Abstract
Background
Chronic rhinosinusitis (CRS) is strongly associated with comorbid asthma. This study compares early-onset and late-onset asthma in a CRS population using patient-reported and clinical characteristics.
Methods
At enrollment into a clinical registry, CRS patients completed the 22-item Sino-Nasal Outcome Test (SNOT-22), Asthma Control Test (ACT), mini-Asthma Quality of Life Questionnaire (miniAQLQ), the 29-item Patient-Reported Outcomes Measurement Information System (PROMIS-29), and medication use questionnaires. Patients also reported comorbid asthma and age at first asthma diagnosis. Early-onset (<18 years) and late-onset (>18 years) asthma groups were defined. Analysis of variance (ANOVA), chi-square, and Kruskal-Wallis tests were used to compare patient responses.
Results
A total of 199 non-asthmatic (56.1%), 71 early-onset asthmatic (20.0%), and 85 late-onset asthmatic (23.9%) CRS patients completed the survey. Body mass index (BMI) was significantly higher in late-onset asthmatic (p = 0.046) while age, gender, race, and smoking history did not differ with time of asthma onset. SNOT-22, ACT, and miniAQLQ were not different between asthma groups, but late-onset asthmatics had significantly lower physical function than non-asthmatics (p = 0.008). Compared to non-asthmatics, late-onset asthmatics showed increased rates of nasal polyps (p < 0.001), higher Lund-Mackay scores (p = 0.005), and had received more oral steroid courses (p < 0.001) and endoscopic surgeries (p = 0.008) for CRS management. Late-onset asthmatics compared to early-onset asthmatics showed increased nasal polyposis (p =0.011) and oral steroid courses for CRS (p = 0.003).
Conclusion
While CRS-specific and asthma-specific patient-reported outcome measures (PROMs) were not significantly different among groups, CRS patients with late-onset asthma had poorer physical function, more frequent nasal polyposis, and required increased treatment for CRS. Late-onset asthma may predict more severe disease in CRS.
Keywords: adult onset asthma, chronic rhinosinusitis, disease severity, nasal polyps, quality of life
Chronic rhinosinusitis (CRS) is a prevalent condition with approximately 9.3% to 11.9% of the general population meeting the epidemiologic criteria for CRS in Europe and the United States.1, 2 Compared to the general population, CRS patients show a higher risk of asthma with up to 20% experiencing comorbid asthma.3 The frequent coexistence of these conditions has led to the concept of a unified airway disease, linking these two disorders.4 Research on CRS and asthma provides growing evidence for this theory, with findings that upper and lower airway epithelial cells react similarly to common environmental and inflammatory stimuli.5 Population-based studies have confirmed the association between the 2 conditions across all age ranges and have shown this relationship to be bidirectional with CRS patients having higher incident asthma and asthmatic patients having higher incident CRS.6–8
In asthmatic cohorts like the Unbiased BIOmarkers in PREDiction of respiratory disease outcomes (U-BIOPRED) and Severe Asthma Research Program (SARP), studies have found increased rates of nasal polyps and more severe sinus radiographic severity among severe asthmatics.9, 10 One cluster analysis of an asthma population identified four groups with severe asthma, two of which were associated with sinusitis. One of these showed characteristic features of severe CRS, namely peripheral eosinophilia and the need for surgery to treat polyposis.9 Other lines of research have reported that endoscopic sinus surgery (ESS) results in reductions in incident asthma and improvements in asthma-associated quality of life, suggesting that CRS influences asthma severity and treatment of CRS may even prevent the onset of asthma.11–14
While CRS is generally an adult-onset disease with peak incidence in the 4th to 5th decades of life,7 asthma has 2 patterns of onset, roughly broken down to early-onset and late-onset asthma, with a multitude of subtypes in each category.15 Asthma incidence was highest in childhood in 1 Italian cohort peaking at 5.17 cases per 1000 persons/year between ages 0 to 5 years and dropping steadily to a trough of 1.4 cases per 1000 persons/year between ages 20 to 25 years.16 Wheezing illness (not limited to asthma) was present in 18% of a British population-based cohort by age 7 years, increasing to 24% by 16 years old and 43% by age 33 years.17 While the risk for asthma onset in early childhood is highest, this research shows that the risk of developing asthma exists well into adulthood. Asthma onset in early childhood has largely been associated with atopy (eg, allergic rhinitis) with this association remaining until adulthood. In addition to atopy, environmental factors such as smoking are commonly associated with late-onset as opposed to early-onset asthma.17, 18 In the same vein, late-onset asthma also demonstrates multiple phenotypes, which have been associated with different immune responses, symptoms, lifestyle, or demographic factors.15 A well-known phenomenon is that the gender most at risk for asthma switches from males in childhood to females in adulthood, with the female predominant, obese, and non-eosinophilic groups being a recognizable constellation within the late-onset asthmatics.15, 19
Overall, the present study seeks to examine the associations between asthma onset, defining early-onset (before 18 years of age) or late-onset (after 18 years of age) groups, and CRS severity assessed using clinical parameters and patient-reported outcome measures (PROMs). Clinical CRS parameters have been studied previously in asthmatic populations showing that severe late-onset asthma is associated with ESS for removal of polyps.9 Other severe asthma populations have shown similar association with extensive sinus disease in asthma patients with later age of asthma onset.20 The present study seeks to validate these findings in a CRS population while expanding the examination to include patient outcomes through PROMs to gain a more complete understanding of asthma onset in relation to sinus disease and symptom severity.
