Abstract
Esophageal adenocarcinoma (EAC) is characterized by rapidly increasing incidence and mortality rates and poor survival. Efficacious preventive and treatment options are urgently needed. An increasing number of pharmacologic agents targeting cancer cell death via autophagy mechanisms are being evaluated in hopes of circumventing apoptotic and therapeutic resistance. We report for the first time, loss of Beclin-1, a key mediator of autophagy, was significantly linked to prognostic factors in EAC. Specifically, Beclin-1 expression loss occurred in 49.0% of EAC patients versus 4.8% of controls. There was a significant inverse correlation between loss of Beclin-1 with histologic grade and tumor stage supporting a tumor suppressive role for Beclin-1. Autophagy modulation linked to cell death was examined in EAC cell lines following treatment with a proanthocyanidin-rich cranberry extract, C-PAC, and the commonly used autophagy inducer, rapamycin. C-PAC induced Beclin-1-independent autophagy in EAC cells characterized by reduced phosphorylation at serine 15 and 93, and significant cell death induction. In contrast, rapamycin-induced autophagy resulted in concomitant, increases in total Beclin-1 levels as well as Beclin-1-phosphorylation in a cell line specific manner, leading to long-term cell survival. Furthermore, autophagic LC3-II was induced by C-PAC following siRNA suppression of Beclin-1 in EAC cells. Together these data support a prognostic role of Beclin-1 in EAC with evidence that Beclin-dependent autophagy induction is agent specific. Future studies are necessary to fully interrogate the role autophagy plays in the progression of normal tissue to EAC and how specific agents targeting autophagic mechanisms can be efficaciously applied for cancer prevention or treatment.
Keywords: autophagy, esophagus, rapamycin, natural product
INTRODUCTION
The incidence of esophageal adenocarcinoma (EAC) has been sharply rising over the past four decades in the United States and many other developed countries [1,2]. Alarmingly, mortality remains high with 5 year survival rates consistently below 20% [3]. EAC is now the seventh leading cause of cancer related death among Caucasian males in the US supporting the growing need for improved screening, preventive measures, and treatment modalities [4].
The strongest and best understood risk factor for EAC is gastroesophageal reflux disease (GERD) [5,6]. GERD associated reflux of bile and acid into the esophagus contributes to local irritation and inflammation which if left unchecked can lead to the development of Barrett’s esophagus, the only identified precursor lesion of EAC [7]. Systemic inflammation and accumulated genetic abnormalities support the progression of BE precursor lesions to EAC development in a subgroup of patients. There is strong in vitro and in vivo evidence supporting that acid exposure increases oxidative injury, DNA damage and induces apoptosis resistance in esophageal cells [8,9]. Apoptotic resistance provides cancer cells with a growth advantage and is linked to therapeutic resistance. Thus, there is growing interest in targeting alternative mechanisms of cell death induction, including autophagy as a mode to circumvent apoptosis and drug resistance. Autophagy is a major catabolic process for directing cytoplasmic cellular components to the lysosome for degradation or recycling [10]. A number of pharmacologic agents targeting autophagy are currently under therapeutic evaluation for different cancers despite unresolved issues regarding the paradoxical roles for autophagy in cancer [11–13]. Activation of autophagy has been reported to be a pro-death mechanism leading to cancer suppression as well as a pro-survival mechanism leading to tumor promotion [14]. Thus, improved understanding of autophagy signaling networks in the context of specific cancers is necessary to determine whether and when autophagy inhibitors or inducers mitigate neoplastic progression.
The role of autophagy in esophageal adenocarcinogenesis is understudied and poorly understood with few published reports on autophagy and EAC. In 2012, Dvorak and colleagues reported a significant decrease of Beclin-1 expression, a key autophagy regulator, in dysplastic BE and EAC cases compared to non-dysplastic BE tissues and normal squamous epithelium [9]. They also showed that acute bile acid exposure increased Beclin-1 levels and induced autophagy; whereas, chronic bile acid exposure led to reduced Beclin-1 expression and autophagy inhibition supporting modulation of Beclin-1 and the autophagic process during progression from premalignancy to EAC. Beclin-1 expression has been linked to improved survival in patients with squamous cell cancer of the esophagus, but to date autophagy markers have not been evaluated in the context of EAC prognosis.
In this study, we sought to determine if altered Beclin-1 expression was linked to clinicopathologic variables considered prognostic in EAC and to characterize Beclin-1 expression profiles linked to cell death in EAC cell lines following treatment with autophagy inducers. We utilized tissue microarrays for immunohistochemical examination of Beclin-1. Consistent with the only published report, our results showed a significant decrease of Beclin-1 expression in EAC cases [9]. Importantly, for the first time we report loss of Beclin-1 expression correlated significantly with tumor grade and stage. Second, we treated JHAD1 and OE19 EAC cell lines with an autophagy inducing proanthocyanidin-rich cranberry extract, C-PAC, and rapamycin, a widely used pharmacologic inducer of autophagy. C-PAC induced autophagy in EAC cells, but surprisingly was accompanied by slight reductions in total Beclin-1 levels and larger magnitude reductions in phospho-specific forms, raising the question of Beclin-1 independent autophagy induction. siRNA studies directed towards Beclin-1 showed that C-PAC treatment of EAC cells resulted in increased levels of autophagic LC3-II. Importantly, C-PAC associated autophagy induction was linked to cell death and not cancer cell survival. In contrast, rapamycin induced increased levels of LC3-II and total Beclin-1 which coincided with increases in phospho-specific Beclin-1, but a lack of cell death supporting a pro-survival role for rapamycin in EAC.
