Abstract
Objectives
This study aimed to assess the effect of age and osteoporosis on the proliferative and differentiating capacity of bone-marrow-derived mesenchymal stem cells (MSCs) in female rats. We also discuss the role of these factors on expression and migration of cells along the C-X-C chemokine receptor type 4 (CXCR-4) / stromal derived factor 1 (SDF-1) axis.
Methods
Mesenchymal stem cells were harvested from the femora of young, adult, and osteopenic Wistar rats. Cluster of differentiation (CD) marker and CXCR-4 expression was measured using flow cytometry. Cellular proliferation was measured using Alamar Blue, osteogenic differentiation was measured using alkaline phosphatase expression and alizarin red production, and adipogenic differentiation was measured using Oil red O. Cells were incubated in Boyden chambers to quantify their migration towards SDF-1. Data was analyzed using a Student’s t-test, where p-values < 0.05 were considered significant.
Results
CD marker expression and proliferation of the MSCs from the three groups was not significantly different. The young MSCs demonstrated significantly increased differentiation into bone and fat and superior migration towards SDF-1. The migration of SDF-1 doubled with young rats compared with the adult rats (p = 0.023) and it was four times higher when compared with cells isolated from ovariectomized (OVX) osteopenic rats (p = 0.013).
Conclusion
Young rat MSCs are significantly more responsive to osteogenic differentiation, and, contrary to other studies, also demonstrated increased adipogenic differentiation compared with cells from adult and ostopenic rats. Young-rat-derived cells also showed superior migration towards SDF-1 compared with MSCs from OVX and adult control rats.
Cite this article: A. Sanghani-Kerai, L. Osagie-Clouard, G. Blunn, M. Coathup. The influence of age and osteoporosis on bone marrow stem cells from rats. Bone Joint Res 2018;7:289–297. DOI: 10.1302/2046-3758.74.BJR-2017-0302.R1.
Keywords: Osteoporosis, Mesenchymal stem cells, Bone formation, Differentiation, Migration, Stem cells
Article focus
The aim of this study was to isolate stem cells from osteopenic rats, and to investigate and compare their cluster of differentiation marker expression, proliferation, migration, osteogenic, and adipogenic differentiation potential.
The hypothesis of this study is that these characteristics will differ between mesenchymal stem cells (MSCs) harvested from young, adult, and ovariectomized rats.
Key messages
The poor bone formation in postmenopausal women could be due to poor retention and function of mesenchymal stem cells resulting into delayed unions.
Our study found significant differences in both the osteogenic and adipogenic potential of stem cells derived from young and osteopenic animals. Similarly, cells derived from osteopenic rats also demonstrated reduced migrative capacity to stromal cell-derived factor 1 (SDF-1).
Strengths and limitations
This study examines the effect of age and osteopenia on cell characteristics, using both quantitative and qualitative analysis.
The study would benefit from further investigation of the underlying mechanisms that reduce differentiating capacity and C-X-C chemokine receptor type 4 (CXCR-4) expression from cells derived from osteopenic animals, and from resultantly addressing the potential link with age-related deficits in bone repair.
Introduction
Osteoporosis causes over 8.9 million fractures annually worldwide; characterized by loss of trabecular architecture and bone mass, the disease has a 40% lifetime risk for sustaining a fragility fracture.1-3 Type I osteoporosis, also known as postmenopausal osteoporosis, is characterized by increased bone turnover and accelerated cancellous bone loss, resulting in an increased risk of vertebral fractures.3 Type II osteoporosis affects both men and women and leads to an increased incidence of fractures; mortality and morbidity thus account for nearly three million disability-adjusted life years. Regardless of type, the underlying pathology of osteoporosis is aberrant bone turnover, secondary to imbalanced bone resorption and formation.4 Bone disorders developed due to osteoporosis are often challenging to treat and therefore new techniques involving gene therapy, cell therapy, and tissue engineering are being explored to improve bone regeneration.5
Osteoporosis is a consequence of the imbalance between bone formation by the osteoblast and bone resorption carried out by the osteoclast, resulting in net bone loss.6 The number of mesenchymal stem cells (MSCs) within bone marrow decreases with age and this may be associated with a reduction in bone formation.7-10 The number of MSCs, as well as their ability to differentiate into osteoprogenitor cells and mature osteoblasts, may be related to this imbalance.11-14
In addition to the differentiating ability of MSCs, their ability to mobilize from their niche to the tissue endothelium, and to mature into active cell types that modulate the fracture environment, is dependent upon their migration capacity. The stromal derived factor-1 (SDF-1) / C-X-C chemokine receptor type 4 (CXCR-4) axis has been found to be an important regulator of stem cell migration. SDF-1 is produced by a multitude of tissue types, including fracture endosteum, and in its active form is bound to the CXCR-4 receptor found on MSCs. Among others, Granero-Molto et al15 have demonstrated that dynamic stem cell migration to the fracture site in a stabilized tibia osteotomy model was CXCR-4 dependent. The overexpression of CXCR-4 on mesenchymal stem cells led to significant increases in bone mineral density in an osteopenic mouse model, indicating the clinical significance of the SDF-1/CXCR-4 axis in the treatment of osteoporosis. The role of ageing and osteoporosis on CXCR-4 cell expression, and thus migration to SDF-1, is yet to be fully elucidated.