Patients and methods
Patients
CRS patients aged 18 to 89 years without ESS in the last 18 months were recruited from the Otolaryngology practice of Northwestern Medicine, a tertiary care center, before initiating medical treatment or undergoing ESS. All patients had either an existing or new diagnosis of CRS as de-fined by the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis.21 The study was approved by the institutional review board (IRB) of Northwestern University.
Enrolled patients completed a patient-reported survey while their treating physician recorded their body mass index (BMI), endoscopy and computed tomography (CT) findings. Endoscopy was quantified by a modified polyp grading system previously used by Gevaert et al.,22 examining a single nostril and grading polyps, when present, as follows: 1, confined to the middle meatus; 2, extending below the middle turbinate; 3, extending to the floor of the nose; 4, completely filling the nasal cavity.23 CT findings were scored according to the Lund-Mackay system.23, 24 For ease of analysis and to limit the effects of minor differences in scoring, the Lund-Mackay raw scores were reported in groups with score ranges of 1 to 8, 8 to 16, or greater than 16. The patient-reported portion of the survey included demographic factors and medical history including but not limited to: sex; age; gender; race; smoking history; and history of asthma, nasal allergies, atopy and respiratory symptoms triggered by aspirin. History of nasal allergies was self-reported; patients were asked to report prior diagnosis by a physician of nasal allergies as a child, adult, or both. This was recorded as a history of nasal allergies and age of onset. Atopy was also self-reported as prior positive testing for aeroallergens by any method. Patient self-report was used to determine the age of asthma onset, if present. Further medical history was collected regarding CRS and asthma treatment. CRS treatment information included previous endoscopic sinus surgery as well as medication used to treat sinus or nasal condition and number of courses (antibiotics, steroid pills, nasal steroid sprays, and over the counter pills) over the previous year. Asthma treatment information included general treatment for asthma in the past 5 years as well as steroid courses taken for asthma over the past year.
CRS-specific PROMs included the 22-item Sino-Nasal Outcome Test (SNOT-22) and the European Position Statement on Rhinosinusitis (EPOS) visual analog scale (VAS).25, 26 Patients who self-reported a prior physician diagnosis of asthma completed the Asthma Control Test (ACT) and mini-Asthma Quality of Life Questionnaire (miniAQLQ), which represent asthma-specific PROMs included in the registry survey.27, 28 The ACT is a validated 5-item measure of asthma control over the previous 4 weeks with higher results correlating with better asthma control. The miniAQLQ asks patients 15 asthma-related questions regarding symptoms, activity limitation, emotional function, and environmental exposure with a 7-point response scale. Each survey result is calculated as an average of the response on all questions with a scale of 1 to 7. The 29-item Patient-Reported Outcomes Measurement Information System (PROMIS-29) was used to assess general quality of life across anxiety, depression, fatigue, sleep disturbance, satisfaction with social roles, pain interference, and physical function domains. PROM data were collected and managed using REDCap electronic data capture tools hosted at Northwestern University Feinberg School of Medicine. Per protocol, the scores of each domain were calculated individually and converted to a T-score, where a score of 50 represented the census-representative population mean with standard deviation of 10.
