Abstract
Large bone defects remain a tremendous clinical challenge. There is growing evidence in support of treatment strategies that direct defect repair through an endochondral route, involving a cartilage intermediate. While culture-expanded stem/progenitor cells are being evaluated for this purpose, these cells would compete with endogenous repair cells for limited oxygen and nutrients within ischaemic defects. Alternatively, it may be possible to employ extracellular vesicles (EVs) secreted by culture-expanded cells for overcoming key bottlenecks to endochondral repair, such as defect vascularization, chondrogenesis, and osseous remodelling. While mesenchymal stromal/stem cells are a promising source of therapeutic EVs, other donor cells should also be considered. The efficacy of an EV-based therapeutic will likely depend on the design of companion scaffolds for controlled delivery to specific target cells. Ultimately, the knowledge gained from studies of EVs could one day inform the long-term development of synthetic, engineered nanovesicles. In the meantime, EVs harnessed from in vitro cell culture have near-term promise for use in bone regenerative medicine. This narrative review presents a rationale for using EVs to improve the repair of large bone defects, highlights promising cell sources and likely therapeutic targets for directing repair through an endochondral pathway, and discusses current barriers to clinical translation.
Cite this article: E. Ferreira, R. M. Porter. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res 2018;7:263–273. DOI: 10.1302/2046-3758.74.BJR-2018-0006.
Keywords: Extracellular vesicles, Critical-sized bone defects, Endochondral ossification, Mesenchymal stromal cells
Introduction
Unlike many fractures, larger bone defects resulting from high-energy trauma or osseous tumour removal do not heal without intervention. Current treatment options include the use of autologous or allogeneic bone grafts,1,2 sometimes preceded by an induced membrane procedure to improve defect vascularization.3 In the United States, for example, it was estimated in 2009 that half a million bone grafts were performed per year at a cost of $2.5 billion; this number was expected to double by 2020.4 For problematic defects, such as those in the lower limbs, bone grafts cannot ensure healing.5 Failed treatment necessitates challenging revision surgeries, with associated pain and risk of infection. If these also fail, amputation is a likely outcome. The lifetime healthcare costs of lower limb amputation were estimated to exceed $500 000,6 but lost quality of life for these patients cannot be fully quantified. These realities underscore the need for better strategies to stimulate bone healing.
Among the wide range of biologicals being considered to improve bone repair, there is recent interest in the therapeutic application of extracellular vesicles (EVs). The term EV has been adopted by the research community to describe multiple types of secreted, membrane-enclosed vesicles.7 Although there has been past inconsistency with nomenclature,8 two distinct classes of EVs are known as exosomes (30 nm to 150 nm), which are released from multivesicular bodies when docking with the plasma membrane, and microvesicles (50 nm to 1000 nm), which result from plasma membrane budding at the cell surface. Additional EV subclasses include apoptotic bodies, ectosomes, and oncosomes (all > 1 μm). EVs were once thought to represent cellular waste, although in recent years, their importance to widespread physiological and pathological processes has been recognized.9-11 For example, EVs can either stimulate or suppress immune responses to pathogens and cancer cells. Circulating EVs are thought to play a role in immune tolerance, yet they can also contribute to the progression of autoimmune diseases, including rheumatoid arthritis and diabetes.12 EVs exert their effects on target cells through multiple mechanisms, directly activating recipient cell surface receptors, transferring their membrane contents to the recipient cell plasma membrane, and delivering packaged cargo into the recipient cytosol.13 This cargo includes proteins, mRNAs, and microRNAs that, importantly, reflect the state of the parent cell (Fig. 1).
Fig. 1.
Diagram showing intercellular communication by extracellular vesicles (EVs). Two principle EV fractions are understood to play roles in intercellular communication: exosomes, which are released from multivesicular bodies after fusing with the parent cell plasma membrane; and larger microvesicles, which bud directly from the parent cell membrane. Each fraction contains unique profiles of intravesicular RNAs and protein as well as membrane-bound receptors and lipids. These fractions stimulate responses within recipient cells by direct activation of recipient cell surface receptors, by transfer of vesicle contents to the recipient cell cytosol after fusion with the plasma membrane, and by intracellular trafficking of vesicle contents following endocytosis.
It may be possible to exploit this natural mechanism for intercellular signalling to overcome some important limitations of cell therapies for bone defect repair. It is known that successful repair, which occurs during fracture healing, involves multiple contributing cell populations, including osteochondral progenitors and macrophages. For each of these populations, multiple signalling pathways regulate essential repair activities.14,15 Consequently, delivery of any single pathway regulator may have limited impact on improving repair. Because EVs stably package protein and nucleic acid signals for transfer between cells, they have the potential to activate complementary, pro-regenerative signalling pathways in the same target cells, and to stimulate multiple target populations. This property could make them an efficient therapeutic vehicle for bone regenerative medicine.
EVs are already being evaluated in clinical trials, mainly for the diagnosis and treatment of specific cancers.16 Their potential uses in regenerative medicine are still largely speculative.11,13,17-20 This review focuses on the possibility for harnessing EVs to enhance the endochondral repair of large bone defects. The following sections present the general hypothesis that parent cells can be manipulated in vitro to produce EVs with complementary, pro-regenerative signals that direct endogenous cells to complete one or more limiting steps to bone repair. A rationale for the use of EVs is presented, along with promising cell sources and likely therapeutic targets for directing repair through an endochondral pathway. Finally, barriers to clinical translation are discussed.
The papers included in this narrative review were identified using PubMED and Web of Science prior to 15 December 2017. With the exception of studies describing matrix vesicles, most of the research discussed below has been published since 2014, demonstrating the relatively nascent state of this research area.