Although osteoporosis and ageing are interlinked, and differences between stem cells from young and old patients have been reported, no previous studies have compared the functional differences between MSCs from adolescent, adult, and osteoporotic populations. The aim of this study was to ovariectomize rats (OVX) and establish an osteopenic model, and to examine the influence of age and osteopenia on the morphology, proliferation, differentiation, CXCR-4 expression, and migration of bone marrow derived MSCs. The hypothesis was that MSCs from OVX rats will have a lower proliferative and osteogenic potential, will have a lower CXCR-4 expression and migratory capacity, and will be more likely to differentiate into adipocytes compared with MSCs obtained from young rats.
Materials and Methods
Bone marrow culture
Rat bone marrow mesenchymal stem cells (rBMCs) were harvested from randomized two-week-old to four-week-old, and six-month-old to nine-month-old, female Wistar rat femora (n = 6), with a mean lifespan of 22 months.16 Bone marrow cells were harvested by flushing the femora with Dulbecco’s modified Eagle medium (DMEM) (Sigma-Aldrich, St Louis, Missouri), 20% fetal calf serum (Wolverhampton, United Kingdom), 1% penicillin streptomycin (Thermo Fisher Scientific, San Diego, California) in a 25 cm2 flask. Cells were cultured at 37°C at 5% CO2. Media was changed after four days to remove non-adherent cells and then continuously refreshed twice a week thereafter. After ten to 14 days of primary culture and when the cells were between 70% and 80% confluent, they were passaged using Trypsin-EDTA (Sigma-Aldrich). rBMCs were passaged every seven to eight days. Cells were characterized by tridifferentation and phenotypically with immunocytochemistry.
Rat ovariectomy
Wistar rats (n = 6) between six months and nine months old were weighed, anaesthetized, and shaved over the abdominal area. A transverse incision was made on the centre of the abdomen. The transverse abdominal muscles were exposed and dissected. Thereafter, the peritoneal space and adipose tissue surrounding the ovary were exposed. The ovary was then retracted, the area around the distal uterine horns was sutured to section it off, and the ovaries were removed. The procedure was then repeated on the left ovary through the same skin incision site. The rats were left for four months, after which their bone mineral density was measured using peripheral quantitative CT (pQCT) (Stratec XCT1000) and results were compared with non-OVX control rats of the same age. Husbandry conditions for all animals were the same, whereby animals were housed in pairs in cages without restriction to ambulation on a standard rodent diet. Cages included adequate bedding and unrestricted access to fluids, along with objects for animal enrichment. The rats were then euthanized and stem cells isolated from their femora as described above.
Flow cytometry analysis for cluster of differentiation marker expression
A total of 10 000 rBMCs from young, adult control, and OVX rats were analyzed for their surface expression of cluster of differentiation (CD) CD29, CD90, CD45, and CD34. The cells were labelled with anti-mouse/rat CD29-fluorescein (Thermo Fisher Scientific),17 anti-mouse/rat CD90-APC (Thermo Fisher Scientific), anti-rat CD45-APC (Thermo Fisher Scientific), and CD34-PE (Abcam, Cambridge, United Kingdom). The CD expression was compared with the isotype control. A total of 10 000 cells were fixed in 4% formalin for 15 minutes at room temperature, washed with 0.5% bovine serum albumin (BSA), and stained with the conjugated primary antibody for one hour at room temperature in the dark. After one hour, the cells were washed with 0.5% BSA and analyzed on flow cytometer (Cytoflex, Beckman Coulter, Brea, California).
Cell morphology
Passage 2 and 3 rBMCs from the three groups were cultured and their morphology was assessed by measuring their aspect ratio, whereby the ratio of the length of a cell to its width was calculated using ImageJ software.18 For each group, n = 3 was used.
Cell proliferation
A total of 10 000 rBMCs from young, adult, and OVX rats (n = 3 for each group) were incubated in DMEM, 20% fetal calf serum, 1% penicillin streptomycin and 10% Alamar Blue assay (AbD Serotec, Kidlington, United Kingdom) for four hours; the resultant media was read at an excitation of 560 nm and an emission of 590 nm using a Tecan plate reader (Infinite Pro 200 Series, Tecan, Männedorf, Switzerland). The mean absorbance was determined from the triplicate samples. The absorbance was then normalized to the DNA assays and a comparison was made between the groups.
