Abstract
Inflammation can profoundly impact motivated behavior, as is the case with inflammation-induced depression. By evaluating objectively measurable basic neurobehavioral processes involved in motivation, recent research indicates that inflammation generally reduces approach motivation and enhances avoidance motivation. Increased effort valuation largely mediates the effects of inflammation on approach motivation. Changes in reward valuation are not uniformly observed in approach motivation. However, inflammation increases the averseness of negative stimuli. Within the context of both approach and avoidance motivation, inflammation appears to enhance the contrast between concurrently presented stimuli. While changes in both approach and avoidance motivation appear to be mediated by midbrain dopaminergic neurotransmission to the ventral striatum, it is unclear if the enhanced contrast is mediated by the same system.
Keywords: motivation, depression, fatigue, inflammation, positive valence systems, negative valence systems
Introduction
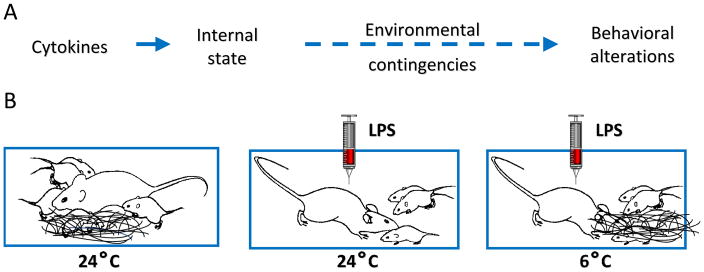
Motivation can be defined as a central state that organizes perception and behavior. In particular it arouses, directs, and maintains behavior. While many factors are capable of affecting motivation, inflammation is the one of interest for the present review [1,2]. Inflammation-induced sickness behavior is one example of inflammation altering motivational states [3]. In response to an infection detected by innate immune cells, the host presents drastic behavioral changes that favor the development of fever and result in environmental withdrawal. However, inflammation is not necessarily associated with reduced motivation. Instead, inflammation reorganizes priorities of the sick individual. As a typical example, rats treated with endotoxin display reduced lever pressing for rewards (e.g., food, water, intracranial self-stimulation) but engage in increased lever pressing in order to get periods of rest when forced to exercise in a running wheel [4]. Further support for inflammation-induced motivational reorganization comes from a study of lactating mice treated with lipopolysaccharide (LPS) [5]. LPS-treated mother mice do not respond to their pups’ solicitations. However, when their pups are dispersed in the home cage and the nest is replaced by cotton wool, they engage in elements of maternal behavior that vary according to ambient temperature (Fig. 1). At normal ambient temperature they move around to retrieve their pups to the cotton wool but do not build a nest. In the cold, they engage in both pup retrieval and nest building. More recently this principle has been illustrated in the context of adaptive cold-seeking behavior in a rat following a high dose of LPS [6].
Figure 1. Motivational interpretation of sickness behavior.
In a situation of motivational competition, the resulting behavior depends on the relative strength of the incentives for each motivated behavior (A). For example, sickness behavior vs. maternal behavior in different temperature contingencies (B). Note that the higher the environmental contingencies for maternal behavior (6 vs. 24°C) the lower the behavioral manifestations of sickness. At 6°C sick lactating mice engage in fast pup retrieval and nest building while at 24°C they only engage in pup retrieval.
While short-term alterations in motivated behavior, as in the context of sickness, are often seen as adaptive, chronic alterations in motivational states are generally considered maladaptive, as would be the case with the motivational deficits that are associated with major depressive disorder (MDD). Inflammation can induce depression [7,8]. This depression is mainly apparent in the form of decreased activity, anergia or fatigue, and decreased response to rewards [9,10]. These symptoms are relatively resistant to antidepressant treatment compared to the affective and cognitive dimensions of depression [11–13].
Understanding the relationship between inflammation and motivation may shed light onto the mechanisms that mediate these effects and lead to new interventions for chronically altered motivational states. Within this brief review we will highlight the literature exploring the components of motivation affected by inflammation and then briefly consider mechanisms. We will do this by focusing on the basic neurobehavioral processes that are involved in motivation and that can be measured objectively in mice and/or humans. This approach, advocated by the NIH Research Domain Criteria (RDoC) framework, places motivational processes primarily within two domains: positive valence systems and negative valence systems [14,15]. Positive valence systems generally govern approach motivation, or motivation guided by a reward. Negative valence systems govern response to aversive stimuli and, therefore, generally guide motivation to avoid discomfort, threat, or loss.
