Abstract
Background
Cyanobacteria of the genus Nostoc are capable of forming symbioses with a wide range of organism, including a diverse assemblage of cyanolichens. Only certain lineages of Nostoc appear to be able to form a close, stable symbiosis, raising the question whether symbiotic competence is determined by specific sets of genes and functionalities.
Results
We present the complete genome sequencing, annotation and analysis of two lichen Nostoc strains. Comparison with other Nostoc genomes allowed identification of genes potentially involved in symbioses with a broad range of partners including lichen mycobionts. The presence of additional genes necessary for symbiotic competence is likely reflected in larger genome sizes of symbiotic Nostoc strains. Some of the identified genes are presumably involved in the initial recognition and establishment of the symbiotic association, while others may confer advantage to cyanobionts during cohabitation with a mycobiont in the lichen symbiosis.
Conclusions
Our study presents the first genome sequencing and genome-scale analysis of lichen-associated Nostoc strains. These data provide insight into the molecular nature of the cyanolichen symbiosis and pinpoint candidate genes for further studies aimed at deciphering the genetic mechanisms behind the symbiotic competence of Nostoc. Since many phylogenetic studies have shown that Nostoc is a polyphyletic group that includes several lineages, this work also provides an improved molecular basis for demarcation of a Nostoc clade with symbiotic competence.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4743-5) contains supplementary material, which is available to authorized users.
Keywords: Cyanobacteria, Nostoc, Lichen, Symbiosis, Symbiotic competence
Background
Lichens are symbiotic associations between a fungus (mycobiont) and a photosynthetic partner (photobiont) that can be an eukaryotic alga (phycobiont), a cyanobacterium (cyanobiont), or both [1]. While the vast majority of lichen fungi (> 13,500 species), mainly from Ascomycota, associate with green algae (Chlorophyta), over 1500 species of lichen-forming fungi form so called “cyanolichens” that have cyanobacteria as primary photobionts (forming “bipartite” lichens) or accessory photobionts (forming “tripartite” lichens) [2]. Cyanobacterial symbioses have evolved repeatedly in different lineages of lichen-forming fungi [3–5], resulting in convergently similar thallus morphology in distantly related cyanolichens [2].
In lichen symbioses cyanobacteria provide mycobionts with photosynthate and/or fixed nitrogen. At the same time, the fungal partners provide the cyanobacteria with moisture, carbon dioxide and inorganic ions, as well as a relatively stable habitat, protected from environmental extremes and predation [1].
The association of fungal mycobiont partner and the photobiont partner (e.g. Nostoc) can either be by codispersal, e.g. in the lecanoromycete lichen Lobaria pulmonaria [6], or de novo by the pairing of a germinating spore and a free-living photobiont, as generally found in the lecanoromycete genus Peltigera [7, 8].
Most lichen symbioses are thought to be obligate as the majority of mycobionts are refractory to propagation in vitro and do not survive without their photosynthetic partners [9, 10]. And, although many cyanobacterial symbionts can be readily isolated and maintained in pure culture [11], they often appear unable to establish aposymbiotic populations outside lichen thalli in nature [12].
Nostoc is common in cyanolichens, especially in the temperate and cold regions of the world. All Nostoc species are filamentous and have complex life cycles involving cellular differentiation. Their non-branching filaments consist of cylindrical or spherical vegetative cells with intercalary heterocysts, large specialized nitrogen-fixing cells developing in mature trichomes [13]. The filaments of Nostoc strains are usually covered with a sheath of mucilage and many free-living Nostoc can form gelatinous macroscopic colonies in nature. The ability to produce mucilage and to form hormogonia, slender motile filaments, is generally used to distinguish Nostoc strains from the closely related genus Anabaena [13, 14], but they can be more reliably differentiated by akinete size and shape, together with other morphological characters [15]. However, some strains of Nostoc only produce hormogonia erratically or do not produce them at all [14, 16, 17].
The taxonomy of the family Nostocaceae is still rather poorly resolved, as exemplified by the placement of Calothrix and Tolypothrix spp. in several phylogenetic clades and the separation of Nostoc spp. into different clades [18]. Symbiotic Nostoc strains of many cyanolichens, including both bi- and tripartite species of Peltigera, have traditionally been called Nostoc punctiforme, but cyanobacterial strains resembling Nostoc muscorum, Nostoc sphaericum and Nostoc linckia have also been cultured from Peltigera species [19, 20].
In the lichen symbiosis cyanobacteria tend to undergo several morphological and structural changes [19–23], confounding phenotypic identification. Thus, molecular techniques offer a powerful addition for studying the diversity of these organisms, and for comparing lichen symbiotic strains as well as free-living cyanobacteria. During the past fifteen years the cyanobacterial symbionts of lichens have been the subject of many molecular investigations which have greatly increased our understanding of symbiont diversity. However, most of these studies have been based on a limited number of marker genes (e.g. 16S rDNA, rbcLX, trnL) and have mainly been focused on phylogenetic relationships of different strains [8, 24–30]. So far little is known about what defines symbiotic competence of cyanobacteria on the genome level.
Genome sequencing of N. punctiforme PCC 73102 [31], a model strain for cyanobacterial symbiosis with plants, together with transposon mutagenesis [32, 33] and insertion of antibiotic resistance cassettes [34] have identified a number of genes involved in the symbiosis [35]. Here we present the complete sequence and analysis of genomes from lichen-symbiotic Nostoc strains - one from the bipartite lichen Peltigera membranacea and one from the tripartite lichen Lobaria pulmonaria, - together with a discussion of genes which appear distinctive for symbiotic Nostoc. In addition we make use of draft genome data from three more Nostoc strains derived from P. membranacea and metagenome data from the lichens P. membranacea and P. malacea, as well as currently available whole genome data from members of the Nostocales.
Results and discussion
Genome properties
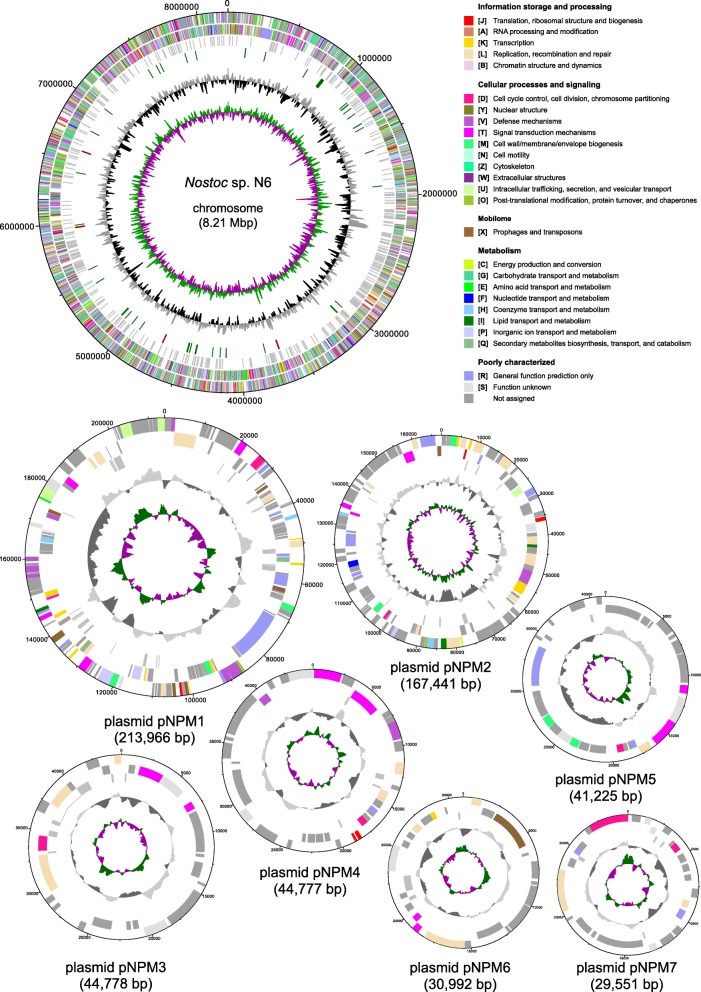
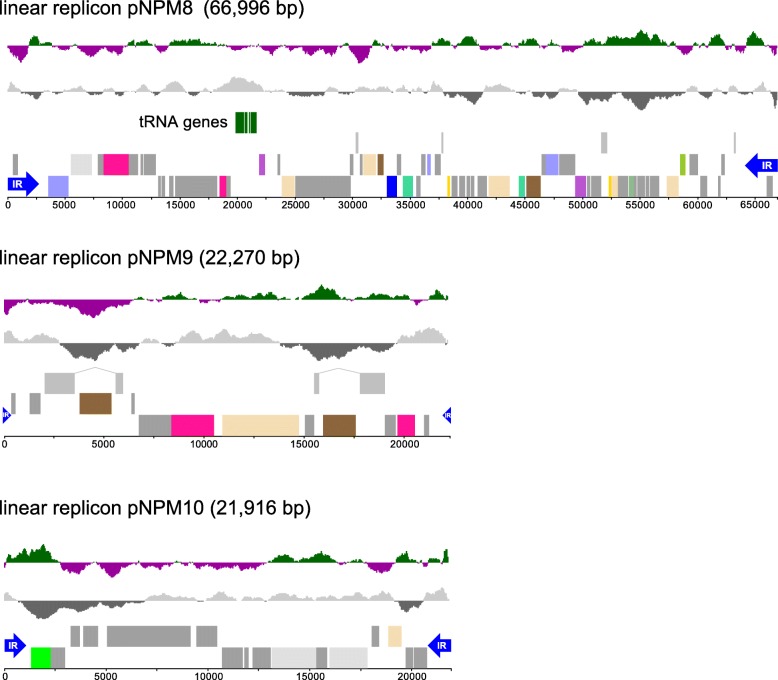
We have shotgun-sequenced DNA from five lichen-associated Nostoc strains and two lichens (Table 1). Draft genome assemblies were generated for three of the strains. The genomes of two strains, namely of the nosperin producer Nostoc sp. N6 [36] and the cyanobiont of L. pulmonaria, were completely assembled and annotated. The genome of Nostoc sp. N6 (8.9 Mb) is similar in size to that of symbiotic N. punctiforme PCC 73102 but it is larger than genomes of free-living Nostoc and Anabaena strains (Table 1 and Additional file 1). It consists of one circular chromosome (8.21 Mb) (Fig. 1) and 10 extrachromosomal replicons – 7 circular (pNPM1, 213,966 bp; pNPM2, 167,441 bp; pNPM3, 44,778 bp; pNPM4, 44,777 bp; pNPM5, 41,255 bp; pNPM6, 30,992 bp; pNPM7, 29,551 bp) (Fig. 1) and 3 linear (pNPM8, 66,996 bp; pNPM9, 22,270 bp; pNPM10, 21,916 bp) (Fig. 2). Based on the sequence coverage obtained, the linear replicons are present in higher copy numbers than the circular ones. pNPM9 and pNPM10 are characterized by a lower GC content (36.6% and 37.7%, respectively) than the rest of the genome (Table 2). The ends of pNPM8, pNPM9 and pNPM10 are in each case composed of identical or nearly identical inverted repeats 2.73, 0.16 and 1.06 kb long, respectively, with a conserved AAATTAACRGAC sequence at each end (Additional file 2: Figure S1). The ends of the linear plasmids form covalently closed hairpins, in which one DNA strand loops around and becomes the complementary strand. This was inferred from two observations: (i) presence of reads with palindrome sequences in the Nextera XT library and (ii) drop in coverage close to the ends of the plasmids and absence of read pairs spanning putative palindromes in the Nextera mate pair library. These methods use different DNA inputs for adapter addition: Nextera XT uses PCR to add adapters to denatured DNA whereas Nextera Mate Pair ligates adapters to blunt ends of double-stranded DNA.