Rationale for directing endochondral repair of large bone defects
The long bones are formed through a developmental programme known as endochondral ossification, which essentially involves the generation of a cartilage template that is remodelled into vascularized bone.21 Bone fractures are repaired through a similar endochondral process: the fracture gap is bridged by a cartilaginous callus formed by progenitor cells migrating from the nearby periosteum; upon chondrocyte hypertrophy and calcification, the callus is remodelled into bone.15 This repair process is often compromised in defects beyond a critical size (critical-sized bone defects, or CSBDs), leading to nonunion. While comorbidities and biomechanical factors (e.g. stability of bone fixation) can influence the success of healing, deficiencies in repair cell numbers and inductive growth factor levels are limiting to the initiation of an endochondral repair pathway.22
There has been increasing focus in the orthopaedic research community on ‘developmental engineering’ strategies for large defect treatment that aim to mimic the endochondral ossification programme of development and fracture repair (Fig. 2).23-25 Common components of these strategies are mesenchymal stromal/stem cells (MSCs), which have the potential to self-renew and differentiate into the constitutive cells of bone, including osteoblasts and hypertrophic chondrocytes (growth plate).26,27 For example, Scotti et al28,29 demonstrated that hypertrophic cartilage engineered in vitro from human MSCs could form a functional bone organ when implanted ectopically in nude mice. Bahney et al30 subsequently demonstrated that cartilaginous grafts, derived either from fracture callus or from MSCs pre-differentiated in vitro, can stimulate bone healing following implantation into murine CSBDs. Other tissue-engineered cartilage constructs have since been tested in various bone formation models in vivo.31-36 While these results are promising, their clinical translation relies on production of cartilage templates outside the body under carefully controlled conditions to ensure safety and efficacy. Alternatively, if the template could be formed within the defect by either exogenous (i.e. culture-expanded) and/or endogenous progenitor cells, this should reduce the complexity – and related cost – of treatment.37
Fig. 2.
Directing endochondral repair of large bone defects. One paradigm for bone regenerative medicine is modelled on the processes of long bone development and successful (fracture) repair. Instead of designing scaffold/biological constructs for the direct stimulation of osteogenesis, constructs can be engineered to undergo an initial chondrogenesis phase, which serves as an efficient template for ordered osteogenic remodelling by successive waves of repair cells. It is noteworthy that cartilage, an avascular tissue, is more resilient to the vascular deficiency within larger bone defects.
Rationale for using EVs versus their parent cells
Relative to fractures, larger bone defects are characterized by an ischaemic microenvironment, with extreme deficiencies in oxygen and nutrients near their core.5,38,39 This harsh microenvironment presents a major challenge for the use of cell-based therapies, because the implanted cells compete with endogenous progenitor cells (i.e. migrating into the defect) for limited oxygen and nutrients (Fig. 3). This should be especially true for repair cells expanded under high serum and normoxia prior to implantation.40,41 It has been demonstrated that most culture-expanded MSCs, for example, die or undergo phagocytosis by macrophages in the first couple weeks after implantation within CSBDs.42,43 Recent studies have demonstrated that these cells cannot adapt to the ischaemic environment, particularly upon the depletion of glucose stores.44 In contrast to culture-expanded cells, their EVs should neither tax the defect for oxygen and nutrients nor actively produce cellular waste.
Fig. 3.
Diagram showing the potential advantage of extracellular vesicles (EVs) within a large bone defect microenvironment. As opposed to simple fractures, bone defects beyond a critical size are characterized by severe nutrient deficiency and near-anoxia within their core. While exogenous cells implanted into these defects may secrete pro-regenerative factors, they also compete with endogenous repair cells migrating into the defect for scarce oxygen and nutrients. In contrast, the same pro-regenerative signals packaged within EVs would not necessarily tax the defect for nutrients and oxygen, potentially permitting enhanced repair by endogenous cells.
If EVs can deliver pro-regenerative paracrine signals produced by their parent cells, they would present a treatment alternative with potential safety advantages. One key advantage would be the inability to undergo malignant transformation. However, because tumour cell EVs have been shown to transfer oncogenic molecules to recipient cells,45 EVs from culture-expanded cells cannot be assumed to be safe in terms of tumourigenicity. Regarding their storage in the absence of cryoprotectants, EVs retain their activity after freezing and thawing better than cells do, although changes to their properties and function have been reported and are dependent on the precise storage conditions.46 While these theoretical advantages are promising, more pre-clinical studies are needed that provide head-to-head comparisons of EVs with their candidate parent cells, in order to demonstrate a clear therapeutic advantage in the context of bone repair.
Cell sources of EVs for bone regenerative medicine
To date, only a handful of studies have evaluated EVs harvested from culture-expanded cells within pre-clinical models of bone injury. These studies are summarized in Table I, along with studies using models of osteochondral defect repair, which may offer insights into the endochondral repair of bone defects. To date, little consideration has been made for strategies to alter EV composition in order to better direct tissue repair.
Table I.
