Summary
With the rapid increase in patients receiving bisphosphonates (BPs) for treating osteoporosis, one of the clinical complications associated with its long-term use is atypical femoral fractures (AFFs). Although the absolute risk for AFFs is low and it was a consensus that AFFs were acceptable compared with the amount of osteoporotic fractures BPs have prevented, epidemiological studies have proved that BPs had a strong association with AFFs and possibly more people were going to suffer from this adverse effect with wide prescriptions of this drug. In addition, AFFs seemed to have impaired ability to heal. Thus, to understand the mechanism(s) behind AFFs is important and desirable for considering preventive measures. This article reviewed the clinical features of AFFs as well as potential underlining pathological characteristics, such as the decreased turnover rate caused by BPs that led to multiple-level alternations, e.g., changes not only at cellular and tissue levels, but also related to changes in bone micro- and macrostructure and organic/inorganic contents, leading to potentially compromised mechanical properties of cortical bone when exposed to prolonged BP therapy. Severely suppressed bone turnover may also be the underlying mechanism for impaired fracture healing in patients with AFFs. The rising concerns about the risk for AFFs in nonosteoporotic patients receiving high-dose BPs to treat cancers were also discussed. Detailed investigation will help develop potential targeted pharmacological treatments such as parathyroid hormone. In addition, potential innovative internal fixation implants were discussed with regard to dynamic and biological fixation for enhancing AFF repair.
Keywords: atypical femoral fractures, bisphosphonates, bone remodelling, fracture healing, parathyroid hormone
Introduction
We are stepping into a society with a significant ageing population, and it is known that one in six women will suffer from osteoporotic fracture at least once during their lifetime [1]. Bisphosphonates (BPs) have been developed and used as potent antiosteoporotic drugs for their bone-protective effect on primary osteoporosis (OP) in both female and male populations, and secondary OP such as glucocorticoid-induced OP. It has been proved that BP usage inhibits the bone remodelling process, elevates bone mineral density (BMD) and bone mechanical properties, and as a consequence, reduces the incidence of vertebral/nonvertebral fractures [2].
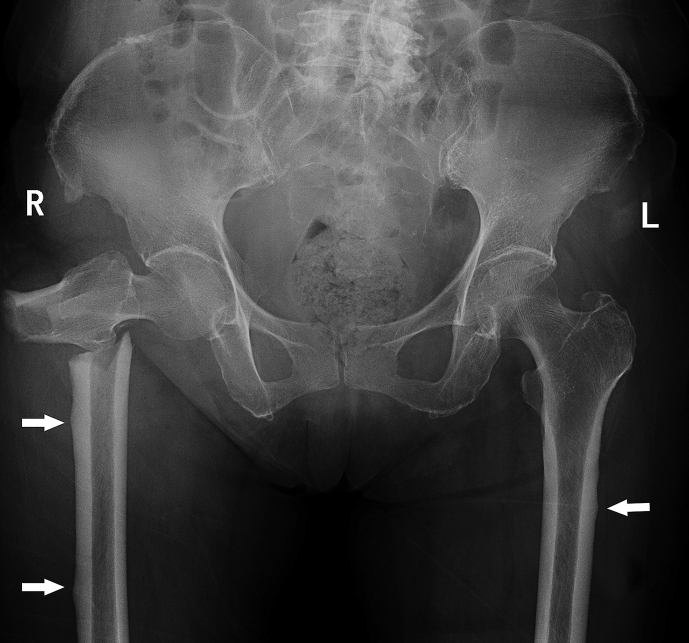
However, atypical femoral fracture (AFF), one of the potential complications of prolonged BP therapy for the treatment of OP, has raised reasonable concerns in recent years. Figure 1 shows the typical features of an AFF that developed in a 74-year-old female presenting to our department with low-energy subtrochanteric (ST) fracture of the right femur after 5 years of BP treatment. The evidence of the association between BP use and AFFs is strong, and the incidence of AFFs among patients taking BPs for over 10 years can be as high as 107.5/100,000 person-y [3]. In addition, it was estimated that in the USA, over 4 million women over 45 years of age were receiving BP treatment [4]. Thus, even though it still remains unknown if and how this long-regarded bone-protective agent causes another new-type fracture, the long-term effects of BPs on either the occurrence or the healing process as well as the treatment of AFFs should not be ignored.
Figure 1.
AFF radiograph of a 74-year-old female with a 5-year BP exposure history. Note the multiple involvement/local cortical thickness of the lateral side of the femurs indicated by white arrows. AFF = atypical femoral fracture; BP = bisphosphonate; L = left; R = right.
In this review, we briefly summarize the epidemiological and pathological features of AFFs and the potential effects of BP usage on the development as well as the healing process of AFFs. The challenges and treatments of osteoporotic fractures, mechanism(s) on impaired healing, and proposed options or treatment protocols for achieving better healing or eventually healing enhancement will be discussed.
Definition of AFFs
In the first publication on AFF in 2005, Odvina et al [5] reported that nine patients receiving long-term alendronate therapy for 3–8 years developed spontaneous nonvertebral fractures later, with six patients having delayed fracture healing or nonunion. Transiliac bone biopsies from the most of the patients showed reduced or absent osteoblastic and osteoclastic activities with decreased or no tetracycline labelling, indicating inhibited bone remodelling either in cancellous or cortical bone. It was suggested that this phenomenon of severe suppression of bone turnover was caused by the long-term use of BPs, leading to increased susceptibility to as well as impaired healing of fractures. After this initial paper, many case reports on rising anxieties about the side effects of BPs on a special type of femoral fracture were published [6], [7]. According to these clinical observations, BP-associated fractures shared similar and unique clinical and pathological features, including chronic pain, transverse fracture line, location in the femoral shaft (FS) or ST area, etc.
In 2009, the task force of the American Society for Bone and Mineral Research (ASBMR) reviewed published literatures about AFFs and developed their case definition [8]. In this report, an AFF was defined as a type of low-energy fracture located typically in the area of distal to the lesser trochanter to proximal to the supracondylar flare of the distal femoral metaphysis. Complete understanding of AFFs is challenging because of not only the low incidence of AFFs compared with other more common fracture types, but also the varying definitions of AFFs by different study groups. Thus, for the purpose of unifying the definition of AFFs, the task force of ASBMR suggested some major features that should all be present when making a diagnosis, and also minor features that were the factors found to be associated with AFFs but should not be necessarily included in the diagnostic criteria [8]. In 2014, an updated version of the case definition of AFFs provided a more precise definition that could better differentiate AFFs from regular ST/FS fractures [9]. In this new version, the localized periosteal reaction of the cortex was upgraded from the minor to major features considering the updated concept of an AFF as a kind of stress/insufficient fracture. Four out of five major features must be present in order to designate a fracture as an AFF, while in the old version all the major features were included.
Epidemiology of AFFs associated with BPs
Prior to the development of a stringent case definition of AFFs by ASBMR, potential AFFs were reported in the context of a more general concept, that is, ST/FS fracture, the incidence of which among women has been reported between 10 and 35 per 100,000 [9]. Since BPs were approved for the treatment of OP at that time, the incidence of femoral neck/intertrochanteric fractures, as typical osteoporotic fractures, has been reported to decrease given the preventive effect of BPs on bone, whereas the rate of ST/FS fractures remained stable [10] or increased [11]. As the case definition of ST/FS fractures was based on the International Classification of Diseases, Ninth Revision classification that might not identify AFFs among ST/FS fractures without radiologic hallmarks of atypia, Wang and Bhattacharyya [12] indicated that the relatively stable or increased rate of ST/FS fractures could be ascribed to an internal shift from typical high-energy-induced ST/FS fractures to AFFs. This fracture pattern shifting was supported by basic science findings that BPs might have better beneficial effect on the cancellous bone region, leading to a decreased incidence of typical low-energy osteoporotic fracture, while for the cortical bone-dominant region, BPs might exert less protective effect [13], [14].
With the development of the consensus criteria of AFFs, AFFs were distinguished from classic ST/FS fractures. Many published studies were reanalysed for the purpose of illustrating the epidemiological features of AFFs as well as the relative risk factors including BP usage [8], [9]. Reports from Dell et al [15] and Neviaser et al [16] declared that among the total number of ST/FS fractures only 17–29% were AFFs. Dell et al [3] indicated that the incidence of AFFs was 1.78/100,000 person-y among patients older than 45 years and receiving BPs for <2 years, whereas for the BP therapy duration of over 10 years the rate could be as high as 107.5/100,000 person-y. This potential duration-dependent detrimental impact was evidenced by the finding that the median of duration of BP usage for patients with AFFs was 7 years (ranging from 1.3 years to 17 years) after reviewing 310 published cases [8]. Schilcher et al [6] reported 12,777 women of 55 years of age or older who sustained fractures of the femurs, and their results indicated the strong relationship between BP exposure and AFFs and confirmed that the risk for AFFs was independent of age. A meta-analysis by Gedmintas et al [17] included five case–control and six cohort studies, and found that the pooled adjusted relative risks based on ASBMR case definition and X-ray confirmation for atypia were 11.78 and 28.16, respectively. While the International Classification of Diseases codes were used to define ST/FS fractures, relative risk was as low as 1.62. Among different subtypes of BPs, oral alendronate was considered to be mostly associated with AFFs, even though cases receiving other type of BPs have also been reported [18]. However, it is still unknown whether this association can be attributed to the pathologically specific effect of alendronate on bone or can simply be a result of variation in the prescription rate of different types of BPs. Regardless of these epidemiological differences, all BPs used for treating OP are required to be prescribed with caution as these are associated with an increased risk for AFF, according to the Food and Drug Administration as well as the European Union [19].