Positive Valence Systems: Approach Motivation
Approach motivation requires action based on integration of information concerning reward valuation and effort valuation. Valuation of the reward involves the assignment of incentive salience to the reward. The assignment of lesser value to an expected reward results in anhedonia, a common feature of MDD. However, reward valuation is insufficient to maintain behavior. Motivation also involves valuation of effort required to obtain the reward. In general individuals make decisions based on the attractiveness of the reward and the cost necessary to obtain it. A reduction in willingness to exert effort is an important component of fatigue, considered as an entity or as a symptom of depression. Apathy, a disorder of motivation that is operationalized as diminished goal oriented behavior and cognition [16], also manifests as reduced willingness to exert effort when rewards are small [17].
It is well accepted that inflammation can reduce approach motivation. Specifically, it has been demonstrated that LPS administration can decrease reward expectancy as measured by a reduction in food-related anticipatory activity [18]. Reduction in reward anticipation also appears to be an important component of the motivational deficits observed in MDD [19,20]. Further, preclinical studies in rodents and monkeys have shown that administration of inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, or interferon-alpha (IFN-α), reduces willingness to work for highly palatable rewards without reducing willingness to eat freely available, less palatable food [21–23]. Similar effects manifest as self-reported fatigue in patients with cancer or hepatitis C who are undergoing treatment with IFN-α or IL-2 [12,24,25] and in patients with MDD [20,26,27]. These studies suggest that effort valuation is impacted by inflammation but not reward sensitivity. In agreement with this interpretation is a human study in which LPS- or placebo-treated volunteers were repeatedly asked to choose whether or not to participate in an effort task after being provided information concerning the required effort level and reward level [28]. LPS treatment reduced selection of high effort tasks (i.e., increased effort valuation) with no significant effect on reward valuation.
However, in another set of studies in which both high and low effort options were available simultaneously, a different pattern of results emerged. Mice subjected to inflammation, in the form of LPS 24 hours prior to testing, showed a reduction in overall effort in a concurrent choice task that contrasts high effort/high reward and low effort/low reward options [29]. This aligns with the previously mentioned studies showing an increase in effort valuation. However, a change in reward sensitivity also emerged. Rather than showing a uniform reduction in reward value, an enhancement in contrast between rewards of different magnitudes was observed such that responding to the less preferred reward was more dramatically suppressed than responding to the more preferred reward, despite the higher work criteria. This enhanced contrast effect generalizes to human participants as demonstrated in a study of volunteers treated with LPS tested in the Effort Expenditure for Rewards Task (EEfRT) [30]. The EEfRT task was developed to assess reward and effort valuation in human subjects [27]. LPS treatment shifted participants toward increased selection of the high effort/high reward trials as opposed to low effort/low reward trials. These findings indicate that if effort is to be expended in an inflamed state, it will be directed toward stimuli that are judged to be “worth the effort”. This is in contrast to the effect observed in patients with MDD, where an overall decrease in effort has been observed [20,27]. This difference is likely due to the fact that inflammation does not induce any anhedonia.
The literature concerning motivation toward social support also helps with understanding the idea of increased motivation in the context of inflammation. Administration of endotoxin to healthy participants leads to enhanced desire for support figures and greater ventral striatum activity in response to images of support figures [31]. This effect appears to be specific to social support figures, which would be adaptive in an inflamed state, as this does not necessarily translate to all social rewards. Another report shows that depression is associated with reduced motivation to work for social feedback yet increases effort toward food rewards [32].
This body of work aligns well with the concept that inflammation shifts motivational priorities rather than blunts all reward cues. As such, inflammation-induced reductions in approach motivation appear to be a result of increased effort valuation, however, it does not necessarily result in a reduction in reward sensitivity [33]. In fact, inflammation can increase the sensitivity of contrast between rewards of differing value.
