Abstract
Background:
Immunosuppressive agents are still inefficient in preventing biopsy-proven acute rejection (BPAR) after expanded criteria donor (ECD) kidney transplantation. The aim of this study was to investigate the relationships between early immunosuppressive exposure and the development of BPAR.
Methods:
We performed a retrospective study of 58 recipients of ECD kidney transplantation treated with enteric-coated-mycophenolate sodium, tacrolimus (Tac), and prednisone. The levels of mycophenolic acid-area under the curve (MPA-AUC)0-12h and Tac C0 were measured at the 1st week and the 1st month posttransplant, respectively. The correlation was assessed by multivariate logistic regression.
Results:
The occurrence rates of BPAR and antibody-mediated rejection were 24.1% and 10.3%, respectively. A low level of MPA-AUC0-12h at the 1st week posttransplant was found in BPAR recipients (38.42 ± 8.37 vs. 50.64 ± 13.22, P < 0.01). In addition, the incidence of BPAR was significantly high (P < 0.05) when the MPA-AUC0-12h level was <30 mg·h-1·L-1 at the 1st week (15.0% vs. 44.4%) or the Tac C0 was <4 ng/ml at the 1st month posttransplant (33.3% vs. 21.6%). Multivariable logistic regression analysis showed that the MPA-AUC0-12h at the 1st week (OR: 0.842, 95% CI: 0.784-0.903) and the Tac C0 at the 1st month (OR: 0.904, 95% CI: 0.822–0.986) had significant inverse correlation with BPAR (P < 0.05).
Conclusions:
Low-level exposure of MPA and Tac C0 in the early weeks posttransplant reflects an increased acute rejection risk, which suggested that MPA-AUC0-12h <30 mg·h-1·L-1 and Tac C0 <4 ng/ml should be avoided in the first few weeks after transplantation.
Keywords: Enteric-Coated-Mycophenolate Sodium, Tacrolimus, Acute Rejection, Expanded Criteria Donor, Kidney Transplantation
摘要
背景:
在扩大标准的供体(expanded criteria donor, ECD)肾移植术后如何有效的应用免疫抑制剂预防活检证实的急性排斥反 应(biopsy-proven acute rejection, BPAR)仍然存在争议。因此,本研究的目的是探讨早期免疫抑制剂暴露与BPAR发生之间的 关系,以期为肾移植术后早期免疫抑制剂的应用提供依据。
方法:
我们回顾性分析了58例ECD肾移植受体早期免疫抑制剂暴露与BPAR的相关性,58例受体的免疫抑制剂方案为麦 考酚钠肠溶片(enteric-coated mycophenolate sodium, EC-MPS)、他克莫司(tacrolimus, Tac)和强的松(prednisone, pred)。MPA-AUC0-12h 和 Tac C0分别在术后1周和1月应用酶联免疫法进行检测。并采用多元logistic回归分析评估其与BPAR 的相关性。
结果:
BPAR和抗体介导排斥反应(antibody-mediated rejection, AMR)的发生率分别为24.1%和10.3%。术后1周发生BPAR 的受体MPA-AUC0-12h水平显著低于未发生BPAR的受体(p<0.01)。并且,在术后1周受体MPA-AUC0-12h水平小于30mg·h-1·L-1 或术后1月受体Tac C0 水平小于4 ng/ml时BPAR发生率显著增加(p<0.05)。多因素Logistic回归分析显示,在术后1周时受体 MPA-AUC0-12h和术后1月受体Tac C0与BPAR呈负相关性(p<0.05)。
结论:
肾移植术后早期免疫抑制剂MPA-AUC0-12h 和 Tac C0低暴露增加BPAR的风险。在肾移植术后早期应避免MPA-AUC0-12h 水平小于30mg h/L和Tac C0 水平小于4 ng/ml。
INTRODUCTION
An increasing number of transplants involving donation after cardiac death (DCD) are being performed to match the demands of a growing waiting list,[1] especially in China.[2,3] Due to a shortage of donor organs, the donor pool required expansion, and many kidneys are being procured from DCD donors who meet the expanded criteria donor (ECD) standard, which was previously considered unacceptable. ECDs as a result of DCD and donation after brain death are defined separately for kidneys according to the United Network for Organ Sharing definition.[4] The recipients of ECD kidneys are often excluded from transplant trials due to the higher rate of delayed graft function (DGF), more biopsy-proven acute rejection (BPAR), decreased long-term graft function, calcineurin inhibitor (CNI)-induced nephrotoxicity, increased incidence of infection, cardiovascular risk, and malignancies.[5,6] For this reason, the ideal immunosuppressive regimen for this population has not yet been defined. Therefore, the aim of this study was to assess the relationship between early immunosuppressive exposure and BPAR in DCD-ECD kidney transplant recipients and to provide a reasonable basis for clinical application.
