applied such as the use of pH indicators and H+-selective microelectrodes (Marschner and Römheld, 1983; Plassard et al., 1999). Rhizosphere acidification would be required to cause the dissolution of HA resulting in efficient phosphate mobilization. Lowest pHs of the growth media were observed for the accessions Cal and Col-0, which showed intermediate or low root organic acid exudation, respectively. Therefore, the main mechanism governing the proton release in Arabidopsis probably is the balancing of cation-anion uptake (Jeong and Lee, 1996; Schöttelndreier and Falkengren-Grerup, 1999). Rhizosphere acidification in response to phosphorus starvation in oilseed rape has been attributed to unbalanced cation-anion uptake (Hedley et al., 1982). The independence of the rate of organic acid anion release and the degree of HA utilization by the Arabidopsis accessions is furthermore supported by the observation that the differences in their PAEs are minor when they are grown in low strength media. The ability to exploit a large substrate volume therefore appears to be of much higher importance than activities related to an enhanced phosphate mobilization from the sparingly soluble HA.
Secretion of organic acids without concomitant release of protons has been observed in phosphorus-efficient plants adapted to acid soils (H. Lambers, personal communication). It remains to be tested whether the Arabidopsis accessions C24 and Co display high PAEs under such conditions.
This study highlights the fact that inherent differences in PAE exist among Arabidopsis accessions. The adaptations evolved in these accessions to cope with low phosphate availability in soils are dominated by root growth and morphology and by phosphate uptake rates per unit root length. Rhizosphere acidification, root organic acid exudation, and affinity of the phosphate transporters appear to be of minor relevance. Two extreme sets of accessions were identified: The first, represented by C24 and Co, develops relatively short roots with high substrate penetration abilities, long root hairs at high densities, and high phosphate uptake efficiencies per unit root length. The second, represented by Col-0 and Te, produces long roots (under favorable conditions) with low substrate penetration abilities, short and sparse root hairs, and low uptake efficiencies per unit root length. Cal appears intermediate for these characteristics, but displays slightly enhanced proton extrusion.
Plants with larger root systems have a greater potential to acquire phosphate than do plants with small roots. However, the uptake experiments show that the accessions with small root systems can be more efficient because they can acquire more phosphate per unit length of root. This finding is in close agreement with the conclusions reached through other studies (Evans 1977; Caradus, 1980; Rao et al., 1997). In most soils the phosphorus available to the plant is concentrated primarily in the upper soil horizons and decreases with the soil depth (Anderson, 1980; Keter and Ahn 1986; Pothuluri et al., 1986; Lynch 1995). When this is the case plants with short roots, but long root hairs at high density and with root systems of branched architecture would be more efficient than accessions with long roots and short and sparse root hairs. The accessions C24 and Co with their slow, but sustainable (high penetration ability) root growth and high phosphate uptake per unit of root are apparently better adapted to compact soils, whereas the less efficient accessions with fast growing roots such as Col-0 and Te are better suited to loose soils. The latter cope with phosphate deficiency through elongation of their roots so that they can access a larger soil volume, which may be of lower richness.
Taking into consideration multiple soil parameters, Barber (1995) predicted that nutrient uptake is likely to be most strongly affected by the root growth rate. This is supported by the observed strong drop in the PAEs of the Col-0 and Te accessions upon increased growth medium density, which caused severe root growth retardation. Under these conditions, the accessions C24 and Co, with their higher root penetration ability and their long root hairs and high uptake per centimeter of root are more efficient.