The available epidemiological evidence implied a potential association between BPs and AFFs. However, such a link needs to be interpreted with caution because it was based mainly on observational studies. There were only two randomized controlled trials (RCTs) providing perspectives about long-term safety of BP consumption for treating OP, both of which failed to find a correlation between prolonged BP therapy (with alendronate and zoledronate) and an increased fracture risk of AFFs [20]. However, as neither of the two studies was initially designed to investigate AFFs, potential atypical features (e.g., incomplete AFFs) might not be properly screened out. Furthermore, as the insufficient statistical power resulted from a low incidence of relevant fractures, the authors did not propose performing a further subgroup analysis. Obviously, no certain conclusion can be drawn if BPs really account for AFFs. Notably, even if there exists a causal relationship, clinical preference for OP treatment using BPs is unlikely to be remarkably changed, as 162 osteoporotic fractures will be treated at a cost of only one AFF [20]. Reassessing the duration and dose of BP treatment to balance between bone-protective effects and possible side events should be the future direction. Screening for risk factors associated with AFFs in patients receiving prolonged BP therapy is also of paramount importance.
AFFs in patients receiving cancer-dose BPs
AFFs have long been regarded as a new type of osteoporotic fractures. However, emerging sporadic evidence indicated that AFFs also occurred in patients receiving higher doses of BPs with more frequent intravenous injections (e.g., at a monthly dose of 4 mg zoledronate) for skeletal malignancies, such as bone metastasis and myeloma, supporting the long-standing opinion that severely decreased bone turnover due to either chronic or higher-dose BP exposure might be the underlying mechanism of AFFs [19], [21], [22]. A retrospective study by Puhaindran et al [21] indentified four patients with AFFs in 327 patients receiving at least 24 doses of intravenous BPs for cancer therapy. Among those four patients, the total number of doses of zoledronate or pamidronate ranged from 48 to 73, with the therapy duration ranging from 68 months to 103 months. Bone biopsies of the cortices showed absence of viable bone cell and no sign of malignant metastasis. The author suggested that the incidence of AFFs in cancer patients receiving high-dose BPs, which was 1.2% as calculated, seemed to be higher than that generally reported in the scenario of OP treatment. A more recent case–control study by Edwards et al [19] indentified 23 AFFs among 10,587 users of BPs for cancer treatment, leading to an incidence of 0.05/100,000 person-y, which seemed to be far lower than what we observed for AFFs occurring under chronic BP exposure for OP treatment [3]. What echoed previous concerns about the safety of cancer-treatment-dose BPs in this study was that odds ratio for AFFs in these cancer patients taking BPs was 355.58 times that in those who did not take BPs. Among the patients who developed AFFs, the median exposure times of zoledronate, pamidronate, alendronate, and ibandronate were 5 months, 14 months, 84 months, and 36 months, respectively. Another study [22] retrospectively indentified six AFFs among 62 patients with femoral fractures after receiving intravenous BPs for breast cancer or myeloma, and found that the mean duration of BP therapy in patients with AFFs was significantly longer than that in those without atypical features (5.9 years vs. 1.6 years). Additionally, patients with AFFs also took much more zoledronate when compared with controls (32 vs. 12 doses). To date, it remains unclear if and how high-dose BPs in cancer patients predispose them to the risks for AFFs because most of the epidemiological evidences were based on observational studies. In addition, in cancer patients with an altered bone metabolism condition, low nutrition is not rare, which may also attribute to compromised bone health [21]. However, this hypothesis might be questioned facing the evidence that a few of the biopsies harvested during surgeries of patients with AFFs showed signs of bone metastasis [22]. In fact, an AFF has been reported in a man with nonmetastatic prostate cancer after receiving a monthly dose of 4 mg intravenous zoledronate for 2 years to prevent potential bone loss resulting from androgen deprivation therapy [23]. Apparently, more clinical trials as well as basic studies are needed to give better guidance to physicians when prescribing BPs for cancer treatment.
Clinical features and pathologies of AFFs
Questions and hypothesis have been developed with regard to the pathological mechanism of AFFs. Bone biopsies from such patients to some extent verified a close relationship between BPs and AFFs. The ASBMR task force reviewed 19 bone biopsies from patients with AFFs and found that nearly all the samples from not only iliac crests, but also areas near fracture sites showed reduced or absent bone turnover, a decreased number of osteoblast and osteoclast, as well as low or no tetracycline labelling [9]. However, there was also a study showing increased bone resorption coupled with decreased bone formation [24]. Whereas, in another report, it was concluded that BPs did not affect normal osteoblastic bone formation in transiliac crest [25]. One important issue that should be kept in mind is that biopsy from near the facture site may be confounded because the fracture itself may accelerate the bone remodelling rate. By contrast, biopsy from the nonfracture site (e.g., transiliac crest) may not reflect the actual pathological features of AFFs. Given the limited biopsy data provided currently, it was suggested that more detailed and standardized histological information from both fracture and nonfracture sites remained to be one of the key research goals for future study [8].
AFFs as stress fractures
There was evidence indicating that AFFs belonged to stress fractures. Firstly, patients with AFFs shared some common clinical symptoms with stress fractures, with prodromal pain for weeks in the lesion side of the leg before the establishment of clinical diagnosis [26]. Secondly, stress fractures had periosteal callus formation, which provided evidence of bone repair prior to confirmed radiographic fractures, and we could also see this type of callus reaction in the lateral side of the femoral cortex in patients with AFFs [8]. Thirdly, the effect of BPs on bones might be an accelerating factor in the development of stress fractures [27]. The reduced bone turnover rate caused by BP exposure may damage the ability of bone to absorb impaired tissue while suffering from microdamages. Considering the pathogenesis of regular stress fractures as a consequence of repetitive and excessive loading on healthy bone, it may be more accurate to ascribe an AFF to an insufficient fracture in order to highlight the incapability of bone itself to heal under the normal loading level [9]. Nevertheless, there are some differences between AFFs and traditional femoral fatigue fractures. Firstly, an AFF shows a more transverse fracture line, while a fatigue fracture of the femur results in a more oblique surface. Secondly, the AFF starts at the lateral cortex of the femur, while the other usually initiates on the medial cortex [9].
Decreased bone remodelling and microcracks/microdamages
To date, we have still not reached a good understanding of the pathology of this rare type of fracture without reviewing available basic studies about the effect of BPs on bone. In 2002, Eriksen et al [28] performed bone transiliac biopsies from women with OP and receiving placebo or 3 years of BP therapy (risedronate). Bone turnover rate was found to decrease in the BP-treated group with reduced mineralizing surface and activation frequency of 58% and 47%, respectively. No structure parameters were found to have significant changes in the BP-treated group compared with the placebo group. The authors speculated that even though suppressed remodelling by long-term BP use might lead to increased bone density and thus reduction in fracture risk, there existed a chance of microdamage accumulation because of the suppressed bone turnover by BPs. Microdamage is a form of microcrack that develops in bone consequent to daily physiological repetitive loading and may increase in old people without any drug usage [29]. Studies showed that BPs might suppress targeted bone resorption to remove the damaged bone and thus lead to the accumulation of microdamages, subsequently accounting for the occurrence of stress fractures, the developing mechanism of which was similar to that of AFFs [30]. Animal studies in rat ulna [27] and dogs (rib and vertebral cancellous bone) [31], [32] confirmed that BPs led to microdamage accumulation by inhibiting bone remodelling (Figure 2). Many clinical studies did not see significant microcrack accumulation in patients receiving long-term BP therapy compared with those who had no BP exposure history [33], while a cross-sectional study by Stepan et al [34] found that patients with alendronate treatment for OP showed significantly higher microcrack accumulation compared with treatment-naïve ones. However, biopsies of this study were harvested from the iliac sites rather from the weight-bearing bones such as femurs, and thus, these are of limited value to understand AFFs. Another issue to be clarified is the association between bone toughness and microdamage. Mashiba et al [31] showed that decreased bone toughness was associated with microdamage accumulation in animals when subjected to long-term BP treatment. However, a study [32] showed decreasing toughness of bone during 3-year BP treatment, while microdamage stopped to grow after 1-year BP treatment. The authors then suggested that it was possible to control microdamage at a very low bone turnover rate and/or the formation of microdamage was decreased in such a situation. As for the continually deteriorated bone mechanical properties, i.e., toughness, other factors or interactions might be responsible for this deterioration. Bone strength is determined by multiple factors including macro-/microarchitecture as well as bone quality and mineral density. It is possible that microcrack accumulation is only one of the multiple ways that BPs influence bone strength. More details on this will be discussed in the following section.
Figure 2.
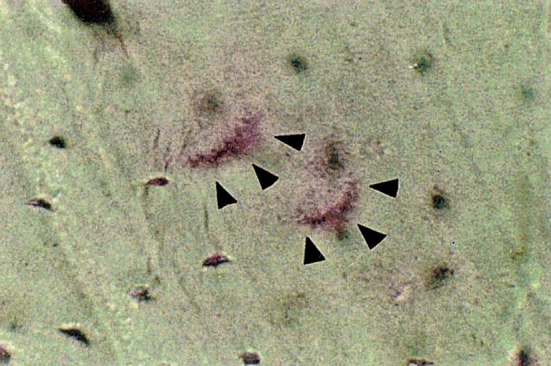
Microdamage in the rib of a dog with long-term BP exposure, as shown by oblique triangles. BP = bisphosphonate. Note. From “Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib,” T. Mashiba et al, 2000, Journal of Bone and Mineral Research, 15, p. 613–20. Copyright 2000, ASBMR. Reprinted with permission.
