Abstract
Objectives
Recently, the field of tissue engineering has made numerous advances towards achieving artificial tendon substitutes with excellent mechanical and histological properties, and has had some promising experimental results. The purpose of this systematic review is to assess the efficacy of tissue engineering in the treatment of tendon injuries.
Methods
We searched MEDLINE, Embase, and the Cochrane Library for the time period 1999 to 2016 for trials investigating tissue engineering used to improve tendon healing in animal models. The studies were screened for inclusion based on randomization, controls, and reported measurable outcomes. The RevMan software package was used for the meta-analysis.
Results
A total of 388 references were retrieved and 35 studies were included in this systematic review. The different biomaterials developed were analyzed and we found that they improve the biomechanical and histological characteristics of the repaired tendon. At meta-analysis, despite a high heterogeneity, it revealed a statistically significant effect in favour of the maximum load, the maximum stress, and the Young’s modulus between experimental and control groups. In the forest plot, the diamond was on the right side of the vertical line and did not intersect with the line, favouring experimental groups.
Conclusions
This review of the literature demonstrates the heterogeneity in the tendon tissue engineering literature. Several biomaterials have been developed and have been shown to enhance tendon healing and regeneration with improved outcomes.
Cite this article: D. González-Quevedo, I. Martínez-Medina, A. Campos, F. Campos, V. Carriel. Tissue engineering strategies for the treatment of tendon injuries: a systematic review and meta-analysis of animal models. Bone Joint Res 2018;7:318–324. DOI: 10.1302/2046-3758.74.BJR-2017-0326.
Keywords: Tissue engineering, Tendon injury, Cell-based therapies, Tendon healing, Biomaterials, Review
Article focus
Tissue engineering has emerged as an interesting alternative in regenerative medicine thanks to its development in recent years.
Researchers have produced bioartificial substitutes that, by combining cells, biomaterials, and growth factors, restore the function of damaged organs.
The objective of this study was to assess the efficacy of tissue engineering in the treatment of tendon injuries in animal models by systematically reviewing the scientific literature.
Key messages
Complete regeneration of the tendon after injury is never achieved and the surgical treatment of these lesions have several limitations.
The use of tissue engineering techniques can accelerate the healing process and produce biomechanical behaviour comparable to that of native tendons.
Strengths and limitations
No previous reviews have produced a meta-analysis of biomechanical results of tissue engineering for the treatment of tendon injuries in animal models.
The included studies were heterogeneous in several ways, including the animal models used and tendon models used.
Introduction
Most large tendons, such as the Achilles tendon, patellar tendon, rotator cuff, or forearm extensors, among others, are vulnerable to overuse, which can lead to pathological changes in the tendon that cause tendinopathy. This pathology is common among athletes and workers, being the main reason for musculoskeletal consult to the general practitioner (about 30%) and accounting for 17% of first consultations to the orthopedic surgeon.1 Acute tendon injury includes partial or complete rupture, altering the continuity of the tendon and causing loss of movement. It is followed by a natural healing process but is less efficient than in other components of the musculoskeletal system. Acute and chronic injuries are facilitated by intrinsic and extrinsic factors. For example, degeneration of collagen and alteration of the orientation of its fibres have been shown in the histology of ruptures of the Achilles tendons.2
Complete regeneration of the tendon after injury is never achieved. The characteristic response is fibroplasia and the tissue that replaces the defect remains hypercellular with thinner collagen fibres. In tendons with tendinopathy or ruptures, there is a lower proportion of type I collagen and an increase in the amount of type III collagen, which reduces the mechanical resistance because this type of collagen has less crosslinking than type I.3
Currently, tendinous repair includes the use of autografts and tendinous transfers because allografts lose their biomechanical properties during the process of sterilization. In this context, tissue engineering emerged as a promising alternative in regenerative medicine, including tendinous repair. Tissue engineering is a multidisciplinary discipline that, through the rational combination of cells, biomaterials, and growth factors, allows the generation of bioartificial substitutes to repair, replace, or even increase the function of damaged tissues or organs.4,5
At present, tissue engineering researchers are developing polymers and 3D bioartificial substitutes that accelerate the healing process with biomechanical behaviour comparable to that of native tendons. However, to date, the studies on tendon repair using animal models have not found an ideal biomaterial and 3D bioartificial substitute for this purpose.6
Because these experimental studies have shown favourable results for the use of tissue engineering in tendon injuries, an update of the current evidence is required. This study presents a systematic review and meta-analysis of the existing progress in tissue engineering combining stem cells, growth factors, and scaffolds to assess the efficacy of treating tendon injuries in animal models.