Table 1.
List of lichen-associated Nostoc strains sequenced in this study
| Strain | Genome status | Genome size | DNA source |
|---|---|---|---|
| Nostoc sp. N6 ‘Peltigera membranacea cyanobiont’ | Complete | 8.9 Mb | Pure culture |
| Nostoc sp. 210A ‘P. membranacea cyanobiont’ | Draft | 8.33 Mb | Pure culture |
| Nostoc sp. 213 ‘P. membranacea cyanobiont’ | Draft | 8.33 Mb | Pure culture |
| Nostoc sp. 232 ‘P. membranacea cyanobiont’ | Draft | 9.16 Mb | Pure culture |
| Nostoc sp. ‘Lobaria pulmonaria cyanobiont’ | Complete | 7.34 Mb | Pure culture |
| Nostoc sp. ‘P. malacea cyanobiont’ | Draft | 8.52 Mb | Metagenomic |
Fig. 1.
Chromosome and seven circular plasmids of the Nostoc sp. N6 genome. The outermost and second circles indicate genes in forward and reverse orientation color-coded by their COG categories. The third circles show pseudogenes. The fourth circle of the chromosome shows the rRNA genes (brown) and tRNA genes (green). The two innermost circles show GC content in gray and black and the GC skew in green (+) and purple (–)
Fig. 2.
Linear replicons of Nostoc sp. N6. The lowermost and second lines indicate genes in forward and reverse orientation color-coded by their COG categories (see Figure 1). The third lines show pseudogenes. The two uppermost lines show GC content in gray and black and the GC skew in green (+) and purple (–). Blue arrows represent terminal inverted repeats (IR)
Table 2.
Summary of the Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’ genomes
| Replicon | GenBank accession | Topology | Size, bp | GC, % | CDS | Pseudogenes | Total | rRNA operons | tRNA genes | CRISPR arrays |
|---|---|---|---|---|---|---|---|---|---|---|
| Nostoc sp. N6 | ||||||||||
| chromosome | CP026681 | circular | 8,214,648 | 41.4 | 6205 | 518 | 6723 | 4 | 103 | 13 |
| pNPM1 | CP026682 | circular | 213,966 | 41.1 | 180 | 20 | 200 | 0 | 0 | 2 |
| pNPM2 | CP026684 | circular | 167,441 | 40.4 | 122 | 10 | 132 | 0 | 0 | 0 |
| pNPM3 | CP026685 | circular | 44,778 | 41.4 | 30 | 5 | 35 | 0 | 0 | 0 |
| pNPM4 | CP026686 | circular | 44,777 | 40.9 | 38 | 4 | 42 | 0 | 0 | 0 |
| pNPM5 | CP026687 | circular | 41,225 | 40.9 | 32 | 1 | 33 | 0 | 0 | 0 |
| pNPM6 | CP026688 | circular | 30,992 | 41.0 | 27 | 3 | 30 | 0 | 0 | 0 |
| pNPM7 | CP026689 | circular | 29,551 | 42.4 | 30 | 4 | 34 | 0 | 0 | 0 |
| pNPM8 | CP026690 | linear | 66,996 | 41.3 | 69 | 4 | 73 | 0 | 24 | 0 |
| pNPM9 | CP026691 | linear | 22,270 | 36.6 | 14 | 2 | 16 | 0 | 0 | 0 |
| pNPM10 | CP026683 | linear | 21,916 | 37.7 | 20 | 0 | 20 | 0 | 0 | 1 |
| Nostoc sp. ‘L. pulmonaria cyanobiont’ | ||||||||||
| chromosome | CP026692 | circular | 7,061,466 | 41.6 | 5311 | 737 | 6048 | 3 | 74 | 7 |
| pNLP1 | CP026693 | circular | 121,770 | 40.3 | 82 | 9 | 91 | 0 | 0 | 0 |
| pNLP2 | CP026694 | circular | 63,064 | 42.2 | 36 | 10 | 46 | 0 | 0 | 0 |
| pNLP3 | CP026695 | circular | 58,727 | 42.0 | 60 | 4 | 64 | 0 | 0 | 0 |
| pNLP4 | CP026696 | circular | 34,881 | 42.4 | 33 | 2 | 35 | 0 | 0 | 0 |
| Other genome features | Nostoc sp. N6 | Nostoc sp. ‘L. pulmonaria cyanobiont’ | ||||||||
| Intron in tRNA fMet gene | Yes | No | ||||||||
| Number of genes with inteins | 5 | 0 | ||||||||
| Number of excision elements in nif cluster | 3 | 1 | ||||||||
Terminal inverted repeats and hairpins are common in linear DNA molecules enabling replication of genome ends [37]. Linear replicons are rarely found in Cyanobacteria, the only known examples being a 429.7 kb linear chromosome of Cyanothece sp. 51142 (accession NC_010547) [38] and a 37.15 kb incision element of Anabaena variabilis ATCC 29413 (accession NC_014000) [39]. An interesting feature of the largest linear plasmid pNPM8 is that it carries 24 tRNA genes out of the minimum of 32 tRNAs required for translation according to Crick’s wobble hypothesis [40]. Genes for tRNAs carrying isoleucine and histidine are not present, while there are 3 different tRNA genes for arginine and 2 for lysine, serine, glutamine and glutamic acid. tRNA genes have frequently been found in phages where they facilitate expression of phage genes with codons that are rare in the host genome [41, 42]. However, codon frequencies in the Nostoc sp. N6 chromosome vs. pNPM8 did not provide support for this (Additional file 2: Figure S2). The Nostoc sp. N6 linear elements might be phage remnants that have lost their structural proteins but retain the ability for self-replication in the host cells. Linear plasmid prophages are uncommon in nature, e.g. N15 in E. coli [43], PY54 in Yersenia enterocolitica [44] and φKO2 in Klebsiella oxytoca [45]. Genes involved in chromosome partitioning and segregation, such as parAB [46] and parM [47], typical of many low copy number plasmids and bacterial chromosomes, were not found on the linear replicons, except for pNPM8 which carries a presumptive parA gene (NPM_80015). Interestingly, a gene encoding a typical phage protein, terminase, involved in DNA packaging into empty phage capsids, was found in pNPM9 (NPM_90012) disrupted by an insertion sequence.
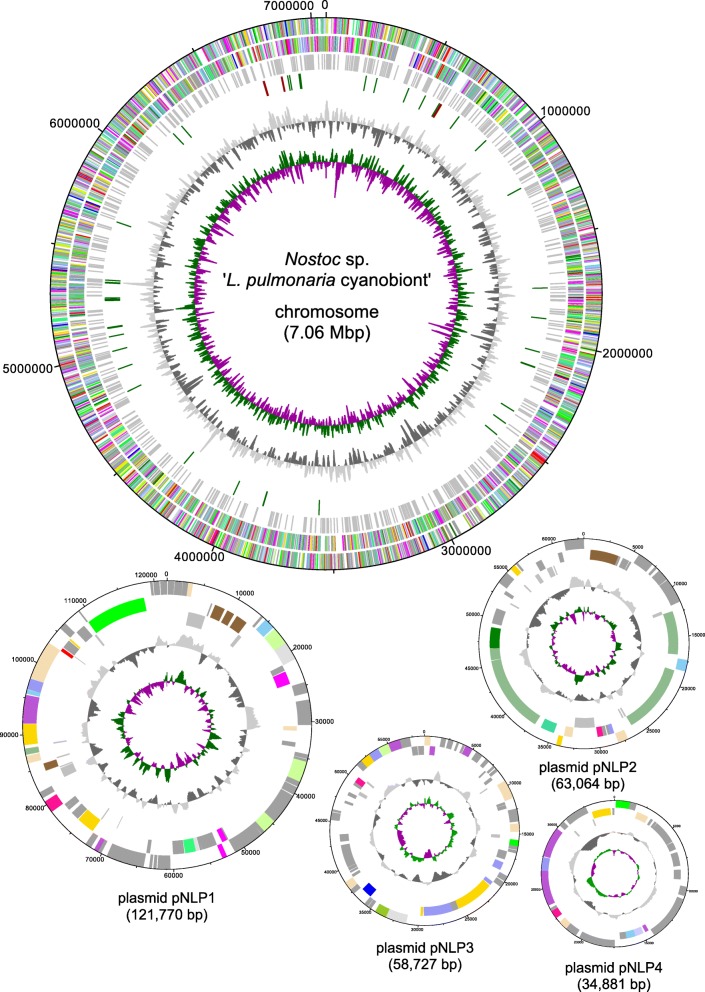
The genome of the L. pulmonaria cyanobiont is nearly 1.5 Mb smaller than that of Nostoc sp. N6 and has a total size of 7.34 Mb. It consists of one circular chromosome (7.06 Mb) and 4 circular extrachromosomal replicons – pNLP1 (121,770 bp), pNLP2 (63,064 bp), pNLP3 (58,727 bp) and pNLP4 (34,881 bp) (Fig. 3). Compared to Nostoc sp. N6, the L. pulmonaria cyanobiont genome contains a smaller number of coding regions, a larger number of pseudogenes and 3 ribosomal DNA (rDNA) operons instead of the 4 copies generally found in Nostocales [48]. These features along with much slower growth observed in pure culture (3–4 times slower than Nostoc sp. N6) suggest genome shrinkage, gene loss and a possible semi-obligate nature of the cyanobiont. Interestingly, cbiM transporter genes, involved in the uptake of cobalt for cobalamin (vitamin B12) biosynthesis (locus tags NLP_0266 and NLP_2774), were found to be pseudo in the L. pulmonaria cyanobiont. Several other essential genes had disabling mutations but had intact functional homologues.
Fig. 3.
Chromosome and four plasmids of the Nostoc sp. ‘L. pulmonaria cyanobiont’ genome. The outermost and second circles indicate genes in forward and reverse orientation color-coded by their COG categories. The third circles show pseudogenes. The fourth circle of the chromosome shows the rRNA genes (brown) and tRNA genes (green). The two innermost circles show GC content in gray and black and the GC skew in green (+) and purple (–)
Inversion of the GC skew ((G −C)/(G+C)) from positive to negative, typically seen at the replication origin of bacterial chromosomes, cannot be applied to predict the location of oriC in Cyanobacteria since their DNA asymmetry is greatly disturbed by mutational pressure [49] and extensive chromosome rearrangements (see below). A putative oriC for the chromosomes was identified in both strains downstream of dnaA, encoding a chromosomal replication initiation protein (locus tags NPM_0001 and NLP_0001) (Additional file 2: Figure S3). Both oriC regions contain 6 DnaA boxes, most with TTTTCCACA, the DnaA box motif specific for Cyanobacteria [50]. Location of oriC adjacent to the dnaN gene encoding the β subunit of DNA polymerase III has been claimed to be universal among Cyanobacteria [50, 51]. In free-living Nostoc strains and in Anabaena variabilis, oriC is located in the intergenic region between the dnaA and dnaN genes. However, in lichen-associated Nostoc strains and in N. punctiforme, dnaA and dnaN genes are not adjacent (∼ 52 kb apart in N. punctiforme). Interestingly, no apparent DnaA boxes were found adjacent to either the dnaA gene (Npun_F0001) or the dnaN gene (Npun_F0034) in N. punctiforme whereas a putative oriC with a cluster of DnaA boxes lies within the Npun_F0036–Npun_F0037 intergenic region.