Decreased bone remodelling and bone tissue mechanical properties (toughness)
Effects of long-term BP therapy on bone mechanical properties may also play an important role in the occurrence AFFs. Regardless of the positive effect of BPs on bone strength and stiffness, animal studies suggested that by reducing bone remodelling, BPs might decrease bone toughness, which was a material-level property of bone calculated by normalizing the amount of energy that bone absorbed prior to a fracture using the parameters of bone geometry [35], [36]. BPs affect the mechanical properties of bone tissue by different mechanisms. The first one is through bone collagen, the organic component of bone. Collagen forms the major part of bone organic matrix and consists of two different components: enzymatic and nonenzymatic collagen cross-links, both of which significantly affect bone mechanical properties [37]. The former one is a trivalent form consisting of pyridinoline, deoxypyridinoline, and pyrroles. Long-term BP treatment (>1 year) increased the pyridinoline/deoxypyridinoline ratio in an animal model (dog) [38], and thus increased bone strength and stiffness [39]. The latter one, which comprises nonenzymatic cross-links, is formed through the interaction of collagen and sugars via oxidation reactions. Reduced bone turnover may increase the levels of pentosidine, a marker of advanced glycation end products (AGEs) [8]. A recent clinical study confirmed a greater serum pentosidine level in patients receiving long-term BP treatment than in BMD-matched controls without BP exposure [40]. AGEs were found to be associated with nonenzymatic cross-links, and brittleness and toughness of bone [8]. Increased AGE accumulation may impair energy dissipation for collagen, leading to fractures under a low level of strain [41]. In general, while BPs increase bone strength and stiffness by altering the pyridinoline/deoxypyridinoline ratio of collagens, bone becomes more vulnerable to fracture because toughness is decreased with the accumulative effect of BPs on AGEs or mature collagen cross-links. The second way BPs affect bone mechanical properties is by affecting its mineral contents. On the one hand, by suppressing bone remodelling, BPs better preserve bone structure and mass. On the other hand, according to Tang et al [39], BPs might make bone a more homogeneous material that is less potent at absorbing energy and easier to initiate as well as accumulate cracks, finally developing clinical fractures. Using Fourier transform infrared imaging to analyse bone biopsies near proximal femoral fracture sites from postmenopausal women with or without a BP history, a group suggested that BPs aid in making bone more uniform not only in mineral, but also in organic contents, possibly accounting for increased susceptibility to fractures [42].
Specific role of BPs in cortical bone
Unlike typical fragility fractures in patients with primary OP, which usually occurs in the cancellous-dominant region, e.g., femoral neck or intertrochanteric region, AFFs usually occur in the cortical dominant ST or diaphyseal femora. Thus, it is logical to speculate that BP treatment plays a role in this shift of fracture patterns. One possible reason is that, unlike cancellous bone that benefits more from BP treatment in terms of a decreased incidence of traditional osteoporotic fractures as these regions become mechanically much stronger than before, cortices receive fewer benefits or even harmful effects after long-term BP exposure [13], [43]. One group reported 3 years of BP treatment, and found impaired intracortical bone architecture with reduced osteon number and enlarged cortical porosity in healthy canine bone [44]. Interestingly, Milovanovic et al [45] found that 6 years of alendronate exposure tended to rejuvenate cortical bone to a younger and healthier status, with evidence of normalized osteon number and better preservation of unmineralized lacunae when compared with ageing or OP bone without BP therapy. To date, it is not clear that BP treatment for how long or at how high a dose would affect bone microstructures as well as molecular signal pathways that are responsible for maintaining the normal physiology status of cortical bone. More importantly, potential mechanisms underlying the pathophysiology of AFFs remain to be further investigated.
BPs and angiogenesis
Angiogenesis has been proved to play an essential role during bone formation and fracture healing. Delay in angiogenesis and vascularization may lead to a decrease in local nutrient supply and accumulation of metabolic wastes, resulting in impaired cell function and compromised tissue regeneration. Different strategies have been developed to enhance angiogenesis and therefore accelerate fracture healing. To date, the decreased bone remodelling rate is considered the major factor involved in the pathology of AFFs. However, the potential adverse effect of BPs on angiogenesis might be associated with a relatively high incidence of delayed healing or nonunion of AFF repair. An available in vitro study showed that BPs had negative effects on angiogenesis, including reduced proliferation and induced apoptosis of endothelial cells [46]. In addition, BPs showed an inhibitory effect on matrix metalloproteinases, which was essential for angiogenesis and vascularization [47]. A recent study [48] reported a significant decline of serum concentration of vascular endothelial growth factor and angiopoietin-1 in patients receiving BP therapy for 1 year. These in vitro studies suggested that long-term and high-dose BP treatment might explain the in vivo results with both high incidence and delayed healing of AFFs through impaired angiogenesis.
Other risk factors of AFFs
Except for these factors, some specific low limb geometric features were supposed to predispose patients to AFFs. The bent shape of the femur allowed concentration of stress on its lateral cortical side, and this high-level tensile tress might precipitate the development of stress fractures [49]. A study [50] using computed tomography-based finite element analysis verified increased tensile stress on the lateral side of the healthy FS in patients diagnosed with preclinical or clinical AFFs and with significant femoral bowing, while in control patients with thigh pain and normal femoral curvatures, no increase in tensile stress was found. The authors then suggested that stress fractures of the bowed FS should be considered as a class of AFFs. However, this study did not consider the potential role of BPs in femoral bone mechanical properties including Young's modulus when conducting finite element analysis. Moreover, sample size of the patient group with AFFs was too small (n = 5). Thus, the result might not be powerful enough. According to this, it is reasonable to speculate that all the potential processes/mechanisms mentioned above can be accelerated by applying excess tensile stress to the lateral femoral cortical bone, together with other specific effects of BPs on the cortical region, e.g., less BMD elevation or intracortical microarchitecture alternation and severely decreased bone turnover that result in impaired healing of stress fracture, leading to a specific fracture pattern of AFFs.
In summary, long-term BP exposure in patients with AFFs affects the mechanical properties of bone by decreasing bone toughness through affecting both organic and inorganic components in bone tissue, making bones prone to fracture. In addition, considering that maintaining normal turnover rate is essential for osteoclasts to absorb microcracks that develop during daily loading conditions, the effects of inhibiting bone remodelling by BPs might account for the accumulation of microdamage and inhibit the repairing of impending fracture, which finally results in an overt one. Ettinger et al [49] suggested that decreased remodelling owing to chronic BP exposure caused changes of bone at micro- (fully mineralized osteons) and submicroscopic (more nonenzymatic collagen cross-links and adverse crystalline alternations) levels, and made it easier to initiate microcracks in bone tissue because bone became more homogeneous, resulting in a reduction in its ability to dissipate energy during daily loading. Once a microcrack was developed, it might penetrate through homogenous cortices, adding to the inability to repair this microfracture due to the absence of targeted bone remodelling by BPs, finally accounting for the transverse fracture pattern of AFFs. This effect of BPs on impairing fracture healing may be exacerbated by the antivasculogenetic effect of BPs [8]. Furthermore, while BPs increase bone mass in the cancellous region and, as a result, reduce the risk of typical osteoporotic fractures, it may shift its risk of fragility fractures to the cortical bone compartment, which may hypothetically be responsible for atypical fractures. Some specific impacts of BPs on the cortical bone region may be the underlying mechanisms, as supported by the epidemiological evidence that typical osteoporotic fractures could be prevented by BP exposure, while atypical fractures in the cortical bone region could be increased among patients receiving BP treatment [9]. In addition, the specific geometry of the low limb of AFF patients logically leading to the hypothesis that tensile stress/strain of the lateral cortex of the femur may be an important anatomic factor accounting for the shifted fracture pattern. It is important to notice that bone strength is determined not only by mineral contents, but also by bone micro- and macroarchitecture, organic and inorganic component profiles, etc. Apparently BPs may affect all these factors by different pathways, and how these processes are orchestrated to develop clinical AFFs still requires more research.
Potential animal model for AFFs
It is imperative to build an appropriate animal model of AFFs, with the goals of illuminating the underlying mechanism and ways to prevent it. Clinical AFFs encompass a wide range of risk factors, and to apply all these to animals would be a tough task. The first and preferable step should be investigating the long-term effects of BPs on the changes of cortical bone quality. Report from ASBMR suggested that dogs or rabbits may be a good choice for the existence of Haversian remodelling, while the consistency and reliability of these attempts for inducing postmenopausal OP have not been well proved [8]. Pennypacker et al [51] used OVX rabbits to induce osteopenia and found that BMD of the lumber vertebrae significantly decreased by 9.8–12.8% 13 weeks after surgery. However, as for the parameters of the mechanical test, no difference was found between the OVX and shame groups. Rats as a suitable animal model for AFFs have the advantages of much cheaper price and widely accepted ovariectomy-induced OP condition [8]. However, multiple factors including oestrogen level, and BP types and duration account for controversial results of different studies. In order to build an appropriate animal model for AFFs, we should first investigate if and how these factors are orchestrated to induce detrimental effects on the cortical bone in vivo. Unfortunately, rats are also not an ideal choice for the lack of the Haversian system. Nonhuman primates and mini pigs might be alternatives, but relatively high cost would be another problem [8]. In addition, regarding to the close relationship between AFFs and stress fractures, studying the effects of long-term BPs on how bone responds to repetitive loading shall be of great value for future preclinical studies.
Challenges of AFFs in clinical practice
Clinical reports showed delayed healing or nonunion as well as the existence of multiple operations in patients with AFFs [5], [52], [53], [54]. Sasaki et al [52] reported a retrospective study of a total of 12 low-energy diaphyseal femoral fractures in nine patients receiving long-term antiosteoporotic drugs (8 patients took BPs and 1 patient took raloxifene; 3.6 years of mean treatment duration). All the fractures were treated surgically [1 with a locking plate and the others with intramedullary nail (IMN) fixation] and low-intensity pulsed ultrasound (LIPUS) was applied for three fractures for the purpose of accelerating healing. As a result, 50% of AFFs (6/12) showed delayed or partial union. Among the three patients receiving LIPUS, two showed partial union and one delayed union. Considering the relatively small sample size of this study, no conclusion can be drawn as regards the efficacy of LIPUS. Egol et al [54] found that 98% (40/41) of AFFs identified healed after surgery within a mean time of 8.3 months, which was longer than the healing time of traditional typical fractures (8 months vs. 3–6 months). In addition, though most AFFs healed (98%), 34% and 36% of these patients reported having persistent pain and limited functional recovery, respectively, 1 year postoperation [54]. Weil et al [53] reported 17 AFFs in 15 patients receiving BP treatment for a mean duration of 7.8 years. It was suggested that only 54% of the IMN-treated patients achieved normal healing, with 46% of patients undergoing secondary procedures (e.g., nail dynamization, exchange nailing, etc.). As a comparison, the authors indicated, IMN fixation applied to typical femoral fractures might achieve a very satisfactory result, generally with a healing rate of 98–99%. The delayed healing in bone treated with long-term BPs was also noted in a report by Odvina et al [5], with six of nine patients showing unsatisfactory union. Not in all cases AFFs are linked with BP usage. Notably, a retrospective study from Singapore [55] identified 20 patients with ST fractures presenting atypia, with only 12 patients having a history of BP exposure for a mean duration of 4.6 years prior to fractures. Three patients in the BP-treated group showed nonunion at the fracture site and underwent secondary operations, with a healing rate of 75%, while for the atypical fracture group without BP exposure, the corresponding rate was 87.5% with one nonunion among eight cases, indicating that BP therapy might prevent fractures from healing. However, caution must be taken when interpreting this result because the diagnostic criteria used in this study were different from the more stringent case definition by ASBMR [9], which may better distinguish AFFs from normal femoral fractures and provide a higher BP exposure rate among patients with AFFs. Recently, a systemic review [56] analysing the data of lower limb fractures from 14 eligible papers found that BP usage indeed prolonged the mean healing time to 8.5 months. The delayed union rate among patients receiving BPs for more than 3 years could be 67%, compared with 26% for <3 years.