Materials and Methods
Search strategy
PRISMA guidelines were followed for this systematic review.
MEDLINE, Embase, and the Cochrane Library were searched for articles published between 1999 and 2016 about the use of tissue engineering in tendon injuries. The keywords used to conduct the research were “tendon injuries” and “tissue engineering”.
Two researchers (DGQ, IMM) reviewed all of the potential abstracts and full texts independently. If there was any disagreement, it was resolved by consultation with another researcher (AC).
Article selection
The studies were included if they investigated tissue engineering techniques (e.g. biomaterials, growth factors) for repairing a tendon lesion. Trials where required to have been conducted in animal models without restriction of specie. Ex vivo experimental studies were excluded. The in vivo studies that investigated biomechanical or histological outcomes by tissue engineering strategies in injured tendons were also included. Due to the nature of intervention, it was agreed trials without blinding design.
We excluded duplicated studies, review articles, case reports, editorials and studies that were not published in English and: which were not reported as full-text articles; which reported on a molecular or genetic level; which reported without a control group (e.g. normal tendons or tendons repaired with or without a tissue engineering approach); and experimental trials without an animal model of tendon injury.
Quality assessment
The ARRIVE Guidelines for reporting animal research were used to measure the methodological quality of all included studies.7 This checklist of 20 items considers characteristics of animal used and the experimental, statistical, and analytical methods of each study. We set a low, medium, and high quality of the papers with a ARRIVE checklist of 12 or less, 13 to 15, and 16 or more respectively.
Statistical analysis
All analyses were performed using the RevMan software (version 5.0, The Cochrane Collaboration, Oxford, United Kingdom). Standard mean difference with 95% confidence interval (CI) was calculated. Heterogeneity between studies was examined using the I2 statistical test. When the I2 value was greater than 50%, we considered that heterogeneity was significant. A random effect model was chosen as the main analysis method. Funnel plots were used to check for the potential of publication bias. All the p-values were two-sided; statistical significance was defined as p < 0.05.
Results
Literature search and study characteristics
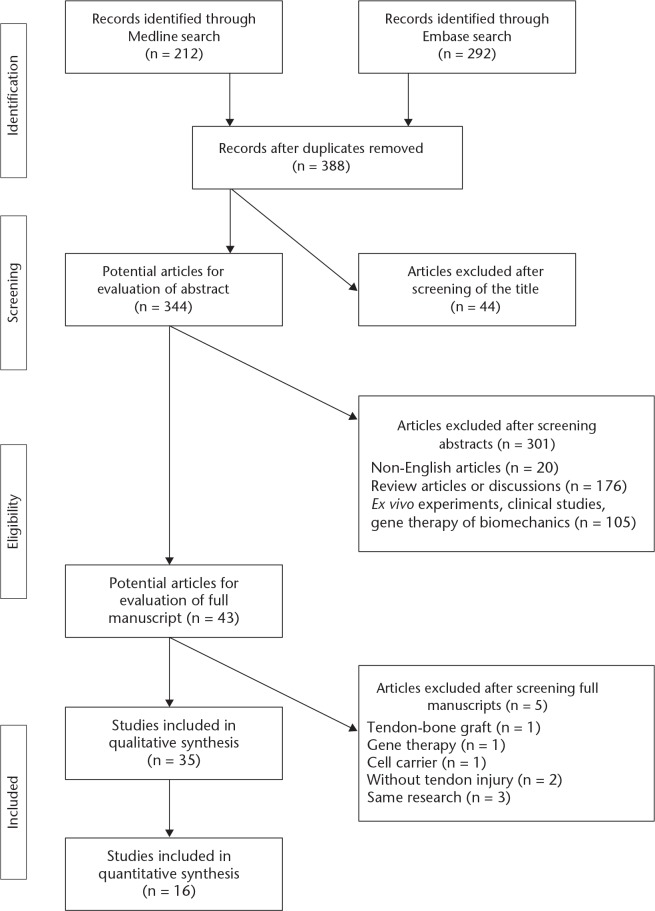
A total of 388 records were identified for abstract review after removal of duplicates. A total of 105 studies were excluded because of their focus on gene therapy, biomechanics, ex vivo experiments, or clinical studies. A total of 176 studies were not included due to their being review articles or discussions, and 20 records were excluded for not being published in English. Of the remaining 43 potential articles, two studies were excluded because the animal model did not include a tendon injury and three articles were excluded due to their focus on gene therapy, tendon-bone graft, and cell carriers, respectively (without animal model). A further three articles were excluded for being part of the same research.