METHODS
Ethical approval
This is a single-center, retrospective, observational cohort study approved by the local institutional review board of the First Affiliated Hospital of Xi'an Jiaotong University, which was in compliance with the provisions of the current Declaration of Helsinki principles and Good Clinical Practice guidelines. Written informed consent was obtained from all participants.
Design
All patients who received an ECD kidney-only first transplant between July 2012 and June 2016 were included in this study. Follow-up was until May 2017. End points studied were as follows: (1) patient survival at 1st year, (2) graft survival at 1st year, (3) DGF, (4) 1-year serum creatinine and estimated glomerular filtration rate (eGFR), and (5) the BPAR rate in the 1st year.
Clinical definitions
ECDs were identified according to the United Network for Organ Sharing definition (age ≥60 years or 50–59 years with at least two of the following: hypertension, death from cerebrovascular accident, and terminal creatinine ≥132 μmol/L).[4] The diagnostic criterion for DGF is dialysis needed in the 1st week after kidney transplantation. Allograft biopsy was performed for suspected acute rejection (AR) cases. AR was identified on biopsy and classified according to the Banff 2013 classification.[7] Graft failure was established when long-term dialysis treatment was reestablished or upon death.
Immunosuppressive regimen
A triple immunosuppressive regimen of enteric-coated- mycophenolate sodium (EC-MPS; myfortic®, Novartis, Basel, Switzerland), tacrolimus (Tac), and prednisone (pred) was the initial maintenance immunosuppressive regimen used in all patients. The initial EC-MPS dose was 1440 mg/d administered within 24 h posttransplantation; Tac was administered at 0.06 mg·kg−1·d−1 beginning on the 3rd day after transplantation. The target Tac C0 level was 4–10 ng/ml from the beginning of transplantation. Oral pred was administered at 10 mg/d after transplantation. The dosage of immunosuppressive agents was adjusted according to clinical experience, biochemical results, and effective exposure of the drug. All recipients were induced with rabbit antithymocyte globulin (ATG; Thymoglobulin®; Genzyme, Waterford, Ireland; 1.25 mg·kg−1·d−1 between days 0 and 4 after transplantation).
Tacrolimus C0 monitoring and pharmacokinetic assessment of enteric-coated-mycophenolate sodium
Blood samples for Tac C0 were drawn before morning medication. All samples were anticoagulated with ethylenediaminetetraacetic acid. Whole-blood Tac concentration levels were measured using a fluorescence polarization immunoassay on an AxSYM analyzer (Abbott Diagnostic, Chicago, IL, USA). The mycophenolic acid (MPA) concentration levels were measured by an enzyme-multiplied immunoassay technique (Mycophenolic Acid Assay; Siemens Healthcare Diagnostic, Camberley, UK) at the 1st week and 1st month posttransplantation. Blood samples were collected before the morning medication and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h after medication. The area under the curve (AUC) of MPA from 0 to 12 h (MPA-AUC0-12h) was calculated using the linear trapezoidal method.
Management of biopsy-proven acute rejection
BPAR cases were treated with 500 mg methylprednisolone administered intravenously for 3 consecutive days combined with optimized Tac and EC-MPS therapy. ATG was administered for 5–10 days for steroid-resistant and early high-grade ARs.[8]
Statistical analysis
The Chi-square test was used to analyze categorical data. Student's t-tests were used as appropriate for continuous data. Multivariate logistic regression analysis was used to determine the correlation between MPA-AUC0-12h and Tac C0 level in the early weeks and BPAR during first 12 months posttransplant; A value of P < 0.05 was considered statistically significant. SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis.