Mechanism of impaired osteoporotic fracture healing in AFFs
In order to better understand the healing process of AFFs, we first need to investigate the process of stress fractures, considering similar pathological features between AFFs and stress fractures [8]. Generally, fracture healing can be categorized into two different patterns: indirect healing and direct healing. The former can be seen in complete fractures and be roughly divided into four overlapping stages: inflammatory, soft callus, hard callus, and bone remodelling stages. Osteoclast was proved to play a vital role in the bone callus remodelling stage in order to convert woven bone to mature lamellar bone. In animal models, BP, a potent inhibitor of osteoclast activity, has been proved to inhibit the remodelling stage significantly, resulting in delayed bone turnover and increased callus volume with immature woven bone [57]. As for the early callus formation stage, BP was reported to have no significant influence on it [58]. The direct healing process, which forms less callus and usually happens in case of absolute rigid fixation of a fracture or in the scenario of healing of a stress fracture, was characterized by the pivotal role of osteoclast activation at the early stage of repair to remove the necrotic or damaged tissue; thus, drugs impairing osteoclast function or reducing bone remodelling (e.g., BPs) might delay or impair this healing process [57]. A recent report confirmed that 3 weeks' injection of ibandronate prior to a fracture indeed inhibited direct fracture healing of tibia of a rat fixed with rigid compression plating [58]. Barrett et al [27] found that in rats, BPs might induce microcrack accumulation in ulnae under fatigue loading and prevent stress fractures from healing by inhibiting targeted remodelling of microdamages. Li et al [29] suggested that in ribs of beagle dogs, BPs not only suppressed stochastic remodelling (remodelling of the whole skeletal system), but also led to complete suppression of targeted bone remodelling (remodelling attributing to the clearance of local lesion of bone), accounting for the rising burden of microdamages. The report from ASBMR [9] indicated that an AFF, as a kind of a stress fracture, healed first by forming periosteal and endosteal surface calluses that bridged the cracks, the process of which could not be affected by BPs in animal and clinical studies. Then to continue the repairing process, normal bone remodelling was needed to remove the bone matrix with microdamages in a similar process to that occurring in the early stage of direct fracture repair or the later stage of indirect fracture repair [8]. However, BPs were proved to accumulate at the fracture site and thus suppress targeted bone remodelling [29], finally leading to impaired/delayed healing of AFFs. Reports found that the radiologic ellipsoid thickening observed prior to AFFs in patients contained larger callus and more advanced mineralization but less lamellar bone, and suggested that this phenomenon might be ascribed to the decreased bone remodelling of the immature callus under the influence of BPs [55]. Clinically, increased woven callus volume during the fracture healing process may be relevant to the elevated mechanical properties as a compensation for an inferior callus structure. However, though the formation of periosteal and endosteal woven callus was normal in patients with AFF patient, the callus was considered not sufficient to mechanically stabilize the fracture gap considering that it was interrupted when the periosteal surface reached the fracture line [59]. In addition, BPs have an antiangiogenic effect on bone, which might also impair fracture healing as mentioned in the previous section [8].
Apparently, this mechanism for the impairment of healing of AFFs is discussed under the basic definition of AFFs as stress fractures and in the hypothesis that decreased bone remodelling after BP treatment not only resulted in the development of complete stress/insufficient fracture under physiological loads, but also impaired fracture healing. However, as stated above, to date a causal relationship between BPs and AFFs has not been established, and other risk factors might also contribute to AFFs considering not all AFF patients had a BP exposure history as stated before. Obtaining the histological or electron microscopic profile of the fracture site from patients with AFFs is essential, while many groups harvested biopsies only from nonfracture sites. Notably, a recent report from Schilcher et al [59] analysed cortical biopsies of the fracture site from eight patients, among whom seven had a BP treatment history with a mean duration of 10 years prior to fractures. Histologically, no sign of remodelling was found in the fracture gaps, which were full of amorphous materials (protein precipitates and detritus) without cell or callus mineralization. Both periosteal and endosteal callus formation could be discerned with mainly woven bone formation. The typical histological features are shown in Figure 3, Figure 4, Figure 5 (with copyright permission from the publisher). It was hypothesized that, except for the failure to repair microcracks due to reduced bone turnover caused by BPs, local interfragmentary strains caused by loading might prevent the cell in the gap from living, maintaining the fracture line. Interestingly, bone turnover and even the tendency of woven bone formation could be seen in the bone near the fracture gap. The authors suggested that the defects were created on the fracture surface due to resorption and fragmentation, providing a place for new bone formation. Such precious clinical studies are still limited in other publications. More detailed mechanisms of AFF repair shall be further investigated.
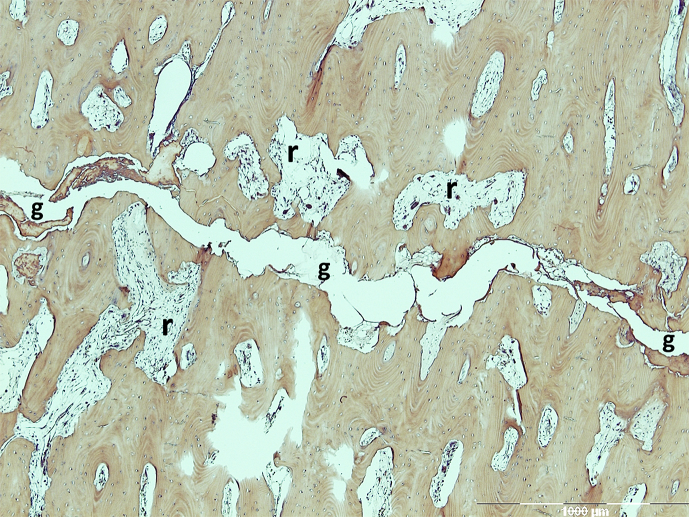
Figure 3.
Histological features of the fracture site in AFF patients. The fracture gap (g) showed no signs of remodelling, while resorptive cavities existed in the bone near the fracture gap with increased osteoclastic activities. AFF = atypical femoral fracture. Note. From “Histology of 8 atypical femoral fractures: remodeling but no healing,” by J. Schilcher et al, 2014, Acta Orthopaedica, 85, p. 280–6. Copyright 2014, Nordic Orthopaedic Federation. Reprinted with permission.
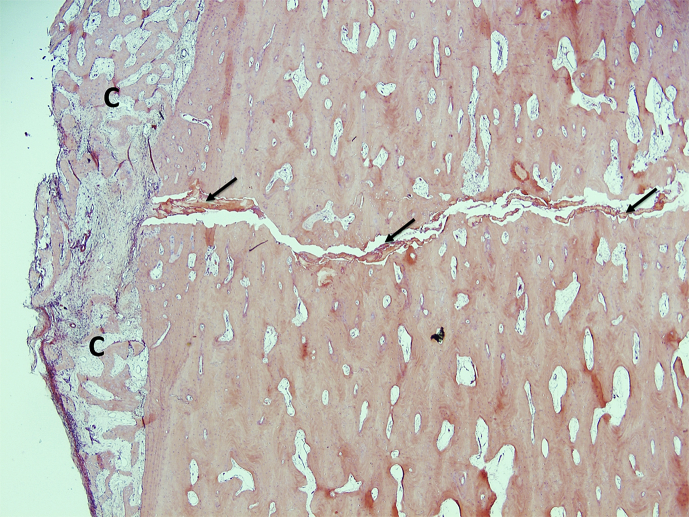
Figure 4.
Histological features of the fracture site in AFF patients. Periosteal callus (c) was formed across the fracture gap, which contained some amorphous materials indicated by oblique arrows. AFF = atypical femoral fracture. Note. From “Histology of 8 atypical femoral fractures: remodeling but no healing,” by J. Schilcher et al, 2014, Acta Orthopaedica, 85, p. 280–6. Copyright 2014, Nordic Orthopaedic Federation. Reprinted with permission.
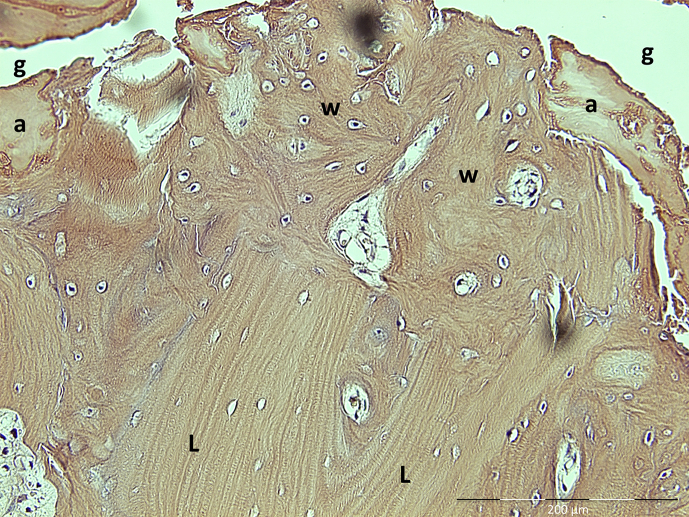
Figure 5.