Therefore, a total of 35 studies were included in this review, as reported in Figure 1. The selected articles are summarized in Supplementary table i. A total of 16 studies were included in the meta-analysis.
Fig. 1.
Flowchart showing the selection process.
A total of 15 studies (42.9%) used rabbit models; the same number of trials used rat models. Two studies (5.7%) used hens. Pig, sheep, and dog animal models were used in one trial (2.9%) each. The tendons selected as lesion prototypes were: Achilles tendon (15, 42.9%); patellar tendon (seven, 20%); digital deep flexor tendon (six, 17.1%); supraspinatus tendon (three, 8.6%); infraspinatus tendon (two, 5.7%); and digital superficial flexor tendon (two, 5.7%).
In terms of tissue engineering strategies for the treatment of tendon injuries in these studies, we found the following breakdown: stem cells (18, 51.4%); biological scaffolds (seven, 20%); growth factors (five, 14.3%); synthetic biomaterials (four, 11.4%); and a combination of these approaches (one, 2.9%).
Meta-analysis
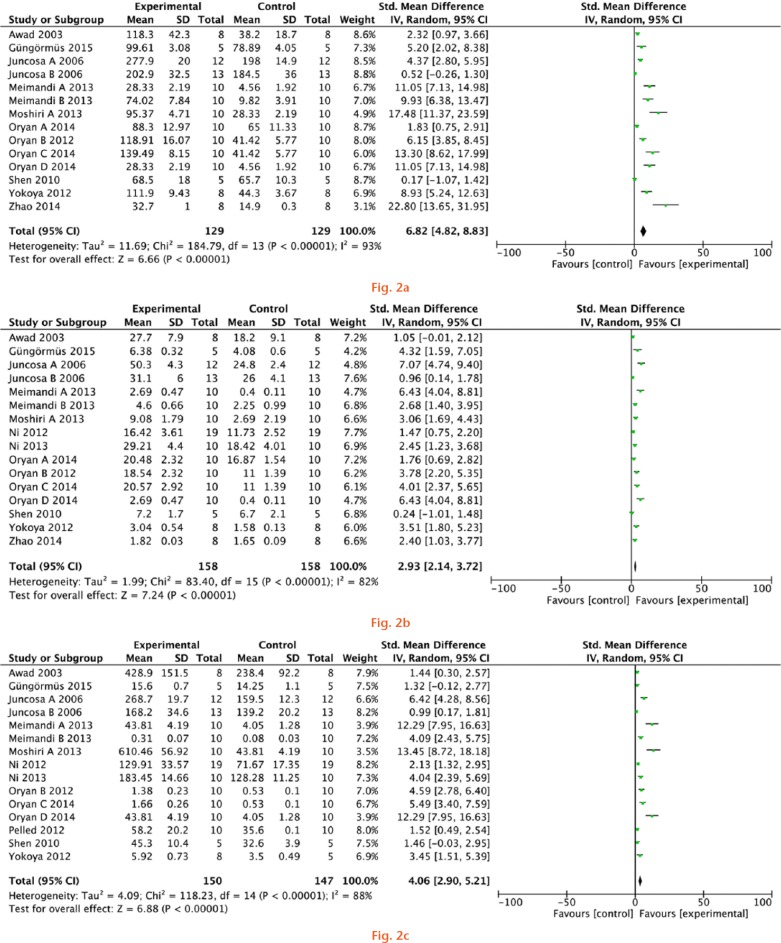
Overall, the meta-analysis revealed a statistically significant effect in favour of the maximum load (structural property; standard mean difference of 6.82 N, 95% CI 4.81 to 8.83), the maximum stress (material property; standard mean difference of 2.93 MPa, 95% CI 2.14 to 3.72), and the Young’s modulus (mechanical property; standard mean difference of 4.06 MPa, 95% CI 2.90 to 5.21) between experimental and control groups (Fig. 2).