MATERIALS AND METHODS
Immunohistochemical Expression of Beclin-1
Immunohistochemical methods were utilized to assess autophagy-related Beclin-1 levels in three tissue microarrays (TMAs) containing EAC tissues which differed by tumor grade and stage for a subset of biopsies (n =54). Specifically, TMAs BS02051, ES208, and ES804 were purchased from US Biomax, Inc. (Rockville, MD). A total of 115 EAC biopsies were evaluated from 51 patients. Additionally, 59 normal squamous esophageal biopsies from 21 patients were assessed for Beclin-1 immunoreactivity. Methods included a 5 min antigen retrieval in sodium citrate, a 3% peroxidase block, 1 h incubation with primary antibody Beclin-1 (Anti-rabbit, NB500-249, 1:1000, Novus Biologicals, Littleton, CO) and detection with Envision +System-HRP/DAB (Dako, Carpenteria, CA). Each Beclin-1 stained biopsy was scored on an intensity scale spanning from “0” or no staining to “+3” for intense staining comprising greater than 30% of the tumor or epithelium in the case of normal tissues. Beclin-1 loss was characterized by an intensity score in the 0–1 range; whereas scores in the 2–3 range were considered positive for Beclin-1. Staining was confirmed using a second Beclin-1 primary antibody (Santa Cruz Biotechnology, Dallas, TX, #sc-11427; 1:100).
Cranberry Proanthocyanidin Preparation and Dose Determination
Cranberry fruit (Vaccinium macrocarpon Ait.) of the “Early Black” cultivar were collected at the Marucci Center for Blueberry and Cranberry Research, Chats-worth, NJ. Purified C-PAC extract was isolated from cranberries utilizing solid-phase chromatography according to well established previously published methodology [15–18]. Briefly, cranberries were homogenized in 70% aqueous acetone, filtered and the pulp discarded. Collected cranberry-derived proanthocyanidins were concentrated under reduced pressure and purified extract isolated using bioassay-directed fractionation. The absence of absorption at 360 nm and 450 nm confirmed all but proanthocyanidins were removed. Additional methods including 13C NMR, electrospray mass spectrometry, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and acid catalyzed degradation with phloroglucinol were utilized to further verify the presence of A-type linkages as well as to determine the concentration of proanthocyanidins in the purified extract. C-PAC is comprised of five main proanthocyanidins as previously characterized by Dr. Howell and colleagues [15,18,19]. The proanthocyanidin molecules largely consist of epicatechin units with degrees of polymerization of 4 or 5, as well as epigallocatechin and catechin. C-PAC contains three types of linkages, two common B-type linkages (C4→C6 and C4→C8) and at least one unique A-type ether linkage (C2→O→C7) found in cranberry, chokeberry, plums, and avocado [15–19]. Purified freeze-dried C-PAC was stored at −80°C. C-PAC concentrations chosen for study were informed by our earlier research which determined the LD50 to be in the 50 to 100 μg/ml range in cancer cell lines originating from the esophagus, lung, and colon [20–23]. Consideration was also given to earlier evaluations by Howell and colleagues showing 50 μg/ml of C-PAC inhibits adhesion of p-fimbriated uropathogenic E. coli bacteria in vitro and that 36 mg/day of C-PAC delivered in 10 ounces of juice inhibits bacterial adhesion in the urinary tract wall of humans [17,24]. Recent research by our group has found orally delivered C-PAC to be well tolerated in mice at 250 μg/day [23] and at considerably higher concentrations in long-term studies in rats with no adverse effects. Importantly, the concentrations of C-PAC under evaluation in this series of preclinical investigations are readily achievable in humans and are already under evaluation for targeting the oral cavity, the urinary tract, and cardio-metabolic health benefits.
Cell Lines, Agents, and Viability Determination
JH-EsoAd1 (JHAD1) and OE19 EAC cell lines were utilized in this series of experiments. JHAD1 were isolated from a distal EAC, stage III, N0 in 1997 (kind gift from Dr. James Eshleman, Johns Hopkins University, Baltimore, MD) and OE19 cells isolated in 1993 from an adenocarcinoma at the gastro-esophageal junction, stage III, N1 (ECACC, Wiltshire, UK). EAC cells were cultured in RPMI medium (with 10% FBS; Life Technologies, Grand Island, NY) under standard conditions (37°C and 5% CO2) [15–17]. Normal human esophageal Het-1A cells (ATCC® CRL-2692) were utilized for comparison purposes and were grown under previously described conditions [25]. JHAD1 (6E3 cells/well), OE19 cells (13E3 cells/well) and Het-1A cells (10E3 cells/well) were plated in 96 well plates, treated with C-PAC (50, 75, and 100 μg/ml) or rapamycin (62.5, 125, or 250 nM) and cell viability determined via Life Technologies’ LIVE/Dead Viability/Cytotoxicity kit for Mammalian cells per the manufacturer’s instructions at 24, 48, and 72 h post-treatment. After the 48 h reading, treatments were removed, fresh media replenished and viability was again determined in the same plate at 120 h to assess recovery potential and long-term cell survival. Fluorescence imaging for the viability assay was conducted using the SpectraMax® MiniMax™ Imaging Cytometer with excitation/emission wavelengths of 460/535 nm.