Studies evaluating the use of exogenous extracellular vesicles (EVs) to alter bone or cartilage repair in vivo
| First author | EV fractions studied | Cell source of EVs | Experiment model | Delivery method | Outcome measures | Key findings |
|---|---|---|---|---|---|---|
| Furuta et al (2016)53 | Multiple* | Human BM-MSCs | Murine femoral fracture | Injection (×2) | X-ray, µCT, histology/IHC | Injections of MSC-EVs rescued delayed fracture healing in CD9-/- mice and enhanced normal healing in wild type mice |
| Li et al (2017)77 | Exosomes | Rabbit BM-MSCs (+/- HIF-1α overexpression) | Rabbit steroid-induced avascular necrosis of femoral head | Injection | MRI, histology/IF | Exosomes from HIF-1α-overexpressing MSCs promoted increased trabecular bone generation and neo-vascularization in femoral heads compared with unmodified MSC-EVs and saline-treated groups |
| Qi et al (2016)63 | Exosomes | Human iPSC-derived MSCs | Rat critical-sized cranial defects (×2) | TCP scaffold | µCT, histology/histomorphometry/IHC | EVs from iPSC-derived MSCs dose-dependently enhanced bone formation and vasculogenesis compared with TCP controls |
| Qin et al (2016)54 | Multiple | Human BM-MSCs | Rat critical-sized cranial defects (×2) | Hydrogel scaffold | µCT, histology | MSC-EVs stimulate bone formation compared with hydrogel controls |
| Xie et al (2017)79 | Multiple | Rat BM-MSCs | Subcutaneous implantation in nude mice | Bovine DBM scaffold | µCT, histology/IHC | EVs from rat MSCs enhanced vessel formation within DBMs implanted subcutaneously, although they did not independently enhance bone formation compared with scaffold-only controls |
| J. Zhang et al (2016)64† | Exosomes | Human iPSC-derived MSCs | Rat critical-sized cranial defects (×2) | TCP scaffold | µCT, histology/IHC | EVs from iPSC-derived MSCs dose-dependently enhanced bone formation compared with TCP controls |
| S. Zhang et al (2016)57 | Exosomes | Human ESC-derived MSCs | Rat osteochondral defects | Weekly injections | Histology/IHC | EVs from ESC cell-line-derived MSCs enhanced cartilage repair score (O’Driscoll) and cartilage marker deposition by six weeks |
| S. Zhang et al (2018)58‡ | Exosomes | Human ESC-derived MSCs | Rat osteochondral defects | Weekly injections | Histology/IHC | EVs from ESC cell-line-derived MSCs enhanced cartilage repair score (Wakitani) as early as two weeks |
Based on the described isolation method, the specification of exosomes does not seem consistent with criteria established by a position paper from the International Society of Extracellular Vesicles7
Follow-up study to Qi et al (2016)63
Follow-up study to S. Zhang et al (2016)57
BM-MSC, bone-marrow-derived mesenchymal stem cells; µCT, micro-computed tomography; IHC, immunohistochemistry; MSC-EVs, mesenchymal stem cell extracellular vesicles; HIF-1α, hypoxia-inducible factor 1-alpha; MRI, magnetic resonance imaging; IF, immunofluorescence; iPSC, induced pluripotent stem cell; TCP, tricalcium phosphate; DBM, demineralized bone matrix; ESC, embryonic stem cell
Mesenchymal stromal/stem cells
MSCs from human adults have been shown to generate bone tissue in a variety of experimental models.47 These laboratory observations have encouraged the development of bone graft substitutes that incorporate MSCs into osteoconductive scaffolds, with the idea that the MSCs will form new bone upon implantation. There are several completed and ongoing clinical trials applying autogenous or allogeneic MSCs for treatment of complex fractures or nonunions. While the first clinical studies using culture-expanded MSCs were promising,48 the cumulative results have been mixed.22 Pre-clinical studies of MSC fate following implantation or systemic delivery have shown that the large majority of culture-expanded cells contribute to repair indirectly, through their paracrine effects on endogenous cells at the site of injury. For example, MSCs enhance recruitment of endogenous repair cells through secretion of angiogenic and chemotactic factors.49 But if MSCs do not directly participate in new bone formation, their implantation might be inefficient for stimulating repair, since they compete with endogenous repair cells for limited oxygen and nutrients (Fig. 3). In place of MSCs, the mediators of their paracrine effects might be delivered to bone defects.
Pro-regenerative effects by MSC-derived EVs have been extensively reported in pre-clinical models of acute kidney injury, liver and lung injury, myocardial infarction, and hindlimb ischaemia.11,13 In some studies, the effects of MSC-EVs were comparable to those of direct MSC administration, suggesting that EVs relay essential paracrine effects of their parent MSCs. Specific effects attributed to MSC-EVs include increased angiogenesis, inhibition of apoptosis, and reduction of oxidative stress. MSC-EVs have been reported to contribute adenosine triphosphate (ATP) production through their surface kinases, which is thought to improve endogenous cell survival at sites of injury.50,51 MSC-EVs also have established immunomodulatory effects that could impact bone repair.13 For example, EVs from adipose-derived MSCs cultured under hypoxia caused an M1-to-M2 shift in bone marrow macrophage phenotype, which was associated with pro-regenerative EV effects in a muscle injury model.52
Many of the studies to date that have evaluated exogenous EVs for stimulating bone and cartilage repair have used primary MSCs as the donor cells (Table I). In one of the most promising studies, Furuta et al53 first demonstrate delayed endochondral ossification during fracture repair in CD9 global knockout mice, which display reduced exosome secretion, suggesting a role for exosomes in normal fracture healing. When the authors injected exogenous EVs from human bone marrow MSCs into the fracture site of CD9-/- mice, they observed a rescue in delayed healing; moreover, MSC-EVs enhanced fracture repair in wild type mice. Another study has shown that human bone marrow MSC-derived EVs stimulate bone formation within critical-sized calvarial defects made in immunocompetent rats.54