Meta-analysis for a) the maximum load, b) the maximum stress, and c) the Young’s modulus of experimental groups and controls.
Nevertheless, there was evidence for significant heterogeneity among groups (the I2 values were > 80%, p < 0.01). For this reason, we used a random-effects model for analysis.
In these analyses, in the forest plot, the diamonds were on the right side of the vertical line and did not intersect with the line, favouring experimental groups.
Publication bias assessment
Funnel plot indicated low likelihood of publication bias (Fig. 3).
Fig. 3.
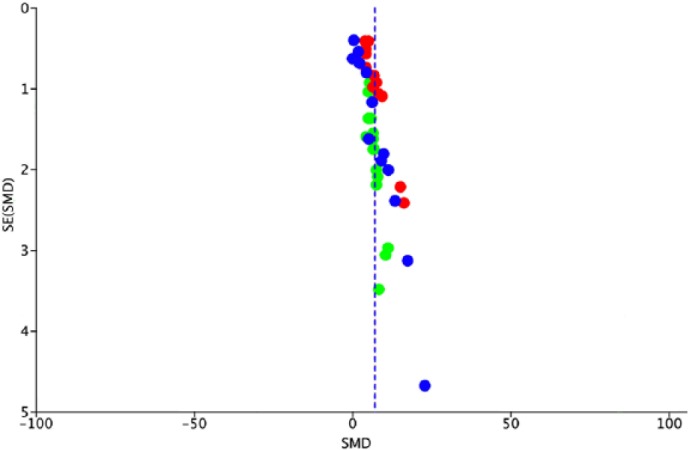
Funnel plot analysis for the publication bias of maximum load (blue), maximum stress (green), and Young’s modulus (red) outcomes. Funnel plot analysis indicated a low likelihood of publication bias.
Discussion
Animal models
The selection of animal models is essential to ensure a correct translation to clinical practice. Rats are less expensive and easier to handle and maintain than other laboratory animals. However, their small size reduces the clinical translation of the developed regenerative strategies, but working with them facilitated the histological, functional and biomechanical analyses. Large animals, such as rabbits, dogs, or cows, have high costs and require specialized personnel to handle them, but they are more suitable for testing surgical approaches, techniques, and rehabilitation protocols.8
The rat rotator cuff model does not replicate the anatomy and kinematics of the human shoulder, but do share some resemblance. The Achilles and patellar tendons are easy to study using animal models because of their simple surgical approach and the possibility of reproducing tendon injuries and cyclic loads.9 However, animal models can never replicate human conditions because many of them are quadrupeds and their tendons carry different loads with molecular differences. In addition to anatomical and size differences, histological, potential molecular, and regenerative differences should be considered.