Lysate Collection and Western Blot Analysis of EAC Cells Treated With Autophagy Inducers
JHAD1 (10E5) and OE19 (15E5) cells were seeded in T-25 flasks and adhered overnight prior to treatment with C-PAC (75 μg/ml) or rapamycin (250 nM; Sigma, Saint Louis, MO) dissolved in medium with 5% FBS. Cell lysates were harvested at 0, 6, 24, and 48 h post-treatment using lysis buffer (Cell Signaling Technology, Inc., Beverly, MA). Protein was quantified using the DC protein assay (Bio-Rad, Hercules, CA) and 20 μg/lane loaded on precast 4–20% or 7.5% Mini-Protean TGX gels (Bio-Rad). Immunoblotting was performed using commercially available antibodies from Cell Signaling: Beclin-1 (#3738; 1:750), LC3AB (#4108; 1:1000), Rab7 (#9367; 1:1000), phospho-Beclin-1 at Ser15 (#13825; 1:750), and Ser93 (#14717; 1:750). Phospho-Beclin-1 antibodies at Ser234 (#p117-234; 1:250) and Ser295 (#p117-295; 1:250) were from Phospho-Solutions (Aurora, CO). Expression values were determined by chemiluminescent immunodetection and analyzed using Image Lab software with normalization to GAPDH. Fold-change from baseline or first detection level was calculated.
siRNA Transfection of EAC Cells
ON-TARGETplus human Beclin-1 SMARTpool siRNAs were purchased from Thermo Scientific Dharmacon (L-010552-00-0005) and transfected using Lipofectamine 2000 (Life Technologies) into JHAD1 (12E3 cells/well) and OE19 (15E3) cells seeded into a 96-well plate at a final concentration of 75 nM. After 72 h, the supernatant was removed, cells were washed and subsequently, treated with C-PAC (75 μg/ml) or rapamycin (250 nM) for 48 h. Lysate samples were harvested from 8 wells per treatment and western blot analysis using Beclin-1, LC3AB, and GAPDH antibodies was conducted as described above. ON-TARGETplus Non-targeting pool siRNA was used as the negative control for transfection. Transfection efficiency was assessed using the siGLO green transfection indicator (25 nM) and was estimated to be above 90% for these experiments.
Statistical Analysis
GraphPad Prism software was used for evaluating statistical differences by treatment. Inhibition of viability data were evaluated for statistical significance using ANOVA with Tukey’s post-hoc test where multiple conditions were assessed. A Student’s t-test was applied for pairwise comparisons. Statistical differences between control and EAC patients were determined using a Student’s t-test and Chi-square for trend tests. P-values of <0.05 were considered significant.
RESULTS AND DISCUSSION
Beclin-1 is Frequently Inactivated in EAC and Correlates Significantly With Tumor Grade and Stage
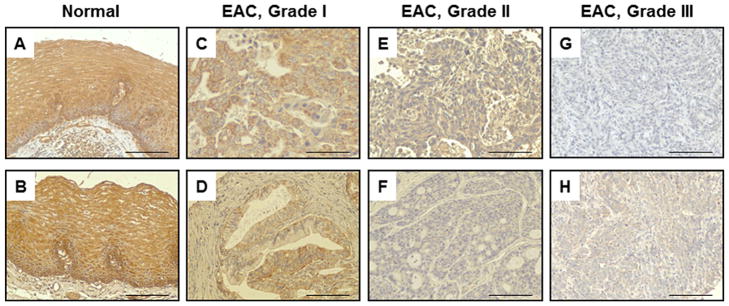
The role of autophagy in EAC is largely unknown which lead to our evaluation of select autophagy-related (ATG) proteins, including Beclin-1 (an ortholog of yeast Atg-6) which functions to either promote autophagy induction or to regulate maturation of autophagosomes [26]. We assessed Beclin-1 levels via immunohistochemical detection in 115 EAC biopsies from 51 patients (Figure 1A–H). As summarized in Table 1, loss of Beclin-1 expression occurred in about half of all EAC patients, comprising 38.26% of all EAC biopsies assessed, compared to less than 2% loss of Beclin-1 in histologically normal esophageal biopsies. Eight non-cancer controls contributed normal esophageal tissue (n =21 biopsies) and the remaining 13 histologically normal esophageal biopsies (n =38 biopsies), originated from non-adjacent esophageal cancers. Interestingly, 90.5% of normal esophageal tissue from non-cancer controls were highly positive for Beclin-1 (+3 intensity) supporting the marker is ubiquitously expressed in normal esophageal epithelium. Correspondingly, Beclin-1 staining was highest in normal esophageal tissues (Figure 1A and B) with a mean intensity score of 2.67 compared to 1.59 in all EACs, a statistically significant reduction. Moreover, this appears to be the first report linking Beclin-1 loss in EAC to prognostic factors, including cancer stage and grade. Beclin-1 expression was progressively reduced with advancing stage and grade (Table 1). Beclin-1 loss occurred in 25.0, 37.5, and 63.3% of patients with well differentiated grade I tumors, moderately differentiated grade II, and poorly differentiated grade III biopsies, respectively. Differences in Beclin-1 staining were not gender-linked, nor were any clear patterns detected based upon patient age. Nodal involvement and metastasis could not be assessed due to the limited reporting of cases with positive nodes or metastasis.
Figure 1.
Immunohistochemical Expression of Beclin-1. (A) and (B) Normal esophageal mucosa with strong Beclin-1 staining (+3); (C) and (D) Grade I EAC biopsies with moderately strong Beclin-1 staining (+2); (E) and (F) Grade II EAC biopsies with reduced Beclin-1 expression, (+1) and (0), respectively; (G) and (H) Grade III EACs displaying low Beclin-1 levels (+0.5) in H and a total loss of Beclin-1 in G (0); 200× magnification. Scale bars are set to 100 μm.