Genome and proteome comparison
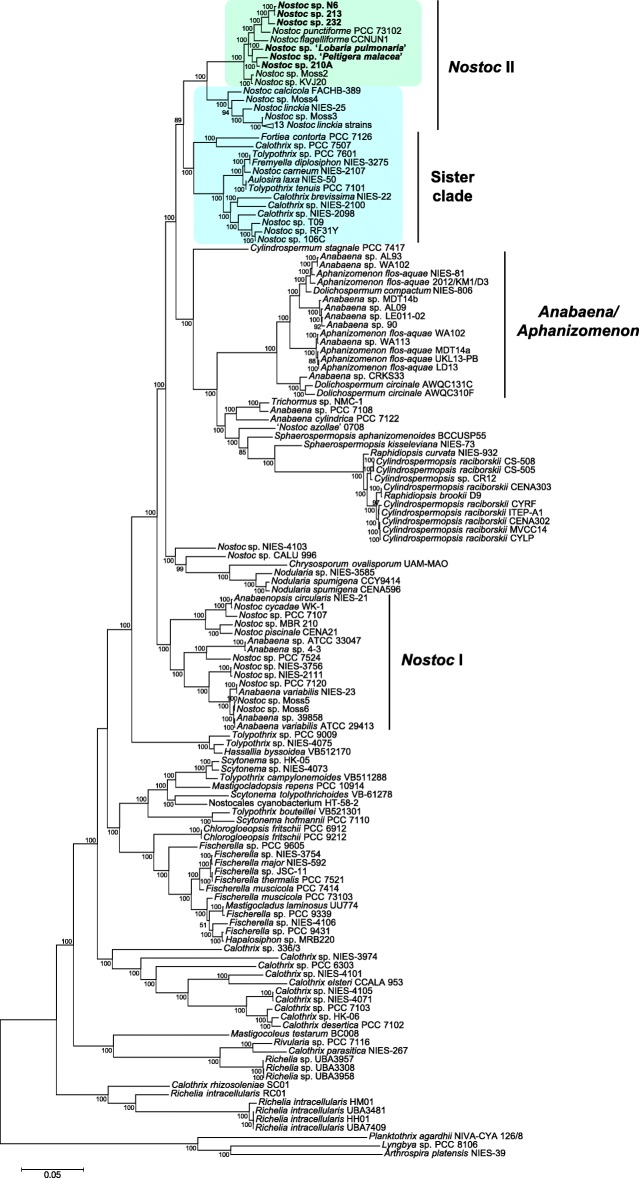
Phylogenetic analysis of 31 conserved single copy protein genes from Nostocales strains available in GenBank and JGI-IMG showed that lichen-associated Nostoc strains and N. punctiforme group together in a clade (Nostoc II; Fig. 4) suggesting a monophyletic origin of these symbiotic Nostoc strains. The recently sequenced symbiotic strains Nostoc sp. KVJ20 [52] and Nostoc sp. Moss 2 [30] also associate with this clade, whereas two other moss derived isolates together with terrestrial soil isolates of Nostoccalcicola and Nostoclinckia form a subclade within the Nostoc II clade. The free-living aquatic Nostoc strains group together with some Anabaena strains (clade Nostoc I; Fig. 4) while other Anabaena strains group with members of the genera Aphanizomenon and Dolichospermum (clade Anabaena/Aphanizomenon). These major phylogenetic relationships are in accord with what has been found by O’Brien and coworkers [29] and by Warshan [30]. Although O’Brien’s clade Nostoc II contains three free-living terrestrial isolates – N. punctiforme SAG 71.79, N. commune 02011101 and N. muscorum SAG 57.79 (currently known as Desmonostoc muscorum) – none of them have been tested for symbiotic competence and two of the strains have P. membranacea cyanobionts as their closest phylogenetic relatives. Nostoc strains with specificity for symbiosis with Gunnera also fall within clade II [53] but their genome sequences are currently not available.
Fig. 4.
Maximum liklelihood phylogenomic tree of Nostocales strains based on 31 single-copy core bacterial phylogenetic markers [135]. Arthrospira platensis NIES-39, Lyngbya sp. PCC 8106 and Planktothrix agardhii NIVA-CYA 126/8 from the order Oscillatoriales were used as the outgroup. Numbers at branch nodes are bootstrap percentages based on 100 replicates (only values >50 are shown). Scale bar indicates 5% sequence divergence. Selected clades are named according to [29]. Predominantly symbiotic clade is highlighted with green, paraphyletic group is highlighted with blue. Lichen-associated strains are shown in bold
Large scale genome comparisons of lichen-associated strains with N. punctiforme PCC 73102 reveal a low level of synteny between them (Additional file 2: Figure S4) indicating high genome plasticity and genome shuffling in these strains (Additional file 2: Figure S5). The ten most prominent regions of synteny include the whole set of genes involved in nitrogen fixation (the nif gene cluster; locally collinear block 1), some photosynthetic genes (locally collinear block 3), and genes encoding the majority of ribosomal proteins (Clusters of Orthologous Groups (COG) category J; locally collinear block 7) (Additional file 2: Figure S5, Additional file 3), which are known to be syntenic across species [54, 55], as well as many genes involved in carbohydrate transport and metabolism (G) and cell wall/membrane/envelope biogenesis (M).
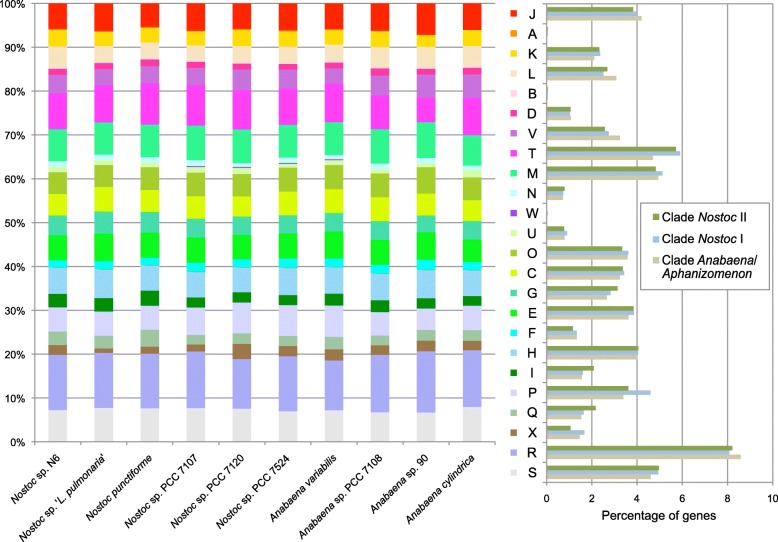
Although symbiotic Nostoc strains N6 and N. punctiforme show a higher number of encoded proteins in most COG categories than the free-living strains (mean total 4344 vs. 3587; Additional file 2: Table S5), the fraction assigned to COG categories was similar (63-68%) and the distribution among categories was similar for all analyzed Nostoc and Anabaena strains (Fig. 5). On the average, clade II of symbiotic Nostoc strains has a higher proportion of genes devoted to carbohydrate transport and metabolism (G), lipid transport and metabolism (I) and secondary metabolite biosynthesis, transport and catabolism (Q) compared to clade I Nostoc and Anabaena strains. Interestingly, clade I, comprised of free-living Nostoc and Anabaena strains (Additional file 1), exhibits the highest number of genes for inorganic ion transport and metabolism (P) (Fig. 5). In contrast, the Nostoc strains in symbiosis may benefit from host (plant or fungus) provision of inorganic ions, e.g. by the action of mycobiont siderophores [56].
Fig. 5.
COG category distribution of the proteins encoded in the genomes of selected Nostoc and Anabaena strains. The ordinate axes indicate the percentage of genes in each COG functional category relative to the genes of all COG categories (left) and percentage COG category distribution among different clades (right)
The genome of Nostoc sp. N6 was found to encode the highest number of COG category L proteins (DNA replication, recombination and repair) (Additional file 2: Table S3). One possible explanation for this is that terrestrial cyanobacteria are generally subject to ultraviolet (UV) irradiation, and therefore are expected to possess efficient mechanisms for repair of UV-induced DNA damage [57]. Nevertheless, one of the genes involved in biosynthesis of the cyanobacterial sunscreen scytonemin (tyrosinase, tyrP) [58–60] is missing in the Nostoc sp. N6 genome, and another one (DSBA oxidoreductase, frnE) was found to be a pseudogene due to an in-frame stop codon (Additional file 2: Table S6). Both genes are thought to participate in oxidative dimerization of precursors to form scytonemin [61]. The cyanobionts of L. pulmonaria and P. malacea appear to have all genes necessary for scytonemin biosynthesis (Additional file 2: Table S6). It is possible that Nostoc sp. N6 compensates for the lack of scytonemin with a larger repertoire of enzymes for DNA repair.
Nostoc sp. N6 has a high number of transposable elements and inteins (Additional file 2: Tables S7-S9). The best studied case of inteins in Cyanobacteria is in DnaE (the α subunit of DNA polymerase III) encoded by two different ORFs and assembled by trans-splicing [62]. More information on transposons and inteins can be found in Supporting Information.
The majority of lichen associated Nostoc strains studied appears to have an alternative vanadium-based nitrogenase in addition to the standard molybdenum-based nitrogenase. This includes three of the strains studied here, Nostoc spp. 210A, 213 and 232, as well as the P. malacea lichen cyanobiont [63]. The reason for the common occurrence of this alternative nitrogenase in lichen-associated cyanobacteria is not clear, but may relate to low availability of molybdenum in cyanolichens and/or a functional advantage at relatively low growth temperatures [64]. A novel finding is that these lichen Nostoc strains carry a near complete duplication of VnfD, with a cyanobacterial aminoacyl-tRNA synthetase domain (CAAD; pfam14159) inserted at the carboxy end, similar to peptide insertions found in GluRS, ValRS, LeuRS and IleRS amino acid tRNA synthetases in a variety of cyanobacteria, where this domain is thought to direct the proteins to thylakoid membranes, a key source of reducing power and ATP [65]. Further information on the molybdenum-based (nif) and the vanadium-based gene clusters (vnf) is provided in Supporting Information (Additional file 2: Figures S9 and S10).
Comparison to minimal bacterial and cyanobacterial gene sets
In order to see what pathways might differ, be incomplete or deteriorating in lichen cyanobionts, we performed comparative analyses with the minimal bacterial [66] and the cyanobacterial “core” and “shell” [67] gene sets, represented by 206 and 682 genes respectively (Additional files 4 and 5). The most prominent differences were observed for pyrimidine metabolism, in split ribonucleotide reductase enzymes, carbohydrate catabolism and potassium transport, as described in the Supporting Information.
Identification of genes specific to symbiotic Nostoc strains
To identify functions enriched in symbiotic Nostoc genomes (present in over 80% of group), we performed an all-by-all BLASTP search of all the proteomes from the Nostoc I and II clades plus the sister clade (Fig. 4) and assigned identified hits into orthologous groups. For the lichen-associated Nostoc strains 152 protein orthologs satisfied the criteria set (see Methods), 189 orthologs for the predominantly symbiotic clade, 399 for the Nostoc II clade and 385 for the combined Nostoc II clade and sister clade (see Additional file 6 for listing). A few of the most prominent gene collectives associated with symbiotic Nostoc are discussed below.
Hormogonium regulating locus.
Hormogonia are relatively short motile filaments, lacking heterocysts, formed by cyanobacteria from the orders Nostocales and Stigonematales. A hormogonium-inducing factor (HIF) secreted by plant hosts induces symbiotic cyanobacteria to differentiate hormogonia and they then dedifferentiate back into nitrogen-fixing filaments after about 48 h [68]. The capacity of Nostoc strains to form hormogonia has been found to be necessary, but not singularly sufficient, for symbiotic competence [69, 70]. An aqueous extract of the hosting hornwort Anthoceros punctatus appears to contain a hormogonium repressing factor (HRF) because it suppresses HIF-induced hormogonia formation. Analysis ofN. punctiforme mutants led to proposal of the following model of HRF-dependent modulation of HrmR transcriptional regulation [71]: HRF enters the Nostoc cell and it, or a derivative similar to galacturonate, binds to the repressor protein HrmR, decreasing affinity for the hrmR and hrmE promoter regions. This derepresses transcription of these genes, somehow leading to inhibition of hormogonia formation and return to the vegetative state [72].