In conclusion this study represents a detailed morphological and physiological analysis of the mechanisms governing PAEs of Arabidopsis accessions. Based on the results presented here the excellent resources available for this molecular genetic model system can be used to isolate genes that determine phosphate efficiency traits. Furthermore, favorable/unfavorable allelic variants of these genes may be identified that shall provide detailed insight into the molecular mechanisms underlying the PAE phenomenon.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of the Arabidopsis accessions Ag-0, An, Bch-1, Bla, Bur, Cal, Cen, Co, Cvi, Ei, Eil-0, En, Gr, Hi, Kil, LaIII, Lip-0, Lm, Lu, M4, No, Ob-0, Old-1, Oy, Pa, Per, Pr, Rsch, Sue, Sg-1, Te, and Yo were obtained from Dr. Simon Misera (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany). Further accessions were represented by the lab strains C24, Col-0, Ler, and Ws. To identify the Arabidopsis accessions with high and low PAEs, seeds of these 36 accessions from a range of different geographical origins were germinated in modified MS medium (Murashige and Skoog, 1962) solidified with 0.4% (w/v) agarose. Ammonium nitrate was replaced with potassium nitrate to avoid acidification resulting from ammonium uptake. For HA [Ca5(PO4)3OH] medium or soluble phosphate medium, HA (0.5 g L−1) or monopotassium dihydrogen phosphate (1 mm), respectively, was included as the sole phosphate source. Through the addition of 10 mm MOPS, the growth medium was buffered to pH 7.0. HA is sparingly soluble at pH 7.0 and was therefore added as a suspension. The seedlings were germinated and cultivated at 22°C and a 16-h light period at 60 to 90 μmol m−2 s−1. After 24 d of culture, 10 shoots of each accession were pooled, blotted between tissue paper, dried overnight at 60°C, weighed, and their phosphate contents were determined according to Ames (1966). On the basis of these data the five most divergent accessions (C24, Co, and Cal with high phosphate contents, and Col-0 and Te with low phosphate contents) were selected for detailed analysis.
Analysis of Five Arabidopsis Accessions with Divergent PAEs
Twenty-five seedlings of accessions C24, Co, Cal, Col-0, and Te were grown for 24 d on 80 mL of modified MS medium (pH 7.0), solidified with 0.4% or 0.15% (w/v) agarose, and their phosphate contents were determined as described earlier.
Measurement of Root Parameters Using Image Analysis
To characterize divergent root parameters, plants were grown for 16 d on agarose media lacking phosphate. The roots of seedlings were examined using a light microscope (AX70, Olympus, Japan) and the root regions with root hairs showing uniform maximum lengths were used for analysis. The root hair images were captured and relayed to a monitor using a charge-coupled device camera (UAFCB1, Olympus) attached to the microscope. Root hairs parameters were measured using soft image analysis software (SiS GmbH, Münster, Germany) by recalling the images.
Phosphate Uptake Experiments
For uptake studies, the plants were grown in modified 0.5× Hoagland (Hoagland and Arnon, 1938) solution in which ammonium nitrate was replaced with potassium nitrate. The phosphate sufficient and phosphate deficient media contained 500 and 2.5 μm KH2PO4, respectively. Surface sterilized seeds of the different Arabidopsis accessions were soaked over night in 0.08% (w/v) agarose solution. An approximate 100 seeds were sown on each 300-μm nylon mesh (2 × 2 cm). Seedlings established on the mesh were floated on the surface of the medium and were incubated with constant shaking (20 rpm) at 20°C until the experiments were performed (7–8 d). Under these conditions the roots of the seedlings grew through the openings of the nylon screen into the nutrient solution. At this developmental stage seedlings have a well-developed main root, but no lateral roots.
Phosphate uptake was measured according to Honda et al. (1998) with minor modifications. Appropriate conditions for uptake and removal of externally adsorbed phosphate were determined before uptake experiments were performed. First the optimal duration of uptake was determined by allowing the plants to absorb phosphate for different intervals. For at least 2 h phosphate uptake was linear (data not shown). For this reason, uptake time was set to 1 h. Second the duration for the removal of externally adsorbed phosphate was determined: After washing with PI buffer (0.1 mm CaCl2, 5 mm MES [2-(N-morpholino)-ethanesulfonic acid], pH 5.75, and KH2PO4 added in the same concentration as in uptake solution), 8-d-old seedlings (or 7-d-old in case of 2-(N-morpholino)-ethanesulfonic acid-sufficient medium) were allowed to absorb 32P-labeled phosphate for 15 min. These seedlings were then washed twice with ice-cold PII buffer (0.1 mm CaCl2, 5 mm MES, pH 5.75, and 1 mm KH2PO4) and incubated in 100 mL of PII buffer for different intervals. Incubation in ice-cold PII buffer for up to 6 h showed that minimal-labeled phosphate desorption occurred after 30 min. For experimental convenience the duration of externally adsorbed phosphate desorption was set to 2 h.