RESULTS
Graft outcomes
Fifty-eight patients who received kidney grafts from DCD-ECD donors during the study time period were enrolled in this study. The average follow-up time was 10.7 month (range: 0.8–12.0 months). Demographic and clinical characteristics of the donors and recipients are shown in Tables 1 and 2. The incidence of BPAR and antibody-mediated rejection was 24.1% and 10.3%, respectively. DGF was observed in 13 patients (22.4%). The allograft function of eGFR was 58.4 ± 13.9 ml·min−1·1.73 m−2 at 12 months posttransplant. The graft survival and patient survival were 86.2% and 89.7%, respectively, in the 12 months following kidney transplantation [Table 3]. The graft loss (n = 8) included rejection unrevised (n = 3), DGF was unretired (n = 3), renal artery stenosis (n = 1), and chronic allograft nephropathy (n = 1). The cause of death (n = 6) included cardiovascular disease (n = 3), multiple organ dysfunction syndrome (n = 2), and lung infection (n = 1).
Table 1.
Recipient characteristics (n = 58)
| Characteristics | Values |
|---|---|
| Age (year) | 38.6 ± 7.3 |
| Sex, n (%) | |
| Male | 41 (70.7) |
| Female | 17 (29.3) |
| Weight (kg) | 66.3 ± 9.5 |
| Body mass index (kg/m2) | 22.4 ± 3.8 |
| Hemodialysis, n (%) | 21 (91.3) |
| Peritoneal dialysis, n (%) | 2 (8.7) |
| Duration of dialysis prior to transplantation (months) | 35.2 ± 9.4 |
| HLA mismatches (loci) | 2.1 ± 1.3 |
| First transplantation, n (%) | 58 (100.0) |
| Pretransplant PRA, n (%) | |
| <10% | 52 (89.7) |
| 10≤ PRA <20% | 5 (8.6) |
| 20≤ PRA <30% | 1 (1.7) |
| Primary diseases, n (%) | |
| Chronic glomerulonephritis | 44 (75.8) |
| Hypertensive nephrosclerosis | 8 (13.8) |
| Diabetic nephropathy | 3 (5.2) |
| Other | 3 (5.2) |
Data was presented as mean ± standard deviation or n (%). HLA: Human leukocyte antigen; PRA: Panel reactive antibody.
Table 2.
Donor characteristics (n = 33)
| Characteristics | Values |
|---|---|
| Age (years) | 45.2 ± 10.4 |
| Sex, n (%) | |
| Male | 21 (63.6) |
| Female | 12 (36.4) |
| Weight (kg) | 66.7 ± 7.5 |
| Body mass index (kg/m2) | 23.1 ± 4.2 |
| Donation after cardiac death, n (%) | 33 (100.0) |
| Primary cause of death, n (%) | |
| Head trauma | 10 (30.3) |
| Cerebrovascular accident/stroke | 16 (48.5) |
| Anoxia | 5 (15.1) |
| Other | 2 (6.1) |
| Hypertension history, n (%) | 20 (60.6) |
| Terminal serum creatinine (µmol/L) | 141.9 ± 33.2 |
| Cold ischemia time (h) | 7.8 ± 2.4 |
| Warm ischemia time (min) | 9.6 ± 2.8 |
Data was presented as mean ± standard deviation or n (%).
Table 3.
Efficacy results during the first 12 months after kidney transplantation (n = 58)
| Parameters | Values |
|---|---|
| BPAR, n (%) | 14 (24.1) |
| IA | 2 (3.4) |
| IB | 4 (6.9) |
| IIA | 3 (5.2) |
| IIB | 3 (5.2) |
| III | 2 (3.4) |
| AMR, n (%) | 6 (10.3) |
| AR treatment failure, n (%) | 4 (6.9) |
| DGF, n (%) | 13 (22.4) |
| Graft loss, n (%) | 8 (13.8) |
| Death, n (%) | 6 (10.3) |
| Graft survival (%) | 50 (86.2) |
| Patient survival (%) | 52 (89.7) |
| Serum creatinine (µmol/L) | 112 ± 23.2 |
| eGFR (ml/min; Cockcroft–Gault) | 58.4 ± 13.9 |
Data was presented as mean ± SD or n (%). AR: Acute rejection; AMR: Antibody-mediated rejection; BPAR: Biopsy-proven acute rejection; DGF: Delayed graft function; eGFR: Estimated glomerular filtration rate.