Histological features of the fracture site in AFF patients; amorphous material (a) within the fracture gap (g) and woven bone (w) replacing old lamellar bone (L). AFF = atypical femoral fracture. Note. From “Histology of 8 atypical femoral fractures: remodeling but no healing,” by J. Schilcher et al, 2014, Acta Orthopaedica, 85, p. 280–6. Copyright 2014, Nordic Orthopaedic Federation. Reprinted with permission.
Management of AFFs and proposed options for healing enhancement
In 2014, the task force report from ASBMR [8] reviewed the clinical dates published and suggested treatment recommendations for patients with AFFs: (1) discontinue BP therapy once the diagnosis of AFFs is established and reaccess the initiation of calcium and vitamin D treatment; (2) conduct prophylactic IMN operation for patients with incomplete fractures who are suffering from pain; and (3) undertake conservative therapy for patients with incomplete fractures who are suffering from mild pain or are without pain. IMN operations must be conducted if there is no radiologic or symptom improvement during two 2–3 months of conservative therapy because of the possible progression to complete fractures. Surgical intervention is one of the most important approaches to treat AFFs, which has been emphasized and investigated for choosing potentially better methods with good efficacy. Bearing in mind the fact that an AFF remains a type of a stress fracture with suppressed bone remodelling after long-term BP exposure, IMN stabilization is preferred for its proendochondral healing potential as a nonrigid fixation method [60]. In fact, IMN fixation triumphed over extramedullary plates and screws for its better union rate, and less revision or low failure rate reported in some retrospective studies [53], [61]. However, one must note that these suggestions are only opinion based because of the lack of RCT-based evidence. It was also a fact that patients with AFFs showed delayed healing or nonunion with implant fixation. This implies new challenges for physicians to develop dedicated surgical and/or conservative methods for specific indication of AFF repair or healing enhancement. Apparently, a multidisciplinary approach other than conventional surgical methods is desired to deal with AFFs, as the task force recommended parathyroid hormone (PTH) therapy to be considered when conservative treatment does not result in successful healing [61], [62], [63].
PTH treatment for AFFs
The overall delayed healing rate for AFFs was reported to be 26% [8]. Though it was recommended that most of AFFs should be managed by surgical stabilization in case of potential progression, the delayed healing and nonunion rates in patients receiving IMN fixation could be as high as 50% and 46%, respectively [52], [53]. Hence, endeavours to accelerate AFF healing have drawn much attention. A representative one is teriparatide (recombinant human PTH 1-34), currently the only potent bone-forming agent approved for treatment of OP in both men and postmenopausal women. Intermittent PTH injection results in activation of osteoblasts and rising of bone formation markers in the early period, followed by increased bone resorption markers in the latter period as an indication of osteoclast activation [64]. Studies proved that PTH might accelerate fracture healing in healthy individuals or OP patients. Komrakova et al [65] indicated that PTH increased fracture callus density and biomechanical properties both in healthy and in osteoporotic rats, while this bone stimulation effect was more effective in healthy rats than in OP rats. It was suggested by the authors that normal gonadal hormone level was essential for the effective stimulation of PTH for bone healing, given the fact that PTH in combination with oestrogen results in better bone mass improvement than PTH treatment alone. While relevant human studies are rare, the positive effect of PTH on fracture healing was also suggested in patients with fractures at distal radius [66]. In addition, successful treatment of PTH in patients with nonunion postfracture was reported [67]. Nakajima et al [68] suggested that the mechanism by which PTH accelerated bone healing included increased proliferation and differentiation of osteoprogenitor, synthesis of bone matrix proteins, and enhanced osteoclastogenesis. Interestingly, Sloan et al [69] reported that while BPs delayed stress fracture healing, PTH accelerated this process due to a stimulating bone turnover rate through not only better osteoblast proliferation and function, but also increases in the production of receptor activator of the nuclear factor kappa-B ligand and macrophage colony stimulating factor in response to PTH treatment.
Based on the findings that PTH might accelerate repair of tensile stress fractures the of femoral neck in humans by increased bone remodelling [70], it is logically expected that PTH may also enhance AFF repair. Tarazona-Santabalbina and Aguilella-Fernández [71] provided a case where a patient developed a spontaneous incomplete fracture (lytic lesion in the external cortex) of the right femoral diaphysis with discomfort in the right thigh months after IMN fixation of AFFs in the right femur. According to the recommendation of ASBMR, prophylactic surgery is indicated for such medical condition [8]. However, the fracture healed and symptoms disappeared after discontinuation of BPs and initiation of PTH therapy. The authors therefore suggested a possible role of PTH in treating AFFs conservatively. PTH was also used as an adjuvant therapy after surgical intervention. Miyakoshi et al [72] retrospectively studied 45 consecutive AFFs in 34 patients, with 37 AFFs being treated surgically and eight conservatively. The results showed that for surgically treated fractures, PTH significantly reduced the average time for fracture healing compared with the non-PTH group (5.4 ± 1.5 months vs. 8.6 ± 4.7 months). In addition, the delayed healing or nonunion rate was also lower in the PTH group. However, not all patients responded to PTH positively [9]. According to the report by Miyakoshi et al [72], other factors such as vitamin D might also impact AFF repair because successful healing of AFFs was reported in patients receiving a combination therapy of vitamin D and PTH. This study suggested that the efficacy of PTH under the influence of vitamin D should be clarified considering that sufficient vitamin D status might serve as an essential factor for successful repair. In fact, it was indicated that vitamin D deficiency had a close relationship with AFFs, and maintaining optimum serum vitamin D level was important to reduce the risk of AFF [48]. Given the fact that vitamin D not only secures sufficient serum calcium concentration, which is essential for bone formation, but also directly or indirectly modulates both osteoclast and osteoblast activities to maintain normal bone homeostasis, e.g., bone remodelling [73], we might logically propose the following hypothesis that vitamin D could get involved in both occurrence and healing of AFFs. Nevertheless, a case–controlled study by Goh et al [74] reported the opposite findings that serum vitamin D level in patients with AFFs was actually higher compared with that in controls. Another uncertainty for using PTH is that the effective dose, dosing regimen, and indication of this kind of drug for fracture repair remain issues to be addressed. Last but not the least, PTH therapy for enhancement of AFF healing should be reaccessed for patients with a PTH therapy history prior to fracture because long-term and high-dose PTH was reported to be strongly associated with osteosarcoma in animal models, and thus, clinically, the time for PTH treatment was recommended to be no longer than 24 months [75]. The task force report from ASBMR in 2014 indicated that no conclusion could be reached with regard to the efficacy of PTH in AFF healing, and high-quality RCT research was still desirable [9].
Other potential therapies for AFFs
Insights into other strategies or treatments being used to accelerate fracture healing or reduce the nonunion rate may shed light on effective management of AFFs. We summarized these methods into four categories: biological agents, pharmacological methods, physical methods, and biomaterials (Table 1).
Table 1.
Summary of current nonsurgical strategies to enhance fracture healing.
| Category | Agent/method | (Putative) action and mechanism | Used in atypical femoral fracture | Shortcomings |
|---|---|---|---|---|
| Pharmacological [76], [77], [78], [79], [80] | Strontium ranelate | Has anabolic effect on OB and inhibit OC | Yes | High active dose, side effect of thrombosis and diarrhoea |
| Prostaglandin E2 receptor agonist | Stimulates bone forming (dominantly) and resorption (partially) | No | Side effects, e.g., diarrhoea | |
| Statins | Increases local BMP and osteocalcin expression | No | Better local delivery system needed | |
| PTH | Activates OB (initial) and OC (latter phase) when intermittently given Increases bone remodelling. |
Yes | Side effects, e.g., osteosarcoma | |
| Physical [52], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90] |
LIPUS | Induces micromechanical strains that result in biochemical events at cellular level Affects migration, proliferation, differentiation of many cell types Thermal effect leads to bioreaction |
Yes | Bone forming effect not clear; more clinical studies needed Individual variance in efficiency |
| LMHFV | Upregulates the expression of chondrogenesis-, osteogenesis-, and remodelling-related genes Induces angiogenesis |
No | ||
| PEMFs | Stimulates proliferation and osteogenic differentiation of osteoprogenitor cells | No | ||
| Biological [79], [85], [90], [91], [92], [93], [94], [95], [99] |
BMP, PGRN | Activates transcription of genes responsible for the cellular migration, proliferation, and differentiation | No | Short half-life time, lack of proper BMP delivery system, ectopic bone formation, and immunogenic reaction |
| Other growth factors (e.g., PDGF, etc) | Activates related gene expression, and induces bone formation or angiogenesis-related cell proliferation and/or differentiation | No | Better delivery system needed in combination with other therapeutic methods | |
| Antagonism of sclerostin or DKK-1 | Elevates endogenous Wnt signalling pathway | No | Clinical application is not clear |
BMP = bone morphogenetic protein; DKK-1 = Dickkopf-1; LIPUS = low impulsive ultrasound stimulation; LMHFV = low-magnitude high-frequency vibration; OB = osteoblast; OC = osteoclast; PDGF = platelet-derived growth factor; PEMF = pulsed electromagnetic field; PGRN = progranulin; PTH = parathyroid hormone.