The roots of the 7- to 8-d-old seedlings were rinsed with deionized water and incubated twice for 1 h with an excess volume of PI buffer supplemented with KH2PO4 at the same concentration as it was used in the following step (2.5–100 μm) to minimize the contamination of Pi from the solution culture. After this preincubation, the roots were carefully immersed in 7.0 mL of uptake solution (in a 12-well tissue culture plate) PI buffer, with KH2 32PO4 (ICN, Irvine, CA). Pi absorption was allowed for 1 h. The plants on the mesh were transferred into 100 mL of ice-cold PII buffer for 2 h to desorb nonincorporated 32P. After the wash the plants were blotted dry between tissue paper and fresh weights were measured. The plants were then dried overnight in scintillation vials in a drier at 60°C. After drying, 2 mL of scintillation liquid (Readysafe liquid scintillation cocktail, Beckman Instruments, Fullerton, CA) was added and the incorporated radioactivity was measured by a liquid scintillation counter (Beckman Instruments).
The root lengths were determined by growing seedlings separately under similar conditions. Phosphate uptake was calculated as nmol phosphate h−1 cm−1 root length and nmol phosphate h−1 g−1 fresh mass plants.
For the determination of Cmin, the seedlings were grown aseptically in Weck tissue culture vessels on nylon meshes prepared as described for the uptake experiments. The meshes (three meshes in each Weck vessel) were floated on the surface of 200 mL of the modified sterile MS medium lacking phosphate and supplemented with 1% (w/v) Suc. These seedlings were incubated at 20°C for 10 d. Ten-day-old seedlings were rinsed in 500 μm CaCl2, blotted dry, and placed on 7.0 mL of solution containing 0.5 μm 32P-labeled KH2PO4. Samples were taken from the medium every hour for the first 3 h and every 2 h thereafter, for up to 9 h. Most of the phosphate (90%) was taken up in the first 3 h and thereafter uptake was reduced. The Cmin was determined as the concentration that remained in the solution after 7 h.
pH Change Measurement
To determine the role of acidification in HA mobilization, the pH changes in the growth medium were measured. For pH measurements, the plants were grown under sterile conditions on modified MS medium without phosphate. An approximate 300 seedlings from each accession were germinated in 200 mL of MS medium on three nylon mesh (pH was adjusted to 5.7 in the unbuffered medium before autoclaving; after autoclaving the pH was 4.76). After 10 d of growth the plants were removed, dried, and weighed. The pH of the medium was determined using a pH meter. As a control the pH of the medium without plants was also determined after 10 d of exposure to identical conditions.
Collection of Exudates and Organic Acid Measurements
For the collection of root exudates, plants were grown in 0.5× modified Hoagland solution in the same way as for the uptake studies. The phosphate-sufficient and phosphate-deficient media contained 500 and 10 μm KH2PO4, respectively. After 8 d of growth, seedlings were rinsed in deionized water and transferred to 12-well tissue culture plates containing 7.0 mL of deionized water per well. Meshes containing 100 seedlings per mesh were placed on wells in such a way that the roots were completely immersed in water. After 1-h incubations at room temperature the plants were removed and the exudate solutions were collected, frozen, lyophilized, and resuspended in 1.4 mL of deionized water. Fresh and dry masses of the plants were determined. The root exudates were analyzed using an anion-exchange chromatography system (DX 500, Dionex, Sunnyvale, CA) with a conductivity detector. The anions loaded on the anion-exchange column (Ionpac AS11 Analytical Column) were eluted using a solution gradient of NaOH (supplemented with methanol). The organic acid peaks were identified through retention time and were quantified according to the calibration of standard solutions.
Statistical Analysis
Analysis of variance was performed with the procedure GLM (General Linear model, type III) of SAS Release 6.12 (SAS-Institute Inc., Cary, NC). In those cases where ANOVA indicated a significant effect of the accession on the tested variable, means were compared by lsd test. Significant different means (P ≤ 0.05) were marked with different letters in the tables or stated as significant in the text.
ACKNOWLEDGMENTS
We would like to thank Dr. Karin Köhl for assistance in the statistical analysis of the data and Megan McKenzie for critical reading of the manuscript, and we express our gratitude to Prof. Lothar Willmitzer for his continuous support.