Tissue-engineered strategies
Nowadays, an ideal engineered tendon substitute must be biodegradable, biocompatible, and biomechanically stable to support the tension during healing. Furthermore, the combination of biomaterials and cells must reassemble the native structure of tendon and support the regeneration without adhesions, toxicity, or, tissue rejection.10
Tissue engineering approaches to repair and improve tendon healing include: biological and decellularized tissues, the use of natural and synthetic biomaterials, the use of growth factors, stem cell-based therapies, or a combination of these strategies.11 In addition, there is a growing interest in the use of nanomaterials in tendon tissue engineering, and they could play a key role in tendon healing, as they can act as a carrier for gene therapy or growth factors, and thus help modulate the regenerative function of cells.12
An example of a biologic scaffold is the use of porcine small intestinal submucosa. When this biomaterial is implanted, it induces a site-specific tissue repair with a tendon histologically similar to native tendon. However, the use of this scaffold may generate a foreign body and inflammatory reaction.13 Some authors are focused on the decellularization of tissues and organs in order to reduce the immunogenicity of these grafts, while maintaining the 3D structure and molecular composition of the extracellular matrix.14,15 In this sense, a reduction of the inflammatory response was observed in vivo.16
Concerning the use of biomaterials, synthetic ones (such as polyglycolic acid) resulted in biomechanically and structurally stable tendon graft that supports tendon cell migration, especially those subjected to crosslinking.17 Similarly, chitosan-based hyaluronan hybrid fibre scaffolds have been used in tendon repair with better collagen type I production.18 Polyhydroxyalkanoates are a family of biopolymers with adaptable mechanical properties and delayed biodegradability.19
Collagen type I, the main component of the tendon, has been shown to have an excellent biocompatibility and biodegradability; with nanostructured and crosslinking technologies, it is possible to polymerize the type I collagen to produce effective scaffolds.20 Electrospinning of collagen fibres produces elaborate nanofibres with the desirable size, density, and alignment.21
Bone marrow and adipose mesenchymal stem cells (MSCs) are a commonly used cell source in musculoskeletal and peripheral nerve tissue engineering due to their ability to differentiate and their easy isolation and culture.4,22,23 When these cells were used in tendon repair, they improved tendinous healing in short-term studies. However, these studies suggest that no significant differences can be observed in long-term comparative studies; this could be related to the high regeneration capability of the animal models used.24-26 Tendon-derived stem cells have higher mRNA expression of tenomodulin, scleraxis, type I collagen and decorin than that of MSCs, and could promote earlier and better tendon healing. On the other hand, in published studies, the biomechanical properties of the native tendon have never been restored successfully using tendon-derived stem cells.27 In addition to the regenerative potential of adipose-derived stromal cells, it was recently reported that these cells promote neoangiogenesis, cell proliferation and extracellular matrix (ECM) remodelling, and that they protect the regenerative microenvironment from macrophages.28
Growth factors, which are produced by anti-inflammatory macrophages, play a key role in the regulation of all phases of tendon healing because they are needed to activate different cellular processes (such as proliferation, differentiation, and migration) and to increase the synthesis of crucial ECM.29 These factors can be delivered to the regenerative microenvironment by direct application or by using impregnated sutures for slow release, or a scaffold-based delivery system.30 Basic fibroblast growth factor (bFGF) has a beneficial effect on tendon healing by regulating cellular migration, promoting angiogenesis, and inhibiting inflammatory processes.31 Bone morphogenetic protein 2 (BMP2) was involved in tendon differentiation and maintenance, modulating collagen formation, and tendon remodelling.32,33 Another example is the connective tissue growth factor (CTGF) that is highly expressed in the early stage of tendon repair and can promote tendon repair.34 Platelet-rich plasma (PRP) was recently introduced as a novel treatment for tendon injuries that would enhance the synthesis of type I and type III collagen and would limit matrix degradation.35,36 Stromal cell-derived factor-1 alpha (SDF-1α) is a cytokine that regulates inflammatory cell recruitment and could improve the structural and biomechanical characteristics of repaired tendon.37
Gene therapy is another attractive field in tissue engineering but it is still necessary to define optimal cell targets and identify genes whose modification has a significant therapeutic response.38
There are several strengths in this meta-analysis. This is the first study that showed favourable outcomes when using tissue engineering strategies in the treatment of tendon injuries in animal models. Therefore, the quality assessment score for most of the included studies was high, favouring the results of the meta-analysis. Nevertheless, there are a series of limitations associated with this work. First, like any systematic review, the conclusions of our work are affected by the quality of studies included. However, most included studies in this work had a high quality according to ARRIVE guidelines. Second, our search strategy may associate search bias due to this is an English language-only revision, which entails language bias. On the other hand, the results on animal model have a limited value for a human application.