Analysis
Patients were divided into 3 groups: early-onset asthmatics (<18 years), late-onset asthmatics (>18 years), and non-asthmatics based on survey responses. All data analysis was carried out using SPSS Statistics 24 (IBM, Armonk, NY). Missing data on the SNOT-22, ACT, miniAQLQ, and PROMIS-29 surveys were estimated by mean imputation when greater than 80% of the individual survey was completed by the patient. Demographic factors (age, gender, BMI, and race), comorbidities, nasal polyp status, medication use, and Lund-Mackay score were categorized by asthma onset and analyzed by analysis of variance (ANOVA) or chi-square test where appropriate. Individual pairwise comparisons by asthma onset was carried out for all analyses when the chi-square test had a p < 0.05. Likewise, pairwise comparisons of SNOT-22, EPOS-VAS, and PROMIS-29 scores were carried out using the Tukey’s honestly significant difference (HSD) when ANOVA was significant (p < 0.05). The ACT and miniAQLQ were compared using the Kruskal-Wallis test by asthma onset status. A p < 0.05 was considered statistically significant for all analyses.
Results
Asthma group characteristics
A total of 355 CRS patients completed at least 80% of a PROM and answered the necessary asthma response questions. Of these, 199 were non-asthmatics (56.1%), 71 were early-onset asthmatics (20.0%), and 85 were late-onset asthmatics (23.9%). Table 1 provides a summary of the baseline characteristics and comorbidities of each group. Age, race, smoking status, and atopy status (by skin-prick testing) did not differ between asthma groups while BMI (p = 0.046), frequency of nasal allergies (p < 0.001), and frequency of respiratory reactions to nonsteroidal anti-inflammatory drugs (NSAIDs) suggestive of aspirin-exacerbated respiratory disease (AERD) (p = 0.008) differed significantly between groups. Individual pairwise comparisons found that higher BMI (p = 0.035) and nasal allergy prevalence (p < 0.001) were reported more frequently by the late-onset asthma population compared to non-asthmatics. Late-onset asthmatics reported significantly higher rates of respiratory reactions to NSAIDs compared to both non-asthmatics (p = 0.008) and early-onset asthmatics (p = 0.01).
TABLE 1.
Asthma group characteristics
| Overall | Asthma status
|
p | |||
|---|---|---|---|---|---|
| None | Early onset | Late onset | |||
|
| |||||
| n (%) | 355 (100.0) | 199 (56.1) | 71 (20.0) | 85 (23.9) | – |
|
| |||||
| Age (years), mean | 45.21 | 45.01 | 42.75 | 47.75 | 0.101 |
|
| |||||
| Sex (male), n (%) | 190 (54.3) | 109 (55.6) | 44 (62.0) | 37 (44.6) | 0.083 |
|
| |||||
| BMI, mean | 27.33 | 26.81 | 27.25 | 28.57 | 0.046a |
|
| |||||
| BMI category, n (%) | 0.494 | ||||
|
| |||||
| Normal | 129 (52.0) | 78 (56.1) | 26 (51.0) | 25 (43.1) | |
|
| |||||
| Overweight | 27 (10.9) | 15 (10.8) | 6 (11.8) | 6 (10.3) | |
|
| |||||
| Obese | 92 (37.1) | 46 (33.1) | 19 (37.3) | 27 (46.6) | |
|
| |||||
| Race, n (%) | 0.152 | ||||
|
| |||||
| White | 287 (84.2) | 170 (87.2) | 57 (83.8) | 60 (76.9) | |
|
| |||||
| Black | 27 (7.9) | 9 (4.6) | 6 (8.8) | 12 (15.4) | |
|
| |||||
| Hispanic | 14 (4.1) | 9 (4.6) | 2 (2.9) | 3 (3.8) | |
|
| |||||
| Other | 13 (3.8) | 7 (3.6) | 3 (4.4) | 3 (3.8) | |
|
| |||||
| Past/current smoker, n (%) | 107 (30.9) | 62 (31.6) | 19 (27.5) | 26 (32.1) | 0.791 |
| Nasal allergies, n (%) | 190 (55.1) | 92 (46.9) | 41 (59.4) | 57 (71.3) | 0.001b |
|
| |||||
| Atopy, n (%) | 182 (74.3) | 78 (67.8) | 51 (83.6) | 53 (76.8) | 0.063 |
|
| |||||
| Aspirin sensitivity, n (%) | 59 (17.0) | 28 (14.4) | 8 (11.3) | 23 (28.0) | 0.008c |
|
| |||||
| Prior sinus surgery, n (%) | 100 (41.2) | 43 (35.8) | 21 (38.2) | 36 (52.9) | 0.064 |
p = 0.035: none vs late-onset asthma.
p < 0.001: none vs late-onset asthma.
p = 0.008: none vs late-onset asthma, p = 0.01: early vs late-onset asthma.