Osteogenic differentiation
A total of 30 000 cells from each of the three experimental groups were cultured in a 48-well plate in osteogenic media that consisted of DMEM supplemented with 100 nM dexamethasone, 50 ug/ml L-ascorbic acid 2-phosphate, and 10 mM beta-glycerol phosphate (n = 3 from each group). The cells were grown at 37°C at 5% CO2 and their alkaline phosphatase (ALP) activity was measured at three, seven, 14, and 21 days. This was then normalized against the DNA Hoescht assay (Sigma-Aldrich). Hoechst 33258 fluorimeteric dye (Sigma-14530, Sigma-Aldrich) was added to the cell lysates and fluorescence was measured at a wavelength of 460 nm.19 Additionally, calcium phosphate deposition was measured by quantification of Alizarin Red staining using cetylpyridium chloride (CPC) on days seven, 14, and 21. The cells were washed in phosphate buffered saline (PBS), fixed in formalin for 15 minutes, and then stained with Alizarin Red solution (pH 4.2) for ten minutes at room temperature. The cultures were then rinsed five times with PBS.20 The stained samples were photographed and then quantitatively de-stained using 10% CPC in 10 mM sodium phosphate, pH 7.0, for 15 minutes at room temperature. The Alizarin Red concentration was then read on a plate reader at a wavelength of 570 nm (Infinite Pro 200 Series, Tecan). Concentrations were determined using a standard curve obtained from tenfold serial dilutions of alizarin red.
Adipogenic differentiation
A total of 30 000 cells were cultured in a 48-well plate in adipogenic media that consisted of DMEM supplemented with 0.1 mM dexamethasone, 50 mM indomethacin, 0.45 mM 3-isobutyl-1-methylxanthine, and 10 mg/ml insulin. The cells were grown at 37°C at 5% CO2 and the presence of fat was stained using Oil Red O at seven, 14, and 21 days. Additionally, Oil Red O staining was quantified using 100% isopropanol. The cells were washed in PBS, fixed in paraformaldehyde for five minutes, washed with 60% isopropanol for ten minutes, and then stained with Oil Red O solution for 15 minutes at room temperature. The cultures were then rinsed five times with PBS. The stained samples were photographed and then quantitatively de-stained using 100% isopropanol for 15 minutes at room temperature. The Oil Red O concentration was read on a plate reader at a wavelength of 510 nm (Infinite Pro 200 Series, Tecan). Again, concentrations were determined using a standard curve obtained from tenfold serial dilution of Oil Red O stain.
Flowcytometry analysis to measure CXCR-4 expression
Young rBMCs from the third passage were trypsinized and centrifuged at 2000 rpm for ten minutes before 100 000 cells were resuspended in PBS. Cell aliquots were incubated with primary CXCR-4 antibody (Abcam) for 30 minutes at room temperature. The cells were washed in PBS and then incubated in secondary goat anti-rabbit antibody (Abcam) for 30 minutes at room temperature. The negative control consisted of cells incubated in the secondary antibody only. Then, 10 000 cells were analyzed using flow cytometry (Guava easyCyte system, Merck, Kenilworth, New Jersey).
Cell migration
A chemoinvasion assay was used to evaluate the ability of rBMCs from young, adult, and OVX rats to migrate towards SDF-1. rBMCs from the three groups were loaded separately in serum free medium in upper compartments of the Boyden chamber, 5 µm pore size (Corning Inc., Corning, New York). The lower compartment of the Boyden chamber was filled with 100 ng/ml SDF-1 (Peprotech, London, United Kingdom) in DMEM and penicillin streptomycin. The chambers were incubated at 37°C, 5% CO2, for 16 hours to allow for cell migration through the chamber. After 16 hours, the cells that migrated to the opposite side of the membrane were fixed in 10% formaldehyde (Sigma-Aldrich) and stained with crystal violet (Sigma-Aldrich). The migrated cells were counted by selecting six random fields at ×20 magnification and calculating the percentage mean number of cells; the counter was blinded to the study group when analyzing the fields. For each cell type, the experiment was repeated in triplicate. For the control, both the top and bottom of the chamber were filled with normal media with no SDF-1 and the cells were loaded in the upper chamber as described.
Statistical analysis
Data was analyzed using multiple two-tailed Student’s t-tests to make comparisons between specific groups. All data was compared using SPSS software (IBM Inc., Armonk, New York); results were considered significant at p < 0.05 level and are expressed where appropriate with standard deviations.
Results
Establishment of the rat osteopenic model
There were no surgical complications and no macroscopic signs of infection. At four months post-surgery, pQCT measurements demonstrated that the mean bone mineral density (BMD) of the OVX rats was 538.2 g/cm3 (sd 23.3) in comparison to 666.9 g/cm3 (sd 46) in aged matched rats, which was a reduction of 19.3% (sd 9) in BMD (p < 0.001).
Expression of CD markers
There was no mean significant difference in CD marker expression between rBMCs from young, adult control, and OVX rats. Flow cytometry showed high expression of CD29 (mean 95.1% (sd 3.2)), CD90 (mean 96.6% (sd 2.1)) and low expression of CD34 (mean 2.6% (sd 1.1)) and CD45 (mean 10.9% (sd 9.6)) for all three groups of MSCs. These CD marker expressions were similar for MSCs from all three groups of rats.
Cell morphology
MSCs from young rats at passage 2 and 3 were smaller with more spindle-like features compared with MSCs from adult control and OVX rats. The mean aspect ratio measured in the young MSC group was 18.66 (sd 13.45), which was significantly larger than cells obtained from adult (mean 4.99 (sd 4.67)) and OVX rats (mean 2.25 (sd 0.94)).