Mycophenolic acid exposure and the risk of biopsy-proven acute rejection
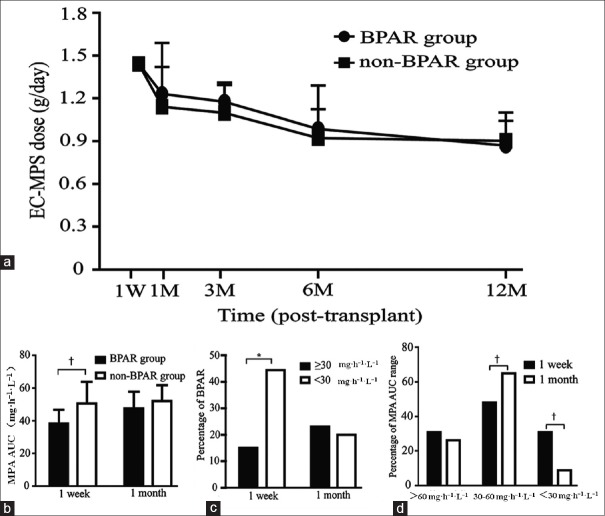
It had been shown that the BPAR was associated with the dose of MPA and its exposure. To definitively prove this, we divided the patients into two groups: BPAR and non-BPAR and analyzed the relationship between EC-MPS dose and its exposure with BPAR. The dose of EC-MPS was similar in the two groups during the study period [Figure 1a], but the MPA-AUC0-12h in the BPAR group was significantly lower than that in the non-BPAR group at the 1st week posttransplantation (38.42 ± 8.37 vs. 50.64 ± 13.22, P < 0.01) [Figure 1b]. Further analysis showed that the incidence of BPAR was significantly increased when the MPA-AUC0-12h was <30 mg·h-1·L-1 at the 1st week posttransplant (15.0% vs. 44.4%, P < 0.05) [Figure 1c]. After comparing the MPA-AUC0-12h range in the 1st week and 1st month posttransplant, we found that the percentage of MPA-AUC0-12h <30 mg·h-1·L-1 at the 1st month was significantly lower than at the 1st week (31.0% vs. 8.8%, P < 0.01), whereas the MPA-AUC0-12h of 30–60 mg·h-1·L-1 at the 1st month was significantly higher than that at the 1st week (P < 0.01) [Figure 1d].
Figure 1.
MPA exposure and the risk of BPAR. (a) EC-MPS dose in BPAR (n=14) and non-BPAR (n=44) groups during the first 12 months. (b) MPA-AUC0-12h in BPAR and non-BPAR groups at the 1st week and 1st month posttransplantation. (c) The percentage of BPAR when MPA-AUC0-12h <30 mg·h-1·L-1 and at ≥30 mg·h-1· L-1 at the 1st week and 1st month posttransplantation. (d) The percentage of MPA-AUC0-12h range at the 1st week and 1st month posttransplantation. *P < 0.05, †P < 0.01. Produced by GraphPad Prism version 6.02 (GraphPad Software Inc., La Jolla, CA, USA). BPAR: Biopsy-proven acute rejection; EC-MPS: Enteric-coated-mycophenolate sodium; MAP: Mycophenolic acid; AUC: Area under the curve.
Tacrolimus C0 and the risk of biopsy-proven acute rejection
We analyzed the relationship between the Tac dosage and BPAR. Our data showed that the Tac dosage was similar in the BPAR and non-BPAR groups during the study period [Figure 2a], but the Tac C0 level in the BPAR group was significantly lower than that in the non-BPAR group at the 1st month posttransplantation (P < 0.05) [Figure 2b]. We used the cut-points Tac C0 <4.0 versus ≥4.0 ng/ml and Tac C0 <7.0 versus ≥7.0 ng/ml to analyze their association with the BPAR rate and found that the incidence of BPAR was significantly higher when Tac C0 was <4 ng/ml compared with Tac C0 ≥4.0 ng/ml at the 1st month posttransplant (7.2 ± 1.2 vs. 8.3 ± 2.7, P < 0.05) [Figure 2c]. This phenomenon recurred when Tac C0 <7.0 versus ≥7.0 ng/ml at the 1st week and 1st month after transplantation, although it was not statistically significant [Figure 2d].
Figure 2.