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant nos. Al 387/2–1, 2–2).
LITERATURE CITED
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- Anderson G. Assessing organic phosphorus in soils. In: Khasawneh FE, Sample EC, Kamprath EJ, editors. The Role of Phosphorus in Agriculture. Madison, WI: American Society of Agronomy; 1980. , 411–431. [Google Scholar]
- Anghinoni I, Barber SA. Phosphorus influx and growth characteristics of corn roots influenced by phosphorous supply. Agron J. 1980;72:685–688. [Google Scholar]
- Barber SA. Soil Nutrient Bioavailability: A Mechanistic Approach. Ed 2. New York: John Wiley & Sons; 1995. [Google Scholar]
- Barber SA, Silberbrush M. Plant root morphology and nutrient uptake. In: Kral DM, editor. Root Nutrient and Water Influx, and Plant Growth. American Society of Agronomy Special Publication No. 49, Symposium, November 28–December 3, 1982, Anaheim, CA. 1984. [Google Scholar]
- Barber SA, Walkner JM, Vasey EH. Mechanisms for the movement of plant nutrients from the soil and fertilizer to the plant root. J Agric Food Chem. 1963;11:204–207. [Google Scholar]
- Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ. The Arabidopsis ribonuclease gene RNSI is tightly controlled in response to phosphate limitation. Plant J. 1994;6:673–685. doi: 10.1046/j.1365-313x.1994.6050673.x. [DOI] [PubMed] [Google Scholar]
- Bar-Yosef B. Root excretions and their environmental effects: influence on availability of phosphorus. In: Weisel Y, Eshel A, Kafkafi U, editors. Plant Roots: The Hidden Half. Ed 2. New York: Marcel Dekker; 1996. [Google Scholar]
- Bates TR, Lynch JP. Simulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. [Google Scholar]
- Bengough AG, Croser C, Pritchard J. A biophysical analysis of root growth under mechanical stress. Plant Soil. 1997;189:155–164. [Google Scholar]
- Boeuf-Tremblay V, Plantureux S, Guckert A. Influence of mechanical impedance on root exudation of maize seedlings at two development stages. Plant Soil. 1995;172:279–287. [Google Scholar]
- Caradus JR. Distinguishing between grass and legume species for efficiency of phosphorus use. N Z J Agric Res. 1980;23:75–81. [Google Scholar]
- Chung HJ, Ferl RJ. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 1999;121:429–436. doi: 10.1104/pp.121.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daram P, Brunner S, Persson BL, Amrhein N, Bucher M. Functional analysis and cell-specific expression of phosphate transporter from tomato. Planta. 1998;206:225–233. doi: 10.1007/s004250050394. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ. Characterization of phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupine (Lupinus albus L.) Plant Cell Environ. 1989;12:285–292. [Google Scholar]
- Dong B, Ryan PR, Rengel Z, Delhaize E. Phosphate uptake in Arabidopsis thaliana: dependence of uptake on the expression of transporter genes and internal phosphate concentrations. Plant Cell Environ. 1999;22:1455–1461. [Google Scholar]
- Drew MC, Saker LR, Barber SA, Jenkins W. Changes in the kinetics of phosphate and potassium absorption in nutrient deficient barley roots measured by a solution-depletion technique. Planta. 1984;160:490–499. doi: 10.1007/BF00411136. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Moorthead GBG, Lefebvre DD, Plaxton WC. Phosphate starvation inducible bypasses of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiol. 1989;90:1275–1278. doi: 10.1104/pp.90.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Phung HT, Meeking R, White DWR. The kinetics associated with phosphate absorption by Arabidopsis and its regulation by phosphorus status. Austr J Plant Physiol. 1997;24:623–629. [Google Scholar]
- Evans PS. Comparative root morphology of some pasture grasses and clovers. N Z J Agric Res. 1977;20:331–335. [Google Scholar]
- Föhse D, Claassen N, Jungk A. Phosphorus efficiency of plants: II. Significance of root radius, root hairs and cation anion balance for phosphorus influx in seven plant species. Plant Soil. 1991;132:261–272. [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil. 1997;191:181–188. [Google Scholar]