Pluripotent stem cells
While the extensive literature on MSCs supports their evaluation as a potential source of therapeutic EVs, alternative cell sources should also be considered. There has been longstanding interest in the use of embryonic stem cell (ESCs) for bone regenerative medicine.55 ESC lines have been used to generate cells with MSC properties.56 Using human ESC-derived MSCs as a source of exosomes, Zhang et al57 demonstrated that weekly injection of these vesicles enhanced cartilage repair in a rat osteochondral defect model. A follow-up to this original report demonstrated that enhanced cartilage repair was associated with enhanced proliferation, reduced apoptosis, and increased M2 macrophage polarization within the defects.58 It is noteworthy that there is significant osseous injury within these 1 mm deep osteochondral defects (thickness of rat trochlear cartilage is ~ 200 microns). The early histology shown by the authors suggests that enhanced repair of subchondral bone occurred through an endochondral pathway.58
Recent work in bone regenerative medicine has focused on the application of induced pluripotent stem cells (iPSCs), which avoid sourcing barriers associated with using ESCs and autologous MSCs.59 As with ESCs, protocols have been established for generating cartilage- or bone-forming cells from iPSCs,60,61 sometimes through the induction of an intermediate MSC phenotype.62 Exosomes harvested from human iPSC-derived MSCs have been reported to dose-dependently enhance neovascularization and/or bone formation within rat cranial defects.63,64
Monocytes/macrophages
Another possible source of therapeutic EVs are cells of the monocyte/macrophage lineage, which play prominent roles in bone repair, including endochondral fracture repair.65 Macrophage-derived EVs have been shown to improve intestinal regeneration in a mouse model of radiation injury through the delivery of multiple wingless-related integration site (WNT) proteins to intestinal stem cells.66 An important question related to the application of macrophage-derived EVs concerns what source phenotype is best suited to enhance the repair of larger bone defects. While anti-inflammatory (M2) macrophages are known to mediate regenerative effects in wound healing, pro-inflammatory (M1) macrophages can also contribute to bone repair. For example, Zhan et al67 reported that EVs from M2 macrophages increased the proliferation and migration of Schwann cells in vitro compared with EVs from M1 macrophages; this was associated with enhanced Schwann cell infiltration and axon formation by M2 macrophage EVs in a rat sciatic nerve injury model. However, Seebach et al42 have demonstrated that CSBD repair enhanced by exogenous MSC delivery is associated with the recruitment of M1, but not M2, macrophages. The most suitable macrophage phenotype for therapeutic EV production may depend on the specific regenerative activities to be stimulated.
Engineering EVs through their parent cells
The therapeutic efficacy of EVs harvested in vitro will not only be determined by the choice of parent cells, but also by how those cells are conditioned prior to EV harvest. Multiple studies have reported that pre-conditioning various stem cell populations under low oxygen tension improves the pro-angiogenic activity of their EVs both in vitro and in vivo.52,68 EVs collected from MSCs while they were stimulated with pro-inflammatory cytokines or with lipopolysaccharide displayed anti-inflammatory effects on recipient lymphocytes and macrophages, respectively.69,70
In addition to pre-conditioning, parent cells can also be genetically modified in order to enrich their EVs with pro-regenerative signals.71 Horizontal transfer of transgene products have been demonstrated following parent cell modification using plasmid, adenoviral, and lentiviral vectors.71,72 In addition to transgene mRNA or cDNA, microRNAs can also be introduced to EVs using this approach.73,74 Which signals to introduce or enrich within EVs would depend on the specific target activities to be stimulated in the recipient population(s).
Therapeutic targets for an endochondral repair strategy
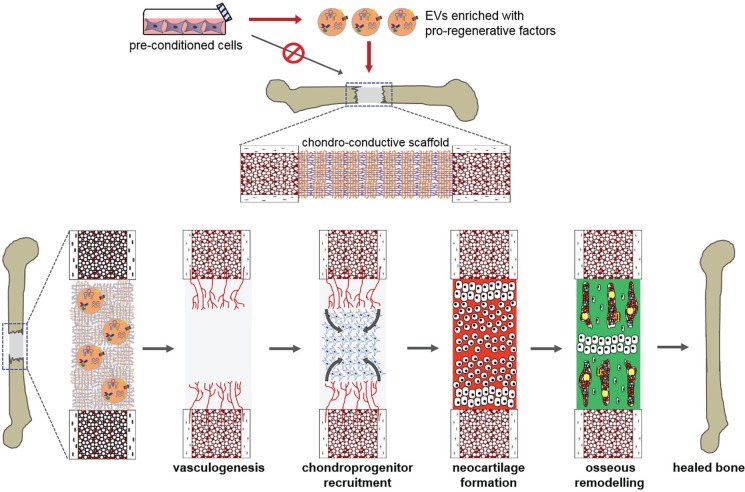
For the repair of large bone defects through an endochondral pathway, three interrelated bottlenecks to repair are hypothesized (Fig. 4): 1) the establishment of a vascular network that supports mesenchymal progenitor recruitment deeper within the defect; 2) the chondrogenic differentiation and hypertrophic maturation of these endogenous progenitors; and 3) the osseous remodelling of the hypertrophic cartilage template by subsequent waves of osteoclast and osteoblast progenitors. It may be possible to deliver EV populations that address one or more of these bottlenecks.
Fig. 4.
Diagram showing therapeutic targets for endochondral repair. Bottlenecks to endochondral bone repair include progressive vascularization, chondroprogenitor recruitment, neocartilage formation, and osseous remodelling. It may be possible to deliver extracellular vesicles (EVs) harvested in vitro from promising parent cell cultures that stimulate endogenous cells to overcome one or more of these bottlenecks. An ideal scaffold for this approach would not only be chondro-conductive but would also control the release of therapeutic EVs, in order to match the migration timeframe of target repair cells (endothelial, chondroprogenitor) into the defect.