Clinical measures of sinus disease
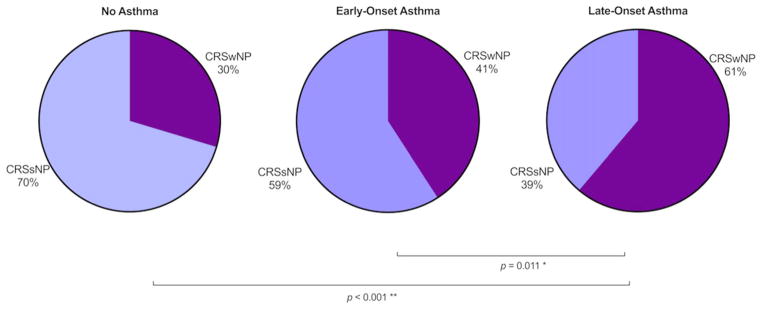
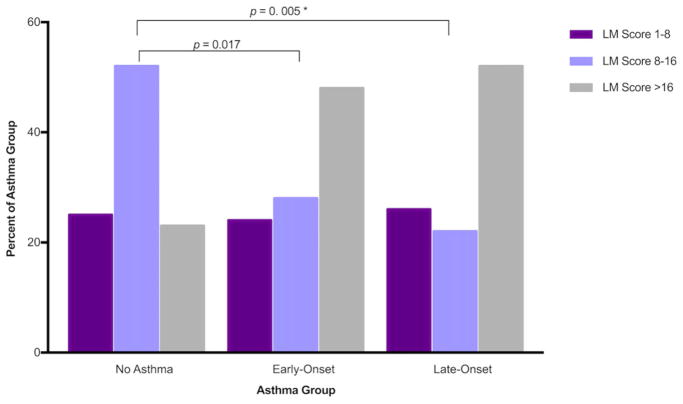
Nasal polyposis (p < 0.001) and Lund-Mackay scores (p = 0.005) were significantly different between asthma onset groups. The frequency of nasal polyps was higher in late-onset asthmatics compared to early-onset (p = 0.011) and non-asthmatics (p < 0.001) (Fig. 1). CT findings quan-tified by Lund-Mackay scores also showed more severe disease in those with early-onset (p = 0.017) or late-onset asthma (p = 0.005) compared to non-asthmatics, but no statistically significant differences were observed between early-onset and late-onset asthmatics (Fig. 2). Polyp grade did not differ between groups.
FIGURE 1.
Nasal polyp status by asthma group. Clinician-reported nasal polyp status was gathered from the CRS Registry Survey. Late-onset asthmatics show higher rates of nasal polyposis than early-onset asthmatics and non-asthmatics. CRS = chronic rhinosinusitis; CRSsNP = CRS without nasal polyps; CRSwNP = CRS with nasal polyps.
FIGURE 2.
LM scores by asthma status. Physician-reported Lund-Mackay scores were reported for patients with an available CT scan. Early-onset and late-onset asthmatics showed more severe sinus disease than those without asthma. CT = computed tomography; LM = Lund-Mackay.
PROMs
The SNOT-22 total, EPOS-VAS, and asthma-specific quality of life measures did not differ significantly between the asthma groups (Table 2). While the PROMIS-29 domains of anxiety, depression, fatigue, sleep disturbance, satisfaction with social roles, and pain interference did not differ based on asthma status or asthma onset, average physical function was significantly different (p = 0.008), with the late-onset asthmatics having worse physical function than non-asthmatics (p = 0.011) (Table 3).
TABLE 2.