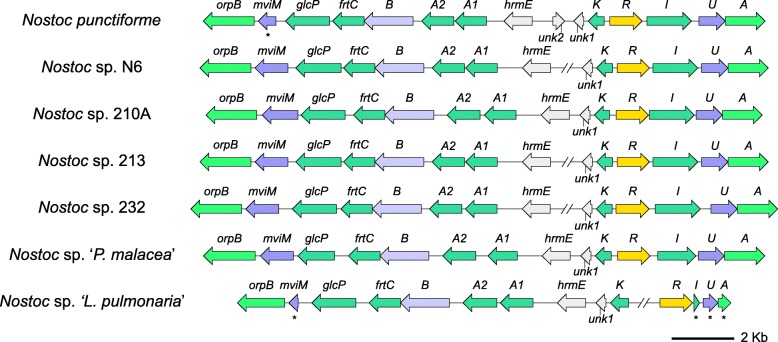
In N. punctiforme the hormogonium regulating locus is linked to genes involved in sugar transport (Fig. 6) [73]. It has been hypothesized that these genes are involved in HRF-induced synthesis of a metabolite inhibitor of hormogonium differentiation, rather than a carbon catabolic function [72]. This metabolite, probably similar to galacturonate [72], binding to the HrmR protein, may act in a positive feedback loop alleviating repression of the hrm locus, leading to increased production of the metabolite and at the same time facilitating increased import of sugars such as glucose, fructose and sucrose. Since PfkA (6-phosphofructokinase) appears to be nonessential in symbiotic Nostoc, these sugars must be channeled through the oxidative pentose phosphate (OPP) pathway or the Entner-Doudoroff (ED) pathway, both producing NADPH reducing equivalents facilitating biosynthesis and decreasing dependence on the non-oxidative pentose phosphate reactions (Calvin cycle). This catabolic shift may simultaneously induce development from hormogonia to vegetative cells and heterocysts. The shift from vegetative cells to heterocysts is accompanied by an increase of the OPP-specific Gnd and an even greater increase in Zwf [74], indicating increased carbon flow via the ED pathway. The hrm locus is restricted to the Nostoc II clade and its sister clade.
Fig. 6.
Hormogonium regulating and sugar transporter loci in symbiotic Nostoc strains. Pseudogenes are denoted with an asterisk. orpB, carbohydrate-selective porin; mviM, inositol-2-dehydrogenase; glpC, glucose permease; frtA1A2BC, ABC-type fructose transporters; hrmE, inositol oxygenase; hrmK, gluconate kinase; hrmR, LacI family transcriptional regulator; hrmI, glucuronate isomerase; hrmU, D-mannonate oxidoreductase; hrmA and unk, unknown. A broken genome line indicates 2 separate loci
D-alanine-D-alanine ligase operon. In addition to a conventional cell-wall specific D-Ala-D-Ala ligase (DdlA), the lichen associated Nostoc strains uniquely harbour another D-Ala-D-Ala ligase, of type 3, thought to be involved in modification of peptide moieties in peptidoglycans as described in Supporting Information (Additional file 2: Figures S14 and S15).
Phosphonate biosynthetic genes.
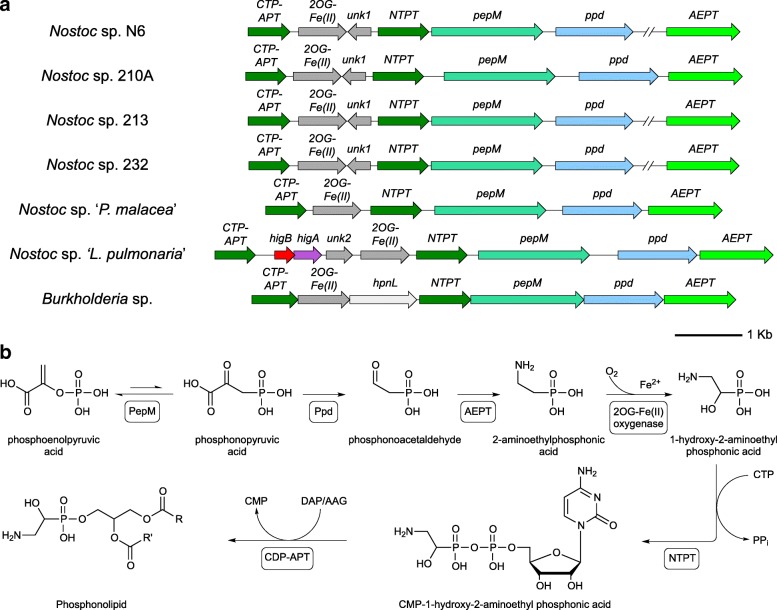
Phosphonates are organophosphorus compounds containing direct carbon-phosphorus bonds, e.g. in phosphonolipids where they can not be cleaved by regular phospholipases. The biochemical pathways and gene clusters for phosphonolipid synthesis are well studied [75], facilitating recognition in new settings as in the case of the lichen-associated Nostoc strains in this study (Fig. 7). This cluster is characteristic of the Nostoc II clade. Extended information is provided in the Supporting Information.
Fig. 7.
Phosphonate biosynthetic gene clusters of lichen cyanobionts (a) and proposed encoded biosynthetic pathway (b) (adapted from [75]). A homologous gene cluster from Burkholderia is shown for comparison. CTP-APT, CDP-alcohol phosphatidyltransferase; OG-Fe(II), 2-oxoglutarate non-heme Fe(II) dependent oxidase; unk, conserved hypothetical proteins; NTPT, NTP transferase; pepM, phosphoenolpyruvate phosphomutase; ppd, phosphonopyruvate decarboxylase; AEPT, 2-aminoethylphosphonate aminotransferase; hpnL, putative membrane protein; higBA, toxin-antitoxin module. A broken genome line indicates separate loci
The additional peptidoglycan and phosphonate lipid functions may lead to cell wall modifications that are well tailored to the intrathalline environment, as well as being recognized as compatible by a mycobiont during establishment of symbiosis. Despite being sheltered by a mycobiont, lichen cyanobionts are subjected to extracellular enzymes and metabolites produced by both the mycobiont and intrathalline bacteria. Therefore, a specific ability to withstand some unfavorable aspects of this cohabitation is expected from lichen associated Nostoc strains.
Chloramphenicol phosphotransferase. Chloramphenicol is an antibiotic produced by Streptomyces venezuelae ATCC 10712 and several other actinomycetes [76]. The bacteriostatic activity of chloramphenicol results from its binding to the 50S subunit of the bacterial ribosome blocking peptidyl transferase [77]. S. venezuelae escapes the toxicity of its own lethal secondary metabolite by expressing a chloramphenicol phosphotransferase (CPT) that phosphorylates the primary (C-3) hydroxyl of chloramphenicol (Additional file 2: Figure S16) [78]. Genes encoding CPT were found almost exclusively in the Nostoc II clade.
Gas vesicles, sulfur metabolism. Genes encoding gas vesicle proteins have been shown to be involved in hormogonium function and establishment of the N. punctiforme symbiosis [79, 80] as well as in the symbiosis of Nostoc with feathermoss [81]. Gas vesicle proteins GvpC, GvpV and GvpW appear to be characteristic for the Nostoc II clade and its sister clade. Several genes associated with assimilation of alkane sulfonates in the moss-Nostoc association [82] were also found to be enriched in the Nostoc II clade.
Sensory mechanisms. All the comparison groups were found to have differences related to sensory mechanisms and motility, including signal transduction histidine kinases, methyl-accepting proteins as well as diguanylate cyclases, thought to be involved in regulating motility in cyanobacteria [83]. The diversity and rapid divergence of sensory mechanisms underlines the great variety of ecotypes found in the genus Nostoc, especially in strains with symbiotic capacity [84]. Differences in genes involved in sensory mechanisms were also found in the comparison made by Warshan et al. [82].
Secondary metabolites
Cyanobacteria produce a multitude of secondary metabolites, many of them toxic [85, 86]. In a recent study, Liaimer et al. [52] found that Nostoc symbionts of the liverwort Blasia pusilla more frequently produce nodularin and microcystin type compounds antagonistic to other Nostoc strains than free living Nostoc from the same locality. Most types of secondary compounds were detected in only 1 to 4 out of the 20 strains examined. The occurrence of the main secondary metabolite pathways in Nostoc punctiforme, in the Nostoc strains from the Blasia habitats [52] and in the lichen-derived strains of the present study shows little overlap. One of the secondary compounds detected by Liaimer et al. [52] is the polyketide synthase plus non-ribosomal peptide synthase (PKS-NRPS) product nosperin [36]. We previously suggested that nosperin might have cytotoxic properties analogous to cyanobiont microcystins [87, 88] which can serve as protective compounds in cyanolichens, e.g. against grazers. Interestingly, Nostoc sp. 232 was found to be devoid of nsp genes encoding nosperin, but it has a putative microcystin gene cluster not found in the nsp containing Nostoc sp. N6 strain. Similarly, the single Blasia-habitat Nostoc strain showing nosperin does not exhibit any of the other metabolites under study [52]. Remnants of the nsp gene cluster were found on the chromosome of the L. pulmonaria cyanobiont (Additional file 2: Figure S19), where almost the entire cluster has been deleted, probably due to the absence of selective pressure.
Whole genome sequencing of the nosperin producer Nostoc sp. N6 revealed that the nsp gene cluster is located on the chromosome. The abundance of insertion sequences surrounding the cluster and the apparent mixed gene origin suggests that it has been acquired as a genomic island through horizontal transfer and undergone several intragenomic recombination events [36]. The genome of Nostoc sp. N6 was also found to encode pathways for the biosynthesis of nostopeptolide- [89] and banyaside/suomilide-like [52] compounds as well as nostocyclopeptide [90] (Additional file 2: Table S14). Nostopeptolide in Nostoc punctiforme has been found to be a major hormogonium-repressing factor and is therefore considered responsible for cellular differentiation of Nostoc [91].
Nostocyclopeptides are cyclic heptapeptides with a unique imino linkage in the macrocyclic ring, isolated from the lichen cyanobiont Nostoc sp. ATCC 53789 [92]. Two homologous NRPS functions (locus tags NPM_1843 and NPM_1844) were found in the genome of Nostoc sp. N6. A nostoclide-like compound with a very similar structure, cyanobacterin, produced by the cyanobacterium Tolypothrix sp. PCC 9009 (Scytonema hofmanni UTEX 2349) [93, 94], was found to inhibit the growth of many cyanobacteria, as well as green algae and angiosperms [95, 96]. Based on the homology with Tolypothrix sp. PCC 9009, we identified putative gene clusters for biosynthesis of nostoclide-like compounds in the genomes of Nostoc spp. 210A and 232 (Additional file 2: Figure S20b). More extensive information on secondary products can be found in the Supporting Information.