Negative Valence Systems: Motivation to Avoid
The effects of inflammation on negative valence systems are much less studied than their effects on positive valence systems. Inflammation by itself is aversive. This can be experimentally demonstrated using a conditioned taste aversion procedure or a conditioned place avoidance test; even a low dose of LPS or IL-1β is able to induce avoidance behavior in mice [34–38]. Not only is inflammation inherently aversive, it also appears to increase the averseness of negative motivational stimuli. For example, treatment with endotoxin enhances brain reactivity to negative feedback concerning social appraisal in several threat-related regions (amygdala, dorsal anterior cingulate cortex) as well as mentalizing-related regions (dorsomedial PFC) [39]. Further, this effect appears to be more pronounced when the negative stimuli are presented concomitantly with positive motivational stimuli. For instance, in a taste-reactivity task, LPS-treated rats responded normally to brief intra-oral infusions of sucrose or quinine solutions. However, they were more sensitive to the aversive component of the mixed sweet/bitter taste of a saccharin solution [40]. This inflammation-induced enhanced contrast has also been noted in human studies. For example, in a human probabilistic instrumental task in which participants learned to select high probability reward and avoid high probability loss, typhoid vaccination enhanced the averseness to loss while simultaneously making rewards less attractive [41]. The enhanced salience of loss rather than reward was also demonstrated in a study of depressed patients who showed higher connectivity between the ventral striatum and midline cortical regions during loss as compared to reward trials [42].
Mechanisms
Dopaminergic neurons terminating in the ventral striatum appear to play a key role in motivated behavior. Dopaminergic agonists, but not classical antidepressants, abrogate inflammation-induced deficits in incentive motivation [43]. A recent study elegantly demonstrated the importance of the type 2 dopamine receptor (D2) within the ventrolateral striatum in mediating motivated behavior [44]. Conditionally knocking out this receptor resulted in a chronic reduction in breakpoint on a progressive ratio task while optogenetic inhibition of these neurons caused transient reduction in breakpoint. Further, there is research indicating that the type 3 dopamine receptor within the striatum may also be involved in motivated behavior [45]. Clinical imaging studies evaluating experimental induced inflammation or natural variations in inflammation also point to the importance of the ventral striatum in mediating these findings [46–50]. For example, increased inflammation in either patients treated with IFN-α or in those with depression show increased glutamate levels in the basal ganglia [51,52], while mean diffusivity imaging reveals that only the motivational symptoms of depression show changes related to the dopaminergic system [53].
Inflammation-induced aversion also appears to be modulated by the nigro-striatal dopaminergic system. Recent experiments carried out in mice have helped to define the cellular network responsible for inflammation-induced conditioned place aversion. Peripheral upregulation of interferon signaling activates brain endothelial cells to cause the release of prostaglandin (PGE2), via a MyD88 dependent process [34,35]. PGE2 act on the dopaminergic system to induce aversion. Further, while imaging studies of IFN-α treated patients revealed broad impacts on functional brain connectivity networks, changes within nodes of the ventral striatum and thalamus were specifically associated with motivationally oriented symptoms, including negative mood [50]. Further, the neural representation of both reward and punishment prediction errors was located within the ventral striatum and anterior insula [41]. Therefore, we can provisionally propose that the neurobiological underpinnings of inflammation-induced motivational changes involve a dual role of midbrain dopamine neurons in modulating both motivational approach and avoidance behaviors.
Further work is needed to confirm the role of the dopaminergic system in inflammation-induced changes in motivational contrast. Additionally, while the role of the dopaminergic system is well established, the data is still unclear concerning the mechanism by which inflammation alters its function. The work that has been done in this area is primarily in the context of Parkinson’s disease. This literature indicates that non-neuronal cells, such as microglia and astrocytes, play a pivotal role in neuroinflammation, leading to oxidative stress and mitochondrial damage that result in neurodegeneration of dopaminergic neurons [54,55]. However, the severity of these effects is modulated by genetic mutations (e.g., parkin, DJ-1 and α-synuclein) that are probably absent in inflammation-induced motivational changes. Therefore, much work is still needed to understand the mechanisms by which neuroinflammation negatively impacts the functionality of dopaminergic neurons projecting to the ventral striatum.
A variety of pathways have been proposed to mediate the effects of inflammation on dopaminergic activity. Brain cytokines can have direct effects on neural function, such as through oxidative stress induced mitochondrial damage or upregulation of dopamine reuptake transporter. They can also have indirect effects, such as via activation of indoleamine 2,3-dioxygenase (IDO1). IDO1 activity in the brain results in the generation of neurotoxic kynurenine metabolites and/or interference with the metabolism of extracellular glutamate [1]. Recent data indicate that activation of the kynurenine pathway is not required for inflammation-induced effect on positive valence systems [18], therefore, future work focusing on the impact of inflammation on oxidative stress, antioxidant response, and mitochondria may be warranted.