- Gahoonia TS, Nielsen NE. Direct evidence of participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil. 1998;198:147–152. [Google Scholar]
- Goldstein AH. Phosphate starvation-inducible enzymes and proteins in higher plants. In: Wray JL, editor. Society for Experimental Biology Seminar Series 49: Inducible Plant Proteins. Cambridge, UK: Cambridge University Press; 1992. pp. 25–44. [Google Scholar]
- Green PJ. The ribonucleases of higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:421–445. [Google Scholar]
- Hedley MJ, Nye PH, White RE. Plant induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings: II. Origin of the pH change. New Phytol. 1982;91:31–44. [Google Scholar]
- Hoagland DR, Arnon DJ (1938) The water culture method for growing plants without soils. Circ Calif Agric Exp Stn No. 347
- Hoffland E, Findenegg GR, Nelemans JA. Solubilization of rock phosphate by rape. Plant Soil. 1989;113:155–165. [Google Scholar]
- Hoffland E, Van Den Boogaard R, Nelemans J, Findenegg G. Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol. 1992;122:675–680. [Google Scholar]
- Holford ICR. Soil phosphorus: its measurement and its uptake by plants. Austr J Soil Res. 1997;35:227–239. [Google Scholar]
- Honda C, Fujiwara T, Chino M. Sulphate uptake in Arabidopsis thaliana. J Plant Nutr. 1998;21:601–604. [Google Scholar]
- Horst WJ, Abdou M, Wiesler F. Differences between wheat cultivars in acquisition and utilization of phosphorus. Z Pflanzenernaehr Bodenkd. 1996;159:155–161. [Google Scholar]
- Jeong BR, Lee CW. Influence of ammonium, nitrate, and chloride on solution pH and ion uptake by ageratum and salvia in hydroponic culture. J Plant Nutr. 1996;19:1343–1360. [Google Scholar]
- Johnson JF, Allan DL, Vance CP, Weiblen G. Root carbon dioxide fixation by phosphorus deficient Lupinus albus: contribution to organic acid exudation by proteoid roots. Plant Physiol. 1996;112:19–30. doi: 10.1104/pp.112.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Adwards AC, Donachie K, Darrah PR. Role of proteinaceous amino acids released in root exudates in nutrient acquisition from the rhizosphere. Plant Soil. 1994;158:183–192. [Google Scholar]
- Jungk A, Asher CJ, Edwards DG, Meyer D. Influence of phosphate status on phosphate uptake kinetics of maize and soybean. Plant Soil. 1990;124:175–182. [Google Scholar]
- Karlsson BH, Silis GR, Nienhuis J. Effects of photoperiod and vernalization on the number of leaves at flowering in 32 Arabidopsis thaliana (Brassicaceae) ecotypes. Am J Bot. 1993;80:646–648. [Google Scholar]
- Keter JKA, Ahn PA. Profile characteristics, and form and surface activity of inorganic phosphorus in a deep red Kenya coffee soil (Nitosol) J Soil Sci. 1986;37:89–97. [Google Scholar]
- Krannitz PG, Aarssen LW, Lefebvre DD. Relationship between physiological and morphological attributes related to phosphate uptake in 25 genotypes of Arabidopsis thaliana. Plant Soil. 1991;133:169–175. [Google Scholar]
- Larsen PB, Tai CY, Kochian LV, Howell SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol. 1996;110:743–751. doi: 10.1104/pp.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RB. Selectivity and kinetics of ion uptake by barley plants following nutrient deficiency. Ann Bot. 1982;50:199–206. [Google Scholar]
- Lewis DG, Quirk JP. Phosphate diffusion in soil and uptake by plants: III. P31 movement and uptake by plants as indicated by autoradiograph. Plant Soil. 1967;27:445–453. [Google Scholar]
- Liao H, Yan X. Seed size is closely related to phosphorus use efficiency and photosynthetic phosphorus use efficiency in common bean. J Plant Nutr. 1999;22:877–888. [Google Scholar]