Conclusions
The complete genome sequences and comparative genomic analyses of two lichen-associated Nostoc strains are presented here. The finished genomes, manually curated, are appropriate for all types of detailed analyses and act as high-quality references for comparative purposes [97]. Comparative genome analysis of symbiotic and free-living cyanobacteria allowed the identification of several pathways that may contribute to symbiotic competence of Nostoc strains. One pathway, encoded by the hormogonium regulating (hrm) locus, was previously identified in symbiotically competent N. punctiforme and plays a central role in abrogating hormogonia formation. This pathway is similar to pathways of sugar uronate metabolism in heterotrophic non-cyanobacterial prokaryotes [71, 72]. Although the hrm locus has been shown to be important in the Nostoc-plant symbiosis, its presence in all of the lichen-associated Nostoc strains from this study suggests it is also relevant to establishing Nostoc-mycobiont symbioses. Pathways that may be involved in cell wall biogenesis of lichen cyanobionts were also identified, including novel gene clusters encoding synthesis of phosphonate lipids and an MXAN_4097-like amidoligase (D-Ala-D-Ala ligase).
It is apparent that the ability to form and maintain symbiosis is a complex trait governed by many factors and different combinations of these factors may result in different symbiotic associations – from loose to the most intimate. The study presented here is the first attempt to determine, on a whole genome level, what genes and features may contribute to symbiotic competence of Nostoc cyanobionts in lichens. Although we have pinpointed candidate symbiotic genes in the lichen-associated Nostoc genomes, a more thorough analysis, e.g. with targeted mutations and resynthesis of symbiosis, is required to verify the importance and involvement of individual genes and pathways. Some progress has been achieved in studying plant-cyanobacterial symbioses using the readily cultured hornwort Anthoceros and the liverwort Blasia as model organisms. However, there are substantial differences between plant- and mycobiont-cyanobacterial symbioses, e.g. due to the heterotrophic nature of fungi. In contrast to many lichens with green algal photobionts, the bionts of cyanolichens are difficult to culture and synthesize in the laboratory. Problems include slow growth or unculturability of most mycobionts, difficulties in obtaining axenic cultures of photobionts, and in maintaining resynthesized biont cultures for long periods of time. Few attempts have been documented of cyanolichen resynthesis under laboratory conditions [98–103] and currently there are no available models to study mycobiont-Nostoc symbiosis. The use of the glomeromycete Geosiphon pyriforme, which is easily culturable and capable of forming symbiosis with Nostoc strains, can help to overcome some of these limitations [104].
Recent studies of ten genomes and proteomes from moss-associated Nostoc strains compared to the non-symbiotic Nostoc sp. CALU 996, identified a number of gene families present in the symbiotic strains but not in the comparison strain [81], [82]. Several of these, including the hrm locus, genes encoding gas vesicle proteins, genes connected with sulfur metabolism and genes linked to sensory mechanisms were identical or similar to symbiotic-specific gene clusters identified in the lichen-associated Nostoc.
In addition to Nostoc, several other nostocean cyanobacteria have been reported in lichen symbioses. Members of the genera Scytonema, Calothrix, Dichothrix, and Tolypothrix have also been found in lichens as cyanobionts [20, 105, 106]. Isolation and genome sequencing of these lichen-associated strains can add more support and knowledge to our current understanding of what determines symbiotic competence in Nostoc and other cyanobacteria.
Methods
Isolation and culture of Nostoc strains
Peltigera membranacea thalli for cyanobiont isolation were collected from a moss carpet (Hylocomium splendens and Pleurozium schreiberi) at Keldur, Reykjavik, Iceland, and Lobaria pulmonaria thallus was collected from a maple tree trunk (Acer macrophyllum) at Cedar Road, Vancouver Island, British Columbia, Canada. Nostoc strains were isolated on BG-110 agar medium as previously described [107], purified by repeated streaking on the same medium and maintained at room temperature.
DNA extraction, library construction and sequencing
Genomic DNA was prepared from Nostoc cultures grown in liquid BG-110 medium at an illumination of 50 μmol photons m −2 s −1 as described in [108]. Sequencing libraries were prepared using Nextera XT and, for some strains, Nextera Mate Pair Sample Preparation Kits (Illumina) according to the manufacturer’s protocols and sequenced using MiSeq Reagent Kits v2 with 2 ×250 and 2 ×150 cycles, respectively (Additional file 2: Table S1). Roche 454 reads of P. membranacea and P. malacea metagenomes generated previously [109] were also used in this study to increase the number of lichen-associated Nostoc strains.
Genome assembly
Draft assemblies of Nostoc spp. N6 and ‘Lobaria pulmonaria cyanobiont’ genomes were constructed using MIRA v3.2.1 (www.chevreux.org/projects_mira.html) and further processed and verified using GAP5 (Staden package) [110] (Additional file 2: Table S1). Remaining gaps were closed by PCR and Sanger sequencing. Draft genomes of Nostoc spp. 210A, 213, 232 and the P. malacea metagenome were assembled using SPAdes v3.10.1 [111] with default parameters. Prior to assembly Illumina reads were processed with Trimmomatic v0.36 [112] with “LEADING:20 TRAILING:20 SLIDINGWINDOW:4:15 MINLEN:20” parameters. SPAdes contigs >1 kb were binned using MaxBin v2.2.4 [113] and those belonging to Cyanobacteria were scaffolded using BESST v2.2.6 [114, 115]. The resulting assemblies were improved with FinishM v0.0.9 (https://github.com/wwood/finishm) and Pilon v2.11.6 [116]. Scaffolds were taxonomically classified using Kaiju (http://kaiju.binf.ku.dk/) [117] and PhyloPythiaS+ (http://phylopythias.bifo.helmholtz-hzi.de/) [118] web servers. Those not assigned to Cyanobacteria were manually checked using a BLAST search [119], and contaminating scaffolds were removed. Completeness and contamination of the assemblies were assessed with CheckM v1.0.7 [120] (Additional file 2: Table S2).
Genome annotation
Draft genome assemblies were annotated using the NCBI Prokaryotic Genome Annotation Pipeline [121]. For complete genomes ORFs were predicted with Prodigal [122], followed by manual correction in Artemis [123] using the gene prediction improvement pipeline GenePRIMP [124]. All encoded proteins were assigned functions by combining results from InterProScan [125], CDD [126] and BLAST searches [119] against the NCBI nonredundant (nr) database. Transfer RNA genes were identified with tRNAScan-SE-1.23 [127] and ribosomal RNA genes (5S, 16S, 23S) were predicted using RNAmmer [128]. Other non-coding RNAs were identified with Infernal (v.1.1) [129] using RFAM convariance models (http://ftp.ebi.ac.uk/pub/databases/Rfam). Identification of CRISPR elements was performed using CRISPRfinder [130] and PILER-CR [131]. Pseudogenes were annotated using the GenePRIMP pipeline and rechecked manually in Artemis. Single in-frame stop codons and frameshifts were confirmed in the original assemblies. Ribosomal slippage was annotated according to standard operating procedures (SOP) at the GenePRIMP website (http://studylib.net/doc/7260119). Finally, short ORFs (encoding < 100 aa) without any significant homology (E-value >10−2) to the nr database, and ORFs represented solely by low-complexity sequences (e.g. spanning micro- and minisatellite regions) were removed from the annotation. Intein-containing proteins were identified by the presence of an intein/homing endonuclease domain (COG1372). Excision of nifD and fdxN excision elements in Nostoc sp. N6 was confirmed by previously generated RNA-Seq data [36] mapped with Bowtie 2 [132]. Origins of replication (oriC) were identified by locating DnaA boxes (TT A/ TTNCACA) [133]. The location of a cluster of DnaA boxes, especially adjacent to dnaA and/or dnaN genes, is considered an indicator for the location of oriC. Transposases were classified into IS families using ISfinder (https://www-is.biotoul.fr/; [134]).
Phylogenomic analysis
Available genomes of Nostocales strains along with Arthrospira platensis NIES-39, Lyngbya sp. PCC 8106 and Planktothrix agardhii NIVA-CYA 126/8 (order Oscillatoriales) as an outgroup were retrieved from GenBank and the Joint Genome Institute’s Integrated Microbial Genomes database (JGI-IMG) in January 2018. Thirty-one marker proteins that are universally conserved across the bacterial domain (dnaG, frr, infC, nusA, pgk, pyrG, rplA, rplB, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoB, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB and tsf) were extracted from the genomes using the AMPHORA2 pipeline [135] and aligned with MUSCLE [136]. An alignment mask was generated using Zorro [137]. The marker alignments were further concatenated into a single partitioned alignment and the best protein substitution model for each of the markers was predicted using the concat_align.pl script of phylogenomics-tools (https://github.com/kbseah/phylogenomics-tools; 10.5281/zenodo.46122). A maximum-likelihood phylogeny was derived using the PROTCATWAG model for tree search in RAxML v8.2.4 [138] automated by the tree_calculations.pl script of phylogenomics-tools. Branch support was assessed using the approximate likelihood ratio test for branches (SH-like aLRT) [139] with 100 replicates.
Genome and proteome comparison
Whole genome comparisons were performed using PROmer (MUMmer 3.0 package; [140]) and Mauve [141]. To identify orthologous groups specific to different clades (Fig. 4) an all-by-all BLASTP search was performed on proteomes of 56 strains belonging to a) Nostoc I clade (16 strains), b) Nostoc II clade (27 strains), c) sister clade to Nostoc II clade (13 strains) with soft masking and thresholds: E-value < 10−10, percentage identity 50% and percentage match 50%. The resulting hits were clustered into orthologous groups using OrthoMCL [142, 143]. Orthologous groups specific to different clades were extracted as shown in Additional file 2: Figure S21. For COG category distribution comparison proteins encoded in the genomes of selected Nostoc and Anabaena strains were classified into COG functional categories [144] using RPS-BLAST against PSSMs (Position-Specific Scoring Matrices) from the updated COG database [145] with an E-value < 10−2 and the top hit retained.
Additional files
Nostocales strains used in this study. (XLS 44 kb)
Supporting Information (SI) Appendix. (PDF 8156 kb)
Proteins encoded in 10 most prominent locally collinear blocks in Nostoc punctiforme PCC 73102, Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’, identified by Mauve. (XLS 84 kb)
Comparison of the minimal bacterial gene set to Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’. (XLS 61 kb)
Comparison of the cyanobacterial core and shell gene set to Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’. (XLS 172 kb)
Orthologous groups enriched in different clades. (XLS 756 kb)
Acknowledgements
The authors are grateful to Ólafur Þ. Magnússon (deCODE genetics, Inc.) for technical support, and to Vivian Miao, Denis Warshan and anonymous reviewers for comments.
Funding
This research was funded by the Icelandic Research Fund RANNIS (project number 134055-051) and by the University of Iceland. Sample collection, study design, analysis, data interpretation and manuscript writing was carried out by the authors.
Availability of data and materials
The genomic sequences generated and analyzed in this study were deposited in the GenBank database under Bioproject numbers PRJNA279350 (Nostoc sp. N6), PRJNA275880 (Nostoc sp. ‘L. pulmonaria’), PRJNA389199 (Nostoc sp. 210A), PRJNA389200 (Nostoc sp. 213), PRJNA389202 (Nostoc sp. 232) and PRJNA389205 (Nostoc sp. ‘P. malacea’). Additional accession numbers and data can be found in Supporting Information.