Vasculogenesis
The importance of defect vascularization to successful bone repair is well known.75 Because the MSC secretome includes chemotactic and vasculogenic factors,49 there has been much interest in using these cells to stimulate vasculogenesis within CSBDs.76 However, the standard conditions for MSC culture expansion, including atmospheric oxygen and abundant nutrients, are very different from the hypoxic and nutrient-deficient defect microenvironment. Switching MSCs to hypoxia and reduced serum in vitro has been shown to increase their vasculogenic and chemotactic factor secretion, potentially enhancing their indirect contribution to bone repair.40,76,77
When Anderson et al78 exposed MSCs to ischaemic conditions, their EVs were enriched with downstream mediators of the platelet derived growth factor (PDGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF) signalling pathways. This translated to an EV-dose-dependent increase in tubule formation by endothelial cells in vitro. More recently, Gonzalez-King et al68 genetically modified MSCs to overexpress hypoxia inducible factor (HIF)-1α, a transcription factor that regulates cell responses to hypoxia. HIF-1α-MSC-EVs stimulated increased tubule formation in vitro as well as enhanced subcutaneous angiogenesis of EV-laden hydrogels within nude mice. These effects were attributed to increased Jagged1 levels on the HIF-MSC-EVs, as the pro-vasculogenic effects were blunted with a neutralizing Ab against Jagged1. While pro-vasculogenic effects have been shown for EVs from MSCs grown under standard culture conditions,63,79 EVs from hypoxia pre-conditioned MSCs have yet to be tested in a CSBD animal model.
Chondrogenesis
Prior studies that tested recombinant vascular endothelial growth factor (VEGF), a potent angiogenic factor important for fracture repair,15 within rodent CSBDs suggest that vasculogenic stimulation alone may not be sufficient to achieve defect repair.80,81 During endochondral bone formation, chondrogenesis by recruited mesenchymal progenitors is critical for priming subsequent osteogenesis. Moreover, the formation of hypertrophic cartilage is necessary, as opposed to a fibrocartilage repair tissue that would not support ossification, likely resulting in nonunion.
Martins et al82 recently reported that EVs harvested from osteogenically differentiated MSCs were able to independently stimulate the osteogenic commitment of naïve, recipient MSCs. A similar observation was made in the context of MSC neurogenic differentiation.83 These studies suggest that the differentiation of endogenous progenitor cells in a bone defect can be guided by EVs from culture-expanded stem/progenitors directed along a similar pathway in vitro. Such lineage guidance using EVs has not yet been described for chondrogenesis. However, it is known that co-culture of MSCs with articular chondrocytes improves chondrogenic differentiation of the MSCs;84 it is possible that chondrocyte-derived EVs contribute, in part, to the co-culture effect.
Osseous remodelling
During the repair of mechanically stable fractures, hypertrophic cartilage within the fracture callus undergoes mineralization, vascularization, and remodelling into woven bone. Matrix vesicles have long been observed in the hypertrophic cartilage of the callus during fracture repair.85 These vesicles, which have been proposed to be matrix-anchored exosomes,86 are secreted by hypertrophic chondrocytes and function to nucleate cartilage calcification.87 More recent studies have suggested a functional role for the microRNAs enriched within matrix vesicles.88 Of note, it has been shown that these vesicles contain growth factors that regulate bone formation, such as BMP-2 and VEGF.89
It may be possible to produce EVs that mimic matrix vesicles in composition for the purpose of stimulating repair tissue ossification. A possible parent cell population for such EVs would be hypertrophic chondrocytes, which can be derived from MSCs.28,90 To produce calcification-nucleating, matrix vesicle-mimetic EVs, however, the normal culture conditions for MSC differentiation may require additional optimization.
Challenges for clinical application
A recent position paper from the International Society of Extracellular Vesicles (ISEV) has provided a comprehensive review of the many translational considerations for EV-based therapeutics.16 Much of the uncertainty about how EVs can be applied clinically is related to persisting gaps in understanding about their biogenesis and function. As with other biological medicinal products, successful translation of EVs to skeletal regenerative medicine will hinge on continued basic science studies that fill in these knowledge gaps.
EV mechanisms of action
The role of EVs in successful endochondral bone repair (e.g. fracture healing) is not completely understood. As early as the 1960s, matrix vesicles were described as playing a role in mineralization of the growth plate.91 The recent study by Furuta et al53 described above suggests that EVs have a significant role to play in fracture healing: the authors demonstrated reduced healing in CD9 knockout mice and partial rescue following injection of exogenous, MSC-derived EVs. As the mechanisms governing EV biogenesis and cell uptake become better understood, more conclusive studies into their roles in bone repair may be completed. For example, identifying key protein mediators of biogenesis and uptake should permit genetic engineering tools to be employed for studies of EV knockout and overexpression. Similarly, a better understanding of how pro-regenerative factors are sorted into specific EV subpopulations could be used to block or enhance their loading and demonstrate the importance of their EV-specific delivery in vivo. In the absence of this knowledge, however, early therapeutic approaches can be guided by studies of how EVs isolated from candidate parent cells in vitro stimulate repair in pre-clinical injury models.