Table 1.
Clinicopathologic Features Associated With Beclin-1 Loss in EAC
| Variable | Beclin-1 reactivity | Beclin-1 loss | |
|---|---|---|---|
|
|
|
||
| (mean intensity score) | Patients (%) | Biopsies (%) | |
| Histopathology | |||
| Normal esophagus | 2.67 | 4.76 | 1.69 |
| All EACs | 1.59a | 49.01 | 38.26 |
| Stage | |||
| T1, T2 | 2.47 | ||
| T3, T4 | 1.42b | ||
| Grade | |||
| I, well differentiated | 2.09a | 25.00 | 25.00 |
| II, moderately differentiated | 1.63a | 37.50 | 40.00 |
| III, poorly differentiated | 1.46a | 63.30c | 40.58 |
Significantly different from normal esophageal biopsy specimens (t-test, P <0.005).
Significantly different from combined Stage 1 and Stage 2 biopsy specimens (t-test, P <0.005).
Significantly different based on test for trend, Beclin-1 loss and increasing grade (Chi-square test, P <0.05); 115 biopsies from 51 patients with EAC were assessed and 59 biopsies from 21 patients with histologically normal esophageal epithelium included.
Our data are consistent with and build upon earlier research showing decreased Beclin-1 expression with progression from normal tissue to BE with dysplasia and in EAC cases [9]. The total number of EAC patients and biopsies analyzed for Beclin-1 expression in our study was considerably larger adding strength to the association first described by Roesly et al. Down-regulation of autophagy has been implicated in the development of breast, prostate, ovarian and more recently, esophageal squamous cell carcinoma, where positive Beclin-1 expression was associated with improved patient survival [27]. Our data supports that Beclin-1 is linked to EAC prognostic indicators for the first time, but further research is warranted to directly assess the correlation of Beclin-1 to survival and its possible role given pharmacologic interventions targeting autophagic pathways.
Preventative Agents Induce Beclin-1-Dependent and Beclin-1-Independent Autophagy
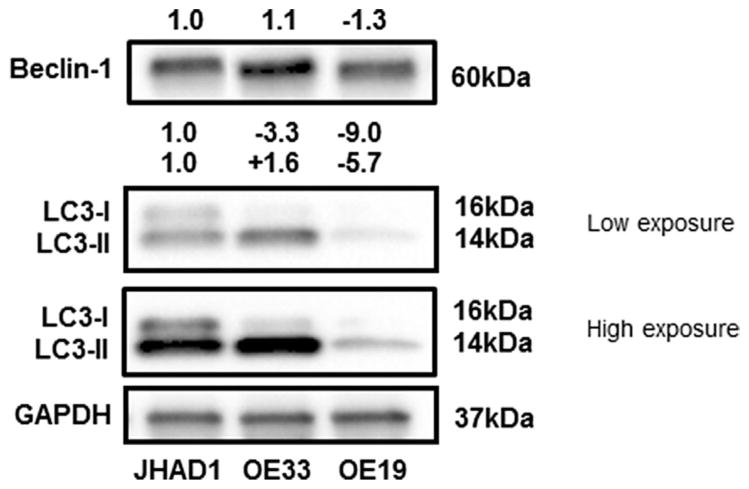
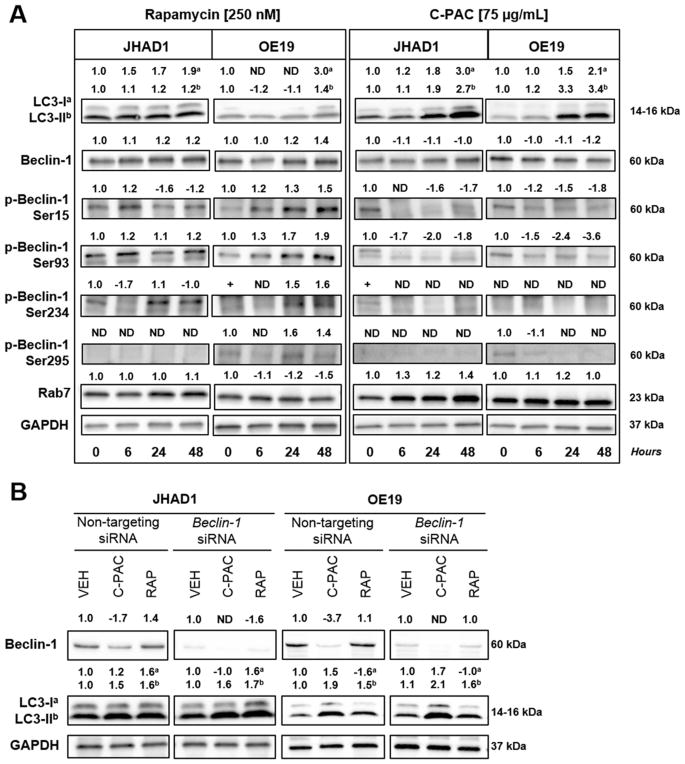
To better understand the role of Beclin-1 in autophagy induction and EAC, we evaluated an autophagy inducing cranberry extract, C-PAC, and rapamycin, a well-documented pharmacologic inducer of autophagy in JHAD1 and OE19 cells [28–30]. These two lines were chosen because, of three cell lines tested, constitutive levels of Beclin-1 and a second autophagy marker, LC3, differed to the greatest extent between JHAD1 and OE19 cells (Figure 2). Furthermore, OE19 cells are resistant to caspase-dependent cell death and we have found OE19 cells more readily form tumor xenografts in nude mice, supporting they are phenotypically aggressive compared to OE33 and JHAD1 cells [23]. Thus, we investigated autophagy as an alternative death pathway given apoptosis resistance of EAC cells which in turn is linked to therapeutic resistance. As a proof of principle, we treated JHAD1 and OE19 cells with 250 nM rapamycin, a similar concentration shown to induce autophagy in other cell lines and esophageal squamous cells [28–31]. Rapamycin-induced autophagy was supported by increased levels of LC3-II, the pro-autophagic lipidated form of LC3 (Figure 3A) [32,33], in both JHAD1 (P <0.016; 24 and 48 h) and OE19 cells, with maximum induction 24 to 48 h post-treatment. Similarly, rapamycin treatment resulted in 1.2 to 1.4-fold increased Beclin-1 levels at 24 and 48 h, with the greatest magnitude of increase occurring in OE19 cells and a significant increase in JHAD1 cells after 24 h (P <0.048).