Abbreviations
- AAG
Alkyl-acylglycerol
- ATP
Adenosine triphosphate
- CAAD
Cyanobacterial Aminoacyl-tRNA synthetase domain
- CDP
Cytidine Diphosphate
- CDP-APT
CDP-alcohol Phosphatidyltransferase
- CMP
Cytidine monophosphate
- COG
Clusters of Orthologous group
- CPT
Chloramphenicol Phosphotransferase
- CTP
Cytidine triphosphate
- DAG
Ddiacylglycerol
- dCMP
Deoxycytidine monophosphate
- dCTP
Deoxycytidine triphosphate
- dNTP
Nucleoside triphosphate
- dTTP
Thymidine triphosphate
- dUMP
Deoxyuridine monophosphate
- dUTP
Uridine diphosphate
- ED
Entner–Doudoroff
- FAD
Flavin adenine dinucleotide
- Gnd
6-Gluconophosphate dehydrogenase
- HAEP
1-hydroxy-2-aminoethylphosphonate
- HIF
Hormogonium inducing factor
- HRF
Hormogonium repressing factor
- IS
Insertion sequence
- KEGG
Kyoto encyclopedia of genes and genomes
- LAM
Lysine 2,3-Aminomutase
- NADP
Nicotinamide adenine dinucleotide phosphate
- NNI
Nearest neighbor interchange
- NRPS
Non-ribosomal peptide synthetase
- OPP
Oxidative pentose pathway
- ORF
Open reading frame
- PEP
Phosphoenolpyruvate
- PepM
Phosphoenolpyruvate mutase
- PKS
Polyketide synthase
- Ppd
Phosphonopyruvate decarboxylase
- PSSM
Position-specific scoring matrix
- RNR
Ribonucleoside reductase
- SET
Lysine Methyltransferase
- UDP
Uridine diphosphate
- UV
Ultraviolet
- Zwf
Glucose-6-phosphate 1-Dehydrogenase
Authors’ contributions
ANG isolated Nostoc strains, performed DNA isolation, library construction and whole genome sequencing, carried out genome assembly and annotation. ANG and ÓSA analyzed data and wrote the paper. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
All samples were collected in Iceland with appropriate permissions and in collaboration with the Icelandic Institute of Natural History.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4743-5) contains supplementary material, which is available to authorized users.
Contributor Information
Andrey N. Gagunashvili, Email: a.gagunashvili@ucl.ac.uk
Ólafur S. Andrésson, Email: osa@hi.is
References
- 1.Nash TH. Lichen Biology. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 2.Rikkinen J. Cyanolichens: an evolutionary overview. In: Rai AK, editor. Cyanobacteria in Symbiosis. New York: Springer; 2002. [Google Scholar]
- 3.Gargas A, DePriest PT, Grube M, Tehler A. Multiple origins of lichen symbioses in fungi suggested by ssu rdna phylogeny. Science. 1995;268(5216):1492–5. doi: 10.1126/science.7770775. [DOI] [PubMed] [Google Scholar]
- 4.Aptroot A. Aspects of the integration of the taxonomy of lichenized and non-lichenized pyrenocarpous ascomycetes. The Lichenologist. 1998;30(4-5):501–14. doi: 10.1006/lich.1998.0151. [DOI] [Google Scholar]
- 5.Lutzoni F, Pagel M, Reeb V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature. 2001;411(6840):937–40. doi: 10.1038/35082053. [DOI] [PubMed] [Google Scholar]
- 6.Dal Grande F, Widmer I, Wagner HH, Scheidegger C. Vertical and horizontal photobiont transmission within populations of a lichen symbiosis. Molecular Ecology. 2012;21(13):3159–72. doi: 10.1111/j.1365-294X.2012.05482.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien HE, Miadlikowska J, Lutzoni F. Assessing reproductive isolation in highly diverse communities of the lichen-forming fungal genus Peltigera. Evolution. 2009;63(8):2076–86. doi: 10.1111/j.1558-5646.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien HE, Miadlikowska J, Lutzoni F. Assessing population structure and host specialization in lichenized cyanobacteria. New Phytologist. 2013;198(2):557–66. doi: 10.1111/nph.12165. [DOI] [PubMed] [Google Scholar]
- 9.Crittenden P, David J, Hawksworth D, Campbell F. Attempted isolation and success in the culturing of a broad spectrum of lichen-forming and lichenicolous fungi. New Phytologist. 1995;130:267–97. doi: 10.1111/j.1469-8137.1995.tb03048.x. [DOI] [Google Scholar]
- 10.Stocker-Wörgötter E, Hager A. Culture methods for lichens and lichen symbionts. In: Nash TH, editor. Lichen Biology. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 11.Ahmadjian V. The Lichen Symbiosis. Hoboken: Wiley; 1993. [Google Scholar]
- 12.Rikkinen J. Molecular studies on cyanobacterial diversity in lichen symbioses. MycoKeys. 2013;6:3–32. doi: 10.3897/mycokeys.6.3869. [DOI] [Google Scholar]
- 13.Waterbury J. B. The cyanobacteria—isolation, purification and identification. In: Dworkin M, Falkow S, editors. The Prokaryotes: Vol. 4: Bacteria: Firmicutes, Cyanobacteria. New York: Springer; 2006. [Google Scholar]
- 14.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111(1):1–61. [Google Scholar]
- 15.Rajaniemi P, Hrouzek P, Kaštovska K, Willame R, Rantala A, Hoffmann L, Komárek J, Sivonen K. Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (nostocales, cyanobacteria) Int J Syst Evol Microbiol. 2005;55(1):11–26. doi: 10.1099/ijs.0.63276-0. [DOI] [PubMed] [Google Scholar]
- 16.Lachance M-A. Genetic relatedness of heterocystous cyanobacteria by deoxyribonucleic acid-deoxyribonucleic acid reassociation. Int J Syst Bacteriol. 1981;31(2):139–47. doi: 10.1099/00207713-31-2-139. [DOI] [Google Scholar]
- 17.Rippka R. Recognition and identification of cyanobacteria. Methods Enzymol. 1988;167:28–67. doi: 10.1016/0076-6879(88)67005-4. [DOI] [PubMed] [Google Scholar]
- 18.Dvořák P, Casamatta DA, Hašler P, Jahodářová E, Norwich AR, Poulíčková A. Modern Topics in the Phototrophic Prokaryotes. New York: Springer; 2017. Diversity of the cyanobacteria. [Google Scholar]
- 19.Bergman B, Hällbom L. Nostoc of Peltigera canina when lichenized and isolated. Can J Botany. 1982;60(10):2092–8. doi: 10.1139/b82-256. [DOI] [Google Scholar]
- 20.Tschermak-Woess E. The algal partner. In: Galun M, editor. CRC Handbook of Lichenology. Boca Raton: CRC Press Inc.; 1988. [Google Scholar]
- 21.Koriem A, Ahmadjian V. An ultrastructural-study of lichenized and cultured nostoc photobionts of Peltigera canina, Peltigera rufescens, and Peltigera spuria. Endocytobiosis and Cell Research. 1986;3(1):65–78. [Google Scholar]
- 22.Boissière M-C. Ultrastructural evidence for polyglucosidic reserves in Nostoc cells in Peltigera and Collema and the effect of thallus hydratation. Bibliotheca Lichenologica. 1987;25:109–16. [Google Scholar]
- 23.Bergman B, Rai AN, Rasmussen U. Cyanobaterial associations In: Elmerich C, William Edward Newton WE, editors. Associative and Endophytic Nitrogen-fixing Bacteria and Cyanobacterial Associations. Springer: 2007. p. 257–301.
- 24.Paulsrud P, Rikkinen J, Lindblad P. Spatial patterns of photobiont diversity in some Nostoc-containing lichens. New Phytologist. 2000;146(2):291–9. doi: 10.1046/j.1469-8137.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- 25.Paulsrud P, Rikkinen J, Lindblad P. Cyanobiont specificity in some Nostoc-containing lichens and in a Peltigera aphthosa photosymbiodeme. New Phytologist. 1998;139(3):517–24. doi: 10.1046/j.1469-8137.1998.00220.x. [DOI] [Google Scholar]
- 26.Paulsrud P, Lindblad P. Sequence variation of the trnaleu intron as a marker for genetic diversity and specificity of symbiotic cyanobacteria in some lichens. Appl Environ Microbiol. 1998;64(1):310–5. doi: 10.1128/aem.64.1.310-315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rikkinen J, Oksanen I, Lohtander K. Lichen guilds share related cyanobacterial symbionts. Science. 2002;297(5580):357. doi: 10.1126/science.1072961. [DOI] [PubMed] [Google Scholar]
- 28.Lohtander K, Oksanen I, Rikkinen J. Genetic diversity of green algal and cyanobacterial photobionts in Nephroma (Peltigerales) Lichenologist. 2003;35(4):325–39. doi: 10.1016/S0024-2829(03)00051-3. [DOI] [Google Scholar]
- 29.O’Brien H, Miadlikowska J, Lutzoni F. Assessing host specialization in symbiotic cyanobacteria associated with four closely related species of the lichen fungus Peltigera. European J Phycology. 2005;40(4):363–78. doi: 10.1080/09670260500342647. [DOI] [Google Scholar]
- 30.Warshan D. Cyanobacteria in symbiosis with boreal forest feathermosses: from genome evolution and gene regulation to impact on the ecosystem. PhD thesis. 2017.
- 31.Meeks JC. The genome of the filamentous cyanobacterium Nostoc punctiforme, what can we learn from it about free-living and symbiotic nitrogen fixation? In: Palacios R, Newton WE, editors. Genomes and Genomics of Nitrogen-fixing Organisms. Nitrogen Fixation: Origins, Applications, and Research Progress. Springer: 2005. p. 27–70.