Disease-specific PROMs
| n | Overall | Asthma status | p | |||
|---|---|---|---|---|---|---|
| None | Early-onset | Late-onset | ||||
| Chronic rhinosinusitis | ||||||
| SNOT-22 total, mean ± SD | 353 | 43.9 ± 17.9 | 42.8 ± 17.6 | 43.1 ± 17.7 | 47.5 ± 18.6 | 0.109 |
| EPOS-VAS, mean ± SD | 353 | 64.5 ± 23.1 | 63.3 ± 22.7 | 64.2 ± 24.5 | 67.5 ± 22.6 | 0.365 |
| Asthma | ||||||
| ACT, mean ± SD | 122 | 19.4 ± 4.6 | – | 19.1 ± 4.5 | 19.6 ± 4.7 | 0.479 |
| mAQLQ, mean ± SD | 108 | 5.4 ± 1.2 | – | 5.5 ± 1.1 | 5.4 ± 1.3 | 0.830 |
ACT = Asthma Control Test; EPOS-VAS = European Position Statement on Rhinosinusitis-visual analog scale; mAQLQ = mini-Asthma Quality of Life Questionnaire; PROM = patient-reported outcome measure; SD = standard deviation; SNOT-22 = 22-item Sino-Nasal Outcome Test.
TABLE 3.
PROMIS-29 patient outcomes
| n | Overall | Asthma status | p | |||
|---|---|---|---|---|---|---|
| None | Early-onset | Late-onset | ||||
| Anxiety, mean ± SD | 345 | 49.6 ± 9.9 | 48.9 ± 9.9 | 50.6 ± 9.7 | 50.4 ± 10.0 | 0.335 |
| Depression, mean ± SD | 346 | 47.8 ± 8.5 | 47.2 ± 8.5 | 48.7 ± 8.2 | 48.4 ± 8.8 | 0.323 |
| Fatigue, mean ± SD | 347 | 52.3 ± 11.1 | 51.5 ± 10.5 | 52.4 ± 13.1 | 53.9 ± 10.5 | 0.269 |
| Sleep disturbance, mean ± SD | 345 | 53.2 ± 7.5 | 53.1 ± 7.7 | 52.0 ± 7.4 | 54.3 ± 7.1 | 0.170 |
| Satisfaction with social roles, mean ± SD | 343 | 50.1 ± 9.4 | 51.0 ± 9.4 | 49.6 ± 8.5 | 48.4 ± 10.0 | 0.107 |
| Pain interference, mean ± SD | 342 | 51.1 ± 9.0 | 50.7 ± 8.9 | 51.8 ± 9.0 | 51.2 ± 9.5 | 0.689 |
| Physical function, mean ± SD | 348 | 50.7 ± 7.5 | 51.8 ± 6.8 | 49.7 ± 6.9 | 49.0 ± 8.9 | 0.008a |
p = 0.011: none vs late-onset asthma.
PROMIS-29 = 29-item Patient-Reported Outcomes Measurement Information System; SD = standard deviation.
Patient-reported CRS treatment information
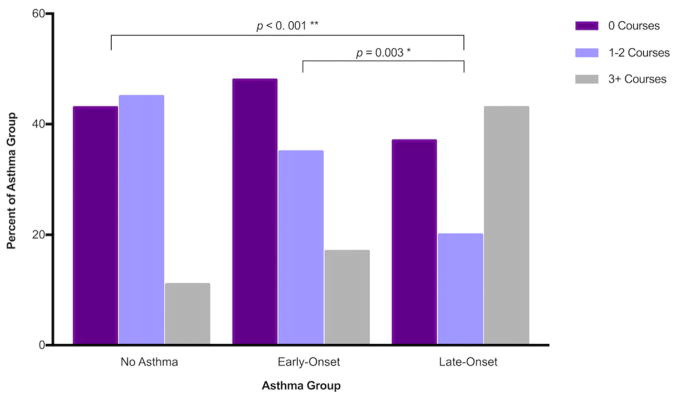
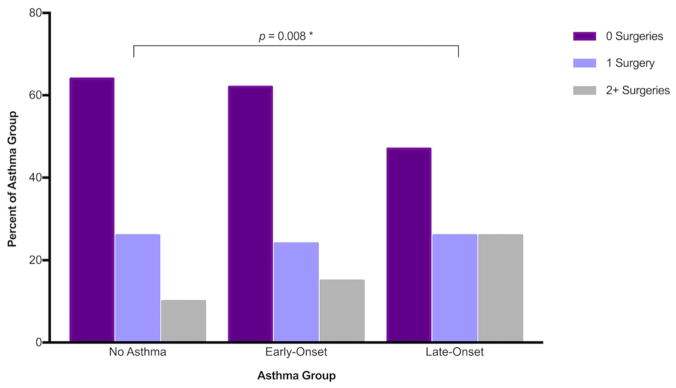
Self-reported antibiotic courses and over-the-counter (OTC) pill use were not significantly different between CRS patients among the different asthma groups. Use of oral steroids (p < 0.001) and nasal steroid sprays (p = 0.034) as well as sinus surgery history (p = 0.041) did vary between asthma groups. Oral steroids were used significantly more in the late-onset asthma group compared to both the early-onset asthmatics (p = 0.003) and non-asthmatics (p < 0.001) with a high proportion of late asthmatics reporting 3 or more courses of oral steroids (Fig. 3). Nasal steroid sprays were also used more frequently by late-onset asthmatics compared to non-asthmatics (p = 0.004). Previous sinus surgery was also reported more frequently in late-onset asthmatics compared to non-asthmatics (p = 0.008, Fig. 4).