In conclusion, at present, there are numerous gaps in the basic science of tendon healing. As a result, the orthopaedic surgeon has few solutions at his fingertips in the event of a tendon injury. In recent years, thanks to tissue engineering techniques and the use of animal models, we have growth factors, scaffolds, and stem cells that can improve the histological and biomechanical characteristics of repair tissue, but do not fully recapitulate the native tendon.
Footnotes
Author Contributions: D. González-Quevedo: Online research and abstract selection, Analyzing and interpreting the data, Writing the manuscript.
I. Martínez-Medina: Online research and abstract selection.
A. Campos: Supervising the study.
F. Campos: Critical revision of the manuscript.
V. Carriel: Critical revision of the manuscript.
Conflict of Interest Statement: None declared.
Follow us @BoneJointRes
Supplementary material
A table showing benefits and limitations of tissue engineering strategies for tendon injuries
Funding Statement
The research and publication of research were supported by the Spanish Society of Orthopedics and Traumatology (SECOT) Foundation.
References
- 1. Kaux JF, Forthomme B, Goff CL, Crielaard JM, Croisier JL. Current opinions on tendinopathy. J Sports Sci Med 2011;10:238-253. [PMC free article] [PubMed] [Google Scholar]
- 2. Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Am J Sports Med 2000;28:857-863. [DOI] [PubMed] [Google Scholar]
- 3. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med 2009;37:1855-1867. [DOI] [PubMed] [Google Scholar]
- 4. Carriel V, Alaminos M, Garzón I, Campos A, Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev Neurother 2014;14:301-318. [DOI] [PubMed] [Google Scholar]
- 5. Alaminos M, Del Carmen Sánchez-Quevedo M, Muñoz-Avila JI, et al. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci 2006;47:3311-3317. [DOI] [PubMed] [Google Scholar]
- 6. Hogan MV, Bagayoko N, James R, et al. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg 2011;19:134-142. [DOI] [PubMed] [Google Scholar]
- 7. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The arrive guidelines for reporting animal research. Animals 2013;4:35-44. [DOI] [PubMed] [Google Scholar]
- 8. Bottagisio M, Lovati AB. A review on animal models and treatments for the reconstruction of Achilles and flexor tendons. J Mater Sci Mater Med 2017;28:45. [DOI] [PubMed] [Google Scholar]
- 9. Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res 2014;3:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol 2010;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Longo UG, Lamberti A, Petrillo S, Maffulli N, Denaro V. Scaffolds in tendon tissue engineering. Stem Cells Int 2012;2012:517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parchi PD, Vittorio O, Andreani L, et al. Nanoparticles for tendon healing and regeneration: literature review. Front Aging Neurosci 2016;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy KD, Mushkudiani IA, Kao D, et al. Successful incorporation of tissue-engineered porcine small-intestinal submucosa as substitute flexor tendon graft is mediated by elevated TGF-beta1 expression in the rabbit. J Hand Surg Am 2008;33:1168-1178. [DOI] [PubMed] [Google Scholar]
- 14. Roosens A, Somers P, De Somer F, et al. Impact of detergent-based decellularization methods on porcine tissues for heart valve engineering. Ann Biomed Eng 2016;44:2827-2839. [DOI] [PubMed] [Google Scholar]
- 15. Oliveira AC, Garzón I, Ionescu AM, et al. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One 2013;8:e66538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Güngörmüş C, Kolankaya D, Aydin E. Histopathological and biomechanical evaluation of tenocyte seeded allografts on rat Achilles tendon regeneration. Biomaterials 2015;51:108-118. [DOI] [PubMed] [Google Scholar]