Conclusion
Inflammation is able to exert a profound effect on motivated behaviors (Table 1). These alterations can express themselves in the form of fatigue, depression, and other symptoms. Understanding how inflammation alters motivation is critical to identifying treatments. A look at the currently available literature indicates that inflammation generally reduces approach motivation and enhances avoidance motivation via midbrain dopaminergic neurotransmission to the ventral striatum. The reduction in approach motivation is largely mediated by increased effort valuation resulting in a reduced willingness to work for rewards. Rather than uniformly impacting reward valuation, inflammation appears to enhance the contrast between rewarding stimuli of the same hedonic valence (making the less rewarding stimuli much less attractive) or of different hedonic value (increasing the averseness of negative stimuli compared to positive stimuli). Future work in this area would benefit from exploring the mechanisms by which inflammation affects dopaminergic neurotransmission, with a possible emphasis on oxidative stress and mitochondrial damage, as well as to determine whether the enhanced contrast noted above is also mediated by alterations in dopaminergic neurotransmission.
Table 1.
Summary of effects of inflammation on components of motivated behavior.
| Effects of Inflammation | |
|---|---|
| Reward Evaluation | ↑ ↓ |
| Effort Evaluation | ↑ |
| Reward Expectancy | ↓ |
| Loss Evaluation | ↑ |
| Reward Contrast | ↑ |
↑ =increase; ↓ = decrease
Highlights.
Inflammation is associated with a reorganization of motivational priorities
Reduced approach motivation is associated primarily with increased effort valuation
Alterations in reward valuation largely depend on the nature of the reward
Negative stimuli are perceived as more aversive
The contrast between concurrently presented stimuli is enhanced
Acknowledgments
Grant Funding
This research was supported by the National Institutes of Health (R01 CA193522 and R21 MH104694 to R. Dantzer as well as an MD Anderson Cancer Center Support Grant, P30 CA016672).
Footnotes
Declarations of interest: R. Dantzer received honoraria from Danone Nutricia Research unrelated to the present study. E.G. Vichaya has no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karshikoff B, Sundelin T, Lasselin J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front Immunol. 2017;8:21. doi: 10.3389/fimmu.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller NE. Some psychophysiological studies of motivation and of the behavioural effects of illness. Bulletin of the British Psychological Society. 1964;17:1–20. [Google Scholar]
- 5.Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–118. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- 6.Wanner SP, Almeida MC, Shimansky YP, Oliveira DL, Eales JR, Coimbra CC, Romanovsky AA. Cold-Induced Thermogenesis and Inflammation-Associated Cold-Seeking Behavior Are Represented by Different Dorsomedial Hypothalamic Sites: A Three-Dimensional Functional Topography Study in Conscious Rats. J Neurosci. 2017;37:6956–6971. doi: 10.1523/JNEUROSCI.0100-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer R. Psychiatric Disorders and Inflammation. In: Cavaillon JM, Singer M, editors. Inflammation: From Mechanisms to the Clinic. Wiley-VCH; 2018. [Google Scholar]
- *8.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. doi: 10.1038/mp.2016.167. Epub ahead of print. This review presents the results of a systematic meta-analysis of 20 clinical trials of anti-cytokine treatment in which antidepressant activity was a secondary outcome. The analyses revealed a significant benefit of the anti-cytokine treatments and point to a possible causal role for cytokines in depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38:1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- *10.White J, Kivimaki M, Jokela M, Batty GD. Association of inflammation with specific symptoms of depression in a general population of older people: The English Longitudinal Study of Ageing. Brain Behav Immun. 2017;61:27–30. doi: 10.1016/j.bbi.2016.08.012. By using data from the English Longitudinal Study of Ageing, White and colleagues performed a cross-sectional analysis on symptoms of depression (I.e., fatigue, sleep, energy) and C-reactive protein (CRP) levels in a sample of 5909 participants. Somatic symptoms of depression (such as fatigue, sleep, and energy) were more likely to be associated with CRP levels than the psychologically oriented symptoms of depression. [DOI] [PubMed] [Google Scholar]