Pharmacological methods
Oral intake of strontium ranelate (SR) has been regarded as an anabolic anti-OP agent that leads to increased BMD and decreased fracture risk due to its anabolic effect on osteoblast modulation and inhibitory effect on osteoclast [76]. Though Habermann et al [77] indicated that SR might enhance bone repair in animal models, relevant clinical studies investigating its effect on fracture healing are however limited. The inhibitory effect of SR on osteoclast may hinder its use for treatment of AFFs, considering that normal bone resorption is needed to trigger the repair of microcracks and subsequent bone remodelling. Notably, a case report showed successful healing in two AFF cases after SR treatment, with increases of serum osteocalcin and serum β-carboxyterminal levels [78]. Prostaglandin E2 receptor agonist is currently a promising agent that stimulates fracture healing, considering its effect on both bone forming (dominantly) and bone resorption (partially), with increased bone formation and fracture healing in rats receiving prostaglandin E2 receptor agonist therapy [79]. Relatively elevated remodelling after administration of this drug may be beneficial to AFF healing. However, systemic administration of prostaglandin E2may also result in adverse effects, including diarrhoea, which limits its clinical application. Another potentially useful drug is statin, which may not only lower the cholesterol level, attributed to its inhibitory effect on the 3-hydroxy-3-glutaryl-coenzyme A reductase activity, but also induce bone formation by elevating bone morphogenetic protein (BMP)-2 and osteocalcin [80]. For the application in AFFs, it may be worth testing the efficacies of these agents in clinical condition. In addition, generally speaking, developing a better delivery system and overcoming the first pass effect of the liver are essential for further clinical use.
Biophysical strategies
As noninvasive approaches, biophysical treatments are cost effective. In addition, biological factors, such as growth factors and novel scaffold, can be applied to patients simultaneously with physical treatment considering better cooperative bone-forming effect. Physical stimulation has been combined with surgical fixation to treat AFF before, while there was no further discussion about its efficacy [52]. Ultrasound plays a positive role in fracture healing in animal and human fracture healing studies, and LIPUS is the most commonly studied form of it, which is reported to influence all the stages of fracture healing processes including inflammation, callus formation, and bone remodelling [81]. Both thermal effect and micromechanical strains induced by LIPUS can influence biological response on cellular or tissue level [82]. LIPUS was reported to affect migration, proliferation, and differentiation of many cell types, and have a positive effect on bone mineralization in in vitro studies [83], as well as bone healing in animal models [82]. However, though LIPUS was reported to have the advantage of minimal side effects [81], to date, clinical efficacy of LIPUS in fracture repair is not well established because high-quality RCT research is lacking [82]. Other commonly used physical methods include pulsed electromagnetic fields and low-magnitude high-frequency vibration (LMHFV); the latter one was considered to accelerate normal or osteoporotic fracture healing [84], [85]. In our previous study [86], concomitant LMHFV treatment was found to partially reverse the decreased bone remodelling rate caused by ibandronate therapy after an osteoporotic fracture of rat, suggesting a potential role of this physical stimulation for AFFs. In other studies from our laboratory, LMHFV was found to increase both osteoporotic and healthy rat fracture healing processes with better mechanical test outcomes [87], [88]. Moreover, angiogenesis was also found to be stimulated by LMHFV [89]. A study [84] suggested that pulsed electromagnetic fields stimulated bone healing, while Einhorn [85] concluded that electromagnetic fields had no significant impact on bone healing considering the limitation of the methodology and heterogeneity of study.
Biological strategies
The most investigated biological strategy to enhance bone repair is BMPs, which contain a large subfamily of transforming growth factor-β produced in bone and partially have osteogenic/bone-forming effect through activation of the transcription of genes responsible for cellular migration, proliferation, and differentiation [90]. The bone-forming mechanism of BMPs depends on the various BMP activity-regulating inhibitors and stimulators [91]. Recombinant human BMP-2 and BMP-7 are most commonly used, and their positive roles on fracture healing enhancement have been proved not only in animal studies but also in clinical trials [91]. For their application in AFFs, it seems reasonable that such kind of growth factors can be injected locally at the fracture site or combined with promising scaffold, even osteoprogenitor cells, when one embarks on optimizing clinical outcome of patients with AFFs using the so-called tissue engineering technologies. However, some clinical trials showed no significant difference in the healing time between the control and BMP-treated groups [84], the reason of which is considered to be the short half-life time of BMPs in specific fracture sites and the lack of proper BMP delivery systems. Other factors that hinder wider clinical applications of BMPs are their potential side effects, including ectopic bone formation and immunogenic reaction to the BMPs administrated [91]. In addition, cost effectiveness of BMPs is also debatable because manufacturing of BMPs is expensive, and these are also needed in relatively high doses to overcome the rapid tissue clearance. Currently, factors inhibiting BMP antagonists are supposed to have a positive effect on fracture healing by upregulating endogenous BMP activity, while relevant clinical trials are limited [92]. Progranulin, a downstream molecule of BMPs, is reported to enhance bone healing through mediating BMP-2 and tumour necrosis factor signalling [93]. Others promising biological strategies mainly are platelet-derived growth factor and recombinant human fibroblast growth factor-2, both of which may stimulate fracture healing [85]. Gene therapy and mobilization or activation of stem cell to fracture sites are also promising strategies with advantages of being combined with other therapeutic methods, such as physical stimulation [94]. Further studies are also needed to confirm their efficacies. Antagonism of sclerostin or Dickkopf-1 by monoclonal antibodies leads to an anabolic effect on bone and then increases bone mass in animals through elevation of the endogenous Wnt signalling pathway [79]. Alaee et al [95] suggested that systemic administration of antagonists of sclerostin may enhance bone repair for higher callus density and strength. However, for all the strategies mentioned above, clinical applications call for not only further investigation on a better delivery system, but also better clinical RCT studies.
Biometal magnesium as an orthopaedic implant
IMN used for AFFs has the advantages of preventing soft tissue damage during operation and maintaining stabilization of the fragment. Titanium and its alloys are the most currently used biometals with the advantages of enough mechanical properties to stabilize the fracture gap, corrosion resistance, and good biocompatibility such that foreign body reaction may be prevented. However, the nonunion and delayed union rates of AFFs were high in clinical cases with conventional Ti or stainless-steel IMN for long bone fixation. This is, therefore, desirable for us to modify our current IMNs with inclusion of potential bone-forming biomaterials or invent new devices for better healing. Scientists and physicians are seeking synthetic/man-made biomaterials that are supposed to be, ideally, both osteoconductive and osteoinductive. In addition, for internal fixation, this ideal biomaterial is preferred to be not only mechanically strong to fix or stabilize the fracture fragments before reaching bony connection, but also biodegradable to prevent secondary operation for implant removal. Apparently traditional Ti or stainless steels do not meet these requirements. Polymers, especially those made of lactic acid and glycolic acid, have been used for orthopaedic applications with the advantages of biodegradation and good biocompatibility [96]. However, polymers are bioinert and do not provide anabolic bioeffects for fracture healing apart from providing a good mechanical support. This suggests that the current polymer-based implants do not provide beneficial effects to patients with medical conditions that are not favourable for fracture repair, such as patients with osteoporotic fractures or AFFs. Mg has been tested as a bone implant that has attracted great attention for inclusion into implant design, which can be attributed to its unique beneficial effects over other traditional materials in the following aspects. Firstly, owing to its excellent mechanical properties Mg has an elastic modulus of 45 GPa, which is similar to normal human bone; hence, internal fixation devices developed using Mg for fracture fixation of weight-bearing bone may prevent the stress shielding effect compared with other permanent metals, such as currently used Ti or stainless steels, with a higher Young's modulus [97]. Secondly, Mg is an essential mineral element in human body and plays an important role in maintaining normal bone metabolism, and thus it is biocompatible and normally does not cause cytotoxicity [98]. Thirdly, Mg is biodegradable and therefore desirable for developing Mg-based medical implants for clinical applications [99]. However, considering the corrosion property of Mg or its alloys and the hydrogen gas released during corrosion in vivo, the problems of internal fixation loosening and deterioration of the mechanical properties of the device before bony bridging of the fracture gap(s) remain to be addressed [100]. Last, the degraded mineral elements of Mg or its alloys were found to be biologically active and could promote bone formation by enhancing not only osteogenic differentiation of bone marrow stem cells, but also osteoblast proliferation and function through activation of some intracellular signalling pathways [101]. This bone-forming effect of Mg alloy made it a promising material for fracture healing enhancement, and to date several preclinical studies have indicated its potential role as an internal fixation device [102], [103]. However, the reported Mg-containing metallic implants or devices were mainly screws and plates, which might not be adequate for application in weight-bearing bones. Combination implants made of conventional permanent metal and Mg-based implants might open up a new area for developing biological dynamic hybrid implant systems for orthopaedic applications, such as Mg-containing IMN. In addition, to date there is no report investigating the role of Mg implants for fixation of AFFs or general fracture in patients or even animals receiving long-term BP therapy. Based on the anabolic effects of Mg, it is possible that Mg and/or its alloys may have a positive bioeffect on the enhancement of AFF repair. In the future, relevant experimental studies at molecular, cellular, and tissue levels are highly desirable.
In conclusion, with the wide prescription of BPs for the treatment of OP, AFF, as one of the side effects resulting from low bone turnover after long-term BP treatment, is worthy of a physician's attention. Pathophysiologically speaking, decreased remodelling has side impacts on bone tissue at different levels, especially for the cortical bone region, leading to a fracture pattern shifted from typical osteoporotic fracture. Apart from this, the bowed geometric feature of femurs and antiangiogenesis effect of BPs on bone is highly likely to be involved in the development of AFFs. Decreased bone remodelling after BP exposure may also be the key reason for impaired healing in such patients, posing huge challenges for surgeons. For future studies, to build an appropriate animal model to mimic clinical AFFs is a prerequisite to understand the mechanism of not only how AFFs develop, but also the delayed healing process. In addition, except for investigating new methods or optimizing therapeutic regimens of BPs to prevent AFFs, exploring novel conservative therapy (e.g., PTH) and surgical instruments (e.g. Mg-containing IMN) to enhance the healing of already occurring AFFs is imperative.
Conflicts of interest
All contributing authors have no conflicts of interest to declare.
Funding/support
This work was supported by Hong Kong RGC Collaborative Research Fund (CRF 2014/2015, C4028-14GF), General Research Fund (Ref. No.: 14112714, 14114415), NSFC/RGC (N_CUHK449/13), and Innovation and Technology Fund (ITF, Ref. No.: ITS/350/13).