- 32.Cohen MF, Wallis JG, Campbell EL, Meeks JC. Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology. 1994;140(12):3233–40. doi: 10.1099/13500872-140-12-3233. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MF, Meeks JC, Cai YA, Wolk CP. Transposon mutagenesis of heterocyst-forming filamentous cyanobacteria. Methods Enzymol. 1998;297:3–17. doi: 10.1016/S0076-6879(98)97003-3. [DOI] [Google Scholar]
- 34.Cai Y, Wolk C. P. Use of a conditionally lethal gene in Anabaena sp. strain pcc 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990;172(6):3138–45. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams D. G, Duggan P. S. Cyanobacteria–bryophyte symbioses. J Exp Botany. 2008;59(5):1047–58. doi: 10.1093/jxb/ern005. [DOI] [PubMed] [Google Scholar]
- 36.Kampa A, Gagunashvili AN, Gulder TAM, Morinaka BI, Daolio C, Godejohann M, Miao VPW, Piel J, Andresson OS. Metagenomic natural product discovery in lichen provides evidence for a family of biosynthetic pathways in diverse symbioses. Proc Nat Acad Sci USA. 2013;110(33):3129–37. doi: 10.1073/pnas.1305867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meinhardt F, Schaffrath R, Larsen M. Microbial linear plasmids. Appl Microbiol Biotechnol. 1997;47(4):329–36. doi: 10.1007/s002530050936. [DOI] [PubMed] [Google Scholar]
- 38.Welsh EA, Liberton M, Stoeckel J, Loh T, Elvitigala T, Wang C, Wollam A, Fulton RS, Clifton SW, Jacobs JM, Aurora R, Ghosh BK, Sherman LA, Smith RD, Wilson RK, Pakrasi HB. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc Natl Acad Sci USA. 2008;105(39):15094–9. doi: 10.1073/pnas.0805418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiel T, Pratte BS, Zhong J, Goodwin L, Copeland A, Lucas S, Han C, Pitluck S, Land ML, Kyrpides NC, Woyke T. Complete genome sequence of Anabaena variabilis ATCC 29413. Standards Genomic Sci. 2014;9(3):562–73. doi: 10.4056/sigs.3899418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crick F. Codon-anticodon pairing: the wobble hypothesis. J Mole Biol. 1965;19(2):548–55. doi: 10.1016/S0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 41.Bailly-Bechet M, Vergassola M, Rocha E. Causes for the intriguing presence of tRNAs in phages. Genome Research. 2007;17(10):1486–95. doi: 10.1101/gr.6649807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enav H, Beja O, Mandel-Gutfreund Y. Cyanophage tRNAs may have a role in cross-infectivity of oceanic Prochlorococcus and Synechococcus hosts. ISME J. 2012;6(3):619–28. doi: 10.1038/ismej.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravin V, Ravin N, Casjens S, Ford M, Hatfull G, Hendrix R. Genomic sequence and analysis of the atypical temperate bacteriophage N15. J Mol Biol. 2000;299(1):53–73. doi: 10.1006/jmbi.2000.3731. [DOI] [PubMed] [Google Scholar]
- 44.Hertwig S, Klein I, Lurz R, Lanka E, Appel B. PY54, a linear plasmid prophage of Yersinia enterocolitica with covalently closed ends. Molecular Microbiology. 2003;48(4):989–1003. doi: 10.1046/j.1365-2958.2003.03458.x. [DOI] [PubMed] [Google Scholar]
- 45.Casjens S, Gilcrease E, Huang W, Bunny K, Pedulla M, Ford M, Houtz J, Hatfull G, Hendrix R. The pKO2 linear plasmid prophage of Klebsiella oxytoca. J Bacteriol. 2004;186(6):1818–32. doi: 10.1128/JB.186.6.1818-1832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bignell C, Thomas C. The bacterial ParA-ParB partitioning proteins. J Biotechnol. 2001;91(1):1–34. doi: 10.1016/S0168-1656(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 47.Moller-Jensen J, Borch J, Dam M, Jensen R, Roepstorff P, Gerdes K. Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Molecular Cell. 2003;12(6):1477–87. doi: 10.1016/S1097-2765(03)00451-9. [DOI] [PubMed] [Google Scholar]
- 48.Schirrmeister BE, Dalquen DA, Anisimova M, Bagheri H. C. Gene copy number variation and its significance in cyanobacterial phylogeny. BMC Microbiology. 2012; 12. 10.1186/1471-2180-12-17. [DOI] [PMC free article] [PubMed]
- 49.Mackiewicz P, Zakrzewska-Czerwinska J, Zawilak A, Dudek M, Cebrat S. Where does bacterial replication start? Rules for predicting the oriC region. Nucleic Acids Res. 2004;32(13):3781–91. doi: 10.1093/nar/gkh699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao F, Zhang C-T. Origins of replication in Cyanothece 51142. Proc Natl Acad Sci USA. 2008;105(52):125. doi: 10.1073/pnas.0809987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Chen W-L, Wang L, Zhang C-C. Identification of the oriC region and its influence on heterocyst development in the filamentous cyanobacterium Anabaena sp. strain PCC 71 20. Microbiology. 2011;157(7):1910–9. doi: 10.1099/mic.0.047241-0. [DOI] [PubMed] [Google Scholar]
- 52.Liaimer A, Jensen JB, Dittmann E. A genetic and chemical perspective on symbiotic recruitment of cyanobacteria of the genus Nostoc into the host plant Blasia pusilla L. Front Microbiol. 2016;7:1–16. doi: 10.3389/fmicb.2016.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svenning MM, Eriksson T, Rasmussen U. Phylogeny of symbiotic cyanobacteria within the genus Nostoc based on 16S rDNA sequence analyses. Arch Microbiol. 2005;183(1):19–26. doi: 10.1007/s00203-004-0740-y. [DOI] [PubMed] [Google Scholar]
- 54.Ran L, Larsson J, Vigil-Stenman T, Nylander JAA, Ininbergs K, Zheng W-W, Lapidus A, Lowry S, Haselkorn R, Bergman B. Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLOS ONE. 2010; 5(7). 10.1371/journal.pone.001148. [DOI] [PMC free article] [PubMed]
- 55.Wang H, Sivonen K, Rouhiainen L, Fewer DP, Lyra C, Rantala-Ylinen A, Vestola J, Jokela J, Rantasarkka K, Li Z, Liu B. Genome-derived insights into the biology of the hepatotoxic bloom-forming cyanobacterium Anabaena sp. strain 90. BMC Genomics. 2012; 13. 10.1186/1471-2164-13-61. [DOI] [PMC free article] [PubMed]
- 56.Haselwandter K, Winkelmann G. Siderophores of symbiotic fungi. In: Varma A, Chincholkar SB, editors. Microbial Siderophores. New York: Springer; 2007. [Google Scholar]
- 57.Singh SP, Häder D-P, Sinha RP. Cyanobacteria and ultraviolet radiation (uvr) stress: mitigation strategies. Ageing Res Rev. 2010;9(2):79–90. doi: 10.1016/j.arr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Soule T, Stout V, Swingley WD, Meeks JC, Garcia-Pichel F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC 29133. J Bacteriol. 2007;189(12):4465–72. doi: 10.1128/JB.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soule T, Garcia-Pichel F, Stout V. Gene expression patterns associated with the biosynthesis of the sunscreen scytonemin in Nostoc punctiforme ATCC 29133 in response to UVA radiation. J Bacteriol. 2009;191(14):4639–46. doi: 10.1128/JB.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soule T, Palmer K, Gao Q, Potrafka RM, Stout V, Garcia-Pichel F. A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genomics. 2009a; 10(336). 10.1186/1471-2164-10-33. [DOI] [PMC free article] [PubMed]
- 61.Malla S, Sommer MOA. A sustainable route to produce the scytonemin precursor using Escherichia coli. Green Chemistry. 2014;16(6):3255–65. doi: 10.1039/C4GC00118D. [DOI] [Google Scholar]
- 62.Gogarten J, Senejani A, Zhaxybayeva O, Olendzenski L, Hilario E. Inteins: structure, function, and evolution. Annu Rev Microbiol. 2002;56:263–87. doi: 10.1146/annurev.micro.56.012302.160741. [DOI] [PubMed] [Google Scholar]
- 63.Hodkinson BP, Allen JL, Forrest LL, Goffinet B, Sérusiaux E, Andrésson ÓS, Miao V, Bellenger J-P, Lutzoni F. Lichen-symbiotic cyanobacteria associated with Peltigera have an alternative vanadium-dependent nitrogen fixation system. European J Phycol. 2014;49(1):11–9. doi: 10.1080/09670262.2013.873143. [DOI] [Google Scholar]
- 64.Miller R, Eady R. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum, low temperature favours n 2 reduction by vanadium nitrogenase. Biochem J. 1988;256(2):429–32. doi: 10.1042/bj2560429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olmedo-Verd E. Santamaría-Gómez J. de Alda JAO. de Pouplana LR. Luque I. Membrane anchoring of aminoacyl-trna synthetases by convergent acquisition of a novel protein domain. J Biol Chem. 2011;286(47):41057–68. doi: 10.1074/jbc.M111.242461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gil R, Silva F, Pereto J, Moya A. Determination of the core of a minimal bacterial gene set. Microbiol Mole Biol Rev. 2004;68(3):518–37. doi: 10.1128/MMBR.68.3.518-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi T, Falkowski PG. Genome evolution in cyanobacteria: the stable core and the variable shell, Vol. 105; 2008. pp. 2510–5. 10.1073/pnas.071116510. [DOI] [PMC free article] [PubMed]
- 68.Meeks JC, Elhai J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mole Biol Rev. 2002;66(1):94–121. doi: 10.1128/MMBR.66.1.94-121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enderlin C, Meeks J. Pure culture and reconstitution of the Anthoceros-Nostoc symbiotic association. Planta. 1983;158(2):157–65. doi: 10.1007/BF00397709. [DOI] [PubMed] [Google Scholar]
- 70.Johansson C, Bergman B. Reconstitution of the symbiosis of Gunnera manicata Linden: cyanobacterial specificity. New Phytologist. 1994;:643–52.
- 71.Cohen M, Meeks J. A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Molecular Plant-Microbe Interactions. 1997;10(2):280–9. doi: 10.1094/MPMI.1997.10.2.280. [DOI] [PubMed] [Google Scholar]
- 72.Campbell E, Wong F, Meeks J. DNA binding properties of the HrmR protein of Nostoc punctiforme responsible for transcriptional regulation of genes involved in the differentiation of hormogonia. Mole Microbiol. 2003;47(2):573–82. doi: 10.1046/j.1365-2958.2003.03320.x. [DOI] [PubMed] [Google Scholar]
- 73.Ekman M, Picossi S, Campbell E. L, Meeks J. C, Flores E. A Nostoc punctiforme sugar transporter necessary to establish a cyanobacterium-plant symbiosis. Plant Physiology. 2013;161(4):1984–92. doi: 10.1104/pp.112.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ow SY, Noirel J, Cardona T, Taton A, Lindblad P, Stensjö K, Wright PC. Quantitative overview of N 2 fixation in Nostoc punctiforme ATCC 29133 through cellular enrichments and iTRAQ shotgun proteomics. J Proteome Res. 2008;8(1):187–98. doi: 10.1021/pr800285v. [DOI] [PubMed] [Google Scholar]
- 75.Yu X, Doroghazi JR, Janga SC, Zhang JK, Circello B, Griffin BM, Labeda DP, Metcalf WW. Diversity and abundance of phosphonate biosynthetic genes in nature. Proc Natl Acad Sci USA. 2013;110(51):20759–64. doi: 10.1073/pnas.1315107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vining L, Stuttard C. Chloramphenicol. Biotechnology. 1995;28:505–30. doi: 10.1016/b978-0-7506-9095-9.50028-9. [DOI] [PubMed] [Google Scholar]
- 77.Vining L, Westlake D. Chloramphenicol: properties, biosynthesis, and fermentation. In: Vandamme E, editor. Biotechnology of Industrial Antibiotics. New York: Marcel Dekker; 1984. [Google Scholar]
- 78.Mosher RH, Camp DJ, Yang K, Brown MP, Shaw WV, Vining LC. Inactivation of chloramphenicol by O-phosphorylation a novel resistance mechanism in Streptomyces venezuelae ISP5230, a chloramphenicol producer. J Biol Chem. 1995;270(45):27000–6. doi: 10.1074/jbc.270.45.27000. [DOI] [PubMed] [Google Scholar]
- 79.Campbell EL, Christman H, Meeks JC. Dna microarray comparisons of plant factor-and nitrogen deprivation-induced hormogonia reveal decision-making transcriptional regulation patterns in Nostoc punctiforme. J Bacteriol. 2008;190(22):7382–91. doi: 10.1128/JB.00990-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Risser DD, Chew WG, Meeks JC. Genetic characterization of the hmp locus, a chemotaxis-like gene cluster that regulates hormogonium development and motility in Nostoc punctiforme. Mole Microbiol. 2014;92(2):222–33. doi: 10.1111/mmi.12552. [DOI] [PubMed] [Google Scholar]
- 81.Warshan D, Espinoza JL, Stuart RK, Richter RA, Kim S-Y, Shapiro N, Woyke T, Kyrpides NC, Barry K, Singan V, et al. Feathermoss and epiphytic Nostoc cooperate differently: expanding the spectrum of plant–cyanobacteria symbiosis. ISME J. 2017;11(12):2821. doi: 10.1038/ismej.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warshan D, Liaimer A, Pederson E, Kim S-Y, Shapiro N, Woyke T, Altermark B, Pawlowski K, Weyman PD, Dupont CL, Rasmussen U. Genomic changes associated with the evolutionary transitions of Nostoc to a plant symbiont. Mole Biol Evol. 2018; 029. 10.1093/molbev/msy029. [DOI] [PMC free article] [PubMed]