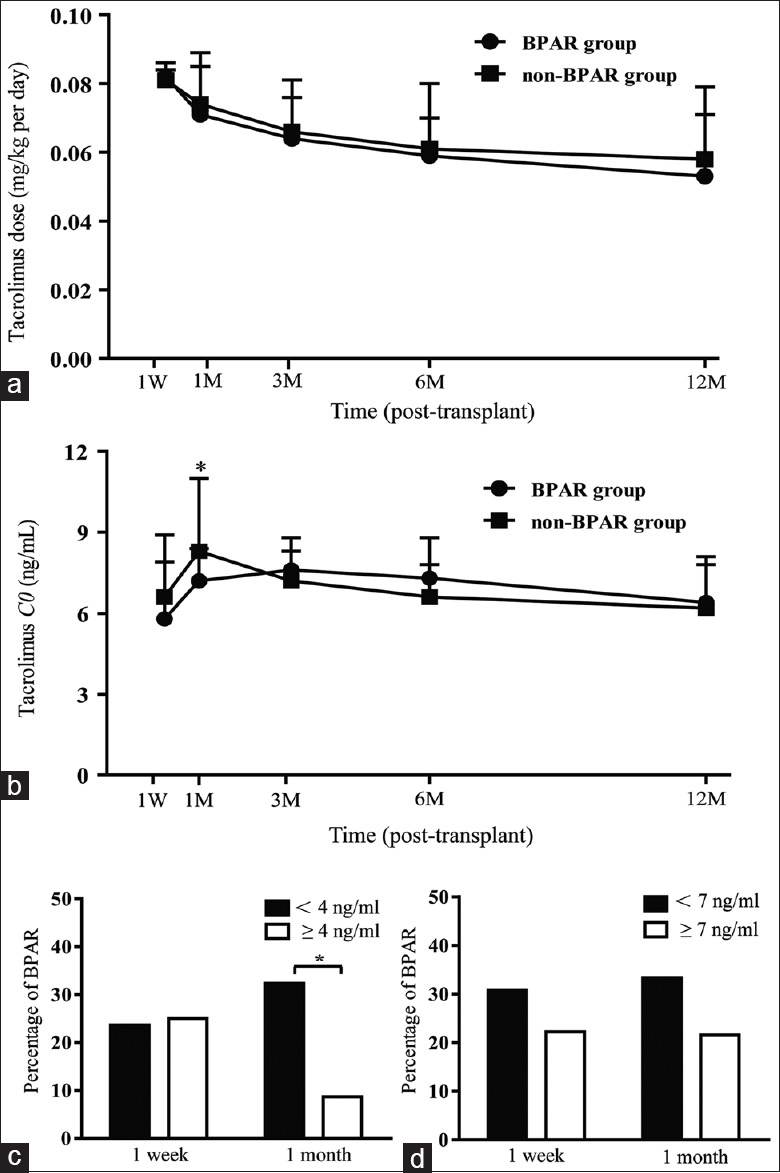
Tac C0 and the risk of BPAR (n=58). (a) Tac dose in BPAR (n=14) and non-BPAR (n=44) groups during the first 12 months (b) Tac C0 in BPAR and non-BPAR groups during the first 12 months. (c) The percentage of BPAR when Tac C0 <4 ng/ml and ≥4 ng/ml at the 1st week and 1st month posttransplantation. (d) The percentage of BPAR when Tac C0 <7 ng/ml and ≥7 ng/ml at the 1st week and 1st month posttransplantation.*P < 0.05. Produced by GraphPad Prism version 6.02 (GraphPad Software Inc., La Jolla, CA, USA). BPAR: Biopsy-proven acute rejection; Tac: Tacrolimus.
Association analysis between mycophenolic acid-area under the curve0-12h/tacrolimus C0 and biopsy-proven acute rejection
To better identify the relationship between immunosuppressive exposure and BPAR, we built a multivariate logistic regression model including all the variables of MPA-AUC0-12h and Tac C0 at the 1st week and 1st month posttransplant. As shown in Table 4, the MPA-AUC0-12h at the 1st week posttransplant (odds ratio [OR], 0.842, 95% confidence interval [CI], 0.784–0.903, P < 0.05) and the Tac C0 at the 1st month posttransplant (OR, 0.904, 95% CI, 0.822–0.986) were identified as an independent risk factor of BPAR (P < 0.05). These results indicated that MPA-AUC0-12h <30 mg·h-1·L-1 and Tac C0 <4.0 ng/ml in the early weeks had a significant correlation with AR.
Table 4.