We acknowledge the Li Ka Shing Institute of Health Sciences (LiHS) for providing a harmonious working space.
References
- 1.Cummings S.R., Black D.M., Rubin S.M. Lifetime risks of hip, Colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445–2448. [PubMed] [Google Scholar]
- 2.Reid I.R. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 3.Dell R.M., Adams A.L., Greene D.F., Funahashi T.T., Silverman S.L., Eisemon E.O. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 4.Siris E.S., Pasquale M.K., Wang Y., Watts N.B. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res. 2011;26:3–11. doi: 10.1002/jbmr.189. [DOI] [PubMed] [Google Scholar]
- 5.Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N., Gottschalk F.A., Pak C.Y.C. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 6.Schilcher J., Michaëlsson K., Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364:1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 7.Lenart B.A., Lorich D.G., Lane J.M. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 8.Shane E., Burr D., Ebeling P.R., Abrahamsen B., Adler R.A., Brown T.D. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 9.Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 10.Nieves J.W., Bilezikian J.P., Lane J.M., Einhorn T.A., Wang Y., Steinbuch M. Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int. 2010;21:399–408. doi: 10.1007/s00198-009-0962-6. [DOI] [PubMed] [Google Scholar]
- 11.Maravic M., Ostertag A., Cohen-Solal M. Subtrochanteric/femoral shaft versus hip fractures: incidences and identification of risk factors. J Bone Miner Res. 2012;27:130–137. doi: 10.1002/jbmr.517. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Bhattacharyya T. Trends in incidence of subtrochanteric fragility fractures and bisphosphonate use among the US elderly, 1996–2007. J Bone Miner Res. 2011;26:553–560. doi: 10.1002/jbmr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin L., Au S.K., Leung P.C., Lau M.C., Woo J., Choy W.Y. Baseline BMD and bone loss at distal radius measured by peripheral quantitative computed tomography in peri- and postmenopausal Hong Kong Chinese women. Osteoporos Int. 2002;13:962–970. doi: 10.1007/s001980200134. [DOI] [PubMed] [Google Scholar]
- 14.Ebetino F., Cornish J., Phipps R., Keck B., Reid I., Callon K. Relative uptake of risedronate in trabecular and cortical bone. Bone. 2010;46(Suppl. 1):S70. [Google Scholar]
- 15.Dell R., Greene D., Ott S., Silverman S., Eisemon E., Funahashi T. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. J Bone Min Res. 2010;25:61. [Google Scholar]
- 16.Neviaser A.S., Lane J.M., Lenart B.A., Edobor-Osula F., Lorich D.G. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 17.Gedmintas L., Solomon D.H., Kim S.C. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28:1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schilcher J., Koeppen V., Aspenberg P., Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371:974–976. doi: 10.1056/NEJMc1403799. [DOI] [PubMed] [Google Scholar]
- 19.Edwards B.J., Sun M., West D.P., Guindani M., Lin Y.H., Lu H. Incidence of atypical femur fractures in cancer patients: the MD Anderson Cancer Center experience. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2818. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Adler R.A., El-Hajj Fuleihan G., Bauer D.C., Camacho P.M., Clarke B.L., Clines G.A. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puhaindran M.E., Farooki A., Steensma M.R., Hameed M., Healey J.H., Boland P.J. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J Bone Jt Surg Am. 2011;93:1235–1242. doi: 10.2106/JBJS.J.01199. [DOI] [PubMed] [Google Scholar]
- 22.Chang S.T., Tenforde A.S., Grimsrud C.D., O'Ryan F.S., Gonzalez J.R., Baer D.M. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone. 2012;51:524–527. doi: 10.1016/j.bone.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Reddy S.V.B., Gupta S.K. Atypical femoral shaft fracture in a patient with nonmetastatic prostate cancer on zoledronic acid therapy: effect of therapy or coincidence? Singapore Med J. 2012;53:e52–4. [PubMed] [Google Scholar]
- 24.Somford M.P., Draijer F.W., Thomassen B.J.W., Chavassieux P.M., Boivin G., Papapoulos S.E. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res. 2009;24:1736–1740. doi: 10.1359/jbmr.090408. [DOI] [PubMed] [Google Scholar]
- 25.Jamal S.A., Dion N., Ste-Marie L.-G. Atypical femoral fractures and bone turnover. N Engl J Med. 2011;365:1261–1262. doi: 10.1056/NEJMc1107029. [DOI] [PubMed] [Google Scholar]
- 26.Chisin R. The role of various imaging modalities in diagnosing stress fractures. In: Burr David B., Milgrom Chuck., editors. Musculoskeletal fatigue and stress fractures. CRC Press; Boca Raton, FL: 2001. pp. 279–294. [Google Scholar]
- 27.Barrett J.G., Sample S.J., McCarthy J., Kalscheur V.L., Muir P., Prokuski L. Effect of short-term treatment with alendronate on ulnar bone adaptation to cyclic fatigue loading in rats. J Orthop Res. 2007;25:1070–1077. doi: 10.1002/jor.20395. [DOI] [PubMed] [Google Scholar]
- 28.Eriksen E.F., Melsen F., Sod E., Barton I., Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Mashiba T., Burr D.B. Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int. 2001;69:281–286. doi: 10.1007/s002230010036. [DOI] [PubMed] [Google Scholar]
- 30.Burr D.B., Forwood M.R., Fyhrie D.P., Martin R.B., Schaffler M.B., Turner C.H. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Mashiba T., Hirano T., Turner C.H., Forwood M.R., Johnston C.C., Burr D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 32.Allen M.R., Burr D.B. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 33.Recker R.R., Delmas P.D., Halse J., Reid I.R., Boonen S., García-Hernandez P.A. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res. 2008;23:6–16. doi: 10.1359/jbmr.070906. [DOI] [PubMed] [Google Scholar]
- 34.Stepan J.J., Burr D.B., Pavo I., Sipos A., Michalska D., Li J. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone. 2007;41:378–385. doi: 10.1016/j.bone.2007.04.198. [DOI] [PubMed] [Google Scholar]
- 35.Allen M.R., Burr D.B. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 36.Smith E.R., Allen M.R. Bisphosphonate-induced reductions in rat femoral bone energy absorption and toughness are testing rate-dependent. J Orthop Res. 2013;31:1317–1322. doi: 10.1002/jor.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Im G.-I., Jeong S.-H. Pathogenesis, management and prevention of atypical femoral fractures. J Bone Metab. 2015;22:1–8. doi: 10.11005/jbm.2015.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen M.R., Gineyts E., Leeming D.J., Burr D.B., Delmas P.D. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 39.Tang S.Y., Zeenath U., Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchiyama S., Ikegami S., Kamimura M., Mukaiyama K., Nakamura Y., Nonaka K. The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations. Bone. 2015;75:84–87. doi: 10.1016/j.bone.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Poundarik A.A., Wu P.-C., Evis Z., Sroga G.E., Ural A., Rubin M. A direct role of collagen glycation in bone fracture. J Mech Behav Biomed Mater. 2015;50:82–92. doi: 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donnelly E., Meredith D.S., Nguyen J.T., Gladnick B.P., Rebolledo B.J., Shaffer A.D. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res. 2012;27:672–678. doi: 10.1002/jbmr.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin L., Choy W.-Y., Hung V.W.Y., Au S.-K., Chan K.-M., Leung K.-S. Age-related vessel calcification at distal extremities is a risk factor of osteoporosis. J Orthop Transl. 2014;2:43–48. [Google Scholar]
- 44.Acevedo C., Bale H., Gludovatz B., Wat A., Tang S.Y., Wang M. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–363. doi: 10.1016/j.bone.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Milovanovic P., Zimmermann E.A., Riedel C., Scheidt A.V., Herzog L., Krause M. Multi-level characterization of human femoral cortices and their underlying osteocyte network reveal trends in quality of young, aged, osteoporotic and antiresorptive-treated bone. Biomaterials. 2015;45:46–55. doi: 10.1016/j.biomaterials.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 46.Fournier P., Boissier S., Filleur S., Guglielmi J., Cabon F., Colombel M. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- 47.Teronen O., Heikkilä P., Konttinen Y.T., Laitinen M., Salo T., Hanemaaijer R. MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci. 1999;878:453–465. doi: 10.1111/j.1749-6632.1999.tb07702.x. [DOI] [PubMed] [Google Scholar]
- 48.Ishtiaq S., Edwards S., Sankaralingam A., Evans B.A.J., Elford C., Frost M.L. The effect of nitrogen containing bisphosphonates, zoledronate and alendronate, on the production of pro-angiogenic factors by osteoblastic cells. Cytokine. 2015;71:154–160. doi: 10.1016/j.cyto.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Ettinger B., Burr D.B., Ritchie R.O. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013;55:495–500. doi: 10.1016/j.bone.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Oh Y., Wakabayashi Y., Kurosa Y., Fujita K., Okawa A. Potential pathogenic mechanism for stress fractures of the bowed femoral shaft in the elderly: mechanical analysis by the CT-based finite element method. Injury. 2014;45:1764–1771. doi: 10.1016/j.injury.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 51.Pennypacker B.L., Duong L.T., Cusick T.E., Masarachia P.J., Gentile M.A., Gauthier J.-Y. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011;26:252–262. doi: 10.1002/jbmr.223. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki S., Miyakoshi N., Hongo M., Kasukawa Y., Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab. 2012;30:561–567. doi: 10.1007/s00774-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 53.Weil Y.A., Rivkin G., Safran O., Liebergall M., Foldes A.J. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J Trauma. 2011;71:186–190. doi: 10.1097/TA.0b013e31821957e3. [DOI] [PubMed] [Google Scholar]