- 11.Papakostas GI, Petersen T, Denninger J, Sonawalla SB, Mahal Y, Alpert JE, Nierenberg AA, Fava M. Somatic symptoms in treatment-resistant depression. Psychiatry Res. 2003;118:39–45. doi: 10.1016/s0165-1781(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 12.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 13.Chung K-F, Yu Y-M, Yeung W-F. Correlates of residual fatigue in patients with major depressive disorder: The role of psychotropic medication. Journal of Affective Disorders. 2015;186:192–197. doi: 10.1016/j.jad.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 16.Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79:1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- 17.Bonnelle V, Veromann KR, Burnett Heyes S, Lo Sterzo E, Manohar S, Husain M. Characterization of reward and effort mechanisms in apathy. J Physiol Paris. 2015;109:16–26. doi: 10.1016/j.jphysparis.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vichaya EG, Laumet G, Christian DL, Grossberg AJ, Estrada DJ, Heijnen CJ, Kavelaars A, Dantzer R. Inflammation alters motivational processes in mice independently of its ability to activate indoleamine 2,3-dioxygenase. Brain, Behavior, and Immunity. 2017;66:e32. [Google Scholar]
- 19.Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC, Xie GR. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220:874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 21.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, et al. Chronic Interferon-[alpha] Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia-Like Behavior in Nonhuman Primates. Neuropsychopharmacology. 2013;38:2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA, Baqi Y, Muller CE, Correa M, Salamone JD. Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/chow feeding choice task. Psychopharmacology (Berl) 2014;231:727–736. doi: 10.1007/s00213-013-3285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Muller CE, Miguel NS, Correa M, Salamone JD. Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology (Berl) 2016;233:3575–3586. doi: 10.1007/s00213-016-4392-9. Using a task evaluating the tendency for rat to work for a preferred reward, Yohn and colleagues showed that IL-6 dose dependently attenuated performance. Dopaminergic mechanisms are indicated in this effect as modulators of dopaminergic activity (I.e., adenosine antagonist MSX-3 and the stimulant methylphenidate) attenuated the IL-6 effects and microdialysis demonstrated that IL-6 decreased dopamine levels within the nucleus accumbens. [DOI] [PubMed] [Google Scholar]
- 24.Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- 25.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershenberg R, Satterthwaite TD, Daldal A, Katchmar N, Moore TM, Kable JW, Wolf DH. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord. 2016;196:97–100. doi: 10.1016/j.jad.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Draper A, Koch RM, van der Meer JW, Apps M, Pickkers P, Husain M, van der Schaaf ME. Effort but not Reward Sensitivity is Altered by Acute Sickness Induced by Experimental Endotoxemia in Humans. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.231. Using a sample of young healthy males, this study evaluated the impact of 2.0 ng/kg LPS (n=14) or placebo (n=15) on effort and reward sensitivity using a task requiring contractions on a hand-held dynamometer. At the 2 h post time point, LPS was associated with a reduction in the percent of high effort options selected but was not associated with an alteration in reward sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vichaya EG, Hunt SC, Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology. 2014;39:2884–2890. doi: 10.1038/npp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Goranson S, Axelsson J, Dantzer R, Lekander M. Lipopolysaccharide Alters Motivated Behavior in a Monetary Reward Task: a Randomized Trial. Neuropsychopharmacology. 2017;42:801–810. doi: 10.1038/npp.2016.191. To evaluate the impact of LPS on motivated behavior, Lasselin and colleagues tested 21 healthy subjects in a double-blinded cross-over design. Participants were tested 4–5 hours post 2.0 ng/kg LPS or saline treatment using the Effort Expenditure for Rewards Task (EEfRT). This task contrasts high effort/high reward trials with low effort/low reward trials on a computer task that has variable levels of reward magnitude and win probability. This task demonstrated that LPS increase incentive motivation for the high-reward trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fussner LM, Mancini KJ, Luebbe AM. Depression and Approach Motivation: Differential Relations to Monetary, Social, and Food Reward. Journal of Psychopathology and Behavioral Assessment. 2017 [Google Scholar]
- 33.Irwin MR, Eisenberger NI. Context-Dependent Effects of Inflammation: Reduced Reward Responding is Not an Invariant Outcome of Sickness. Neuropsychopharmacology. 2017;42:785–786. doi: 10.1038/npp.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritz M, Klawonn AM, Jaarola M, Engblom D. Interferon- mediated signaling in the brain endothelium is critical for inflammation-induced aversion. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Fritz M, Klawonn AM, Nilsson A, Singh AK, Zajdel J, Wilhelms DB, Lazarus M, Lofberg A, Jaarola M, Kugelberg UO, et al. Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J Clin Invest. 2016;126:695–705. doi: 10.1172/JCI83844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross-Mellor SK, Kavaliers M, Ossenkopp KP. The effects of lipopolysaccharide and lithium chloride on the ingestion of a bitter-sweet taste: comparing intake and palatability. Brain Behav Immun. 