Multivariable logistic regression analysis of BPAR based on early MPA-AUC0-12h and Tac C0 variables
| Variables | Comparison | OR (95% CI) | P |
|---|---|---|---|
| MPA-AUC0-12h (mg·h−1·L−1) 1 week | 30> versus ≥30 | 0.842 (0.784–0.903) | 0.021* |
| MPA-AUC0-12h (mg·h−1·L−1) 1 month | 30> versus ≥30 | 1.109 (0.827–1.390) | 0.248 |
| Tac C0 (ng/ml) 1 week | 4> versus ≥4 | 1.052 (0.526–2.103) | 0.186 |
| Tac C0 (ng/ml) 1 month | 4> versus ≥4 | 0.904 (0.822–0.986) | 0.043* |
| Tac C0 (ng/ml) 1 week | 7> versus ≥7 | 1.024 (0.832–1.216) | 0.453 |
| Tac C0 (ng/ml) 1 month | 7> versus ≥7 | 0.916 (0.807–1.040) | 0.096 |
*P<0.05 was considered statistically significant. AUC: Area under the curve; OR: Odds ratio; CI: Confidence interval; Tac: Tacrolimus; MPA: Mycophenolic acid; BPAR: Biopsy-proven acute rejection.
DISCUSSION
ECD transplantations are complicated by increased rates of DGF and AR, especially in the earlier posttransplantation period, and an adequate level of immunosuppressant is desired under these circumstances. Recipients of ECD kidneys were often excluded from transplant trials, and therefore the optimal maintenance regimen for them is unknown. This single-center study retrospectively followed 58 ECD kidney transplant recipients and investigated the association between Tac C0 and MPA-AUC0-12h and AR risk during the first 12 months posttransplant.
For all the study recipients, there was a significant correlation between the MPA-AUC0-12h and the risk of AR after renal transplantation. These results are consistent with the studies that suggest an inverse association between MPA exposure and a risk of AR.[9,10] Adequate MPA exposure in the early posttransplant phases is required because patients are at the highest risk of AR during this period. A therapeutic window of MPA-AUC has been recommended (30–60 mg·h-1·L-1) to achieve optimal efficacy.[10,11] Similarly, our data showed that the incidence of BPAR was significantly higher when MPA-AUC0-12h was <30 mg·h-1·L-1 compared with ≥30 mg·h-1·L-1 at the 1st week posttransplant in DCD-ECD kidney transplant patients (P < 0.05). Moreover, to better identify the association between MPA exposure and BPAR, we built a multivariate logistic regression model including MPA-AUC0-12h (ref <30 mg·h-1·L-1) at the 1st week and 1st month posttransplant. These results suggest that MPA-AUC0-12h <30 mg h/L at the 1st week has a significant correlation with AR during the first 12 months after transplantation (P < 0.05). However, the increase in the AR rate was similar in recipients with an MPA-AUC0-12h <30 mg h/L or ≥30 mg h/L (20.0% vs. 23.1%) at the 1st month. After analyzing the range of MPA-AUC0-12h, we found that the compliance rate was significantly higher at the 1st month than at the 1st week posttransplant (P < 0.01). This implies that the presence of significant difference during the first 12 months is largely due to the differences that were already achieved before the 1st month.[12]
As a result of the extensive application of MPA and CNIs, many studies have demonstrated that CNI exposure can cause acute and chronic nephrotoxicity; thus, low exposure to CNIs has been advocated in the recent years to further improve transplant outcomes, particularly in ECD kidneys.[13,14,15,16,17] However, many studies have shown that lower Tac C0 levels during the 1st week,[18] during the 1st month,[19] after 3 months,[20] and Tac C0 of <4 ng/ml[13] or <7 ng/ml[21] posttransplant were significantly correlated with the subsequent higher BPAR rates. Therefore, we decided to use the cut-point <4.0 versus ≥4.0 ng/ml and <7.0 versus ≥7.0 ng/ml to analyze the association between Tac C0 and BPAR risk during the first 12 months after transplantation. In our results, the percentage of <4 ng/ml was 22.4 and <7 ng/ml was 34.5 at the 1st week posttransplantation. In addition, the percentage of <4 ng/ml was 15.5 and <7 ng/ml was 20.7 at the 1st month posttransplantation. The incidence of BPAR was significantly higher when Tac C0 was <4 ng/ml compared with Tac C0 ≥4.0 ng/ml at the 1st month posttransplantation (P < 0.05). This phenomenon recurred when Tac C0 <7.0 versus ≥7.0 ng/ml at the 1st week and 1st month after transplantation, although it was not statistically significant. The results provide a more accurate description of the correlation between lower Tac C0 levels and subsequent higher BPAR risk, and notably 4.0 ng/ml represented the minimum of the target Tac C0 level range specified in our protocol at the 1st month after transplantation. Research indicates that lower MPA-AUC values were associated with a significantly higher BPAR risk during the first 12 months after transplantation. Reanalysis of the Opticept[22] and FDCC[12] trials supports a lower MPA-AUC at day 3 after transplantation which was associated with a significantly higher AR rate during the first 12 months. However, none of these analyses include the prognostic influence of CNI level, either tested or controlled. More recently, Daher Abdi et al.[10] reported that lower MPA-AUC was more significantly associated with subsequently higher AR risk than a lower Tac level. In fact, our findings further confirm their conclusion.