Cell proliferation
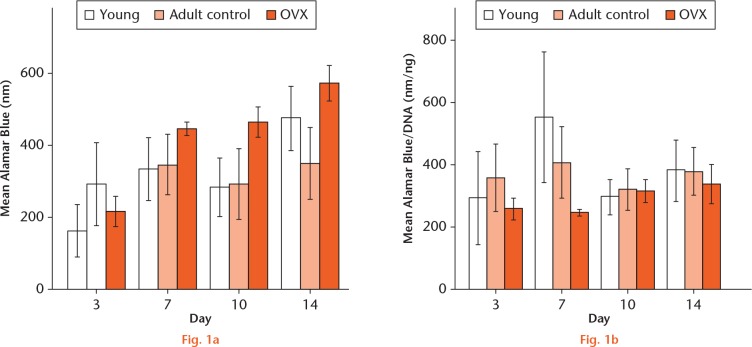
Growth curves were plotted to examine cell proliferation. After three days in culture, the rBMCs showed time-dependent growth in all samples until day seven. Overall, there was no significant difference in proliferation between rBMCs from young, adult control, and OVX rats. All cell groups had an increase in proliferation between days three and days seven (Fig. 1).
Graphs showing: a) the mean Alamar Blue measurements of mesenchymal stem cells from young, adult control, and ovariectomized rats at day three, seven, ten, and 14 (n = 3); and b) mean Alamar Blue readings normalized against DNA to reflect their metabolic activity. No statistical significance was found.
Osteogenic differentiation
Mineralization of the extracellular matrix in young, adult, and OVX rBMCs increased significantly from day seven to day 21 for all the three rat groups (Fig. 2). At day seven, the amount of calcium phosphate produced was similar between the three groups of cells, although by day 14 there was a significant increase in the amount of calcium phosphate produced by all groups; this remained the case up to day 21. At day 14, MSCs from young rats produced nearly three times the amount of mineral compared with cells isolated from ovariectomized animals (p = 0.004). This significant difference in mineralization (p = 0.0018) was still apparent at 21 days.
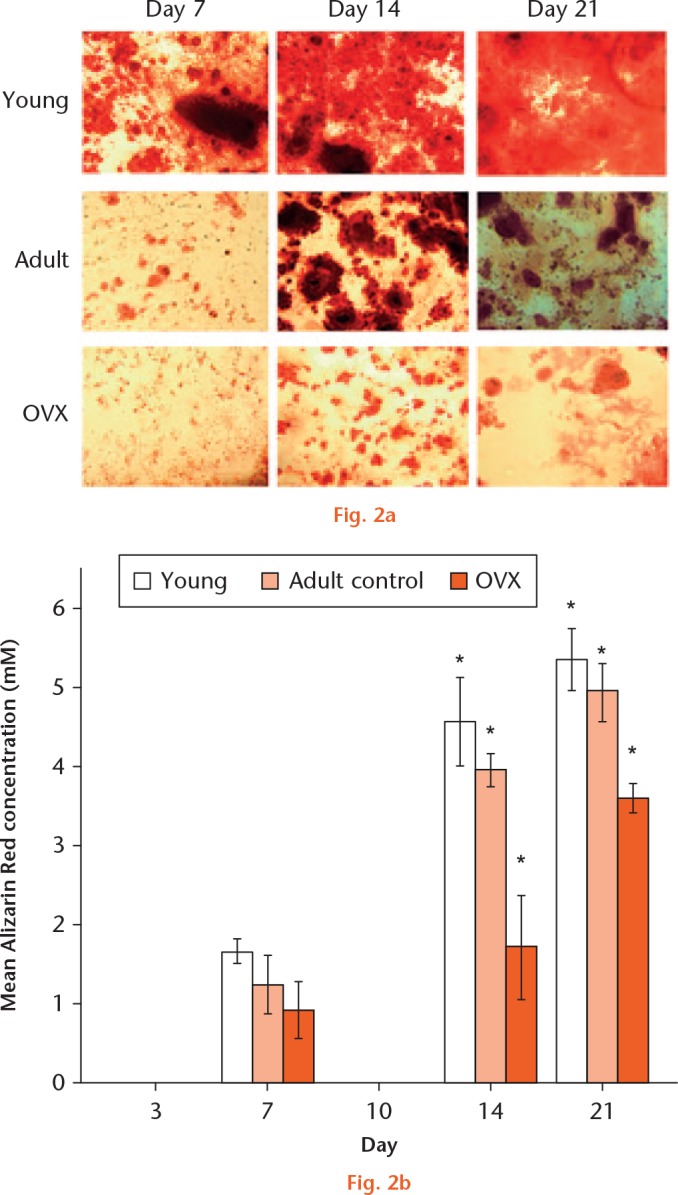
a) Alizarin staining to show osteogenic differentiation of young, adult, and ovariectomized (OVX) mesenchymal stem cells (MSCs) at day seven, 14, and 21. b) Graph showing the mean Alizarin Red production when MSCs from young, adult control, and OVX rats (n = 3) differentiated to osteoblasts at days seven, 14, and 21. *Significant difference of p < 0.05 using a two-tailed Student’s t-test.