Figure 2.
Constitutive expression of autophagic proteins in EAC lines. Three EAC lines were examined for constitutive expression of Beclin-1 and LC3. Commercially available antibodies were used to perform western blot analysis for autophagic proteins as detailed in the Materials and Methods. GAPDH was used as a loading control. Expression values were normalized to GAPDH and a fold change from expression levels in JHAD1 cells calculated utilizing ImageLab software. Positive fold change values indicated increased expression and negative values reflect decreased expression compared to JHAD1 cells.
Figure 3.
Preventative agents induce autophagy in EAC cells. (A) JHAD1 and OE19 cells were treated with rapamycin (250 nM) or C-PAC (75 μg/ml) and lysates were isolated at 0, 6, 24, and 48 h post treatment. Representative images are presented and significance is denoted in the results and discussion. GAPDH was used as a loading control. Expression values were normalized to GAPDH and a fold change from baseline or time of first detection was calculated with ImageLab software. Positive fold change values indicated increased expression and negative values reflect decreased expression. Autophagy induction was assessed by examining total Beclin-1 levels and the lipidation of LC3-I to LC3-II. Beclin 1-dependent autophagy induction was evaluated using phospho-specific Beclin-1 antibodies for phosphorylation at Ser15, Ser93, Ser234, and Ser295. Rab7 was analyzed as a late marker for autophagy induction. (B) JHAD1 and OE19 cells were transfected with Beclin-1 siRNA for 72 h and subsequently, treated with C-PAC (75 μg/ml) or rapamycin (250 nM). Lysates were collected after 48 h of treatment and Western blot analysis conducted. Representative images are presented and GAPDH was used as the loading control. Expression values were normalized as described above. In JHAD1 and OE19 cells, there was a 6.4 and 3.6-fold decrease in Beclin-1 levels following siRNA transfection, respectively.
In contrast, C-PAC treatment of EAC cells resulted in reduced total Beclin-1 levels over time. Beclin-1 levels declined 1.1-fold in JHAD1 (P <0.045; 24 h; Figure 3A) and 1.2-fold in C-PAC treated OE19 cells (P <0.018; 48 h; Figure 3A). Although unexpected, these findings are consistent with a recent study we conducted evaluating C-PAC as an inhibitor of OE19 xenografts. C-PAC treatment resulted in a 67.6% reduction in OE19 xenograft volume which involves LC3-II induction and modulation of AKT/mTOR/MAPK signaling without induction of Beclin-1 [23].
Rab7, considered a marker of endolysosomal activity and late autophagy was also evaluated. Rab7 is associated with late endosomes, lysosomes, and autolysosomes and is required for fusion of autophagosomes to lysosomes. Results were consistent with the other markers evaluated in C-PAC induced expression of Rab7 6, 24, and 48 h post-treatment in JHAD1 cells (1.2 to 1.4-fold) and more modestly in OE19 cells. In addition, we recently published results showing that C-PAC induces autophagic vacuole formation early (6 h) followed by increased formation of degradative autophagic vacuoles (24 h), a late event, in esophageal adenocarcinoma JHAD1 cells as measured by transmission electron microscopy [23].
Next, siRNA was utilized to knockdown Beclin-1 in EAC cells, followed by a 48 h treatment with C-PAC or rapamycin to further investigate the role of Beclin-1 alterations in relation to LC3 expression. Beclin-1 knockdown efficiency was 84% and 72% in JHAD1 and OE19 cells, respectively based on densitometry. C-PAC treatment of JHAD1 cells increased the autophagic or lipidated form of LC3, LC3-II; yet, significantly reduced Beclin-1 levels prior to knockdown as previously demonstrated in time-course experiments (Figure 3A). With siRNA suppression of Beclin-1 (Figure 3B) there was a greater magnitude of LC3-II induction (1.5% or 55.5% vs. 1.6% or 61.5%, respectively) in JHAD1 cells. Conversely, rapamycin treatment of JHAD1 cells increased Beclin-1 levels (1.4-fold) and with Beclin-1 knockdown levels of LC3-I and LC3-II remained in nearly equal proportions (48.5% vs. 51.5%, respectively). C-PAC treatment of OE19 cells with Beclin-1 knockdown resulted in LC3-II levels which increased in magnitude (1.9% or 55.9% vs. 2.1% or 55.2%) following C-PAC treatment, although interestingly, the proportion of LC3-1 to LC3-II remained unchanged in OE19 cells compared to JHAD1 cells. Rapamycin treatment of OE19 cells resulted in an increase in LC3-II levels that were maintained following Beclin-1 knockdown. Results support that C-PAC induces an alternative autophagic pathway which is not dependent on Beclin-1 as a molecular regulator.