Currently, the most common immunosuppressive treatment after de novo renal transplantation is a triple regimen including Tac, MPA, and corticosteroids. In addition, most centers use antibody induction systematically in selected patients at high immunologic risk; for example, ECD kidney, PRA positive. In many recent experimental regimens, and increasingly in clinical practice also, the recommended target concentration for Tac is <10 ng/ml, which is lower than that in previous years, as reflected by the newly revised prescribing information in the United States (recommended target concentration 4–11 ng/ml in combination with MPA and an interleukin-2 receptor antagonist).[23] For ECD kidney transplantation, we recommended Tac target concentration 4–10 ng/ml in combination with MPA and rabbit ATG. In addition, let MPA reach adequate exposure early.
In conclusion, low-level exposure to MPA and Tac C0 in the early weeks posttransplantation reflects an increased AR risk, which suggests that MPA-AUC0-12h <30 mg h/L and Tac C0 <4 ng/ml should be avoided in the first few weeks after transplantation.
Financial support and sponsorship
This work was supported by grants from the major clinical research projects of the First Affiliated Hospital of Xi'an Jiaotong University (No. XJTU1AF-CRF-2015-005) and the National Natural Science Foundation of China (No. 81670681).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Morrissey PE, Monaco AP. Donation after circulatory death: Current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97:258–64. doi: 10.1097/01.TP.0000437178.48174.db. doi: 10.1097/01.TP.0000437178.48174. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Millis JM, Mao Y, Millis MA, Sang X, Zhong S, et al. Voluntary organ donation system adapted to Chinese cultural values and social reality. Liver Transpl. 2015;21:419–22. doi: 10.1002/lt.24069. doi: 10.1002/lt.24069. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Zeng L, Gao X, Wang H, Zhu Y. Transformation of organ donation in China. Transpl Int. 2015;28:410–5. doi: 10.1111/tri.12467. doi: 10.1111/tri.12467. [DOI] [PubMed] [Google Scholar]
- 4.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–6. doi: 10.1097/00007890-200211150-00014. doi: 10.1097/01.TP.0000034060.18738.0B. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Sáez MJ, Montero N, Redondo-Pachón D, Crespo M, Pascual J. Strategies for an expanded use of kidneys from elderly donors. Transplantation. 2017;101:727–45. doi: 10.1097/TP.0000000000001635. doi: 10.1097/TP.0000000000001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querard AH, Foucher Y, Combescure C, Dantan E, Larmet D, Lorent M, et al. Comparison of survival outcomes between expanded criteria donor and standard criteria donor kidney transplant recipients: A systematic review and meta-analysis. Transpl Int. 2016;29:403–15. doi: 10.1111/tri.12736. doi: 10.1111/tri.12736. [DOI] [PubMed] [Google Scholar]
- 7.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–83. doi: 10.1111/ajt.12590. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraja P, Roberts GW, Stephens M, Horvath S, Kaposztas Z, Chavez R, et al. Impact of expanded criteria variables on outcomes of kidney transplantation from donors after cardiac death. Transplantation. 2015;99:226–31. doi: 10.1097/TP.0000000000000304. doi: 10.1097/TP.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Liu L, Li J, Fu Q, Wan J, Deng R, et al. The efficacy and safety of intensified enteric-coated mycophenolate sodium with low exposure of calcineurin inhibitors in Chinese de novo kidney transplant recipients: A prospective study. Int J Clin Pract. 2016;70(Suppl 185):22–30. doi: 10.1111/ijcp.12813. doi: 10.1111/ijcp.12813. [DOI] [PubMed] [Google Scholar]
- 10.Daher Abdi Z, Prémaud A, Essig M, Alain S, Munteanu E, Garnier F, et al. Exposure to mycophenolic acid better predicts immunosuppressive efficacy than exposure to calcineurin inhibitors in renal transplant patients. Clin Pharmacol Ther. 2014;96:508–15. doi: 10.1038/clpt.2014.140. doi: 10.1038/clpt.2014.140. [DOI] [PubMed] [Google Scholar]