- 54.Egol K.A., Park J.H., Rosenberg Z.S., Peck V., Tejwani N.C. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop. 2014;472:2728–2734. doi: 10.1007/s11999-013-2963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das De S., Setiobudi T., Shen L., Das De S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92:679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 56.Yue B., Ng A., Tang H., Joseph S., Richardson M. Delayed healing of lower limb fractures with bisphosphonate therapy. Ann R Coll Surg Engl. 2015;97:333–338. doi: 10.1308/003588415X14181254789321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J., Mori S., Kaji Y., Mashiba T., Kawanishi J., Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999;14:969–979. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- 58.Savaridas T., Wallace R.J., Salter D.M., Simpson A.H.R.W. Do bisphosphonates inhibit direct fracture healing?: a laboratory investigation using an animal model. Bone Jt J. 2013;95-B:1263–1268. doi: 10.1302/0301-620X.95B9.31562. [DOI] [PubMed] [Google Scholar]
- 59.Schilcher J., Sandberg O., Isaksson H., Aspenberg P. Histology of 8 atypical femoral fractures: remodeling but no healing. Acta Orthop. 2014;85:280–286. doi: 10.3109/17453674.2014.916488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balach T., Baldwin P.C., Intravia J. Atypical femur fractures associated with diphosphonate use. J Am Acad Orthop Surg. 2015;23:550–557. doi: 10.5435/JAAOS-D-14-00024. [DOI] [PubMed] [Google Scholar]
- 61.Teo B.J.X., Koh J.S.B., Goh S.K., Png M.A., Chua D.T.C., Howe T.S. Post-operative outcomes of atypical femoral subtrochanteric fracture in patients on bisphosphonate therapy. Bone Jt J. 2014;96-B:658–664. doi: 10.1302/0301-620X.96B5.32887. [DOI] [PubMed] [Google Scholar]
- 62.Blood T., Feller R.J., Cohen E., Born C.T., Hayda R. Atypical fractures of the femur: evaluation and treatment. JBJS Rev. 2015;3:e1. doi: 10.2106/JBJS.RVW.N.00062. [DOI] [PubMed] [Google Scholar]
- 63.Donnelly E., Saleh A., Unnanuntana A., Lane J.M. Atypical femoral fractures: epidemiology, etiology, and patient management. Curr Opin Support Palliat Care. 2012;6:348–354. doi: 10.1097/SPC.0b013e3283552d7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Resmini G., Iolascon G. New insights into the role of teriparatide. Aging Clin Exp Res. 2011;23:30–32. [PubMed] [Google Scholar]
- 65.Komrakova M., Stuermer E.K., Werner C., Wicke M., Kolios L., Sehmisch S. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone. 2010;47:480–492. doi: 10.1016/j.bone.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 66.Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 67.Schober H. Successful treatment with hPTH for 3 patients suffering from non healing fractures. J Bone Min Res. 2009;24:A09002859. [Google Scholar]
- 68.Nakajima A., Shimoji N., Shiomi K., Shimizu S., Moriya H., Einhorn T.A. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17:2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 69.Sloan A.V., Martin J.R., Li S., Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2010;47:235–240. doi: 10.1016/j.bone.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 70.Malhotra R., Meena S., Digge V.K. Tensile type of stress fracture neck of femur: role of teriparatide in the process of healing in a high risk patient for impaired healing of fracture. Clin Cases Miner Bone Metab. 2013;10:210–212. [PMC free article] [PubMed] [Google Scholar]
- 71.Tarazona-Santabalbina F.J., Aguilella-Fernández L. Bisphosphonate long-term treatment related bilateral subtrochanteric femoral fracture. Can teriparatide be useful? Aging Clin Exp Res. 2013;25:605–609. doi: 10.1007/s40520-013-0137-3. [DOI] [PubMed] [Google Scholar]
- 72.Miyakoshi N., Aizawa T., Sasaki S., Ando S., Maekawa S., Aonuma H. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2014;33:553–559. doi: 10.1007/s00774-014-0617-3. [DOI] [PubMed] [Google Scholar]
- 73.Van der Meijden K., Buskermolen J., van Essen H.W., Schuurman T., Steegenga W.T., Brouwer-Brolsma E.M. Long-term vitamin D deficiency in older adult C57BL/6 mice does not affect bone structure, remodeling and mineralization. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.09.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.Goh S.-K., Yang K.Y., Koh J.S.B., Wong M.K., Chua S.Y., Chua D.T.C. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 75.Vahle J.L., Sato M., Long G.G., Young J.K., Francis P.C., Engelhardt J.A. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 76.Baron R., Tsouderos Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol. 2002;450:11–17. doi: 10.1016/s0014-2999(02)02040-x. [DOI] [PubMed] [Google Scholar]
- 77.Habermann B., Kafchitsas K., Olender G., Augat P., Kurth A. Strontium ranelate enhances callus strength more than PTH 1-34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int. 2010;86:82–89. doi: 10.1007/s00223-009-9317-8. [DOI] [PubMed] [Google Scholar]
- 78.Carvalho N.N.C., Voss L.A., Almeida M.O.P., Salgado C.L., Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011;96:2675–2680. doi: 10.1210/jc.2011-0593. [DOI] [PubMed] [Google Scholar]
- 79.Sibai T., Morgan E.F., Einhorn T.A. Anabolic agents and bone quality. Clin Orthop. 2011;469:2215–2224. doi: 10.1007/s11999-010-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohnaka K., Shimoda S., Nawata H., Shimokawa H., Kaibuchi K., Iwamoto Y. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 81.Padilla F., Puts R., Vico L., Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54:1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Martinez de Albornoz P., Khanna A., Longo U.G., Forriol F., Maffulli N. The evidence of low-intensity pulsed ultrasound for in vitro, animal and human fracture healing. Br Med Bull. 2011;100:39–57. doi: 10.1093/bmb/ldr006. [DOI] [PubMed] [Google Scholar]
- 83.Reher P., Harris M., Whiteman M., Hai H.K., Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/s8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- 84.Androjna C., Fort B., Zborowski M., Midura R.J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics. 2014;35:396–405. doi: 10.1002/bem.21855. [DOI] [PubMed] [Google Scholar]
- 85.Einhorn T.A. New technologies for the enhancement of skeletal repair: challenges and opportunities. Indian J Orthop. 2011;45:489–491. doi: 10.4103/0019-5413.87113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chow D.H.-K., Leung K.-S., Qin L., Leung A.H.-C., Cheung W.-H. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res. 2011;29:746–752. doi: 10.1002/jor.21303. [DOI] [PubMed] [Google Scholar]
- 87.Shi H.-F., Cheung W.-H., Qin L., Leung A.H.-C., Leung K.-S. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone. 2010;46:1299–1305. doi: 10.1016/j.bone.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 88.Leung K.S., Shi H.F., Cheung W.H., Qin L., Ng W.K., Tam K.F. Low-magnitude high-frequency vibration accelerates callus formation, mineralization, and fracture healing in rats. J Orthop Res. 2009;27:458–465. doi: 10.1002/jor.20753. [DOI] [PubMed] [Google Scholar]
- 89.Cheung W.-H., Sun M.-H., Zheng Y.-P., Chu W.C.-W., Leung A.H.-C., Qin L. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model—evaluation of pulsed-wave Doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med Biol. 2012;38:2120–2129. doi: 10.1016/j.ultrasmedbio.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 90.Verdonk R., Goubau Y., Almqvist F.K., Verdonk P. Biological methods to enhance bone healing and fracture repair. Arthrosc J Arthrosc Relat Surg. 2015;31:715–718. doi: 10.1016/j.arthro.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 91.Lissenberg-Thunnissen S.N., de Gorter D.J.J., Sier C.F.M., Schipper I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35:1271–1280. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garrison K.R., Shemilt I., Donell S., Ryder J.J., Mugford M., Harvey I. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Libr. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y., Tian Q., Frenkel S., Liu C. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–6421. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho C.-Y., Sanghani A., Hua J., Coathup M., Kalia P., Blunn G. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracture healing. Tissue Eng Part A. 2015;21:594–602. doi: 10.1089/ten.tea.2013.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alaee F., Virk M.S., Tang H., Sugiyama O., Adams D.J., Stolina M. Evaluation of the effects of systemic treatment with a sclerostin neutralizing antibody on bone repair in a rat femoral defect model. J Orthop Res. 2014;32:197–203. doi: 10.1002/jor.22498. [DOI] [PubMed] [Google Scholar]
- 96.Morawska-Chochół A., Chłopek J., Szaraniec B., Domalik-Pyzik P., Balacha E., Boguń M. Influence of the intramedullary nail preparation method on nail's mechanical properties and degradation rate. Mater Sci Eng C Mater Biol Appl. 2015;51:99–106. doi: 10.1016/j.msec.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 97.Li Z., Gu X., Lou S., Zheng Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29:1329–1344. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 98.Reinhart R.A. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch Intern Med. 1988;148:2415–2420. doi: 10.1001/archinte.148.11.2415. [DOI] [PubMed] [Google Scholar]
- 99.Jiang T., Guo L., Ni S., Zhao Y. Upregulation of cell proliferation via Shc and ERK1/2 MAPK signaling in SaOS-2 osteoblasts grown on magnesium alloy surface coating with tricalcium phosphate. J Mater Sci Mater Med. 2015;26:1–9. doi: 10.1007/s10856-015-5479-2. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Jiang H., Bi Y., Sun J.E., Chen M., Liu D. Effects of gas produced by degradation of Mg–Zn–Zr Alloy on cancellous bone tissue. Mater Sci Eng C. 2015;55:556–561. doi: 10.1016/j.msec.2015.05.082. [DOI] [PubMed] [Google Scholar]
- 101.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Role of magnesium ions on osteogenic response in bone marrow stromal cells. Connect Tissue Res. 2014;55:155–159. doi: 10.3109/03008207.2014.923877. [DOI] [PubMed] [Google Scholar]
- 102.Chaya A., Yoshizawa S., Verdelis K., Myers N., Costello B.J., Chou D.-T. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015;18:262–269. doi: 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Han P., Cheng P., Zhang S., Zhao C., Ni J., Zhang Y. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015;64:57–69. doi: 10.1016/j.biomaterials.2015.06.031. [DOI] [PubMed] [Google Scholar]