C-PAC linked cell death appears largely caspase-independent, involves induction of LC3-II and the formation of autophagic vacuoles, particularly in JHAD1 and OE33 cells; whereas, greater necrotic cell death occurs in OE19 cells [23]. In this series of studies, LC3-II accumulation was 2.7 and 3.4-fold higher for JHAD1 and OE19 (P <0.012; 48 h) cells treated with C-PAC after 48 h, respectively (Figure 3A). This supports C-PAC activation of autophagic machinery and to a greater magnitude compared with rapamycin treatment of EAC cells. Furthermore, these data show for the first time that C-PAC induces autophagy without inducing Beclin-1 or increasing phosphorylation specific forms of Beclin-1 in EAC cells. Autophagy induction has classically involved increases in total Beclin-1 levels as occurring post-rapamycin treatment. However, recent evidence has emerged supporting Beclin-1-independent or non-canonical autophagy mechanisms by which autophagosomal degradation occurs through variants of the canonical pathway [34]. The specific functions of non-canonical forms of autophagy are still being unraveled, including the role of phosphorylation specific forms of Beclin-1 and the context in which autophagy leads to cancer cell death versus cancer cell survival. Thus, we next evaluated Beclin-1 phosphorylation patterns (Figure 3) and evaluated whether C-PAC, and rapamycin ultimately induced cancer cell survival or death (Figure 4A–D).
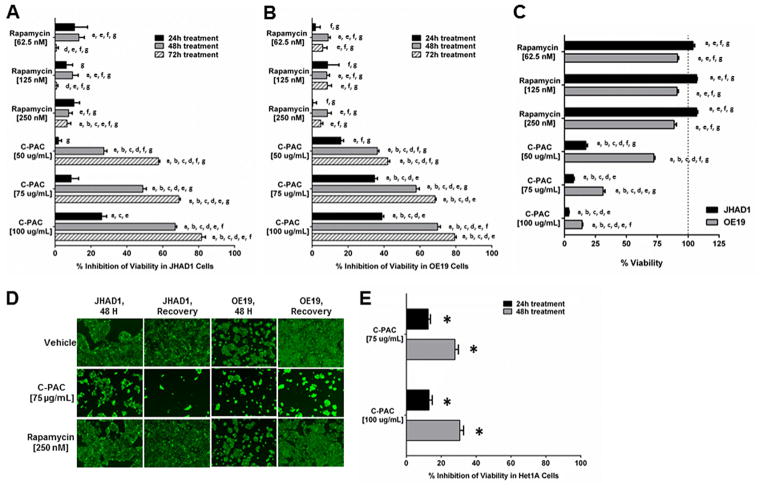
Figure 4.
Cell death modulation by autophagy inducing agents. Inhibition of viability of (A) JHAD1 and (B) OE19 cells was assessed using the live stain Calcein-AM following treatment with C-PAC (50, 75, and 100 μg/ml) and rapamycin (62.5, 125, and 250 nM). The data are reported as the mean ±SEM of at least four wells per treatment. (C) Viability was assessed 72 h after the removal of the 48 h treatment in JHAD1 and OE19 cells to assess recovery of treated cells as compared to the VEH treated wells. (D) Representative fluorescent images of viable JHAD1 and OE19 cells after 48 h of treatment with C-PAC or rapamycin, followed by media replenishment and a 72 h recovery period. Inhibition of viability was analyzed by ANOVA with Tukey’s post-hoc test (P <0.05) where multiple conditions were assessed. Treatments were significantly different from a =vehicle, b =rapamycin [62.5 nM], c =rapamycin [125 nM], d =rapamycin [250 nM], e =C-PAC [50 μg/ml], f =C-PAC [75 μg/ml] and g = C-PAC [100 μg/ml]. (E) Inhibition of Het-1A cell viability was measured 24 h and 48 h after treatment with C-PAC (75 and 100 μg/ml). A two-tailed unpaired Student’s t-test was utilized to determine statistical significance; asterisks denote significance from VEH treated cells (P <0.05).
Preventative Agents Differently Modulate Beclin-1 Phosphorylation
Induction of Beclin-1-dependent autophagy relies on target phosphorylation by kinases in the mTOR pathway including, ULK1, AMPK, and AKT [35–38]. Levels of autophagy-linked LC3-II and total Beclin-1 levels in JHAD1 (LC3-II; P <0.021; 6, 24, and 48 h) and OE19 cells modestly increased with rapamycin treatment (Figure 3A). In parallel, levels of Beclin-1 phosphorylated at Ser15 increased 1.5-fold in OE19 cells following rapamycin treatment, but significantly decreased in JHAD1 cells (P <0.031; 24 h and 48 h), potentially due to cell line specific sensitivity to rapamycin induced mitigation of AMPK and ULK signaling pathways [39]. Up-regulation of the autophagy initiation kinase, ULK1 (Ser15), has been reported via p53 following DNA damage leading to autophagy and cell death [40]. Levels of Beclin-1 phosphorylated at Ser93 significantly increased in JHAD1 (P <0.037; 24 h and 48 h) and OE19 cells (P <0.004; 6, 24, and 48 h). Cell line specific effects were also noted for Ser234 and Ser295, whose phosphorylation has been linked to autophagy inhibition and tumorigenesis [38]. Levels of Ser234 and Ser295, important for AKT-mediated autophagy, increased in OE19 cells; whereas, Ser234 decreased in JHAD1 cells and Ser295 was not detectable supporting constitutive loss. The latter difference may be linked to greater autophagy resistance in OE19 cells compared to JHAD1.