- 11.Stracke S, Shipkova M, Mayer J, Keller F, Zarghom A, Yang L, et al. Pharmacokinetics and pharmacodynamics of mycophenolate sodium (EC-MPS) co-administered with cyclosporine in the early-phase post-kidney transplantation. Clin Transplant. 2012;26:57–66. doi: 10.1111/j.1399-0012.2011.01403.x. doi: 10.1111/j.1399-0012.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- 12.van Gelder T, Tedesco Silva H, de Fijter JW, Budde K, Kuypers D, Arns W, et al. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation. 2010;89:595–9. doi: 10.1097/TP.0b013e3181ca7d84. doi: 10.1097/TP.0b013e3181ca7d84. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor JJ, Ciancio G, Guerra G, Sageshima J, Roth D, Goldstein MJ, et al. Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl Int. 2016;29:216–26. doi: 10.1111/tri.12699. doi: 10.1111/tri.12699. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Xue W, Tian P, Ding X, Pan X, Xiang H, et al. Which is more suitable for kidney transplantation at the early post-transplantation phase in china – Low dosing or standard dosing of enteric-coated mycophenolate sodium? Int J Clin Pract Suppl. 2014;68:10–6. doi: 10.1111/ijcp.12401. doi: 10.1111/ijcp.12401. [DOI] [PubMed] [Google Scholar]
- 15.Ding C, Xue W, Tian P, Ding X, Pan X, Yan H, et al. Outcomes of standard dose EC-MPS with low exposure to CsA in DCD renal transplantation recipients with DGF. Int J Clin Pract Suppl. 2015;69:8–15. doi: 10.1111/ijcp.12661. doi: 10.1111/ijcp.12661. [DOI] [PubMed] [Google Scholar]
- 16.Cortinovis M, Gotti E, Pradini S, Gaspari F, Perico N. Renal graft function and low-dose cyclosporine affect mycophenolic acid pharmacokinetics in kidney transplantation. Transplantation. 2011;92:550–6. doi: 10.1097/TP.0b013e318225dbd0. doi: 10.1097/TP.0b013e318225dbd0. [DOI] [PubMed] [Google Scholar]
- 17.Sommerer C, Glander P, Arns W, Ariatabar T, Kramer S, Vogel EM, et al. Safety and efficacy of intensified versus standard dosing regimens of enteric-coated mycophenolate sodium in de novo renal transplant patients. Transplantation. 2011;91:779–85. doi: 10.1097/TP.0b013e31820d3b9b. doi: 10.1097/TP.0b013e31820d3b9b. [DOI] [PubMed] [Google Scholar]
- 18.Borobia AM, Romero I, Jimenez C, Gil F, Ramirez E, De Gracia R, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31:436–42. doi: 10.1097/FTD.0b013e3181a8f02a. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 19.Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16:1905–9. doi: 10.1093/ndt/16.9.1905. doi: 10.1093/ndt/16.9.1905. [DOI] [PubMed] [Google Scholar]
- 20.Israni AK, Riad SM, Leduc R, Oetting WS, Guan W, Schladt D, et al. Tacrolimus trough levels after month 3 as a predictor of acute rejection following kidney transplantation: A lesson learned from DeKAF genomics. Transpl Int. 2013;26:982–9. doi: 10.1111/tri.12155. doi: 10.1111/tri.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatault P, Kamar N, Büchler M, Colosio C, Bertrand D, Durrbach A, et al. Reduction of extended-release tacrolimus dose in low-immunological-risk kidney transplant recipients increases risk of rejection and appearance of donor-specific antibodies: A randomized study. Am J Transplant. 2017;17:1370–9. doi: 10.1111/ajt.14109. doi: 10.1111/ajt.14109. [DOI] [PubMed] [Google Scholar]
- 22.Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: The opticept trial. Am J Transplant. 2009;9:1607–19. doi: 10.1111/j.1600-6143.2009.02668.x. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 23.Ekberg H, van Gelder T, Kaplan B, Bernasconi C. Relationship of tacrolimus exposure and mycophenolate mofetil dose with renal function after renal transplantation. Transplantation. 2011;92:82–7. doi: 10.1097/TP.0b013e31821fad06. doi: 10.1097/TP.0b013e31821fad06. [DOI] [PubMed] [Google Scholar]