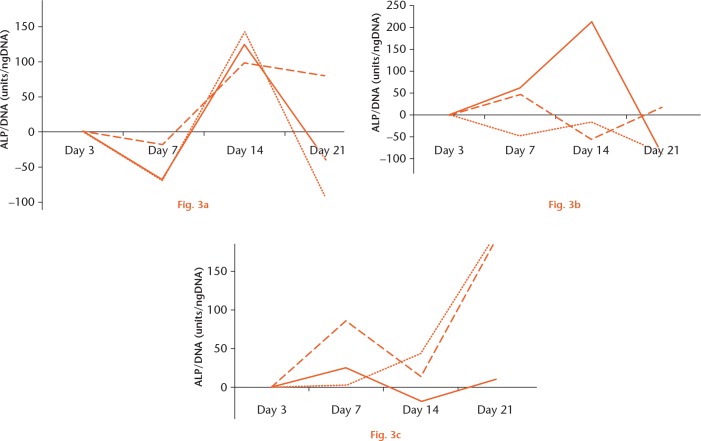
When MSCs from young, adult, and OVX rats were differentiated to osteoblasts, there was a large variability in the ALP expression from day three to day 21 between the three groups of cells. In the young MSCs, the percentage change in ALP was seen to decrease from day three to day seven and increase thereafter up to day 14, after which it dropped significantly at day 21. However, this pattern was not visible for the adult control or OVX MSCs. The OVX MSCs had a peak increase in ALP expression at day seven and a drop at day 14. There was a significant difference in ALP change between young and OVX MSCs at day 14 (p = 0.003). However, due to the large variability in the ALP in adult MSCs, this significance was not observed between young and adult MSCs, or between adult and OVX MSCs (p = 0.21). It is worth noting that two of the adult MSC cultures at day 14 showed very low expression, whereas in one adult culture, the ALP expression was very high. Interestingly, the ALP expression in the young stem cells cultures was less variable over all of the time periods studied. There was a percentage increase in ALP expression in OVX MSCs at day seven (mean 37.5% (sd 3.5), and this was significantly more than in young MSCs (p = 0.02). However, this surge in ALP was still not as high as that expressed by young MSCs at day 14 (mean 123% (sd 21.9) (Fig. 3).
Graphs showing the percentage change in alkaline phosphatase (ALP)/DNA for a) young, b) adult, and c) ovariectomized (OVX) mesenchymal stem cells (MSCs) differentiated to osteoblasts at days three, seven, 14, and 21. Each line represents the results from a single culture. There were three replicates from each group of MSCs isolated from young, adult, and OVX rats.
Adipogenic differentiation
At days 14 and 21, adipocytic differentiation was significantly greater in MSCs isolated from young animals compared with adult control and OVX groups. MSCs from young rats formed lipid droplets significantly faster from day seven compared with the other two groups of cells. Additionally, lipid droplet accumulation significantly accelerated from day 14 to day 21 for young MSCs, while MSCs from adult and OVX rats showed no increase in lipid accumulation at days seven, 14, and, 21 (Fig. 4).
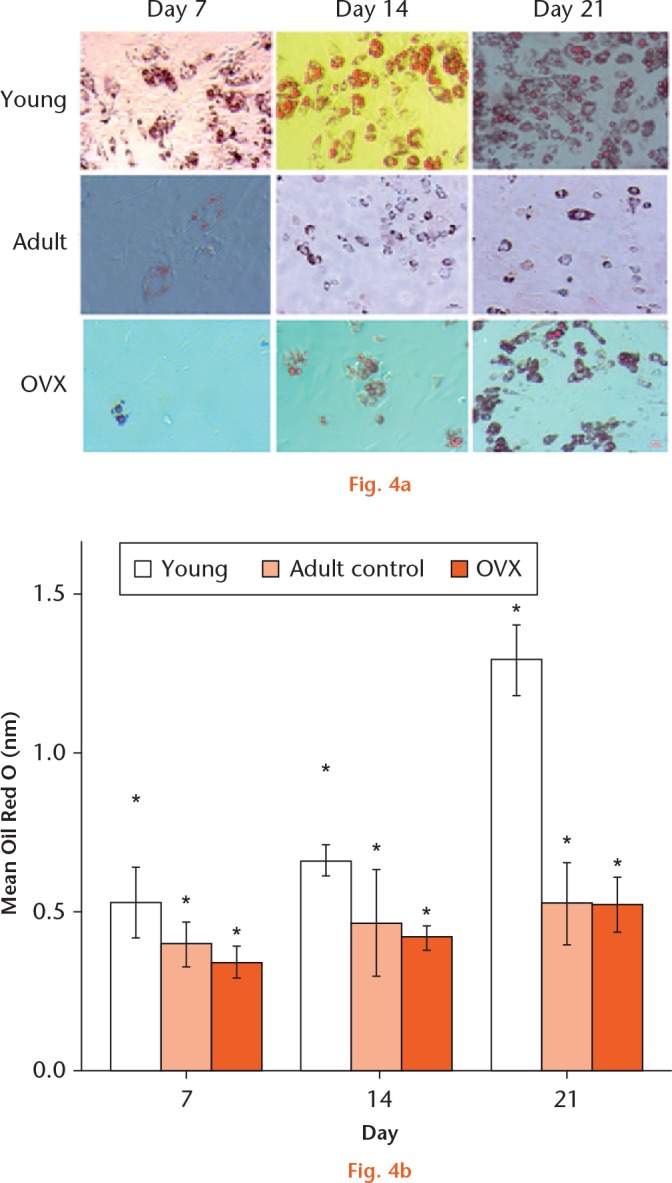
a) Oil Red O staining to show adipogenic differentiation of young, adult, and ovariectomized (OVX) mesenchymal stem cells (MSCs) at day seven, 14, and 21. b) Graph showing the mean Oil Red O production when MSCs from young, adult control, and OVX rats (n = 3) differentiated to adipocytes at days seven, 14, and 21. *Significant difference of p < 0.05 using a two-tailed Student’s t-test.