The mechanisms of action for an EV therapeutic will likely depend on the subtype used. While a greater degree of evidence has been provided for exosomes, pro-regenerative actions have also been demonstrated for the microvesicle fraction of parent-cell-conditioned media.92 While many studies have shown beneficial effects when using a heterogeneous population of EVs,53,54,79 these studies cannot infer whether the different subpopulations were agonistic or antagonistic to one another. Future studies should consider the relative efficacy of exosomes versus microvesicles for each therapeutic application. Their conclusiveness will depend on an improved distinction of these distinct EV subpopulations.7
EV delivery
For bone defect repair, medicinal EVs would ideally be delivered directly to the defect site, as opposed to systemic delivery, which would require higher total doses to obtain the same defect concentration, thereby increasing risk of side effects in off-target tissues. Because large defects typically require implantation of a gap-filling scaffold, which supports ingrowth of endogenous repair cells, a promising solution would be to employ the scaffold as a depot for local release of EVs. Such an approach has been used for the delivery of other osteogenic factors, such as recombinant bone morphogenetic protein (BMP)-2, to bone defects93,94 However, clinical observations from BMP-2 use demonstrate the need for scaffolds that control the release of an osteogenic therapeutic: burst release of BMP-2 at supraphysiological levels from collagen-based scaffolds has been associated with adverse effects, including perioperative inflammation and pain, resorption of nearby intact bone, and formation of heterotopic bone in adjacent soft tissues.95-99 It is possible that EVs could have similar adverse effects if delivered by burst release. Additional research into how biomaterials can be used to control the release of different EV populations will complement mechanistic studies of EV action in order to design therapeutic strategies for large bone defects.
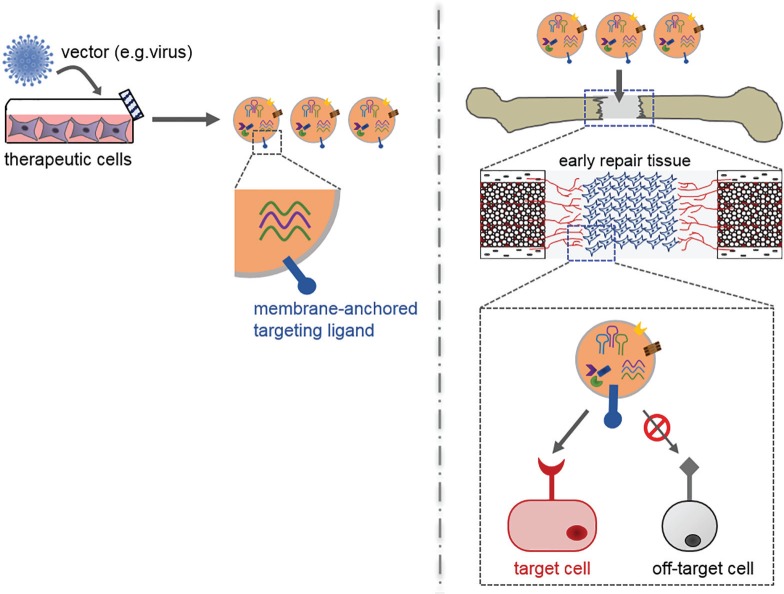
In addition to intra-defect release, the specificity of EV action may be improved by targeting them to endogenous repair populations, such as endothelial or chondroprogenitors cells. During normal intercellular communication, EVs deliver their cargo by direct signalling with target cell receptors, through vesicle docking with the target cell plasma membrane, or through endocytosis of the EV and subsequent cargo release. Accordingly, EVs could be engineered to express targeting ligands on their surface, such as membrane-anchored peptides, that specifically recognize cognate receptors on the target cells of interest100 (Fig. 5). By targeting the vesicles to the repair populations of interest, the per-cell delivery of therapeutic cargo might be enhanced, allowing the total administered EV dose to be lowered. Additional studies are still required to identify the most appropriate target cells and surface receptors for improving bone defect repair. Because these populations may enter the defect over distinct timeframes, information on their migration kinetics will guide the design of scaffolds for controlled release of pre-loaded EVs.
Fig. 5.
Diagram showing the genetic engineering of extracellular vesicles (EVs) for targeting endogenous repair cells. The efficacy of exogenous EVs may be improved by introducing targeting ligands onto their surface that recognize cell surface receptors specific for key repair cells. One way in which to introduce these ligands would be to genetically engineer parent cells to express them within the extracellular domain of a membrane-anchored fusion protein. The fusion protein would then be expressed on the surface of EVs secreted by the parent cells. In addition to potentially improving the efficiency of target cell uptake of EV contents, this strategy could also limit nonspecific or adverse effects in off-target cells.
Approval of medicinal EVs
Widespread clinical use of EVs will depend on approval by regional regulatory bodies, such as the United States Food and Drug Administration. These regulatory bodies will require thorough characterization of specific EV therapeutics, including their essential composition and proposed mode of action (MoA), and the qualification of potency assays that reflect the proposed MoA. Demonstration of safety and efficacy in clinically relevant animal models of bone injury will also be important for translation to the clinic. Finally, procedures must be established for EV isolation, storage, and quality control testing that meet Good Manufacturing/Laboratory Practice (GMP/GLP) standards.16 Due to the complex makeup of EVs, meeting these standards may be more challenging than for biological medicinal products already approved for bone repair, such as BMP-2. Because EVs have a similar degree of complexity as their parent cells, including heterogeneity in composition, EV-based therapeutics may follow a similar regulatory pathway as cell-based therapeutics. For EVs derived from genetically modified cells, the higher degree of manipulation would likely categorize them as Advanced Therapy Medicinal Products,101 requiring more stringent safety testing.
Long-term prospective
Given the inherent complexity of EVs, information gained from ongoing pre-clinical studies may ultimately be used to develop synthetic vesicles containing only those essential components required for the desired effects in target cells. There have been extensive efforts to develop liposomes as drug delivery vehicles, providing a base of knowledge for the production of artificial, EV-mimetic vesicles.102 However, development of an effective EV alternative would require a much better understanding of the mechanisms of EV action, in order to determine which components are essential and at what concentrations they should be loaded into artificial vesicles. It is likely that effective, if imperfect, natural EV products may be employed clinically while the research community learns how to reverse engineer their essential functions.
Footnotes
Author Contribution: E. Ferreira: Reviewing the literature, Preparing and editing the manuscript.