- 17. Bagnaninchi PO, Yang Y, El Haj AJ, Maffulli N, Bosch U. Tissue engineering for tendon repair. Br J Sports Med 2007;41:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majima T, Irie T, Sawaguchi N, et al. Chitosan-based hyaluronan hybrid polymer fibre scaffold for ligament and tendon tissue engineering. Proc Inst Mech Eng H 2007;221:537-546. [DOI] [PubMed] [Google Scholar]
- 19. Webb WR, Dale TP, Lomas AJ, et al. The application of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model. Biomaterials 2013;34:6683-6694. [DOI] [PubMed] [Google Scholar]
- 20. Oryan A, Moshiri A, Meimandi-Parizi A. In vitro characterization of a novel tissue engineered based hybridized nano and micro structured collagen implant and its in vivo role on tenoinduction, tenoconduction, tenogenesis and tenointegration. J Mater Sci Mater Med 2014;25:873-897. [DOI] [PubMed] [Google Scholar]
- 21. Oryan A, Moshiri A, Parizi Meimandi A, Silver IA. A long-term in vivo investigation on the effects of xenogenous based, electrospun, collagen implants on the healing of experimentally-induced large tendon defects. J Musculoskelet Neuronal Interact 2013;13:353-367. [PubMed] [Google Scholar]
- 22. Paredes JJ, Andarawis-Puri N. Therapeutics for tendon regeneration: a multidisciplinary review of tendon research for improved healing. Ann N Y Acad Sci 2016;1383:125-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carriel V, Scionti G, Campos F, et al. In vitro characterization of a nanostructured fibrin agarose bio-artificial nerve substitute. J Tissue Eng Regen Med 2017;11:1412-1426. [DOI] [PubMed] [Google Scholar]
- 24. Juncosa-Melvin N, Boivin GP, Galloway MT, et al. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng 2006;12:681-689. [DOI] [PubMed] [Google Scholar]
- 25. Juncosa-Melvin N, Shearn JT, Boivin GP, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng 2006;12:2291-2300. [DOI] [PubMed] [Google Scholar]
- 26. Chong AK, Ang AD, Goh JC, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg [Am] 2007;89-A:74-81. [DOI] [PubMed] [Google Scholar]
- 27. Ni M, Rui YF, Tan Q, et al. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials 2013;34:2024-2037. [DOI] [PubMed] [Google Scholar]
- 28. Shen H, Kormpakis I, Havlioglu N, et al. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res Ther 2016;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol 2015;11:223-233. [DOI] [PubMed] [Google Scholar]
- 30. Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull 2011;98:31-59. [DOI] [PubMed] [Google Scholar]
- 31. Oryan A, Moshiri A. Recombinant fibroblast growth protein enhances healing ability of experimentally induced tendon injury in vivo. J Tissue Eng Regen Med 2014;8:421-431. [DOI] [PubMed] [Google Scholar]
- 32. Chamberlain CS, Lee J-S, Leiferman EM, et al. Effects of BMP-12-releasing sutures on Achilles tendon healing. Tissue Eng Part A 2015;21:916-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipner J, Shen H, Cavinatto L, et al. In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng Part A 2015;21:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lui PPY, Wong OT, Lee YW. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 2016;18:99-112. [DOI] [PubMed] [Google Scholar]
- 35. Martinello T, Bronzini I, Perazzi A, et al. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 2013;31:306-314. [DOI] [PubMed] [Google Scholar]
- 36. Kaux JF, Drion PV, Colige A, et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound Repair Regen 2012;20:748-756. [DOI] [PubMed] [Google Scholar]
- 37. Shen W, Chen X, Chen J, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010;31:7239-7249. [DOI] [PubMed] [Google Scholar]
- 38. Huang D, Balian G, Chhabra AB. Tendon tissue engineering and gene transfer: the future of surgical treatment. J Hand Surg Am 2006;31:693-704. [DOI] [PubMed] [Google Scholar]