FIGURE 3.
Oral steroid courses for sinus symptoms in the previous year. Patients reported the number of courses of oral steroids taken in the past year for the treatment of sinus symptoms. Late-onset asthmatics took significantly more oral steroid courses compared to non-asthmatics (p < 0.001) and early-onset asthmatics (p = 0.003).
FIGURE 4.
Patient-reported endoscopic sinus surgery history. Patients reported the number of endoscopic sinus surgeries in their lifetime. Late-onset asthmatics showed an increased rate of surgery over the non-asthmatic population (p = 0.008).
Patient-reported asthma treatment information
Asthma treatment information collected by patient-report included steroid courses for asthma or chest symptoms in the past year and treatment of any kind for asthma in the past 5 years, including any occasional inhaler use (Table 4). Steroid courses for asthma in the past year were not significantly different between asthma groups (p = 0.159). Treatment in the past 5 years was significantly higher in the late-onset asthma population (p = 0.002).
TABLE 4.
Patient-reported asthma treatment
| Overall | Asthma status | p | ||
|---|---|---|---|---|
| Early-onset | Late-onset | |||
| Steroid courses for asthma in the past year, n (%) | 0.159 | |||
| 0 | 79 (56.4) | 42 (63.6) | 37 (50.0) | |
| 1–2 | 28 (20.0) | 13 (19.7) | 15 (20.3) | |
| 3 or more | 33 (23.6) | 11 (16.7) | 22 (29.7) | |
| Received asthma treatment in past 5 years (n = 152), n (%) | 126 (82.9) | 50 (72.5) | 76 (91.6) | 0.002 |
Discussion
In comparing CRS patients based on asthma status and age of asthma onset, this study found that late-onset asthma is associated with higher rates of nasal polyposis than early onset asthma and the presence of asthma regardless of age of onset is associated with more severe CT scans. However, Lund-Mackay scores did not differ between early-onset and late-onset asthma. The characteristic comorbidities of allergic rhinitis and AERD were also found at higher rates in late-onset asthma; however, only AERD was significantly higher in late-onset compared to early-onset asthma. While these clinical measures of disease severity were more frequent in the late-onset asthmatics, SNOT-22, miniAQLQ, ACT, and PROMIS-29 outcomes showed no difference between groups apart from the physical function domain of PROMIS-29. While few differences in patient-reported measures were found, late-onset asthmatics reported more oral corticosteroid treatments for CRS when compared to early-onset asthmatics as well as both oral and nasal corticosteroid use when compared to non-asthmatics. Surgical treatment for CRS was also more frequent in late-onset asthmatics compared to non-asthmatics.