R. M. Porter: Reviewing the literature, Preparing and editing the manuscript.
Conflicts of Interest Statement: None declared.
Follow us @BoneJointRes
Funding Statement
The authors’ activity in this area is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under award numbers AR069253 and AR071560. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Stevenson S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res 1998;355S(suppl):S239-S246. [DOI] [PubMed] [Google Scholar]
- 2. Levin LS. Vascularized fibula graft for the traumatically induced long-bone defect. Journal Am Academy Ortho Surgeons 2006;14:S175-176. [DOI] [PubMed] [Google Scholar]
- 3. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 2010;41:27-37. [DOI] [PubMed] [Google Scholar]
- 4. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012;40:363-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mauffrey C, Barlow BT, Smith W. Management of segmental bonedefects. J Am Acad Orthop Surg 2015;23:143-153. [DOI] [PubMed] [Google Scholar]
- 6. MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg [Am] 2007;89-A:1685-1692. [DOI] [PubMed] [Google Scholar]
- 7. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-659. [DOI] [PubMed] [Google Scholar]
- 10. Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol 2015;55:439-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest 2016;126:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum Gene Ther 2015;26:506-517. [DOI] [PubMed] [Google Scholar]
- 14. Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 2003;88:873-884. [DOI] [PubMed] [Google Scholar]
- 15. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2015;11:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malda J, Boere J, van de Lest CH, van Weeren P, Wauben MH. Extracellular vesicles— new tool for joint repair and regeneration. Nat Rev Rheumatol 2016;12:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz M, Cosenza S, Maumus M, Jorgensen C, Noël D. Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin Biol Ther 2016;16:33-42. [DOI] [PubMed] [Google Scholar]
- 19. Burke J, Kolhe R, Hunter M, et al. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int 2016;2016:5802529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen B, Li Q, Zhao B, Wang Y. Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Transl Med 2017;6:1753-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kronenberg HM. Developmental regulation of the growth plate. Nature 2003;423:332-336. [DOI] [PubMed] [Google Scholar]
- 22. Grayson WL, Bunnell BA, Martin E, et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol 2015;11:140-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenas P, Moos M, Jr, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev 2009;15:381-394. [DOI] [PubMed] [Google Scholar]
- 24. Farrell E, van der Jagt OP, Koevoet W, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods 2009;15:285-295. [DOI] [PubMed] [Google Scholar]
- 25. Farrell E, Both SK, Odörfer KI, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 2011;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-147. [DOI] [PubMed] [Google Scholar]
- 27. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998;238:265-272. [DOI] [PubMed] [Google Scholar]
- 28. Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA 2010;107:7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scotti C, Piccinini E, Takizawa H, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA 2013;110:3997-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bahney CS, Hu DP, Taylor AJ, et al. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 2014;29:1269-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang PN, Dwivedi N, Phillips LM, et al. Controlled dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem Cells Transl Med 2016;5:206-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dang PN, Herberg S, Varghai D, et al. Endochondral ossification in critical-sized bone defects via readily implantable scaffold-free stem cell constructs. Stem Cells Transl Med 2017;6:1644-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng J, Wei Y, Zhou B, et al. Extracellular matrix components and culture regimen selectively regulate cartilage formation by self-assembling human mesenchymal stem cells in vitro and in vivo. Stem Cell Res Ther 2016;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheehy EJ, Mesallati T, Vinardell T, Kelly DJ. Engineering cartilage or endochondral bone: a comparison of different naturally derived hydrogels. Acta Biomater 2015;13:245-253. [DOI] [PubMed] [Google Scholar]
- 35. Harada N, Watanabe Y, Sato K, et al. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 2014;35:7800-7810. [DOI] [PubMed] [Google Scholar]
- 36. Cunniffe GM, Vinardell T, Murphy JM, et al. Porous decellularized tissue engineered hypertrophic cartilage as a scaffold for large bone defect healing. Acta Biomater 2015;23:82-90. [DOI] [PubMed] [Google Scholar]
- 37. Evans CH, Palmer GD, Pascher A, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng 2007;13:1987-1993. [DOI] [PubMed] [Google Scholar]
- 38. Carlier A, van Gastel N, Geris L, Carmeliet G, Van Oosterwyck H. Size does matter: an integrative in vivo-in silico approach for the treatment of critical size bone defects. PLOS Comput Biol 2014;10:e1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. OReilly A, Hankenson KD, Kelly DJ. A computational model to explore the role of angiogenic impairment on endochondral ossification during fracture healing. Biomech Model Mechanobiol 2016;15:1279-1294. [DOI] [PubMed] [Google Scholar]
- 40. Potier E, Ferreira E, Andriamanalijaona R, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007;40:1078-1087. [DOI] [PubMed] [Google Scholar]
- 41. Moya A, Larochette N, Paquet J, et al. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells 2017;35:181-196. [DOI] [PubMed] [Google Scholar]
- 42. Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater 2014;10:4730-4741. [DOI] [PubMed] [Google Scholar]
- 43. Manassero M, Paquet J, Deschepper M, et al. Comparison of survival and osteogenic ability of human mesenchymal stem cells in orthotopic and ectopic sites in mice. Tissue Eng Part A 2016;22:534-544. [DOI] [PubMed] [Google Scholar]
- 44. Moya A, Paquet J, Deschepper M, et al. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells 2017;12:21. [DOI] [PubMed] [Google Scholar]
- 45. Al-Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008;10:619-624. [DOI] [PubMed] [Google Scholar]
- 46. Lőrincz AM, Timár CI, Marosvári KA, et al. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles 2014;3:25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344:385-386. [DOI] [PubMed] [Google Scholar]
- 49. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 2011;9:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 2013;10:301-312. [DOI] [PubMed] [Google Scholar]