In general, Beclin-1 is rapidly dephosphorylated at the same 4 residues following C-PAC treatment in JHAD1 and OE19 cells. Ser15 levels in JHAD1 and OE19 cells decreased 1.7 and 1.8-fold at 48 h post-treatment, respectively. C-PAC treatment resulted in 1.8 and 3.6-fold reductions in phosphorylated Ser93 levels in JHAD1 and OE19 cells, respectively, at 48 h post-treatment. Constitutive levels of Beclin-1 phosphorylated at Ser234 are undetectable in JHAD1 cells and barely detectable but become undetectable after C-PAC treatment of OE19 cells. Finally, only OE19 cells constitutively express phosphorylated Beclin-1 at Ser295, likely linked to differential AKT activation [38] and levels decreased post-treatment. These data indicate that C-PAC treatment of EAC lines generally results in dephosphorylation of four serine residues in Beclin-1, further supporting a role for alternative regulators of C-PAC induced autophagy. Unexpectedly, these data indicate that autophagy inducers can have differential effects in the same cell line which has important relevance for clinical applications of autophagy mitigating agents.
Autophagy Inducing Agents Differently Modulate Cell Death
Given the different patterns of phosphorylated and total Beclin following rapamycin and C-PAC treatment, we next evaluated whether treatments contributed to pro-survival or pro-death pathways. C-PAC treatment significantly induced cell death in a time and dose dependent manner (Figure 4). All concentrations of C-PAC treatment [50, 75, and 100 μg/ml] significantly inhibited viability of JHAD1 and OE19 cells 48 h and 72 h post-treatment (22% to 70% at 48 h and 40% to 80% at 72 h). In contrast, rapamycin treatment only weakly impacted EAC viability. The lowest concentration of rapamycin resulted in the greatest reduction in viability of JHAD1 (13%, P <0.001) and OE19 (9%, P <0.02) cells at 48 h. Moreover, when JHAD1 cells treated for 48 h were replenished with fresh media and followed for an additional 72 h to evaluate cell fate (Figure 4A and C), all rapamycin treated JHAD1 cells exhibited significantly increased viability compared to vehicle treated cells (P <0.009). OE19 cells initially treated with rapamycin for 48 h then replenished with fresh media for 72 h maintained their viability but did not surpass vehicle cell viability, as did the JHAD1 cells. Conversely, C-PAC treated (48 h) EAC cells replenished with media continued to die in large numbers when assessed for viability 72 h later and effects were in a dose responsive manner, supporting that C-PAC alterations in autophagic machinery is linked to EAC cell death; whereas, rapamycin induced alterations in autophagic markers supports cancer cell survival, particularly in JHAD1 cells. This is inspite of the fact that both agents are known to modulate AKT/mTOR signaling pathways. Images of data summarized in Figure 4A–C show fluorescent stained viable JHAD1 and OE19 cells following 48 h of treatment with C-PAC or rapamycin, followed by replenishment with fresh media and re-assessment of viability 72 h post-treatment removal to permit assessment of recovery. The images parallel the graphic data clearly illustrating that C-PAC [75 μg/ml] induced potent EAC cell death with little recovery of viability after the removal of C-PAC. In contrast, rapamycin treated EAC cells appear to fully recover with JHAD1 treated cells surpassing vehicle treated cells further supporting induction of JHAD1 cell survival during the recovery period. Normal esophageal Het-1A cells were also assessed for inhibition of viability by C-PAC to ensure effects were not due to a generalized toxicity. Figure 4E shows that C-PAC induced levels of cell death are significantly lower in normal cells compared to esophageal adenocarcinoma cells. Maximum inhibition of viability by C-PAC [100 μg/ml] was 30% in normal esophageal cells compared to approximately 70% in EAC cells. In addition, we have treated Barrett’s cells with C-PAC and noted greater resistance to cell death compared to EAC cells (data not shown) supporting that C-PAC is not highly toxic, but selectively induces cell death in premalignant and cancer cell lines. In addition, recently completed research assessing the inhibitory effects of C-PAC against OE19 esophageal xenografts reported no signs of toxicity in mice up to 250 μg/day [23] and more recently, we have treated rodents with levels up to 1,000 μg/day with no signs of toxicity. Moreover, cranberry food products and juices regularly deliver equivalent amounts of C-PACs and other polyphenols found in cranberries [19].
Future studies involving genetic manipulations of additional members in the autophagy pathway, as well as inhibitors and inducers of the autophagy pathway are necessary to more fully elucidate requisite molecular machinery leading to autophagy induced cancer cell survival versus cancer cell death. Specifically, our results show that LC3 lipidation can be induced in the presence of Beclin-1 repression or knock down supporting potential alternative pathways of autophagy induction. Our results, for the first time, link the autophagy protein Beclin-1 to prognostic factors in EAC, but also illustrate the complexity of autophagy networks and uncover that two inducers of autophagy can differentially impact cancer cell fate.