While this study confirms the frequent comorbidity of CRS and asthma (~44% prevalence) in this tertiary care population, we find that asthma onset is almost evenly distributed between the early-onset (20.0%) and late-onset (23.9%) asthma groups. A previous study by Jarvis et al.6 examining the epidemiology of asthma onset in CRS has found that CRS is positively associated with late-onset asthma, although this study looked specifically at those without allergic rhinitis. While late asthma onset was the more common presentation of asthma in this CRS population, early-onset asthma was almost equally as common in contrast to prior literature. However, epidemiologic studies have shown that early-onset asthma may be more prevalent in the general population, as incidence of asthma in one population study peaked before 5 years of age (over 5 cases per 1000 persons/year) and dropped to a constant level after age 20 (between 1 and 2 cases per 1000 persons/year).16 Thus, our findings suggest that this CRS population is enriched for late-onset asthma. It should be noted that an age threshold of less than 12 years old has previously been used to delineate childhood-onset asthma but there is less consensus on the delineation of late-onset asthma with some studies using ages greater than 20 years of age15 and others using an age of onset of 40 years. For this study, we separated the groups using the legal age of majority 18 years, which should capture these previously defined categories of asthma. Although not statistically significant, we found that 62% of patients with early-onset asthma were male, but this decreased to 44% among late-onset asthmatics. This supports prior descriptions that late-onset asthma is generally female predominant.19
This analysis also demonstrated increased rates of nasal polyposis in late-onset asthmatics compared to early-onset asthmatics. Nasal polyposis is characterized by a pronounced type 2 inflammation with elevated levels of cytokines such as interleukin-4 (IL-4), IL-5, and IL-13, as well as eosinophilia resulting in hyperresponsiveness of the airway.29 This type 2 inflammatory response is also associated with early-onset allergic asthma and late-onset eosinophilic asthma.15 The increased rate of nasal polyposis has previously been connected to comorbid asthma, but our association with late-onset asthma is interesting, as previous studies have suggested that late-onset, severe asthma was similarly associated with nasal polyposis.9, 10 In these studies, severe asthma, with or without an association with smoking, was associated with an increased rate of nasal polyposis compared to mild-moderate asthmatics and non-asthmatics.10 Likewise, in a cluster analysis of severe, late-onset asthmatics, one of the clusters of severe asthmatics had higher frequency of sinusitis and sinus surgery for polyps; however, the rate of polyposis was not specifically measured.9 Another study found that “extensive” sinus disease determined by a cutoff on CT scan scoring was associated with later age of asthma onset, again not accounting for the rate of polyposis.20 Here, we demonstrate that nasal polyposis is associated with late-onset asthma compared to both non-asthmatics and early-onset asthmatics in a CRS population. Our analysis also examined CT scoring by the Lund-Mackay system. While this analysis failed to demonstrate a difference between early-onset and late-onset asthma, both groups of asthmatics had a higher Lund-Mackay score than those without asthma. Overall, these findings augment associations between late-onset asthma and nasal polyposis and suggest that CRS patients with late-onset asthma have more severe sinus disease by clinical measures.
In addition to clinical measures of CRS, analysis of patient-reported outcomes was carried out to equate these findings to patient experience. Measures of CRS-specific, asthma-specific, and general outcomes collected through the SNOT-22, miniAQLQ, ACT, and PROMIS-29 surveys were found to be similar between asthma groups. Due to limitations of a registry-based study, patients received medical and surgical treatment per the standard of care. For example, oral steroid use was significantly different between groups and was higher in the late-onset asthmatics compared to both the non-asthmatics and early-onset asthmatics. Likewise, nasal steroid sprays and ESS were used more frequently in the late-onset asthmatics than non-asthmatics. Asthmatics were also more frequently treated for asthma symptoms if they had late-onset disease compared to early-onset disease. Previous work has also found that both of the SNOT-22 total and PROMIS-29 are responsive to both medical management and ESS.30 Thus, the similarity of PROMs may reflect the more aggressive management of the patients with late-onset asthma.
There were several limitations to the current study. The nature of a tertiary care center patient population may have selected for more severe patients than a general CRS population. The largest treatment group of patients in this study was derived from the population being recommended sinus surgery (56.6%) and thus may be reflective of more severe CRS. Likewise, a significant number (41.1%) of patients had prior sinus surgery. Additionally, most of our data utilized patient self-report, although we hypothesize that a patient’s ability to recall an asthma diagnosis should be reasonably accurate and recall bias about age of asthma onset would not affect clinical measures like Lund-Mackay score and nasal polyp status. Another limitation is that this study utilized only cross-sectional data, making it difficult to ascertain the relative temporal onset of asthma and CRS for each patient. These are dynamic conditions and lacking a longitudinal component also limits the ability to effectively categorize patients who would later go on to develop new symptoms of asthma or nasal polyposis.