- Lipton DG, Blanchar RW, Blevins DG. Citrate, malate, succinate in exudates from P sufficient and P stressed Medicago sativa L. seedlings. Plant Physiol. 1987;85:315–317. doi: 10.1104/pp.85.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorous. Plant Physiol. 1998;116:91–99. doi: 10.1104/pp.116.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler A, Abel S, Jost W, Beintema JJ, Glund K. Phosphate-regulated induction of intracellular ribonucleases in cultured tomato (Lycopersicon esculentum) cells. Plant Physiol. 1992;98:1472–1478. doi: 10.1104/pp.98.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiol. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, van Beem J. Growth and architecture of seedling roots of common bean genotypes. Crop Sci. 1993;33:1253–1257. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press; 1988. [Google Scholar]
- Marschner H, Römheld V. In vivo measurement of root-induced pH changes at the soil-root interface. Z Pflanzenphysiol. 1983;111:241–251. [Google Scholar]
- Moorby H, White RE, Nye PH. The influence of phosphate nutrition on H ion efflux from the roots of young rape plants. Plant Soil. 1988;105:247–256. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Neumann G, Massonneau A, Martinoia E, Römheld V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta. 1999;208:373–382. [Google Scholar]
- Neumann G, Römheld V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil. 1999;211:121–130. [Google Scholar]
- Nye PH. The effect of nutrient intensity and buffer power of a soil, and the absorbing power, size of root hairs, on nutrient uptake by diffusion. Plant Soil. 1966;25:81–105. [Google Scholar]
- Plassard C, Messaoud M, Souche G, Jaillard B. Localization and quantification of net fluxes of H+ along maize roots by combined use of pH-indicator dye videodensitometery and H+-selective microelectrodes. Plant Soil. 1999;158:183–192. [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. A mutant of Arabidopsis thaliana deficient in xylem loading of phosphate. Plant Physiol. 1991;97:1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuluri JV, Kiesel DE, Whitney DA, Thien SJ. Phosphorus uptake from soil layers having different soil test phosphorus levels. Agron J. 1986;78:991–994. [Google Scholar]
- Rao IM, Borrero V, Ricaurte J, Garcia R, Ayarza MA. Adaptive attributes of tropical forage species to acid soils: III. Differences in phosphorus acquisition and utilization as influenced by varying phosphorus supply and soil type. J Plant Nutr. 1997;20:155–180. [Google Scholar]
- Römer W, Schenk H. Influence of genotype on phosphate uptake and utilization efficiencies in spring barley. Eur J Agron. 1998;8:215–224. [Google Scholar]
- Sadeghian S. Influencia de algunas caracteristicas de las semillas y plantulas de frijol (Phaseous vulgaris L.) sobre la tolerencia a la baja disponibilidad de fosforo en el suelo. MS thesis. Palmira: National University of Colombia; 1991. [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. Phosphorus uptake by plants: from soil to cell. Plant Physiol. 1998;116:447–453. doi: 10.1104/pp.116.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttelndreier M, Falkengren-Grerup U. Plant induced alteration in the rhizosphere and the utilization of soil heterogeneity. Plant Soil. 1999;209:297–309. [Google Scholar]
- Shimogawara K, Usuda H. Uptake of inorganic phosphate by suspension-cultured tobacco cells: kinetics and regulation by Pi starvation. Plant Cell Physiol. 1995;36:341–351. [Google Scholar]
- Smith AD, Robinson AD, Abbott LK. The involvement of mycorrhizas in assessment of genetically dependent efficiency of nutrient uptake and use. In: Randall PJ, Delhaize E, Richards RA, Munns R, editors. Genetic Aspects of Plant Mineral Nutrition. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 221–231. [Google Scholar]
- Trull MC, Guiltinan M, Lynch J, Deikman J. The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant Cell Environ. 1997;20:85–92. [Google Scholar]
- Van den Boogaard HAGM, Hoffland E, Findenegg GR. Acquisition of rock phosphate by plant species in relation to their excretion of organic acids from the roots during P deficiency: root ecology and its practical application, 3. In: Kutschera L, Hübl E, Lichtenegger E, Persson H, Sobotik M, editors. Root Ecology and Its Practical Application, 3rd Symposium, verein für Wurzelforschung. Klagenfurt, Austria: Universität Bodenkultur; 1992. pp. 189–191. [Google Scholar]