- 83.Schuergers N, Mullineaux CW, Wilde A. Cyanobacteria in motion. Current Opinion Plant Biol. 2017;37:109–15. doi: 10.1016/j.pbi.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 84.Joneson S, O’Brien H. A molecular investigation of free-living and lichenized Nostoc sp. and symbiotic lifestyle determination. Bryologist. 2017;120(4):371–81. doi: 10.1639/0007-2745-120.4.371. [DOI] [Google Scholar]
- 85.Dittmann E, Gugger M, Sivonen K, Fewer D. P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015;23(10):642–52. doi: 10.1016/j.tim.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Pearson LA, Dittmann E, Mazmouz R, Ongley SE, D’Agostino PM, Neilan BA. The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria. Harmful Algae. 2016;54:98–111. doi: 10.1016/j.hal.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Kaasalainen U, Jokela J, Fewer DP, Sivonen K, Rikkinen J. Microcystin production in the tripartite cyanolichen Peltigera leucophlebia. Molecular Plant-Microbe Interactions. 2009;22(6):695–702. doi: 10.1094/MPMI-22-6-0695. [DOI] [PubMed] [Google Scholar]
- 88.Kaasalainen U, Fewer DP, Jokela J, Wahlsten M, Sivonen K, Rikkinen J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proc Natl Acad Sci USA. 2012;109(15):5886–91. doi: 10.1073/pnas.1200279109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoffmann D, Hevel JM, Moore RE, Moore BS. Sequence analysis and biochemical characterization of the nostopeptolide A biosynthetic gene cluster from Nostoc sp. GSV224. Gene. 2003;311:171–80. doi: 10.1016/S0378-1119(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 90.Becker JE, Moore RE, Moore BS. Cloning, sequencing, and biochemical characterization of the nostocyclopeptide biosynthetic gene cluster: molecular basis for imine macrocyclization. Gene. 2004;325:35–42. doi: 10.1016/j.gene.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 91.Liaimer A, Helfrich EJ, Hinrichs K, Guljamow A, Ishida K, Hertweck C, Dittmann E. Nostopeptolide plays a governing role during cellular differentiation of the symbiotic cyanobacterium Nostoc punctiforme. Proc Natl Acad Sci USA. 2015;112(6):1862–7. doi: 10.1073/pnas.1419543112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Golakoti T, Yoshida WY, Chaganty S, Moore RE. Isolation and structure determination of nostocyclopeptides A1 and A2 from the terrestrial cyanobacterium Nostoc sp. ATCC53789. J Nat Prod. 2001;64(1):54–9. doi: 10.1021/np000316k. [DOI] [PubMed] [Google Scholar]
- 93.Mason C, Edwards K, Carlson R, Pignatello J, Gleason F, Wood J. Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium Scytonema hofmanni. Science. 1982;215(4531):400–2. doi: 10.1126/science.6800032. [DOI] [PubMed] [Google Scholar]
- 94.Pignatello JJ, Porwoll J, Carlson RE, Xavier A, Gleason FK, Wood JM. Structure of the antibiotic cyanobacterin, a chlorine-containing γ-lactone from the freshwater cyanobacterium Scytonema hofmanni. J Org Chem. 1983;48(22):4035–8. doi: 10.1021/jo00170a032. [DOI] [Google Scholar]
- 95.Gleason FK, Baxa CA. Activity of the natural algicide, cyanobacterin, on eukaryotic microorganisms. FEMS Microbiol Letters. 1986;33(1):85–8. doi: 10.1111/j.1574-6968.1986.tb01217.x. [DOI] [Google Scholar]
- 96.Gleason FK, Case DE. Activity of the natural algicide, cyanobacterin, on angiosperms. Plant Physiology. 1986;80(4):834–7. doi: 10.1104/pp.80.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chain P, Grafham D, Fulton R, Fitzgerald M, Hostetler J, Muzny D, Ali J, Birren B, Bruce D, Buhay C, et al. Genome project standards in a new era of sequencing. Science. 2009;326(5950):236–7. doi: 10.1126/science.1180614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmadjian V. Studies on the isolation and synthesis of bionts of the cyanolichen Peltigera canina (Peltigeraceae) Plant Systematics and Evolution. 1989;165(1–2):29–38. doi: 10.1007/BF00936032. [DOI] [Google Scholar]
- 99.Stocker-Wörgötter E, Türk R. Artificial resynthesis of thalli of the cyanobacterial lichen Peltigera praetextata under laboratory conditions. Lichenologist. 1991;23(02):127–38. doi: 10.1017/S0024282991000294. [DOI] [Google Scholar]
- 100.Yoshimura I, Kurokawa T, Yamamoto Y, Kinoshita Y. Development of lichen thalli in vitro. Bryologist. 1993;96:412–21. doi: 10.2307/3243871. [DOI] [Google Scholar]
- 101.Stocker-Worgötter E, Türk R. Artificial resynthesis of the photosymbiodeme Peltigera leucophlebia under laboratory conditions. Cryptogamic Botany. 1994;4:300–8. [Google Scholar]
- 102.Yoshimura I, Kurokawa T, Yamamoto Y, Kinoshita Y. In vitro development of the lichen thallus of some species of Peltigera. Cryptogamic Botany. 1994;4:314. [Google Scholar]
- 103.Stocker-Wörgötter E. Experimental cultivation of lichens and lichen symbionts. Can J Botany. 1995;73(S1):579–89. doi: 10.1139/b95-298. [DOI] [Google Scholar]
- 104.Kluge M, Mollenhauer D, Mollenhauer R. Geosiphon pyriforme (kützing) von wettstein, a promising system for studying endocyanoses. In: Progress in Botany. Springer: 1994. p. 130–41.
- 105.Rai AN, et al. Handbook of Symbiotic Cyanobacteria. Boca Raton: CRC Press, Inc; 1990. [Google Scholar]
- 106.Friedl T, Büdel B. Photobionts. In: Nash TH, editor. Lichen Biology. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 107.Yoshimura I, Yamamoto Y, Nakano T, Finnie J. Isolation and culture of lichen photobionts and mycobionts. In: Kranner I, Beckett R, editors. Protocols in Lichenology—Culturing, Biochemistry, Physiology and Use in Biomonitoring. New York: Springer; 2002. [Google Scholar]
- 108.Nilsson M, Rasmussen U, Bergman B. Cyanobacterial chemotaxis to extracts of host and nonhost plants. FEMS Microbiol Ecol. 2006;55(3):382–90. doi: 10.1111/j.1574-6941.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 109.Xavier BB, Miao VPW, Jonsson ZO, Andresson OS. Mitochondrial genomes from the lichenized fungi Peltigera membranacea and Peltigera malacea: Features and phylogeny. Fungal Biol. 2012;116(7):802–14. doi: 10.1016/j.funbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 110.Bonfield JK, Whitwham A. Gap5 – editing the billion fragment sequence assembly. Bioinformatics. 2010;26(14):1699–1703. doi: 10.1093/bioinformatics/btq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. Spades: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Y-W, Tang Y-H, Tringe SG, Simmons BA, Singer SW. Maxbin: an automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome. 2014;2(1):26. doi: 10.1186/2049-2618-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sahlin K, Vezzi F, Nystedt B, Lundeberg J, Arvestad L. Besst – efficient scaffolding of large fragmented assemblies. BMC Bioinformatics. 2014;15(1):281. doi: 10.1186/1471-2105-15-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sahlin K, Chikhi R, Arvestad L. Assembly scaffolding with pe-contaminated mate-pair libraries. Bioinformatics. 2016;32(13):1925–32. doi: 10.1093/bioinformatics/btw064. [DOI] [PubMed] [Google Scholar]
- 116.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PloS ONE. 2014;9(11):112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menzel P, Ng K. L, Krogh A. Fast and sensitive taxonomic classification for metagenomics with kaiju. Nature Communications. 2016;7. [DOI] [PMC free article] [PubMed]
- 118.Patil K. R, Roune L, McHardy A. C. The phylopythias web server for taxonomic assignment of metagenome sequences. PloS ONE. 2012;7(6):38581. doi: 10.1371/journal.pone.0038581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. Checkm: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–55. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. Ncbi prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44(14):6614–24. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010; 11. 10.1186/1471-2105-11-11. [DOI] [PMC free article] [PubMed]
- 123.Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2012;28(4):464–9. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nature Methods. 2010;7(6):455–62. doi: 10.1038/nmeth.1457. [DOI] [PubMed] [Google Scholar]
- 125.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong S-Y, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–40. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013;41(D1):348–52. doi: 10.1093/nar/gks1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lowe T, Eddy S. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–64. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lagesen K, Hallin P, Rodland EA, Staerfeldt H-H, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–8. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29(22):2933–5. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grissa I, Vergnaud G, Pourcel C. CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Research. 2007;35(S):52–7. doi: 10.1093/nar/gkm360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edgar RC. PILER-CR: Fast and accurate identification of CRISPR repeats. BMC Bioinformatics. 2007; 8. 10.1186/1471-2105-8-1. [DOI] [PMC free article] [PubMed]
- 132.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–4. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schaper S, Messer W. interaction of the initiator protein DnaA of Escherichia coli with its DNA target. J Biol Chem. 1995;270(29):17622–6. doi: 10.1074/jbc.270.29.17622. [DOI] [PubMed] [Google Scholar]
- 134.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(SI):32–6. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu M, Scott AJ. Phylogenomic analysis of bacterial and archaeal sequences with amphora2. Bioinformatics. 2012;28(7):1033–4. doi: 10.1093/bioinformatics/bts079. [DOI] [PubMed] [Google Scholar]
- 136.Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu M, Chatterji S, Eisen JA. Accounting for alignment uncertainty in phylogenomics. PloS ONE. 2012;7(1):30288. doi: 10.1371/journal.pone.0030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stamatakis A. Raxml version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Systematic Biol. 2006;55(4):539–52. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 140.Kurtz S, Phillippy A, Delcher A, Smoot M, Shumway M, Antonescu C, Salzberg S. Versatile and open software for comparing large genomes. Genome Biology. 2004; 5(2). 10.1186/gb-2004-5-2-r1. [DOI] [PMC free article] [PubMed]
- 141.Darling A, Mau B, Blattner F, Perna N. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Research. 2004;14(7):1394–403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li L, Stoeckert CJ, Roos DS. Orthomcl: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–89. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fischer S, Brunk BP, Chen F, Gao X, Harb OS, Iodice JB, Shanmugam D, Roos DS, Stoeckert CJ. Using orthomcl to assign proteins to orthomcl-db groups or to cluster proteomes into new ortholog groups. Current Protocols Bioinforma. 2011;CHAPTER:Unit–6.1219. 10.1002/0471250953.bi0612s35. [DOI] [PMC free article] [PubMed]
- 144.Tatusov R, Koonin E, Lipman D. A genomic perspective on protein families. Science. 1997;278(5338):631–7. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 145.Galperin MY, Makarova KS, Wolf YI, Koonin EV. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015;43(D1):261–9. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nostocales strains used in this study. (XLS 44 kb)
Supporting Information (SI) Appendix. (PDF 8156 kb)
Proteins encoded in 10 most prominent locally collinear blocks in Nostoc punctiforme PCC 73102, Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’, identified by Mauve. (XLS 84 kb)
Comparison of the minimal bacterial gene set to Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’. (XLS 61 kb)
Comparison of the cyanobacterial core and shell gene set to Nostoc sp. N6 and Nostoc sp. ‘Lobaria pulmonaria cyanobiont’. (XLS 172 kb)
Orthologous groups enriched in different clades. (XLS 756 kb)
Data Availability Statement
The genomic sequences generated and analyzed in this study were deposited in the GenBank database under Bioproject numbers PRJNA279350 (Nostoc sp. N6), PRJNA275880 (Nostoc sp. ‘L. pulmonaria’), PRJNA389199 (Nostoc sp. 210A), PRJNA389200 (Nostoc sp. 213), PRJNA389202 (Nostoc sp. 232) and PRJNA389205 (Nostoc sp. ‘P. malacea’). Additional accession numbers and data can be found in Supporting Information.