CXCR-4 expression
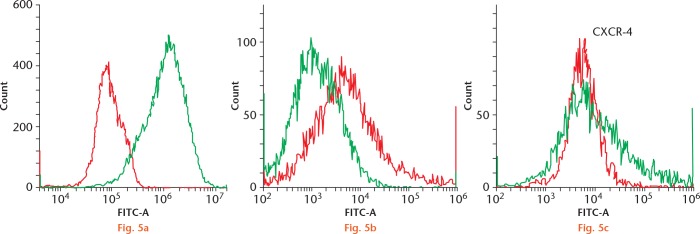
Flow cytometry results demonstrated that rBMCs from adult (p < 0.001) and OVX rats (p < 0.001) expressed significantly lower levels of CXCR-4 compared with rBMCs from young rats (Fig. 5). The rBMCs from adult rats expressed significantly greater CXCR-4 in comparison with the OVX rats (p = 0.04). A mean of 32.1% (sd 6.2) and 19.4% (sd 9.8) of the rBMCS from adult and OVX rats expressed CXCR-4, respectively, while 87.5% (sd 5.1) of the young rBMCs expressed CXCR-4.
Flow cytometry showing C-X-C chemokine receptor type 4 (CXCR-4) surface expression in mesenchymal stem cells (MSCs) labelled with anti-CXCR-4 antibody, from a) young, b) adult control, and c) ovariectomized (OVX) rats. Young MSCs have the highest expression of CXCR-4 (87%), followed by MSCs from adult control (32%); MSCs from OVX rats (19%) had the lowest expression of CXCR-4 (n = 3). In a), the red histogram shows the secondary background control and the green histogram shows the CXCR-4 expression in young MSCs. In figures b) and c), the green histogram demonstrates the background expression and the red histogram shows the CXCR-4 expression. FITC, fluorescein isothiocyanate.
Cell migration
The migration of SDF-1 doubled in the young cell group compared with adult rat cells (p = 0.023) and it was four times higher when compared with cells isolated from OVX rats (p = 0.013) (Fig. 6).
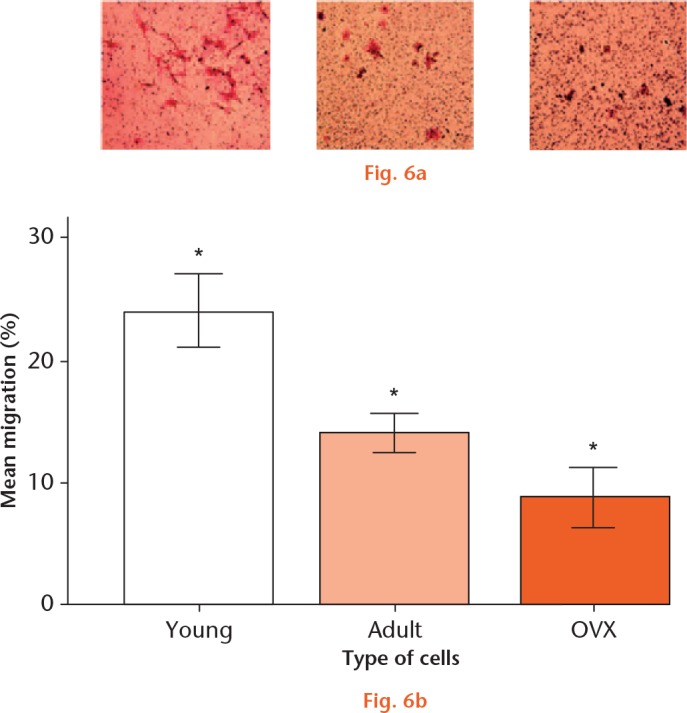
Images of a) young, b) adult control, and c) ovariectomized (OVX) mesenchymal stem cells (MSCs) migrated towards stromal cell-derived factor 1 (SDF-1) in a Boyden chamber and stained with crystal violet. d) Graph showing the mean percentage migration of uninfected MSCs from young, adult control, and OVX rats in a transwell chamber towards SDF-1. *Significant difference of p < 0.05 using a two-tailed Student’s t-test.