2005;19:564–573. doi: 10.1016/j.bbi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Mormede C, Palin K, Kelley KW, Castanon N, Dantzer R. Conditioned taste aversion with lipopolysaccharide and peptidoglycan does not activate cytokine gene expression in the spleen and hypothalamus of mice. Brain Behav Immun. 2004;18:186–200. doi: 10.1016/S0889-1591(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson A, Wilhelms DB, Mirrasekhian E, Jaarola M, Blomqvist A, Engblom D. Inflammation-induced anorexia and fever are elicited by distinct prostaglandin dependent mechanisms, whereas conditioned taste aversion is prostaglandin independent. Brain Behav Immun. 2017;61:236–243. doi: 10.1016/j.bbi.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun. 2016;57:21–29. doi: 10.1016/j.bbi.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubert A, Dantzer R. The taste of sickness: lipopolysaccharide-induced finickiness in rats. Physiol Behav. 2005;84:437–444. doi: 10.1016/j.physbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- *41.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biol Psychiatry. 2016;80:73–81. doi: 10.1016/j.biopsych.2015.07.018. To evaluate the reorientation of motivational states, this study used a cross-over design with 24 healthy volunteers 3 hours after typhoid vaccination or saline in a choice behavioral task during functional magnetic resonance imaging. Typhoid vaccination resulted in orienting behavior toward avoiding punishment (monetary loss), causing rewards (monetary gain) to be less behaviorally attractive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quevedo K, Ng R, Scott H, Kodavaganti S, Smyda G, Diwadkar V, Phillips M. Ventral Striatum Functional Connectivity during Rewards and Losses and Symptomatology in Depressed Patients. Biol Psychol. 2017;123:62–73. doi: 10.1016/j.biopsycho.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, Zafar N, Correa M, Salamone JD. Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: Implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacology. 2016;109:270–280. doi: 10.1016/j.neuropharm.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsui-Kimura I, Takiue H, Yoshida K, Xu M, Yano R, Ohta H, Nishida H, Bouchekioua Y, Okano H, Uchigashima M, et al. Dysfunction of ventrolateral striatal dopamine receptor type 2-expressing medium spiny neurons impairs instrumental motivation. Nature Communications. 2017;8:14304. doi: 10.1038/ncomms14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson EH, Winiger V, Biezonski DK, Haq I, Kandel ER, Kellendonk C. Selective Overexpression of Dopamine D3 Receptors in the Striatum Disrupts Motivation but not Cognition. Biological Psychiatry. 2014;76:823–831. doi: 10.1016/j.biopsych.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. To investigate the relationship between inflammation and cortiostriatal reward circuitry, Felger and colleagues measured CRP levels and conducted whole-brain voxel-wise functional connectivity in 48 unmediated patients with depression. The data revealed that increased levels of CRP were associated with decreased connectivity between the striatum and ventromedial prefrontal cortex. These changes were associated with anhedonia and decreased motor speed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, Olson DP, Cooper JA, Pizzagalli DA. Association Between Interleukin-6 and Striatal Prediction-Error Signals Following Acute Stress in Healthy Female Participants. Biol Psychiatry. 2017;82:570–577. doi: 10.1016/j.biopsych.2017.02.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA. Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol Psychiatry. 2016;79:320–328. doi: 10.1016/j.biopsych.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dipasquale O, Cooper EA, Tibble J, Voon V, Baglio F, Baselli G, Cercignani M, Harrison NA. Interferon- alpha acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain Behav Immun. 2016;58:31–39. doi: 10.1016/j.bbi.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, Hu XP, Miller AH. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39:1777–1785. doi: 10.1038/npp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, Hu XP, Miller AH. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21:1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi H, Taki Y, Sekiguchi A, Nouchi R, Kotozaki Y, Nakagawa S, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T, et al. Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain Struct Funct. 2017;222:1027–1037. doi: 10.1007/s00429-016-1262-5. [DOI] [PubMed] [Google Scholar]
- 54.Niranjan R. The Role of Inflammatory and Oxidative Stress Mechanisms in the Pathogenesis of Parkinson’s Disease: Focus on Astrocytes. Molecular Neurobiology. 2014;49:28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- 55.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Frontiers in Neuroanatomy. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]