Acknowledgments
Grant sponsor:National Institutes of Health; Grant sponsor: National Cancer Institute; Grant number: CA158319; Grant sponsor: Advancing a Healthier Wisconsin; Grant number: 5220251
We thank the National Institutes of Health and National Cancer Institute for funding support via grant (R01-CA158319) and Advancing a Healthier Wisconsin (5220251) for equipment funds, both awarded to Laura A. Kresty.
Abbreviations
- ATG
autophagy related
- BE
barrett’s esophagus
- C-PAC
cranberry proanthocyanidin extract
- EAC
esophageal adeno-carcinoma
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GERD
gastroesophageal reflux disease
- JHAD1
JH-EsoAd1 cells
- TMAs
tissue microarrays
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011. National Cancer Institute; Bethesda, MD: Apr, 2014. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112–120. doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spechler SJ. Barrett esophagus and risk of esophageal cancer: A clinical review. JAMA. 2013;310:627–636. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 7.Dawsey SM, Fagundes RB, Jacobson BC, et al. Diet and esophageal disease. Ann N Y Acad Sci. 2014;1325:127–137. doi: 10.1111/nyas.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng S, Huo X, Rezaei D, et al. Barrett’s esophagus patients and Barrett’s cell lines, ursodeoxycholic acid increases antioxidant expression and prevents DNA damage by bile acids. Am J Physiol Gastrointest Liver Physiol. 2014;307:G129–G139. doi: 10.1152/ajpgi.00085.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roesly HB, Khan MR, Chen HD, et al. The decreased expression of Beclin-1 correlates with progression to esophageal adenocarcinoma: The role of deoxycholic acid. Am J Physiol Gastrointest Liver Physiol. 2012;302:G864–G872. doi: 10.1152/ajpgi.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YC, Guan KL. MTOR: A pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroemer G. Autophagy: A druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125:1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125:14–24. doi: 10.1172/JCI73938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–181. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 16.Foo LY, Lu Y, Howell AB, Vorsa N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 17.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Krueger CG, Reed JD, Feliciano RP, Howell AB. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal Bioanal Chem. 2013;405:4385–4395. doi: 10.1007/s00216-013-6750-3. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg JB, Camesano TA, Cassidy A, et al. Cranberries and their bioactive constituents in human health. Adv Nutr. 2013;4:618–632. doi: 10.3945/an.113.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kresty LA, Clarke J, Ezell K, Exum A, Howell AB, Guettouche T. MicroRNA alterations in Barrett’s esophagus, esophageal adenocarcinoma, and esophageal adenocarcinoma cell lines following cranberry extract treatment: Insights for chemo-prevention. J Carcinog. 2011;10:34. doi: 10.4103/1477-3163.91110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kresty LA, Howell AB, Baird M. Cranberry proanthocyanidins induce apoptosis and inhibit acid-induced proliferation of human esophageal adenocarcinoma cells. J Agric Food Chem. 2008;56:676–680. doi: 10.1021/jf071997t. [DOI] [PubMed] [Google Scholar]
- 22.Kresty LA, Howell AB, Baird M. Cranberry proanthocyanidins mediate growth arrest of lung cancer cells through modulation of gene expression and rapid induction of apoptosis. Molecules. 2011;16:2375–2390. doi: 10.3390/molecules16032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kresty LA, Weh KM, Zeyzus-Johns B, Perez LN, Howell AB. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget. 2015;6:33438–33455. doi: 10.18632/oncotarget.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaspar KL, Howell AB, Khoo C. A randomized, double-blind, placebo-controlled trial to assess the bacterial anti-adhesion effects of cranberry extract beverages. Food Funct. 2015;6:1212–1217. doi: 10.1039/c4fo01018c. [DOI] [PubMed] [Google Scholar]
- 25.Stoner GD, Kaighn ME, Reddel RR, et al. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991;51:365–371. [PubMed] [Google Scholar]
- 26.Botti J, Djavaheri-Mergny M, Pilatte Y, Codogno P. Autophagy signaling and the cogwheels of cancer. Autophagy. 2006;2:67–73. doi: 10.4161/auto.2.2.2458. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Xia X, Pan H. Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis. Oncol Lett. 2013;5:411–416. doi: 10.3892/ol.2012.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai N, Zhao X, Jing Y, et al. Autophagy protects against palmitate-induced apoptosis in hepatocytes. Cell Biosci. 2014;4:28. doi: 10.1186/2045-3701-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkas T, Hoyer-Hansen M, Jaattela M. Identification of novel autophagy regulators by a luciferase-based assay for the kinetics of autophagic flux. Autophagy. 2009;5:1018–1025. doi: 10.4161/auto.5.7.9443. [DOI] [PubMed] [Google Scholar]
- 30.Nyfeler B, Bergman P, Triantafellow E, et al. Relieving autophagy and 4EBP1 from rapamycin resistance. Mol Cell Biol. 2011;31:2867–2876. doi: 10.1128/MCB.05430-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H, Pan B, Yi J, Chen L. Autophagic activation with nimotuzumab enhanced chemosensitivity and radiosensitivity of esophageal squamous cell carcinoma. Exp Biol Med (Maywood) 2014;239:529–541. doi: 10.1177/1535370214525315. [DOI] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 34.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: Variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- 35.Fogel AI, Dlouhy BJ, Wang C, et al. Role of membrane association and Atg14-dependent phosphorylation in beclin-1-mediated autophagy. Mol Cell Biol. 2013;33:3675–3688. doi: 10.1128/MCB.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Kim YC, Fang C, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell RC, Tian Y, Yuan H, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao W, Shen Z, Shang L, Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ. 2011;18:1598–1607. doi: 10.1038/cdd.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]