Discussion
Impaired MSC recruitment from the stem cell niche, reduced differentiation to bone, and decreased proliferative activity of the mature stem cells are all probable causes of reduced bone formation in age-related disease.21 Our data has highlighted that during ageing and osteoporosis, the proliferative potential of the stem cells is maintained but their differentiation ability is compromised. Many studies have looked at the differences in stem cells from old and young animals; however, the novelty in this study includes comparing the stem cell function between young, adult control, and OVX rats. We have demonstrated differences between MSCs isolated from young, adult, and OVX rats; although all cells positively expressed CD90 and CD29 and negatively expressed CD45 and CD34, they showed differences in osteogenic and adipogenic differentiation potential. We also showed that the expression of CXCR-4 and migration of cells towards SDF-1 was much greater with cells derived from young rats, while cells isolated from OVX rats showed a reduction in migration compared with adult cells.
The capability of cells to differentiate to bone and their use in cell-based therapy is vital in many orthopaedic interventions, particularly in the ageing population. There is contradictory data on the differentiation potential of MSCs from young and aged rats. With identifiable differences in gene expression,22,23 we had hypothesized that MSCs from OVX rats would have lower osteogenic potential and would more likely differentiate to adipocytes compared with MSCs from young rats; however, this was not the case. MSCs from OVX rats had lower osteogenic differentiation potential, but they also had a lower adipogenic differentiation ability compared with MSCs from young rats. Asumda and Chase24 did not look at the differentiation potential of MSCs from osteopenic rats, but they showed similar differences to this study in terms of osteogenic and adipogenic differentiation between MSCs from young and old rats. They demonstrated a reduced differentiation ability of MSCs from old rats compared with MSCs from young rats.24 However, Singh et al25 found no observable difference in osteogenic and adipogenic differentiation between cells from young and old mice. Likewise, Beane et al26 showed no differences in ALP expression, as well as alizarin red staining, between bone marrow MSCs from young and old rabbits, but they found that age affected the adipogenic differentiation of the same cells. Moerman27 reported changes in the expression of phenotype specific gene markers in bone marrow MSCs from older mice that encouraged them to differentiate to fat rather than bone in the bone marrow niche. Bone marrow from old mice had increased levels of peroxisome proliferator-activated receptor-gamme (PPAR-γ), which stimulated them to turn to fat rather than bone and inhibited osteoblast function. The bone marrow from the young mice, on the other hand, had increased levels of BMP-2 and TGF-β, which induced MSCs from these mice towards the osteoblastic lineage. Our results were contrary to the main body of literature. For the reasons expressed above, previous studies have found increased adipogenic potential of cells gained from aged and/or osteopenic rats. Conversely, we found that the younger cells showed the greatest differentiating capacity of both the osteogenic and adipogenic lineage compared with the other cell groups. Such results demonstrate the importance of cell origin on differentiating capacity in addition to the cytokine profile in their environment. A further point of interest would be the comparison of ovariectomy in younger rats, comparing characteristics with age-matched animals. This would remove the impact of ageing, and would enable one to look solely at the effect of osteopenia on cell activity.
In the present study, there was no difference in proliferation between the different types of MSCs. This contrasts with other studies. Several studies have shown that MSCs from older rats have significantly lower proliferation compared with MSCs from young rats.26,28 Beane et al26 looked at MSCs from young and old rabbits from the bone marrow, muscle, and fat; they demonstrated a relative reduction in proliferation with age of MSCs from the bone marrow, but not from the other two sources. Georgen29 found that MSCs from OVX rats had a lower proliferation rate than their control counterparts and therefore concluded that the low proliferation rate would correlate with reduced self-renewal capacity, which might cause a gradual depletion of MSC sources in the bone marrow of OVX animals.
This study showed that MSCs from OVX rats have a lower in vitro migration and CXCR-4 expression compared with MSCs from young rats and control adult rats. This is in keeping with our previously published work on CXCR-4 transfection.30 A study limitation may be in the method of flow cytometery for CXCR-4 analysis; if cells were not fully saturated with the primary antibody, levels of expression would not be representative. SDF-1 is a chemokine receptor for CXCR-4 and the SDF-1/CXCR-4 biological axis plays an important role in the migration of stem cells and the wound repair of tissues and organs.31-35 The impaired migration capacity of MSCs from four months post-ovariectomy rats could be due to their low expression of CXCR-4; this could explain the impaired bone formation in osteoporotic patients, as cells from these patients have a reduced capacity to migrate to the site of bone loss. SDF-1 is produced in the periosteum of injured bone and encourages endochondral bone repair by recruiting mesenchymal stem cells to the site of injury. Therefore, mobilization of osteoblastic progenitors to the bone surface is an important step in osteoblast maturation and formation of mineralized tissue.36
This study supports the notion that, although stem cells remain active with age, their differentiation ability is affected, therefore impairing their regenerative and differentiation capacity. The contradictory results from various studies could be because of variability of patients, osteoporotic models, and sources of stem cells. Further animal studies are necessary to clarify whether the in vivo bioactivity of MSCs from young patients is good enough for bone regeneration in osteoporotic patients, and whether using cells from younger hosts could be an option for cellular and genetic therapies for bone degenerative diseases. There are other factors to consider as well in such stem cell therapies; for example, would an allogenic stem cell source from young patients be compatible in osteoporotic patients? Although we have shown that stem cells from OVX rats are similar in their proliferation and expression of CD markers to their younger counterparts, their inability to migrate to the site of bone loss, as well as their reduced capacity to differentiate, renders them incompetent to form bone. In addition, the rat OVX model is an osteopenic model and does not fully represent human osteoporosis. This study could therefore be further validated by using MSCs from human osteoporotic, age-matched non-osteoporotic, and young patients.