The diversity of phenotypes of late-onset asthma could also play a role in these findings, and our study is limited in examining them without immunologic phenotype data. Late-onset eosinophilic asthma has an association with the type 2 immune response and is clinically associated with nasal polyposis as well as AERD, which could explain some of the findings reported.15 Other types of late-onset asthma include female predominant, smoking-associated, and obesity-associated asthma, which have been linked with a non–type 2 immune response.15 Further research with biologic markers of the immune response examining the underlying pathophysiology of late-onset asthma in CRS may better delineate the underpinnings of this association.
Conclusion
In summary, this study illustrates that late-onset asthma in a CRS population is common and strongly associated with nasal polyposis and increased treatment relative to the early-onset asthma population. In terms of clinical measures of disease severity, late-onset asthmatics in this population had more severe CRS than early-onset asthmatics as evidenced by increased rates of nasal polyposis and aspirin sensitivity. Although CRS-specific or asthma-specific PROMs were not worse in the CRS patients with late-onset asthma, late-onset asthmatics with CRS had received more aggressive management, particularly with oral corticosteroids.
Acknowledgments
Funding sources for the study: NIH (NUCATS - Northwestern University Clinical and Translational Sciences Institute UL1TR001422); NIH: Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (BKT, CPEP, ARW, RCK, KEH, AK, RPS) and R37 HL0768546 (RPS); Ernest S. Bazley Foundation (RPS).
REDCap is supported at Feinberg School of Medicine (FSM) by the Northwestern University Clinical and Translational Science (NUCATS) Institute. Research reported herein was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422.
Footnotes
Potential conflict of interest: None provided.
Presented at the Annual ARS Meeting on September 8–9, 2017 in Chicago, IL.
References
- 1.Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72:274–281. doi: 10.1111/all.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomassen P, Newson RB, Hoffmans R, et al. Reliability of EP3OS symptom criteria and nasal endoscopy in the assessment of chronic rhinosinusitis—a GA(2) LEN study. Allergy. 2011;66:556–561. doi: 10.1111/j.1398-9995.2010.02503.x. [DOI] [PubMed] [Google Scholar]
- 3.Joe SA, Thakkar K. Chronic rhinosinusitis and asthma. Otolaryngol Clin North Am. 2008;41:297–309. doi: 10.1016/j.otc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Krouse JH, Veling MC, Ryan MW, et al. Executive summary: asthma and the unified airway. Otolaryngol Head Neck Surg. 2007;136:699–706. doi: 10.1016/j.otohns.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Kelly EAB, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111:79–86. doi: 10.1067/mai.2003.28. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 7.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated pre-morbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch A, Yan X, Sundaresan A, et al. Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy. 2015;70:1613–1621. doi: 10.1111/all.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W, Bleecker E, Moore W, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw DE, Sousa AR, Fowler SJ, et al. U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 11.Schlosser RJ, Smith TL, Mace J, Soler ZM. Asthma quality of life and control after sinus surgery in patients with chronic rhinosinusitis. Allergy. 2017;72:483–491. doi: 10.1111/all.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benninger MS, Sindwani R, Holy CE, Hopkins C. Early versus delayed endoscopic sinus surgery in patients with chronic rhinosinusitis: impact on health care utilization. Otolaryngol Head Neck Surg. 2015;152:546–552. doi: 10.1177/0194599814565606. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins C, Andrews P, Holy C. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Retrospective analysis using the UK clinical practice research data. Rhinology. 2015;53:18–24. doi: 10.4193/Rhino14.077. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins C, Rimmer J, Lund V. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Prospective findings from the National Comparative Audit of Surgery for Nasal Polyposis and Chronic Rhinosinusitis. Rhinology. 2015;53:10–17. doi: 10.4193/Rhino13.217. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 16.de Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 17.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863–869. doi: 10.1016/j.jaci.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 19.De Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women: a retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 20.ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 21.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 22.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti–IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 24.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins C, Gillett S, Slack R, Lund V, Browne J. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 26.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3. 1–298. preceding table of contents. [PubMed] [Google Scholar]
- 27.Juniper E, Guyatt G, Cox F, Ferrie P, King D. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 28.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Hulse K, Stevens W, Tan B, Schleimer R. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lidder AK, Detwiller KY, Price CP, et al. Evaluating metrics of responsiveness using patient-reported outcome measures in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:128–134. doi: 10.1002/alr.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]