- 51. Lai RC, Yeo RW, Tan KH, Lim SK. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med 2013;8:197-209. [DOI] [PubMed] [Google Scholar]
- 52. Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med 2017;6:1018-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Furuta T, Miyaki S, Ishitobi H, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med 2016;5:1620-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 2016;6:21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gamie Z, Tran GT, Vyzas G, et al. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin Biol Ther 2012;12:713-729. [DOI] [PubMed] [Google Scholar]
- 56. Lian Q, Lye E, Suan Yeo K, et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells 2007;25:425-436. [DOI] [PubMed] [Google Scholar]
- 57. Zhang S, Chu WC, Lai RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage 2016;24:2135-2140. [DOI] [PubMed] [Google Scholar]
- 58. Zhang S, Chuah SJ, Lai RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018;156:16-27. [DOI] [PubMed] [Google Scholar]
- 59. Teng S, Liu C, Krettek C, Jagodzinski M. The application of induced pluripotent stem cells for bone regeneration: current progress and prospects. Tissue Eng Part B Rev 2014;20:328-339. [DOI] [PubMed] [Google Scholar]
- 60. Phillips MD, Kuznetsov SA, Cherman N, et al. Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays. Stem Cells Transl Med 2014;3:867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Craft AM, Rockel JS, Nartiss Y, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol 2015;33:638-645. [DOI] [PubMed] [Google Scholar]
- 62. Tran NT, Trinh QM, Lee GM, Han YM. Efficient differentiation of human pluripotent stem cells into mesenchymal stem cells by modulating intracellular signaling pathways in a feeder/serum-free system. Stem Cells Dev 2012;21:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci 2016;12:836-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schlundt C, El Khassawna T, Serra A, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 2018;106:78-89. [DOI] [PubMed] [Google Scholar]
- 66. Saha S, Aranda E, Hayakawa Y, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 2016;7:13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhan C, Ma CB, Yuan HM, Cao BY, Zhu JJ. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun 2015;468:343-348. [DOI] [PubMed] [Google Scholar]
- 68. Gonzalez-King H, García NA, Ontoria-Oviedo I, et al. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 2017;35:1747-1759. [DOI] [PubMed] [Google Scholar]
- 69. Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med 2015;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 2018;36:79-90. [DOI] [PubMed] [Google Scholar]
- 71. Kanada M, Bachmann MH, Hardy JW, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 2015;112:E1433-E1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim SH, Lechman ER, Bianco N, et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol 2005;174:6440-6448. [DOI] [PubMed] [Google Scholar]
- 73. Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 2014;445:381-387. [DOI] [PubMed] [Google Scholar]
- 74. Chen Y, Zhao Y, Chen W, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther 2017;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bahney CS, Hu DP, Miclau T, III, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Paquet J, Deschepper M, Moya A, et al. Oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Transl Med 2015;4:809-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li H, Liu D, Li C, et al. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int 2017;41:1379-1390. [DOI] [PubMed] [Google Scholar]
- 78. Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells 2016;34:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xie H, Wang Z, Zhang L, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep 2017;7:45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Eckardt H, Ding M, Lind M, et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg [Br] 2005;87-B:1434-1438. [DOI] [PubMed] [Google Scholar]
- 81. Patel ZS, Young S, Tabata Y, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008;43:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martins M, Ribeiro D, Martins A, Reis RL, Neves NM. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Reports 2016;6:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Takeda YS, Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One 2015;10:e0135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 2010;62:2696-2706. [DOI] [PubMed] [Google Scholar]
- 85. Ketenjian AY, Arsenis C. Morphological and biochemical studies during differentiation and calcification of fracture callus cartilage. Clin Orthop Relat Res 1975;107:266-273. [DOI] [PubMed] [Google Scholar]
- 86. Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: are they anchored exosomes? Bone 2015;79:29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boyan BD, Schwartz Z, Swain LD. Matrix vesicles as a marker of endochondral ossification. Connect Tissue Res 1990;24:67-75. [DOI] [PubMed] [Google Scholar]
- 88. Lin Z, Rodriguez NE, Zhao J, et al. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone 2016;88:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab 2008;26:514-519. [DOI] [PubMed] [Google Scholar]
- 90. Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 2008;58:1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 1969;41:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep 2017;7:16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Govender S, Csimma C, Genant HK, Valentin-Opran A. BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg [Am] 2002;84-A:2123-2134. [DOI] [PubMed] [Google Scholar]
- 94. Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 2002;27:2396-2408. [DOI] [PubMed] [Google Scholar]
- 95. Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg [Br] 2008;90-B:1617-1622. [DOI] [PubMed] [Google Scholar]
- 96. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471-491. [DOI] [PubMed] [Google Scholar]
- 97. Kang JD. Another complication associated with rhBMP-2? Spine J 2011;11:517-519. [DOI] [PubMed] [Google Scholar]
- 98. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine 2007;32:2885-2890. [DOI] [PubMed] [Google Scholar]
- 99. Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J 2007;7:609-614. [DOI] [PubMed] [Google Scholar]
- 100. Zhao C, Busch DJ, Vershel CP, Stachowiak JC. Multifunctional Transmembrane Protein Ligands for Cell-Specific Targeting of Plasma Membrane-Derived Vesicles. Small 2016;12:3837-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hanna E, Remuzat C, Auquier P, Toumi M. Advanced therapy medicinal products: current and future perspectives. J Mark Access Health Policy 2016:4:31036-31045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. van der Meel R, Fens MH, Vader P, et al. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release 2014;195:72-85. [DOI] [PubMed] [Google Scholar]