Footnotes
Author Contributions: A. Sanghani-Kerai: Cell culture works, Preparing the manuscript.
L. Osagie-Clouard: Cell culture works, Reviewing the manuscript.
G. Blunn: Conception of the study, Reviewing the manuscript.
M. Coathup: Conception of the study, Reviewing the manuscript.
Statement of Animal rights: All applicable institutional and/or national guidelines for the care and use of animals were followed.
Conflict of Interest Statement: None declared
Follow us @BoneJointRes
Funding Statement
This study was supported by the John Scales Endowment Fund and Orthopaedic Research UK.
References
- 1. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005;115:3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Randell A, Sambrook PN, Nguyen TV, et al. Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos Int 1995;5:427-432. [DOI] [PubMed] [Google Scholar]
- 3. Sambrook P, Cooper C. Osteoporosis. Lancet 2006;367:2010-2018. [DOI] [PubMed] [Google Scholar]
- 4. Bonyadi M, Waldman SD, Liu D, et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci USA 2003;100:5840-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HT, Lee MJ, Chen CH, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med 2012;16:582-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandhu SK, Hampson G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J Clin Pathol 2011;64:1042-1050. [DOI] [PubMed] [Google Scholar]
- 7. Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 2006;194(suppl):S3-S11. [DOI] [PubMed] [Google Scholar]
- 8. Lippuner K. The future of osteoporosis treatment - a research update. Swiss Med Wkly 2012;142:w13624. [DOI] [PubMed] [Google Scholar]
- 9. Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 2007;22:1943-1956. [DOI] [PubMed] [Google Scholar]
- 10. Katsara O, Mahaira LG, Iliopoulou EG, et al. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev 2011;20:1549-1561. [DOI] [PubMed] [Google Scholar]
- 11. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91-116. [DOI] [PubMed] [Google Scholar]
- 12. Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 2007;3:226-237. [DOI] [PubMed] [Google Scholar]
- 13. Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 2001;19:117-125. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Goh J, Das De S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng 2006;12:1753-1761. [DOI] [PubMed] [Google Scholar]
- 15. Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009;27:1887-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quinn R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005;21:775-777. [DOI] [PubMed] [Google Scholar]
- 17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:4:315-317. [DOI] [PubMed] [Google Scholar]
- 18. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012;9:671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moe D, Garbarsch C, Kirkeby S. The protein effect on determination of DNA with Hoechst 33258. J Biochem Biophys Methods 1994;28:263-276. [DOI] [PubMed] [Google Scholar]
- 20. Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J Biol Chem 1995;270:9420-9428. [DOI] [PubMed] [Google Scholar]
- 21. Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep 2013;2:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li JJ, Wang BQ, Fei Q, Yang Y, Li D. Identification of candidate genes in osteoporosis by integrated microarray analysis. Bone Joint Res 2016;5:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia B, Li Y, Zhou J, Tian B, Feng L. Identification of potential pathogenic genes associated with osteoporosis. Bone Joint Res 2017;6:640-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asumda FZ, Chase PB. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol 2011;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh L, Brennan TA, Russell E, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 2016;85:29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One 2014;9:e115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004;3:379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kretlow JD, Jin YQ, Liu W, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 2008;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goergen J, Wenisch S, Raabe O, et al. Characterization of bone-marrow-derived stem cells in osteoporotic models of the rat. ISRN Stem Cells, 2013. [Google Scholar]
- 30. Sanghani-Kerai A, Coathup M, Samazideh S, et al. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4. Bone Joint Res 2017;6:358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molyneaux KA, Zinszner H, Kunwar PS, et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003;130:4279-4286. [DOI] [PubMed] [Google Scholar]
- 32. Toupadakis CA, Wong A, Genetos DC, et al. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J Orthop Res 2012;30:1853-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shao H, Xu Q, Wu Q, et al. Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med 2011;15:2046-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lien CY, Chih-Yuan Ho K, Lee OK, Blunn GW, Su Y. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res 2009;24:837-848. [DOI] [PubMed] [Google Scholar]
- 35. Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643-2645. [DOI] [PubMed] [Google Scholar]
- 36. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009;60:813-823. [DOI] [PubMed] [Google Scholar]